Abstract
Switches between different behavioral states of the animal are associated with prominent changes in global brain activity, between sleep and wakefulness or from inattentive to vigilant states. What mechanisms control brain states, and what are the functions of the different states? Here we summarize current understanding of the key neural circuits involved in regulating brain states, with a particular emphasis on the subcortical neuromodulatory systems. At the functional level, arousal and attention can greatly enhance sensory processing, whereas sleep and quiet wakefulness may facilitate learning and memory. Several new techniques developed over the past decade promise great advances in our understanding of the neural control and function of different brain states.
Introduction
In our complex and changing environment, animals constantly switch between different behavioral states. The most conspicuous changes occur at the sleep-wake transitions, and effective neural control of these transitions is critical for the fitness and survival of the animal (Mahowald and Schenck, 2005). Sleep can be further divided into two distinct types: rapid eye movement (REM) sleep with vivid dreams and non-REM (NREM) sleep with dull or lack of sensation (Hobson, 2005). During wakefulness, animals must also dynamically adjust their behavioral states, switching rapidly from quiet, inattentive to aroused, vigilant states upon task demand.
These switches of behavioral states are accompanied by obvious changes in the global pattern of neural activity in many brain areas, which can be measured electrophysiologically (Gervasoni et al., 2004). In 1924, the German psychiatrist Hans Berger first measured the voltage difference between two electrodes placed on the scalp of a human subject (Berger, 1929), which later became known as the electroencephalogram (EEG). He found that the pattern of EEG changes dramatically with the behavioral state of the subject. When the subject is awake, the EEG is fast and low-voltage, and as the subject falls asleep the EEG changes progressively into high-voltage slow patterns. We now know that the high-amplitude slow EEG activity reflects the synchronous alternation between firing and inactivity of a large population of neurons (Steriade et al., 1993a), thus the corresponding brain states are referred to as ‘synchronized states’. The desynchronized states (with low-voltage fast EEG) are often referred to as the ‘activated states’ because of their association with behavioral activation.
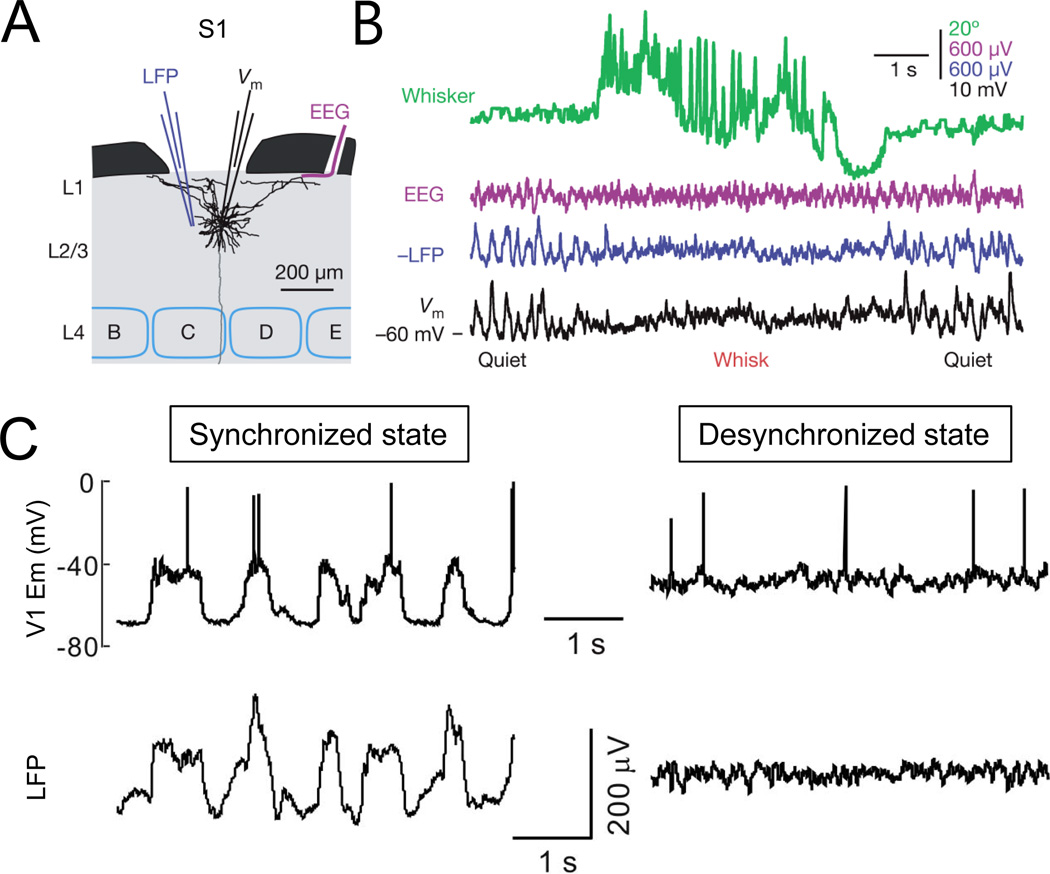
Another commonly used measure of population neural activity is the local field potential (LFP), the low-frequency (<200 Hz) voltage fluctuations recorded by inserting the electrodes into brain tissues. The LFP mainly reflects the excitatory and inhibitory synaptic processes, and compared to EEG it measures activity from a more local brain area (Kajikawa and Schroeder, 2011; Katzner et al., 2009; Xing et al., 2009). Network activity can also be inferred from intracellular recordings, since membrane potential fluctuations in individual cells are strongly correlated with the network activity (Crochet and Petersen, 2006; Li et al., 2009; Okun et al., 2010; Poulet and Petersen, 2008; Steriade et al., 1993b) (Fig. 1). For example, during NREM sleep and under certain anesthesia the EEG and LFP show pronounced slow oscillations (<1 Hz). In individual cells these oscillations manifest as alternating UP and DOWN states of the membrane potential (Steriade et al., 2001), with the UP state characterized by a barrage of excitatory and inhibitory synaptic inputs, and the DOWN state with deep hyperpolarization and little synaptic activity (Fig. 1C).
Figure 1. Different methods for monitoring brain states.
A, Schematic showing the recording configuration for simultaneous measurement of EEG, LFP, and single-cell membrane potential in the S1 barrel cortex. A pyramidal neuron in layer 2/3 was reconstructed. B, EEG, LFP, and whole-cell recordings show large-amplitude, low-frequency activity during quiet wakefulness and synchronous state change during whisking (Figures adapted and reproduced with permission from Poulet and Petersen, 2008). C, Synchronized (left) and desynchronized (right) brain states observed with simultaneous whole-cell patch clamp recording from a visual cortical neuron and LFP recording 2 mm from the patch electrode. Figures reproduced from Li et al., 2009).
There are two fundamental questions concerning brain states: what mechanisms control brain states and what is the function of each state. Lesion studies have identified multiple brain regions important for regulating brain states, including those in the brainstem, hypothalamus, and the basal forebrain/preoptic area, but the specific role of each region and the underlying synaptic circuits are not yet well understood. The striking state-dependent changes of ensemble neuronal activity observed in many brain areas suggest that different brain states are associated with distinct functions, but definitive evidence for some of these functions is still lacking. In this review, we summarize our current understanding of these issues and propose future studies using newly developed techniques.
Neural control of wakefulness and sleep
Wakefulness and sleep can be well distinguished by measuring both EEG and electromyogram (EMG). During wakefulness, the EEG is generally desynchronized, and the EMG indicates high muscle tone. During NREM sleep, the skeletal muscle EMG activity is reduced, and the EEG is dominated by slow (<1 Hz) and delta (1 – 4 Hz) oscillations. Interestingly, during REM sleep the EEG shows a desynchronized pattern that is similar to the awake state. However, the EMG indicates an almost complete loss of muscle tone, thus allowing a clear-cut distinction from the awake state.
Identification of the brain areas controlling sleep and wakefulness began with the work of Constantin von Economo, a Romanian neurologist who studied patients with encephalitis. He found that lesions in the brainstem and posterior hypothalamus cause excessive sleepiness (Von Economo’s sleepy sickness), whereas lesions of the anterior hypothalamus and basal forebrain cause the opposite symptom of insomnia (Von Economo, 1930). Subsequent work by Moruzzi and Magoun showed that the ascending reticular activating system originating in the brainstem is crucial for wakefulness and arousal (Moruzzi and Magoun, 1949). More recent studies have further identified the various cell groups in the brainstem, hypothalamus, and basal forebrain that contribute to sleep-wake regulation (Fig. 2).
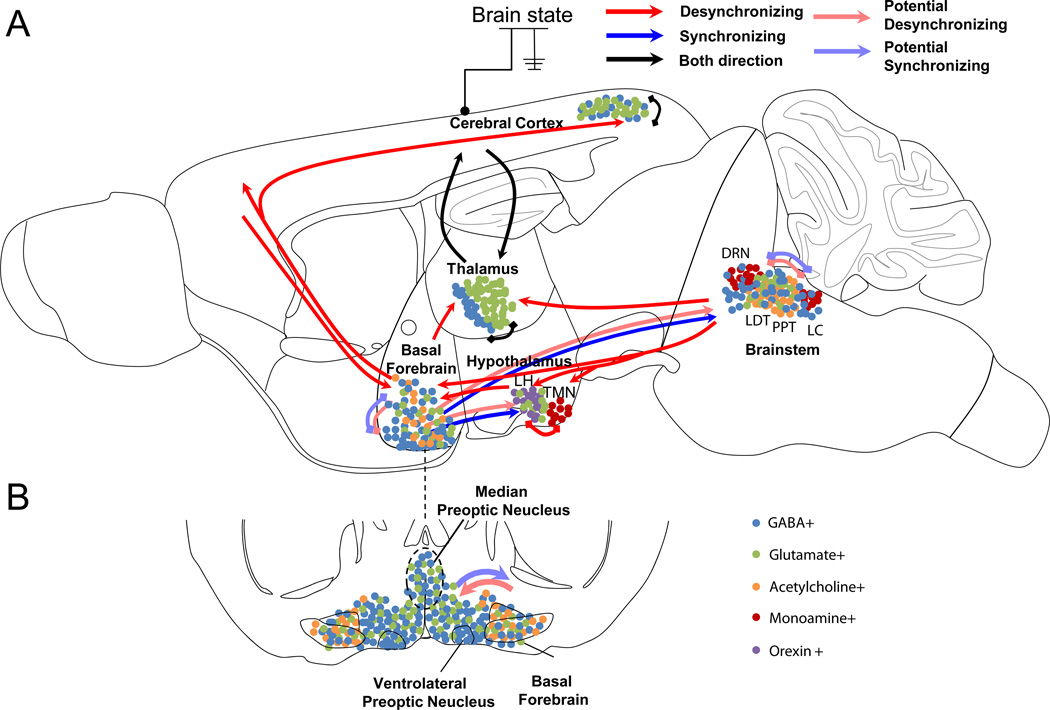
Figure 2. Schematic diagram showing the key circuits involved in regulating brain states.
A, Sagital view. Arrows indicate major pathways connecting the brain areas. Red arrow, pathway inducing cortical desynchronization; blue arrow, pathway inducing cortical synchronization, black arrow; pathway that mediate both synchronization and desynchronization; light red, possible pathway for desynchronization; light blue, possible pathway for synchronization. Each cell types were schematically illustrated by colored dots in each brain area. B, Coronal view of the basal forebrain/preoptic area, at the position indicated by dashed line in A. MnPO is in fact more anterior but outlined here for convenience.
Brainstem and posterior hypothalamus
The brainstem is a key region that regulates both the brain state and muscle tone. In humans and other animals, large damage in the brainstem can cause coma, a prolonged state of unconsciousness and unresponsiveness. From the brainstem, two pathways are critical for maintaining wakefulness: the ascending reticular activating system projecting to the thalamus, hypothalamus, basal forebrain, and neocortex is important for cortical activation, and the descending pathway to the spinal cord is important for maintaining muscle tone (Holstege and Kuypers, 1987; Jones and Yang, 1985). Activity of the two pathways must be coordinated to ensure that voluntary movement is enabled when (and only when) the brain is awake.
The ascending activating system consists of several nuclei in the brainstem and posterior hypothalamus. They contain essential neuromodulators, including acetylcholine (ACh) and several monoamines - norepinephrine (or noradrenaline) from the locus coeruleus (LC), serotonin (5-HT) mostly from the dorsal raphe nucleus (DRN), and histamine from the tuberomammillary nucleus (TMN) (Fig. 2). The LC consists of a very small number of noradrenergic neurons (~1500 in rat), but it projects widely to almost the entire central nervous system (Berridge, 2008; Sara, 2009). Optogenetic stimulation of the noradrenergic neurons can cause an immediate transition from sleep to wakefulness (Carter et al., 2010). Although earlier studies suggested that the effect of LC stimulation on cortical activation is indirect (Dringenberg and Vanderwolf, 1998), probably through its projection to the basal forebrain cholinergic circuit, a recent study showed that pharmacological blockage of noradrenergic signaling within the cortex prevents the desynchronization when the animal is awake (Constantinople and Bruno, 2011), indicating that the intracortical release of noradrenaline is important for the desynchronization. The histaminergic neurons located in the TMN in the posterior hypothalamus show similar projection patterns (Thakkar, 2011). Antihistamine drugs promote sleep, and lesion of the histamine neurons or blockade of histamine synthesis induces hypersomnia (Monti, 1993). The serotonergic neurons in the DRN also project widely to the cortex and subcortical areas. Application of agonists to a variety of 5-HT receptors enhances wakefulness, whereas antagonist application increases sleep (Dugovic et al., 1989; Monti et al., 2008). Genetic knock-out of the 5-HT1B receptors also changes the ratio between REM and NREM sleep (Boutrel et al., 1999). Interestingly, all of these monoaminergic neurons fire at high rates during wakefulness, low rates during NREM sleep, and they virtually stop firing during REM sleep (Aston-Jones and Bloom, 1981; Jacobs and Fornal, 1991; Kocsis et al., 2006; Steininger et al., 1999; Takahashi et al., 2010; Takahashi et al., 2006). Thus, these neurons appear to serve similar functions in promoting cortical desynchronization and behavioral arousal (Jones, 2003).
The cholinergic neurons in the brainstem are clustered in the pedunculopontine tegmental (PPT) and lateral dorsal tegmental (LDT) nuclei, and they project extensively to the thalamus, hypothalamus, and basal forebrain (Hallanger et al., 1987; Jones and Cuello, 1989; Steriade et al., 1988). These neurons fire at high rates during wakefulness. However, unlike the monoaminergic neurons, which cease firing during REM sleep, the cholinergic neurons are also highly active during REM sleep (Maloney et al., 1999; McCarley and Hobson, 1975; Steriade et al., 1990). Since both wakefulness and REM sleep are associated with desynchronized EEG, activity of these cholinergic neurons appears to be linked to cortical activation but not necessarily behavioral arousal. Lesion of the cholinergic neurons in the PPT nucleus significantly reduces REM sleep (Shouse and Siegel, 1992), suggesting their causal role in promoting REM sleep.
While the studies summarized above indicate that both the cholinergic and monoaminergic systems are important for the overall brain activation, their specific impacts on each target area may differ substantially (Edeline, 2012; Hirata et al., 2006). Much remains to be learned about how each neuromodulator affects circuit functions by activating its multiple receptors that are differentially expressed among neuronal subtypes (Bacci et al., 2005; Lee et al., 2010). Furthermore, although the functions of the cholinergic and monoaminergic neurons have been studied extensively by manipulating their outputs, the inputs controlling the activity of these neurons remain poorly understood. For example, in the PPT and LDT nuclei, there are GABAergic and glutamatergic neurons intermingled with the cholinergic neurons (Ford et al., 1995) (Fig. 2). These cell types exhibit complex activity patterns during different brain states (Boucetta and Jones, 2009), but whether and how they modulate the activity of cholinergic neurons are unclear. Microinjection of GABA receptor agonist in the PPT increases REM sleep and decreases wakefulness (Pal and Mallick, 2009; Torterolo et al., 2002), but which neurons mediate these effects is unknown. In addition to the local synaptic interactions, each nucleus also receives long-range inputs from numerous brain regions. Delineating both the local and long-range synaptic inputs to the modulatory neurons will be essential for understanding the neural control of sleep and wake states.
Lateral hypothalamus and basal forebrain/preoptic area
Several forebrain regions are also important for regulating brain states: the lateral hypothalamus containing orexin/hypocretin neurons and the basal forebrain containing cholinergic neurons. These areas also contain many local and long-range projecting GABAergic, glutamatergic, and neuropeptidergic neurons.
Activity of the orexin neurons is high during active waking and low during sleep (Lee et al., 2005b; Mileykovskiy et al., 2005). Optogenetic activation of orexin neurons induces wakefulness (Adamantidis et al., 2007), whereas loss of orexin, orexin receptors, or orexin neurons causes narcolepsy, a sleep disorder characterized by excessive sleepiness and sudden sleep attacks (Chemelli et al., 1999; Hara et al., 2001; Lin et al., 1999; Peyron et al., 2000). Orexin neurons innervate the cortex, basal forebrain, and brainstem, where they provide a strong excitatory input to the ascending arousal system (Sutcliffe and de Lecea, 2002). Glutamatergic neurons within the hypothalamus are also known to be activated by orexin, and they in turn activate the orexin neurons to orchestrate the hypothalamic arousal system (Li et al., 2002).
In the basal forebrain, one of the main cell types is cholinergic (Zaborszky et al., 1999) (Fig. 2). In fact the basal forebrain is the primary source of cholinergic input to the cortex. Similar to the cholinergic neurons in the brainstem, these basal forebrain neurons are also active during both waking and REM sleep but not during NREM sleep (Lee et al., 2005a). Lesion of the basal forebrain can cause a dramatic increase of EEG delta waves during wakefulness (Berntson et al., 2002; Buzsaki et al., 1988; Fuller et al., 2011), and combined cholinergic and serotonergic blockade completely prevents cortical desynchronization (Vanderwolf and Pappas, 1980). Conversely, stimulation of the basal forebrain induces cortical desynchronization, which can be observed from both the reduced EEG/LFP power at low frequencies (Metherate et al., 1992) and the decrease in correlated spiking among cortical neurons (Goard and Dan, 2009) (Fig. 3). At the cellular level, the desynchronization is known to depend on the muscarinic ACh receptors (mAChRs) in the cortex, and a modeling study (Bazhenov et al., 2002) suggests that this may be mediated by both the suppression of excitatory intracortical connections (Gil et al., 1997; Hsieh et al., 2000; Kimura et al., 1999) and the depolarization of cortical pyramidal neurons (McCormick and Prince, 1985; Nishikawa et al., 1994).
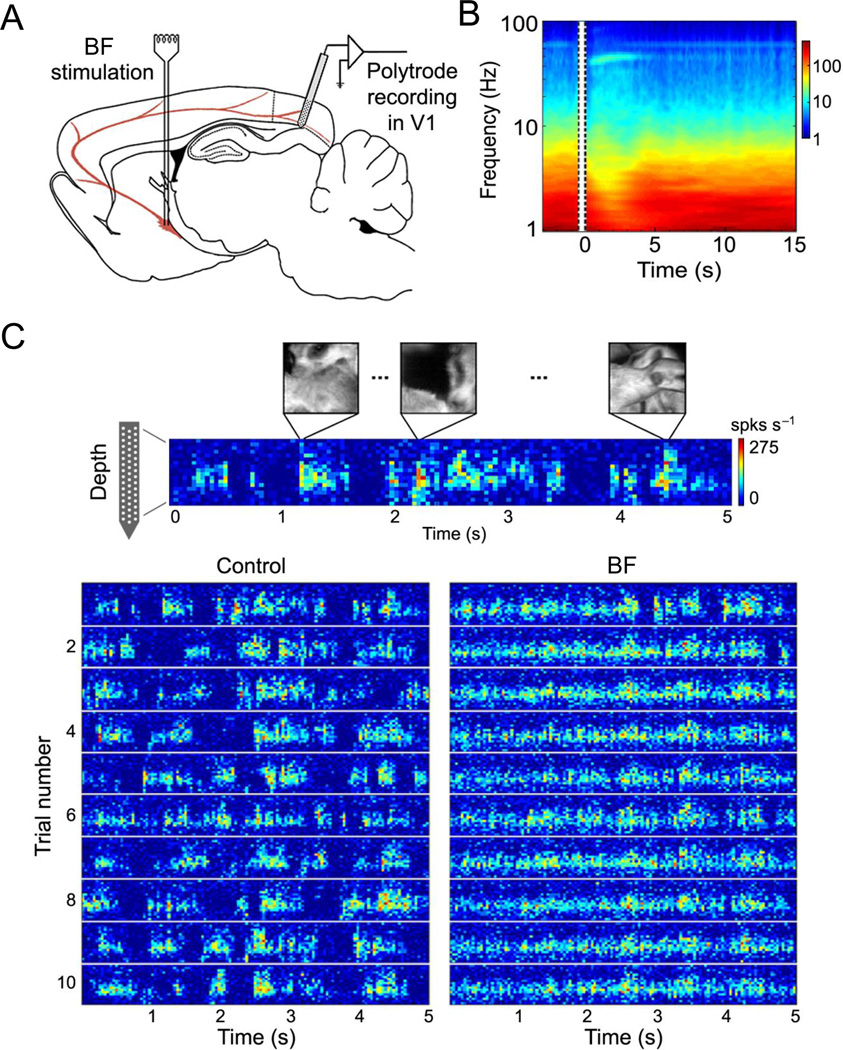
Figure 3. Effect of basal forebrain stimulation on multiunit activity in the visual cortex.
A, Schematic illustration of experimental setup. B, Time-frequency analysis of LFP before and after basal forebrain stimulation from an example experiment, averaged over 30 trials. Amplitude is color coded. Vertical lines indicate the period of basal forebrain stimulation. C, Multiunit spike rate (color coded) in response to the natural movie stimulation recorded by a multichannel silicon probe plotted against cortical depth. Bottom panel shows the responses to 10 trials of visual stimuli before (control) and 0–5 s after basal forebrain (BF) stimulation. Basal forebrain stimulation decreased correlation between cortical neurons and increased response reliability during visual stimulation (adapted and reproduced from Goard and Dan, 2009).
Intermingled with the cholinergic neurons are a large number of GABAergic neurons (up to 60% of all neurons in the basal forebrain/preoptic area). These neurons are likely to play diverse roles in brain state regulation, as some of them are active during wakefulness while others are sleep active (Manns et al., 2000; Szymusiak and McGinty, 1989). Several studies have identified groups of GABAergic neurons in the ventrolateral preoptic area (VLPO) and median preoptic nucleus (MnPO) as sleep-promoting cells (Saper et al., 2005; Sherin et al., 1996; Szymusiak and McGinty, 2008) (Fig. 2B). These neurons are more active during sleep than wakefulness, and lesion of the VLPO causes insomnia (Lu et al., 2000). The projections from VLPO and MnPO to the ascending arousal system and lateral hypothalamus allow them to effectively shut down the activity of the wake-promoting neurons (Sherin et al., 1998; Suntsova et al., 2007), and neuromodulators from the ascending arousal system can in turn inhibit these sleep-active neurons (Gallopin et al., 2000; Manns et al., 2003). This has led to the proposal of an elegant flip-flop circuit for sleep-wake switches based on the mutual inhibition between the VLPO and ascending arousal system (Saper et al., 2010).
Outside of the VLPO and MnPO, however, wake- and sleep-active GABAergic neurons seem largely intermingled in the basal forebrain/preoptic area (Manns et al., 2000; Takahashi et al., 2009). Although several studies in the rat showed that basal forebrain lesion causes behavioral unresponsiveness and EEG synchronization (Berntson et al., 2002; Buzsaki et al., 1988; Fuller et al., 2011), in the cat the lesion was found to induce severe insomnia (Szymusiak and McGinty, 1986). These mixed results may be related to the functional diversity of the basal forebrain neurons. In terms of their outputs, a subset of the GABAergic neurons innervate the cortex along with the cholinergic neurons (Henny and Jones, 2008; Sarter and Bruno, 2002), and others project to the hypothalamus and brainstem (Gritti et al., 1994). Electrophysiological and electron microscopic studies have also shown extensive local synaptic interactions among basal forebrain neurons (Momiyama and Zaborszky, 2006; Zaborszky and Duque, 2003). This raises the possibility that in addition to the long-range connections between the VLPO and ascending arousal system, sleep-wake switches also depend on the local reciprocal inhibition between the sleep- and wake-active GABAergic neurons and between GABAergic and cholinergic neurons within the basal forebrain/preoptic area (Fig. 2B, light red and blue arrows). Furthermore, the wake-active neurons in this region may also project to the brainstem and hypothalamus, innervating the cholinergic, monoaminergic, as well as glutamatergic or GABAergic neurons (Fig. 2A, light red arrows). Thus, the flip-flop circuit for sleep-wake switches may involve multiple loops of excitation and inhibition.
Given the large number of GABAergic neurons in the basal forebrain/preoptic area and the importance of GABAergic transmission in sleep-wake regulation (Monti et al., 2010), delineating the functional organization of these neurons may be a key step towards understanding the subcortical circuits controlling brain states. Studies in the neocortex and hippocampus have shown that GABAergic neurons with distinct molecular markers exhibit different physiological properties and innervation patterns (Ascoli et al., 2008; Fishell and Rudy, 2011), and they play different roles in sensory processing (Lee et al., 2012; Wilson et al., 2012). In the basal forebrain, juxtacellular recordings from a small number of immunocytochemically identified neurons suggest that cells with different sleep-wake activity patterns may also express distinct molecular markers (Duque et al., 2000). Since a large number of Cre driver mouse lines targeting different subtypes of GABAergic neurons have now become available (Taniguchi et al., 2011), a promising approach is to make targeted recording from each cell type to determine their sleep-wake activity patterns. Optogenetic manipulation of their activity in a bi-directional manner (Chow et al., 2010; Deisseroth, 2011), which has been achieved in various neuronal circuits, can further establish the causal role of these neurons in brain-state regulation (Fig. 4). Moreover, recent advances in viral tracing techniques (Wickersham et al., 2007) may greatly facilitate the dissection of synaptic connectivity among the various neuronal subtypes.
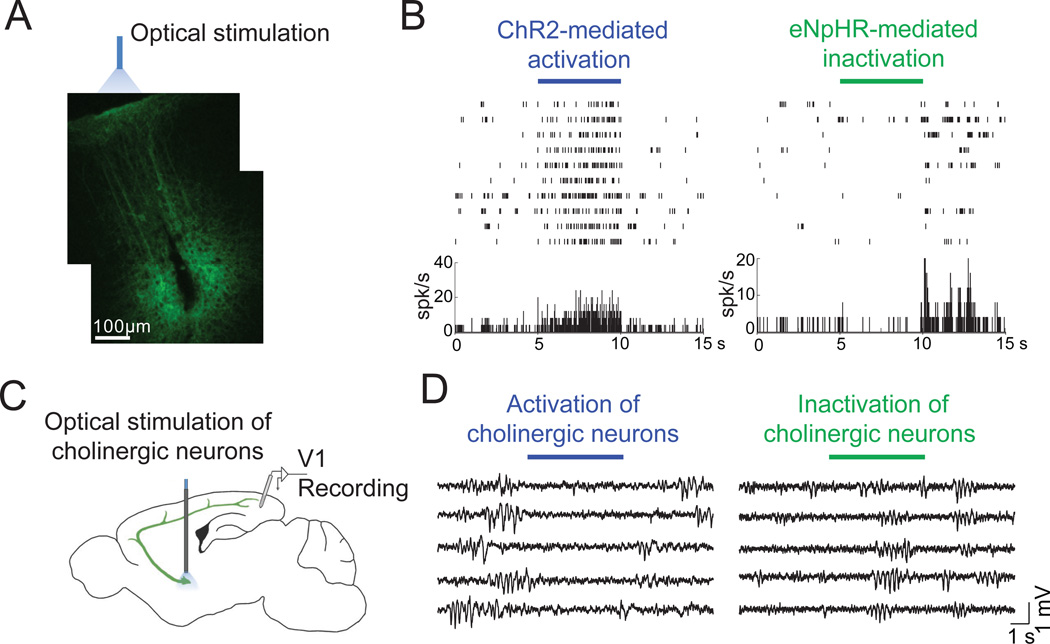
Figure 4. Optogenetic manipulation of neuronal activity in vivo.
A, Channelrhodopsin-2 (ChR2) was expressed in cortical neurons. Optical stimulation was applied to the area of ChR2 expression and recording site (marked by lesion caused by electrode) (Figures reproduced from Lee et al., 2012 and unpublished data from Lee S.H.). B, Bi-directional modulation of neuronal activity by optical stimulation of ChR2 or halorhodopsin (eNpHR) in the same neuron in primary visual cortex expressing both ChR2 and eNpHR. C, Schematic showing simultaneous LFP recording in the visual cortex and optogenetic manipulation of cholinergic neurons in the basal forebrain. D, Activation of cholinergic neurons (left) causes cortical desynchronization while inactivation (right) caused more synchronized activity (unpublished data from Pinto L.).
Thalamus and cortex
The thalamus is the gateway of sensory inputs to the cortex, and it receives massive cortical feedback. The thalamocortical loop, composed of the highly interconnected thalamocortical, thalamic reticular, and cortical neurons, plays a pivotal role in setting the global brain state and controlling the flow of sensory information (Castro-Alamancos, 2004b; Sherman, 2005). The thalamus also receives strong inputs from the ascending activating system and basal forebrain (Bickford et al., 1994; Levey et al., 1987; Manning et al., 1996), and it serves as a major pathway through which the neuromodulatory inputs regulate cortical function.
The thalamic neurons exhibit distinct modes of firing in different brain states, with tonic spiking during alertness and rhythmic bursting during NREM sleep or drowsiness (Bezdudnaya et al., 2006; McCormick and Bal, 1997; Sherman, 2005; Stoelzel et al., 2009). Thalamic activity can directly influence cortical state. Delta and spindle oscillations observed in the cortex during drowsiness/sleep are both generated in the thalamus, by the intrinsic biophysical properties of thalamocortical and thalamic reticular neurons (McCormick and Pape, 1990) and through their synaptic interactions (McCormick and Bal, 1997). Even during wakefulness, a brief activation of the thalamic reticular nucleus is sufficient to evoke thalamic bursts and cortical spindles (Halassa et al., 2011). On the other hand, increasing the tonic activity of thalamocortical neurons by local application of a cholinergic agonist can desynchronize the cortical area receiving their input (Hirata and Castro-Alamancos, 2010). In brain slices, electrical or chemical stimulation of the thalamus can effectively trigger cortical UP states (Rigas and Castro-Alamancos, 2007)(Fig. 5A, B), and in vivo optogenetic activation of thalamocortical neurons during quiet wakefulness leads to desynchronized cortical activity normally observed in an aroused state (Poulet et al., 2012). Surprisingly, extensive lesion in the thalamus does not prevent cortical desynchronization measured by EEG (Buzsaki et al., 1988; Fuller et al., 2011) or intracellular recording from cortical neurons (Constantinople and Bruno, 2011). These experiments suggest that while an intact thalamus is not required for cortical activation, perturbation of thalamic activity is often sufficient to alter the cortical state.
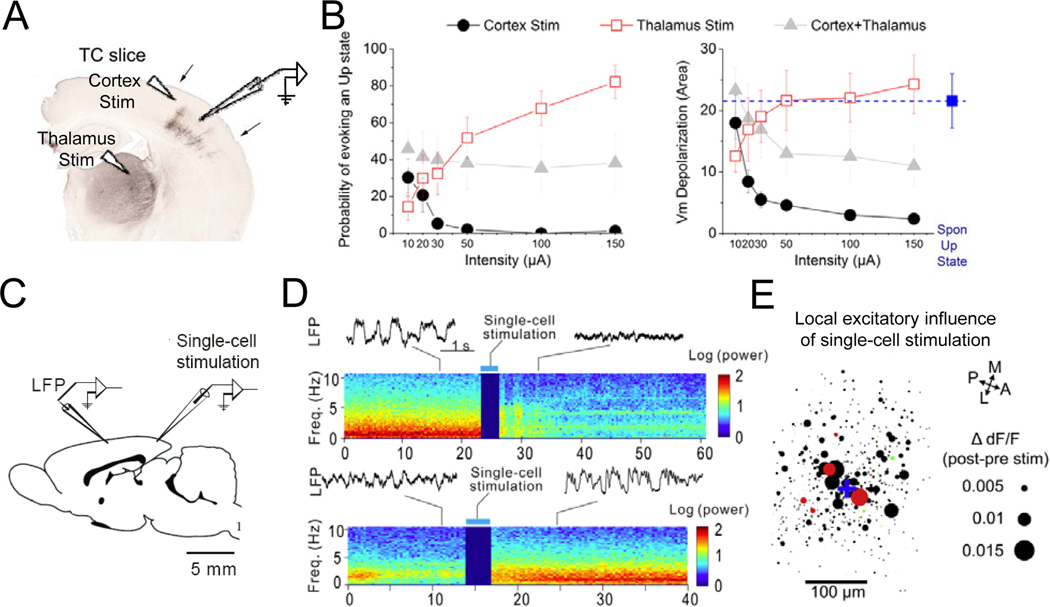
Figure 5. Effects of thalamic and cortical stimulation on brain states.
A, UP or DOWN states were measured in brain slices with intact thalamocortical circuits (TC slice). B, Population data showing probability of triggering a cortical UP state (left) and overall membrane potential depolarization measured by area (right) in response to stimulation of the thalamus, cortex, or both as a function of stimulation intensity. Figures adapted and reproduced with permission from Rigas and Castro-Alamancos, 2007. C, Schematic illustration of simultaneous whole-cell and LFP recordings measuring brain state change in response to single-cell stimulation. D, Brain state switch measured by change in LFP power spectrum from synchronized to desyncrhronized state or vice versa induced by single-cell stimulation (adapted from Li et al., 2009). E, Local excitatory influence of single-cell stimulation in the visual cortex. Blue cross, stimulated cell. Each circle represents a cell and the diameter represents the magnitude of excitation (ΔdF/Fpost-pre) measured by two-photon calcium imaging in layer 2/3 cortical neurons (red, SOM+; green, PV+; black, unidentified neurons; adapted from Kwan and Dan, 2012).
Cortical neurons can also exert strong influences on global brain state. Slow oscillations during NREM sleep originate in the cortex (Sanchez-Vives and McCormick, 2000; Steriade et al., 1993b), and cortico-cortical connections are necessary for synchronizing the oscillations across brain areas (Amzica and Steriade, 1995). In brain slices, low-intensity cortical stimulation can trigger UP state, while high-intensity stimulation suppresses UP state (Rigas and Castro-Alamancos, 2007). Interestingly, in anesthetized rat high-frequency burst firing of a single cortical neuron is sufficient to induce a global brain state transition, either from a synchronized to desynchronized state or vice versa (Li et al., 2009) (Fig. 5C, D). Based on two-photon calcium imaging, burst of a single pyramidal neuron was estimated to activate ~14 nearby excitatory neurons and 3–9 somatostatin-positive GABAergic interneurons (Kwan and Dan, 2012)(Fig. 5E). It would be interesting to find out whether the brain state switches triggered by single neuron burst in vivo is related to the bi-directional effects of cortical stimulation on the occurrence of UP states in slices (Rigas and Castro-Alamancos, 2007) (Fig. 5B). Cortical neurons are also highly interconnected with thalamic neurons, and those from the prefrontal cortex provide strong descending inputs to the neuromodulatory circuits in the basal forebrain (Golmayo et al., 2003; Sarter et al., 2005; Zaborszky et al., 1997) and brainstem (Jodo and AstonJones, 1997). Thus, the brain state switch triggered by single neuron stimulation could also be mediated by the activation of thalamic neurons or the neuromodulatory circuits.
In addition to the areas reviewed above, which are core components of the neural machinery controlling sleep and wake states, many other brain structures also play modulatory roles. For example, sleep is strongly regulated by the circadian rhythms, which are controlled by the suprachiasmatic nucleus (SCN) in the hypothalamus. Dissecting the synaptic pathways between these structures and the core components described above will be essential for understanding how sleep-wake transitions are regulated by both internal and environmental factors.
Mechanisms for arousal and attention
Wakefulness is not a unitary brain state, and the ensemble neural activity exhibits clear changes at different levels of vigilance. When the animal is drowsy or quietly resting, there is considerable delta-band activity in EEG and LFP, although the power is generally lower than that during NREM sleep. When the animal is in an aroused/attentive state (e.g., actively engaged in sensory processing or motor tasks), the cortical activity is highly desynchronized, as measured by both LFP (Bezdudnaya et al., 2006; Niell and Stryker, 2010) and intracellular recordings (Crochet and Petersen, 2006; Okun et al., 2010; Poulet and Petersen, 2008) (Fig. 1A, B). In addition to the general arousal, selective attention to specific stimuli is also associated with changes in ensemble cortical activity, although at a more local level. Attention to visual stimuli within the receptive fields of recorded neurons is accompanied by decreases in the low-frequency LFP activity (Fries et al., 2001; Khayat et al., 2010), and it can cause either increase or decrease in gamma activity (30–80 Hz), depending on the cortical area (Chalk et al., 2010; Fries et al., 2001).
Role of neuromodulatory systems
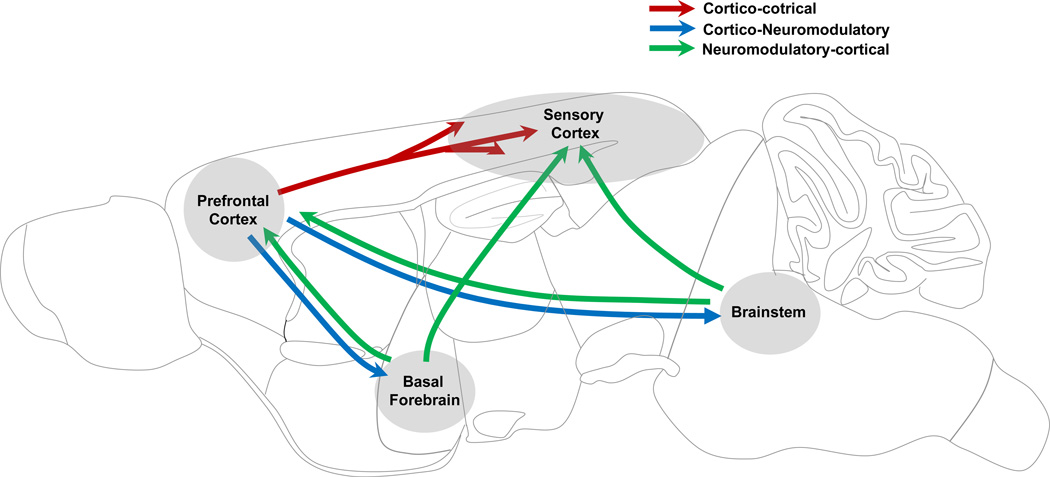
The subcortical neuromodulatory circuits involved in sleep-wake control also play important roles in the regulation of arousal and attention, and malfunctioning of these circuits causes a variety of cognitive impairments. Both the monoaminergic and cholinergic neurons in the brainstem and basal forebrain receive inputs from the prefrontal cortex (Berridge, 2008; Jodo and AstonJones, 1997; Sarter et al., 2005), a key circuit exerting cognitive control of behavior (Miller and Cohen, 2001)(Fig. 6). The activity of these neurons could thus be modulated in a task-dependent manner. For example, while the monkey performs a visual discrimination task, the noradrenergic neurons in the LC exhibit both phasic and tonic modes of firing, which are correlated with good and bad performance (Usher et al., 1999). Subsequent experiments showed that the phasic activity of LC neurons occurs specifically before the behavioral response, and it may serve to facilitate the task-related decision process (Clayton et al., 2004). In a study in the rat performing an odor-guided decision task, serotonergic neurons in the DRN showed transient firing precisely time locked to a variety of task-related events (Ranade and Mainen, 2009). Another study in the monkey showed that firing rates of the DRN neurons were modulated by both the expected and received reward sizes (Nakamura et al., 2008). Neurons in the primate basal forebrain are also modulated by novel or reinforced stimuli (Wilson and Rolls, 1990). In behaving rats, the non-cholinergic basal forebrain neurons showed strong burst responses to both reward- and punishment-predicting stimuli, and the occurrence of the burst is strongly correlated with successful sensory detection (Lin and Nicolelis, 2008).
Figure 6. Schematic diagram showing potential pathways for attentional modulation of sensory processing.
Arrows indicate major pathways connecting brain areas. Red arrows, top-down connections from prefrontal cortex to sensory areas; green arrows, projections from prefrontal cortex to brainstem and basal forebrain neuromodulatory centers; green arrows; projections from neuromodulatory centers to the cortex.
The neuromodulator especially linked to vigilance and attention is ACh. In the rat, behaviorally relevant sensory cues can evoke transient increases in ACh concentration in the prefrontal cortex at the time scale of seconds (Parikh et al., 2007), and activating cholinergic transmission in the prefrontal cortex improves the performance of a sustained attention task (St Peters et al., 2011). A recent study based on genetic manipulation with recombinant viral vectors in the prefrontal cortex further demonstrated the importance of nicotinic ACh receptors (nAChRs) in sustained attention (Guillem et al., 2011). Cholinergic signaling is also involved in selective attention. In the monkey performing a top-down spatial attention task, local application of ACh in the primary visual cortex was found to enhance the attentional modulation of neuronal firing rates, whereas mAChR antagonist had the opposite effect (Herrero et al., 2008). Together, these studies indicate that in addition to the daily sleep-wake cycle, the subcortical neurmodulatory circuits also serve to regulate arousal and attention on a sub-second to second time scale.
Changes in sensory neuron activity
Numerous studies in monkeys performing selective attention tasks have shown increased neuronal responses (Reynolds and Chelazzi, 2004), which are thought to enhance the perceptual saliency of the attended stimuli. Recent studies have shown that attention also causes a decrease in stimulus-independent correlated firing between neurons (Cohen and Maunsell, 2009; Mitchell et al., 2009), which may improve sensory encoding at the ensemble level (Zohary et al., 1994). In fact, analysis of the population signal-to-noise ratio showed that the attention-related improvement in neural coding is attributable more to the decrease in inter-neuronal correlation than to the increase in single neuron firing rate (Cohen and Maunsell, 2009; Mitchell et al., 2009).
Interestingly, basal forebrain activation also causes a decrease in inter-neuronal correlation and increase in sensory-driven response reliability in the visual cortex (Fig. 3C), and both effects contribute to improved coding of natural scenes (Goard and Dan, 2009). The strong similarity between the effects of attention and basal forebrain activation again suggests an involvement of the cholinergic system in selective attention. The decrease in inter-neuronal correlation may be mediated by mAChRs within the cortex (Goard and Dan, 2009; Metherate et al., 1992), whereas the improved visual responses of single cortical neurons could involve enhanced responses of thalamic neurons (Goard and Dan, 2009), nAChR-dependent augmentation of thalamocortical transmission (Disney et al., 2007), and/or mAChR-dependent firing rate increase within the cortex (Herrero et al., 2008; Soma et al., 2012). The recent finding that cholinergic activity can be modulated on a time scale of seconds in a task-dependent manner (Parikh et al., 2007) further supports the plausibility of its involvement in attentional modulation.
Of course, it is possible that the cholinergic input plays a permissive rather than instructive role. Selective attention is associated with local activity changes in neurons encoding the attended stimuli, but the neuromodulatory systems in general project diffusely to multiple brain regions. Although there is some topographical organization of the basal forebrain projections to the cortex (Zaborszky et al., 1999), whether there is sufficient spatial precision to support the local modulation by selective attention remains unclear. Another candidate pathway is the top-down feedback from higher-order cortical areas, such as the frontal eye field (FEF), to the visual cortical areas (Gregoriou et al., 2009; Moore and Fallah, 2004; Zhou and Desimone, 2011) (Fig. 6). Interestingly, firing rate increases in the FEF induced by local application of a dopamine receptor antagonist mimicked the attentional modulation of V4 neuronal responses (Noudoost and Moore, 2011), suggesting that the effect of neuromodulators could also be mediated by activating the cortico-cortical glutamatergic pathways.
While selective attention is typically associated with firing rate increase of the relevant neurons, behavioral arousal or task engagement in general does not always lead to enhanced responses. In the barrel cortex, behavioral arousal or engagement in the learning of a new task was found to suppress whisker-evoked responses (Castro-Alamancos, 2004a; Castro-Alamancos and Oldford, 2002). Similarly, smaller responses to brief tactile stimuli were observed in the rat during exploratory whisker movement than during quite immobility (Fanselow and Nicolelis, 1999). In the auditory cortex, neuronal response to a sound stimulus was also lower when the rat was engaged in an auditory task than when the stimulus was perceived passively (Otazu et al., 2009). These studies suggest that while selective attention can preferentially enhance the responses to the attended stimuli, a general increase in vigilance may in fact reduce the overall response in order to accentuate representation of the relevant stimulus (Atiani et al., 2009).
In contrast to the findings above, a study in mouse visual cortex showed that the neuronal responses to drifting grating stimuli are much higher when the mouse was behaviorally active (running) than inactive (standing still) (Niell and Stryker, 2010). One factor that may contribute to the discrepancy among these experiments is the use of transient (e.g., a brief sound or tactile stimulus) vs. sustained (e.g., drifting gratings) sensory stimuli, which evoke different degrees of neuronal adaptation (Harris and Thiele, 2011), as strong adaptation is observed primarily in behaviorally inactive states (Castro-Alamancos, 2004a). More importantly, the modulation of sensory responses by different behaviors – selective attention to a single stimulus, non-selective increase in vigilance, and general behavioral arousal (e.g., running) – may be mediated by different mechanisms, involving partially overlapping but non-identical sets of neuromodulatory inputs. Testing this hypothesis will require simultaneous measurement of activity of both the neuromodulatory systems and the sensory neurons under the different behavioral paradigms. Optogenetic manipulation of each neuromodulatory system (Fig. 4C, D) will also reveal its impact on the activity of sensory neurons within each behavioral context.
Function of sleep and quiet wakefulness
While it is well accepted that the aroused, attentive states are favorable for sensory processing, what are the functions of the synchronized brain states? In particular, why is sleep so universal in the animal kingdom (Cirelli and Tononi, 2008), given that the loss of responsiveness to environmental stimuli makes the animal more vulnerable to predator attacks?
Function of sleep
The importance of sleep can be appreciated from the severe effects of sleep deprivation on cognitive functions and general health. Prolonged total sleep deprivation is known to be lethal in flies (Shaw et al., 2002) and rats (Rechtschaffen and Bergmann, 2002), although some of the harmful effects may be attributable to the stress induced by the experimental methods of deprivation. Specifically, one function of sleep may be energy conservation or brain recuperation (Siegel, 2005). A recent study showed that the ATP concentration surges in the first few hours of sleep, and the level of surge is correlated with the EEG delta activity during NREM sleep (Dworak et al., 2010). However, the cause for this energy surge may not be a simple reduction of neuronal activity. We know that during NREM sleep many neurons remain highly active, and the difference from the awake state resides more in the spatiotemporal pattern than in the overall level of neural activity.
Another idea that is gaining traction in recent years is that sleep is critical for learning and memory (Diekelmann and Born, 2010; Maquet, 2001; Stickgold, 2005). Several studies on human subjects have shown that sleep shortly after practicing visual discrimination (Gais et al., 2000; Stickgold et al., 2000) or motor skills (Fischer et al., 2002; Walker et al., 2002) can significantly enhance the practice-induced improvement in task performance. Both NREM and REM sleep states seem to contribute to this enhancement, but an equal period of wakefulness after practice has little effect. Task practicing is also found to affect brain activity during subsequent sleep. For example, after training on a visuomotor task, the brain region activated during the training is specifically re-activated during REM sleep (Maquet et al., 2000). Learning of a motor adaptation task can cause a local increase in slow- and delta-wave EEG activity during NREM sleep, and the degree of increase is correlated with the performance improvement after sleep (Huber et al., 2004). To evaluate the role of synchronized slow brain activity per se in learning and memory, Marshall et al. (Marshall et al., 2006) applied transcranial slow oscillating potential (< 1 Hz) to human subjects during NREM sleep. The stimulation, which increased both NREM sleep and slow-wave EEG activity, enhanced the retention of declarative memory, indicating a direct contribution of slow-wave activity to memory consolidation.
How does the neuronal activity during sleep contribute to memory consolidation? An important clue came from the studies of spike sequence replay. Multielectrode recordings in the rat hippocampus have shown that sequential firing among a group of neurons observed during active exploration recurs spontaneously during subsequent sleep (Lee and Wilson, 2002; Louie and Wilson, 2001; Nadasdy et al., 1999; Skaggs and McNaughton, 1996; Wilson and McNaughton, 1994). Similar replay was also observed in the neocortex (Euston et al., 2007; Ji and Wilson, 2007; Ribeiro et al., 2004). During NREM sleep, the temporal order of spiking among the neurons is preserved in each replay, but the sequences occur at a faster time scale than that during active exploration. As a result, the different neurons fire within a few milliseconds from each other. A widely observed form of synaptic plasticity in both the hippocampus and neocortex is spike-timing-dependent plasticity (STDP), in which presynaptic spiking within tens of milliseconds before postsynaptic spiking induces long-term potentiation, whereas spikes in the reverse order result in depression (Dan and Poo, 2004). The time-compressed spike sequence replay observed during NREM sleep is thus well suited for the induction of long-term circuit modifications through STDP.
In addition to the neuronal activity representing exploratory experience, sensory-evoked responses can also reverberate in brain circuits. Voltage-sensitive dye imaging showed that in the visual cortex of anesthetized rats, both spontaneous and visually-evoked activity manifest as propagating waves. Repeated visual stimulation caused an increase in the number of spontaneous waves that resemble the stimulus-evoked waves (Han et al., 2008), reminiscent of the notion of reverberation proposed by Lorente de No (Lorente de No, 1938) and Hebb (Hebb, 1949). Although in this experiment the reverberatory activity was found under anesthesia, the prevalence of spontaneous waves propagating across large cortical areas is similar to that during NREM sleep. Since correlated activation of a large number of neurons is conducive to long-term synaptic modifications (Bi and Poo, 2001; Weliky, 2000), the synchronized brain states may be particularly suited for circuit modification through memory re-activation.
There is also direct evidence that sleep can facilitate activity-dependent synaptic modification. For example, a well-established model for experience-dependent circuit refinement during early development is ocular dominance plasticity, in which monocular deprivation of visual inputs can cause a drastic shift in the relative strengths of inputs from the two eyes to the visual cortex. Studies have shown that sleep significantly enhances the effect of monocular deprivation (Frank et al., 2001), and the degree of enhancement is correlated with the amount of NREM sleep. At the synaptic level, some studies found net synaptic strengthening during wakefulness and depression during sleep (Vyazovskiy et al., 2008). This led to the suggestion that while the potentiation of specific synapses encoding awake experience leads to an imbalance of synaptic strength, a global depression of all synapses during sleep serves to restore the balance. This overall depression may also increase the signal-to-noise ratio of the memory by leaving only the most important connections intact. Furthermore, synaptic plasticity is strongly influenced by neuromodulators (Pawlak et al., 2010; Rasmusson, 2000). A recent study showed that the firing rates of LC noradrenergic neurons are increased during NREM sleep after learning (Eschenko and Sara, 2008), which could in turn enhance synaptic plasticity and facilitate memory consolidation (Sara, 2009).
Quiet wakefulness
Although spike sequence replay was initially discovered during sleep, recent studies have shown that it also occurs during wakefulness, especially during quiet immobility or consummatory behaviors (Diba and Buzsaki, 2007; Foster and Wilson, 2006; Karlsson and Frank, 2009). In both sleep and awake states, the replay events occur during sharp wave ripples in LFP (Buzsaki et al., 1992; O'Neill et al., 2006), which are strongly associated with slow oscillations (Molle et al., 2006). Selective interruption of hippocampal ripple events during wakefulness impairs spatial learning (Jadhav et al., 2012), similar to the effect of ripple disruption during sleep (Ego-Stengel and Wilson, 2010; Girardeau et al., 2009). Thus, the quiet wakeful state may contribute to spatial learning through a similar spike sequence reactivation mechanism.
While the sequential activation of hippocampal place cells is evoked by locomotion of the animal, sequential spiking of visual neurons can be evoked by a moving stimulus sweeping across their receptive fields. Multielectrode recording in the visual cortex of both anesthetized and awake rats showed that stimulation with a moving spot evoked sequential firing of an ensemble of neurons whose receptive fields fell along the motion path. After repeated stimulation with the moving spot, a brief light flash at the starting point of the motion path evoked more sequential firing of these neurons similar to that evoked by the moving spot. Interestingly, in awake animals this cue-triggered recall of spike sequence was observed during a synchronized quiet wakeful state, but not in a desynchronized active state (Xu et al., 2012), reminiscent of the hippocampal replay during quiet immobility (Diba and Buzsaki, 2007; Foster and Wilson, 2006; Karlsson and Frank, 2009).
Together, these studies suggest that while the desynchronized brain state favors faithful representation of sensory inputs, the synchronized state may be more suited for either spontaneous or cue-triggered reactivation of previous experience. Optimal control of behavior depends on the integration of current sensory information with predictions based on prior experience. The relative weights of sensory and memory signals may be adjusted by changing the brain states through neuromodulatory inputs (Yu and Dayan, 2005).
Concluding Remarks
Studies over the last century have led to tremendous progress in our understanding of the neural control and functions of different states. Many key structures regulating brain states have been identified by measuring the effects of their disruption, and the firing patterns of those neurons under different brain states have been characterized. A major new challenge is to dissect the microcircuitry within each structure and the long-range connections between them. These efforts will be greatly facilitated by the newly developed optogenetic and circuit tracing tools. Functionally, the effects of vigilance and attention on sensory processing have been studied extensively through electrophysiological experiments in awake behaving animals. There is also accumulating evidence for the importance of synchronized brain states in learning and memory. Future studies combining the recording and selective manipulation of the reactivated memory traces should provide a definitive test of this hypothesis.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. The Journal of Neuroscience. 1995;15:4658–4677. doi: 10.1523/JNEUROSCI.15-06-04658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, DeFelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Reviews Neuroscience. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. The Journal of Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard J, Prince D. Modulation of neocortical interneurons: extrinsic influences and exercises in self-control. Trends in neurosciences. 2005;28:602–612. doi: 10.1016/j.tins.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. The Journal of Neuroscience. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Electroencephalogram in humans. Archiv Fur Psychiatrie Und Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. European Journal of Neuroscience. 2002;16:2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Gunluk AE, Vanhorn SC, Sherman SM. Gabaergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. Journal of Comparative Neurology. 1994;348:481–510. doi: 10.1002/cne.903480402. [DOI] [PubMed] [Google Scholar]
- Boucetta S, Jones BE. Activity Profiles of Cholinergic and Intermingled GABAergic and Putative Glutamatergic Neurons in the Pontomesencephalic Tegmentum of Urethane-Anesthetized Rats. The Journal of Neuroscience. 2009;29:4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. Journal of Neuroscience. 1999;19:3204–3212. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. Journal of Neuroscience. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nature Neuroscience. 2010;13 doi: 10.1038/nn.2682. 1526-U1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of Rapid Sensory Adaptation in Neocortex during Information Processing States. Neuron. 2004a;41:455–464. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Progress in Neurobiology. 2004b;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. The Journal of physiology. 2002;541:319–331. doi: 10.1113/jphysiol.2002.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention Reduces Stimulus-Driven Gamma Frequency Oscillations and Spike Field Coherence in V1. Neuron. 2010;66:114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong YM, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. Journal of Neuroscience. 2004;24:9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nature Neuroscience. 2009;12 doi: 10.1038/nn.2439. 1594-U1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople Christine ÂM, Bruno Randy  M. Effects and Mechanisms of Wakefulness on Local Cortical Networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat Meth. 2011;8:26. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Involvement of Direct and Indirect Pathways in Electrocorticographic Activation. Neuroscience & Biobehavioral Reviews. 1998;22:243–257. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PAJ. Functional-role of 5-ht2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology. 1989;97:436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of neurophysiology. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. The Journal of Neuroscience. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM. Beyond traditional approaches to understanding the functional role of neuromodulators in sensory cortices. Frontiers in behavioral neuroscience. 2012;6:45. doi: 10.3389/fnbeh.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem-cortex interplay for memory consolidation? Cereb Cortex. 2008;18:2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. The Journal of Neuroscience. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of Inhibition within the Telencephalon: “Where the Wild Things are”. Annual Review of Neuroscience. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. The Journal of comparative neurology. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. The Journal of comparative neurology. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MAL. Global forebrain dynamics predict rat behavioral states and their transitions. Journal of Neuroscience. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nature Neuroscience. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmayo L, Nuñez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical†“prefrontal– basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-Frequency, Long-Range Coupling Between Prefrontal and Visual Cortex During Attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. The Journal of comparative neurology. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic Acetylcholine Receptor Î22 Subunits in the Medial Prefrontal Cortex Control Attention. Science. 2011;333:888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. Journal of Comparative Neurology. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K-i, Goto K, Yanagisawa M, et al. Genetic Ablation of Orexin Neurons in Mice Results in Narcolepsy, Hypophagia, and Obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nature reviews Neuroscience. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. European. Journal of Neuroscience. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. The. Journal of Neuroscience. 2006;26:4426–4436. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. Journal of neurophysiology. 2010;103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–1256. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HGJM. Brainstem projections to spinal motoneurons: An update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain research. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jodo E, AstonJones G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain research. 1997;768:327–332. doi: 10.1016/s0006-8993(97)00703-8. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Frontiers in bioscience : a journal and virtual library. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jones BE, Cuello AC. Afferents to the basal forebrain cholinergic cell area from pontomesencephalic--catecholamine, serotonin, and acetylcholine--neurons. Neuroscience. 1989;31:37–61. doi: 10.1016/0306-4522(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. The Journal of comparative neurology. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder Charles ÂE. How Local Is the Local Field Potential? Neuron. 2011;72:847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local Origin of Field Potentials in Visual Cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat PS, Niebergall R, Martinez-Trujillo JC. Frequency-dependent attentional modulation of local field potential signals in macaque area MT. The Journal of Neuroscience. 2010;30:7037–7048. doi: 10.1523/JNEUROSCI.0404-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Fukuda M, Tsumoto T. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the source of input. The European journal of neuroscience. 1999;11:3597–3609. doi: 10.1046/j.1460-9568.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan AC, Dan Y. Dissection of Cortical Microcircuits by Single-Neuron Stimulation In Vivo. Curr Biol. 2012 doi: 10.1016/j.cub.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. Journal of Neuroscience. 2005a;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. The Journal of Neuroscience. 2005b;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Boyden ES, Deisseroth K, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012 doi: 10.1038/nature11312. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. The Journal of Neuroscience. 2010;30:16796–17604. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Hallanger AE, Wainer BH. Cholinergic nucleus basalis neurons may influence the cortex via the thalamus. Neuroscience Letters. 1987;74:7–13. doi: 10.1016/0304-3940(87)90042-5. [DOI] [PubMed] [Google Scholar]
- Li C-yT, Poo M-m, Dan Y. Burst Spiking of a Single Cortical Neuron Modifies Global Brain State. Science. 2009;324:643–646. doi: 10.1126/science.1169957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao X-B, Sakurai T, van den Pol AN. Hypocretin/Orexin Excites Hypocretin Neurons via a Local Glutamate Neuron—A Potential Mechanism for Orchestrating the Hypothalamic Arousal System. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lin S-C, Nicolelis MAL. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. Analysis of the activity of the chains of internuncial neurons. Journal of neurophysiology. 1938;1:207–244. [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of Lesions of the Ventrolateral Preoptic Nucleus on NREM and REM Sleep. The Journal of Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. Differential c-Fos Expression in Cholinergic, Monoaminergic, and GABAergic Cell Groups of the Pontomesencephalic Tegmentum after Paradoxical Sleep Deprivation and Recovery. The Journal of Neuroscience. 1999;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning KA, Wilson JR, Uhlrich DJ. Histamine-immunoreactive neurons and their innervation of visual regions in the cortex, tectum, and thalamus in the primate Macaca mulatta. Journal of Comparative Neurology. 1996;373:271–282. doi: 10.1002/(SICI)1096-9861(19960916)373:2<271::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. Journal of Neuroscience. 2000;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. The European journal of neuroscience. 2003;18:723–727. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Discharge Patterns of Cat Pontine Brain-Stem Neurons During Desynchronized Sleep. Journal of neurophysiology. 1975;38:751–766. doi: 10.1152/jn.1975.38.4.751. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. The Journal of physiology. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. 2 Types of Muscarinic Response to Acetylcholine in Mammalian Cortical-Neurons. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. The Journal of Neuroscience. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial Attention Decorrelates Intrinsic Activity Fluctuations in Macaque Area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. Journal of neurophysiology. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- Momiyama T, Zaborszky L. Somatostatin presynaptically inhibits both GABA and glutamate release onto rat basal forebrain cholinergic neurons. Journal of neurophysiology. 2006;96:686–694. doi: 10.1152/jn.00507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. Involvement of Histamine in the Control of the Waking State. Life Sciences. 1993;53:1331–1338. doi: 10.1016/0024-3205(93)90592-q. [DOI] [PubMed] [Google Scholar]
- Monti JM, Jantos Hc, Giuseppe Di Giovann VDM, Ennio E. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. In Progress in brain research (Elsevier) 2008:625–646. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- Monti JM, Pandi-Perumal SR, Mohler H SpringerLink (Online service) GABA and sleep molecular, functional and clinical aspects. Basel: Springer Basel AG; 2010. xxiv, 486. [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. Journal of neurophysiology. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. The Journal of Neuroscience. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. The Journal of Neuroscience. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of Visual Responses by Behavioral State in Mouse Visual Cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Munakata M, Akaike N. Muscarinic Acetylcholine Response in Pyramidal Neurons of Rat Cerebral-Cortex. British Journal of Pharmacology. 1994;112:1160–1166. doi: 10.1111/j.1476-5381.1994.tb13205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Senior T, Csicsvari J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron. 2006;49:143–155. doi: 10.1016/j.neuron.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. The Journal of Neuroscience. 2010;30:4440–4448. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otazu GH, Tai L-H, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164:404–414. doi: 10.1016/j.neuroscience.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not Everything: Neuromodulation Opens the STDP Gate. Frontiers in synaptic neuroscience. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Poulet JFA, Fernandez LMJ, Crochet S, Petersen CCH. Thalamic control of cortical states. Nat Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035. [DOI] [PubMed] [Google Scholar]
- Poulet JFA, Petersen CCH. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. Journal of neurophysiology. 2009;102:3026–3037. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behavioural brain research. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional Modulation of Visual Processing. Annual Review of Neuroscience. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical Up States: Differential Effects of Intrinsic and Extrinsic Cortical Inputs on Persistent Activity. The Journal of Neuroscience. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature reviews Neuroscience. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. European Journal of Neuroscience. 2002;15:1867–1873. doi: 10.1046/j.1460-9568.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of Histaminergic Tuberomammillary Neurons by GABAergic and Galaninergic Neurons in the Ventrolateral Preoptic Nucleus of the Rat. The Journal of Neuroscience. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of Ventrolateral Preoptic Neurons During Sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamic relays and cortical functioning. Progress in brain research. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Siegel JM. Pontine Regulation of Rem-Sleep Components in Cats - Integrity of the Pedunculopontine Tegmentum (PPT) is Important for Phasic Events but Unnecessary for Atonia During Rem-Sleep. Brain research. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Soma S, Shimegi S, Osaki H, Sato H. Cholinergic modulation of response gain in the primary visual cortex of the macaque. Journal of neurophysiology. 2012;107:283–291. doi: 10.1152/jn.00330.2011. [DOI] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced Control of Attention by Stimulating Mesolimbic-Corticopetal Cholinergic Circuitry. Journal of Neuroscience. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain research. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. The Journal of Neuroscience. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993a;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. The Journal of Neuroscience. 1993b;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Pare D, Parent A, Smith Y. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: A view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Stoelzel CR, Bereshpolova Y, Swadlow HA. Stability of Thalamocortical Synaptic Transmission across Awake Brain States. Journal of Neuroscience. 2009;29:6851–6859. doi: 10.1523/JNEUROSCI.5983-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. The Journal of Neuroscience. 2007;27:1616–1630. doi: 10.1523/JNEUROSCI.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold. Nature reviews Neuroscience. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep suppression following kainic acid-induced lesions of the basal forebrain. Exp Neurol. 1986;94:598–614. doi: 10.1016/0014-4886(86)90240-2. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res Bull. 1989;22:423–430. doi: 10.1016/0361-9230(89)90069-5. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Annals of the New York Academy of Sciences. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kayama Y, Lin JS, Sakai K. Locus Coeruleus Neuronal Activity During the Sleep-Waking Cycle in Mice. Neuroscience. 2010;169:1115–1126. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. The Journal of Neuroscience. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Characterization and Mapping of Sleep-Waking Specific Neurons in the Basal Forebrain and Preoptic Hypothalamus in Mice. Neuroscience. 2009;161:269–292. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;72:1091–1091. doi: 10.1016/j.neuron.2011.07.026. (vol 71, pg 995 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM. Histamine in the regulation of wakefulness. Sleep Medicine Reviews. 2011;15:65–74. doi: 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Morales FR, Chase MH. GABAergic mechanisms in the pedunculopontine tegmental nucleus of the cat promote active (REM) sleep. Brain research. 2002;944:1–9. doi: 10.1016/s0006-8993(02)02475-7. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Pappas BA. Reserpine abolishes movement-correlated atropine-resistant neocortical low voltage fast activity. Brain research. 1980;202:79–94. [PubMed] [Google Scholar]
- Von Economo C. Sleep as a problem of localization. The Journal of Nervous and Mental Disease. 1930;71:249–259. [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Weliky M. Correlated neuronal activity and visual cortical development. Neuron. 2000;27:427–430. doi: 10.1016/s0896-6273(00)00053-2. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann KK, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FAW, Rolls ET. Learning and Memory is Reflected in the Responses of Reinforcement-Related Neurons in the Primate Basal Forebrain. Journal of Neuroscience. 1990;10:1254–1267. doi: 10.1523/JNEUROSCI.10-04-01254.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh C-I, Shapley RM. Spatial Spread of the Local Field Potential and its Laminar Variation in Visual Cortex. The Journal of Neuroscience. 2009;29:11540–11549. doi: 10.1523/JNEUROSCI.2573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Jiang W, Poo MM, Dan Y. Activity recall in a visual cortical ensemble. Nat Neurosci. 2012;15:449, 455–S441. doi: 10.1038/nn.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Duque A. Sleep-wake mechanisms and basal forebrain circuitry. Frontiers in Bioscience. 2003;8:D1146–D1169. doi: 10.2741/1112. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse: In Honor of Lennart Heimer, J.F. McGinty, ed. 1999. The basal forebrain corticopetal system revisited; pp. 339–367. [DOI] [PubMed] [Google Scholar]
- Zhou H, Desimone R. Feature-Based Attention in the Frontal Eye Field and Area V4 during Visual Search. Neuron. 2011;70:1205–1217. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]