Abstract
Objective
To determine the extent to which levels of membrane eicosapentaenoic (EPA)+docosahexaenoic acids (DHA) (the omega-3 index) were associated with depression in patients with acute coronary syndrome (ACS). Depression is associated with worse cardiovascular (CV) outcomes in patients with ACS. Reduced levels of blood cell membrane omega-3 (n-3) fatty acids (FAs), an emerging risk factor for both CV disease and depression, may help to explain the link between depression and adverse CV outcomes.
Methods
We measured membrane FA composition in 759 patients with confirmed ACS. The analysis included not only EPA and DHA but also the n-6 FAs linoleic and arachidonic acids (LA and AA). Depressive symptoms were measured with the Patient Health Questionnaire-9 (PHQ). Multivariable linear regression was used to adjust for demographic and clinical characteristics.
Results
There was a significant inverse relationship between the n-3 index and depressive symptoms (PHQ) in the fully adjusted model (p = .034). For every 4.54% point rise in the n-3 index, there was a 1-point decline in depressive symptoms. In contrast to the n-3 FAs, membrane levels of the n-6 FAs LA and AA were not different between depressed and nondepressed ACS patients.
Conclusion
We found an inverse relationship between the n-3 index and the prevalence of depressive symptoms in patients with ACS. Therefore, this study supports the hypothesis that reduced n-3 FA tissue levels are a common and potentially modifiable link between depression and adverse CV outcomes.
Keywords: depression, acute coronary syndrome, omega-3 fatty acids
INTRODUCTION
Despite centuries of anecdotal reports linking depression with heart disease, only recently has depression been recognized as a common comorbidity among cardiac patients and an independent predictor of adverse outcomes (1). Over the past decade, numerous studies have documented that depression in patients with acute coronary syndrome (ACS) is associated with a higher incidence of mortality, recurrent cardiovascular (CV) events, and healthcare utilization (2). Although this association is now well established, the underlying mechanism leading to poor CV outcomes in depressed patients is still unknown. Yet, understanding this relationship will improve our ability to manage and minimize the associated risk of depression in cardiac patients. One potential explanation for the poor prognosis of depressed ACS patients is that depression and coronary artery disease (CAD) share a common causal factor. Therefore, deficiencies in essential fatty acids (FAs) may be a part of the causal network linking depression, CAD, and adverse CV events.
Essential FAs, whether of the omega-3 (n-3) or the omega-6 (n-6) classes, are not synthesized in vivo and thus must be consumed in the diet (3–5). These FAs have several physiologic roles, including supporting cell membrane structure, providing substrates for biochemical signaling, and modulating gene expression (3,6,7). In addition, certain n-3 FAs (eicosapentaenoic acid (EPA) and docosahexaenoic acids (DHA)) and n-6 FAs (arachidonic acid (AA)) also serve as substrates for the synthesis of a wide variety of mediators of inflammation and thrombosis, important mechanisms in the development of ACS (8 – 11). In general, AA gives rise to proinflammatory and prothrombotic cascades predominantly through its conversion into 2-series prostaglandins, 4-series leukotrienes, and thromboxane A2 (12,13). However, AA also gives rise to prostacyclin, a highly antithrombotic molecule. EPA and DHA, on the other hand, give rise to a related but physiologically less active set of eicosanoids (e.g., 3-series prostaglandins, 5-series leukotrienes, and thromboxane A3) (6,13). The n-3 derived eicosanoids are typically less inflammatory than the n-6 derived products and do not stimulate platelet aggregation (13,14).
Low levels of n-3 have been shown in cross-sectional and cohort studies to be associated with depression in general medical and/or postpartum populations (7,15–21). Some, but not all, clinical trials treating depression with n-3 FA supplementation have shown improvements in patients’ depressive symptoms (22,23). This combined with over 30 years of research suggesting that high levels of n-3 FAs intake protect against CV disease provide the foundation for exploring the role of n-3 FAs in depressed ACS patients (3,24,25).
Previous studies exploring the relationship between n-3 status and depression in ACS patients have been limited to relatively small samples and have restricted their n-3 analyses to plasma (15,26). In contrast to plasma levels, membrane n-3 FAs content provides a more reliable, biologically relevant, and long-term assessment of n-3 FA status (27). We, therefore, sought to expand the existing literature on the association between n-3 FA status and depressive symptoms by performing a cross-sectional analysis of depressive symptoms and blood cell membrane EPA+DHA levels (hereafter the n-3 index) in a large group of patients with confirmed ACS. Because the n-6 FAs may oppose the actions of the n-3 FAs, we also explored their relationship with depression among patients with ACS.
METHODS
Study Design
From March 2001 through June 2004, ACS patients presenting to two urban hospitals in Kansas City, Missouri were prospectively screened for enrollment in an observational study. Methods for identifying and enrolling patients with ACS were standardized at both institutions and have been previously reported (28). ACS was diagnosed using prespecified criteria for acute myocardial infarction (MI) or unstable angina (UA) (29,30). MI patients were defined by a positive troponin blood test in the setting of symptoms or electrocardiogram changes (both ST segment elevation and non-ST segment elevation) consistent with MI. UA was diagnosed if the patient had a negative troponin blood test and any one of the following: new onset angina (<2 months) of at least Canadian Cardiovascular Society Classification class III, prolonged (>20 minutes) rest angina, recent (<2 months) worsening of angina, or angina that occurred within 2 weeks of an MI.
Patients were excluded if they were demented, deceased, discharged, or transferred before contact, diagnosed with ACS secondary to another event (gastrointestinal bleeding, sepsis, surgery), under the care of hospice, or non-English speaking. All troponin-negative, potential UA patients who had a diagnostic study that excluded obstructive coronary disease (i.e., coronary angiography, nuclear or echocardiographic stress testing) or who had an additional diagnostic study confirming an alternative explanation for the patient’s presentation, were also excluded. Three physicians reviewed the charts of those patients for whom diagnostic uncertainty remained and consensus on the final diagnosis of MI or UA was collaboratively reached. Of the troponin-negative UA patients, 92.5% had a cardiac catheterization, a nuclear stress test, or a stress echocardiogram to corroborate their diagnosis.
Data collection consisted of direct patient interviews at the time of their ACS hospitalization and detailed hospital chart abstractions. Chart abstractions were performed to collect data on medical history, laboratory results, disease severity, and the processes of inpatient care. All data elements conform to established data definitions and the most valid representations of nonclinical domains available (28,31). Detailed patient interviews were conducted to ascertain participants’ demographic, economic, and psychosocial information. To quantify the presence and severity of depressive symptoms, trained interviewers administered a nine-item depression screening tool, the Patient Health Questionnaire-9 (PHQ).
Approval from the Institutional Review Boards of both institutions was obtained before the initiation of the study and each participant provided informed consent before enrollment.
Quantification of Depressive Symptoms
The PHQ is a validated screening tool for quantifying depressive symptoms. It assesses the presence of nine symptoms and their frequency of occurrence in the two preceding weeks (32,33). A raw score is calculated according to whether each of the nine symptoms are present “not at all” (0 points), on “several days” (1 point), on “more than half the days” (2 points), or “nearly every day” (3 points). Total PHQ scores range from 0 to 27. Higher scores indicate a greater severity of depressive symptoms. For presentation of baseline data in Table 1, the PHQ score was dichotomized into depressed (PHQ ≥10) and not depressed (PHQ <10) groups; and for the multivariable linear regression analysis, the PHQ score was included as a continuous variable. A raw score of ≥10 suggests moderate-to-severe depressive symptoms and is 88% sensitive and 88% specific for diagnosing Major Depressive Disorder in general medical populations and is 90% specific for Major Depressive Disorder in outpatients with CAD (32–34).
Table 1.
Baseline characteristics of acute coronary syndrome patients with and without depression#
| Variable | Depression Status | p-value | |
|---|---|---|---|
| Depressed (PHQ≥10) n=118 | Not Depressed (PHQ<10) n=641 | ||
| Demographics | |||
| Age | 55.9 (±10.8) | 61.9 (±12) | <0.001 |
| Male | 50 (42%) | 452 (71%) | <0.001 |
| Caucasian | 97 (82%) | 598 (93%) | <0.001 |
| College Education | 46 (39%) | 318 (50%) | 0.028 |
| Clinical Characteristics | |||
| Hypertension | 93 (79%) | 392 (61%) | <0.001 |
| Diabetes Mellitus | 46 (39%) | 145 (23%) | <0.001 |
| Body Mass Index (kg/m2) | 30.6 (±6.8) | 29.3 (±5.9) | 0.029 |
| Current Smoker | 61 (52%) | 205 (32%) | <0.001 |
Categorical data is presented as frequency (percent) and were compared using chi-square. Continuous data is presented as mean (± standard deviation) and were compared using two-sample t-test or wilcoxon rank sum test as appropriate.
percentages may not add up to 100% because of rounding
PHQ, Patient Health Questionnaire.
Covariates of Interest
In addition to depressive symptoms, several baseline demographic and clinical characteristics were also assessed (Table 1). Race was obtained by patient self-report and categorized according to the Office of Management and Budget. Education level was categorized as any college or no college. Body mass index (BMI, kg/m2) was calculated using height and weight obtained during patients’ index hospitalization. Self-reported smoking status was categorized as current (last use ≤30 days) or not.
Candidate covariates for selection into multivariable models were established a priori based on clinical importance as potential confounders in the relationship between n-3 index and depression. Whereas the underlying causes for depression are multifactorial and likely related to complex biologic, social, and psychological factors, the common risk factors include age, gender, race, education, weight, and chronic medical conditions (35–37). We therefore sought to explore the independent relationship between n-3 FA and depression adjusting for age, gender, race, education, hypertension, diabetes, BMI, and smoking status because these factors have been associated with both depression and n-3 FA levels (3,35 – 41).
Laboratory Methods
Membrane FA composition was measured as previously described (42). Erythrocyte membranes were isolated by ultracentrifugation, and membrane FAs were converted to methyl esters by heating them at 100°C for 10 minutes in 14% boron trifluoride. Extracted methyl esters were analyzed by gas chromatography. The coefficient of variation for membrane, the n-3 index, was 5%, for the two major n-6 FAs LA and AA, 2% and 3%, respectively.
Statistical Methods
Patients were stratified by the presence of depressive symptoms or not (PHQ score of ≥10 or <10, respectively) for comparison of baseline characteristics. Categorical data are reported as frequencies and compared with X2 or Fisher’s exact tests; and continuous data are reported as the mean ± standard deviation and compared with independent sample, two-tailed t tests.
All membrane FAs (the n-3 index and n-6 FAs) were analyzed as continuous variables. Linear regression was performed to create a model with the FA variable of interest as an independent variable and depressive symptoms, quantified by the PHQ, as the outcome. To determine the independent association between the n-3 index and depressive symptoms, multivariable linear regression was performed after adjusting for selected covariates (i.e., age, gender, race, education, hypertension, diabetes, BMI, and smoking status). The adjusted parameter estimates for variables in this final model are presented as β coefficients with their associated 95% confidence interval. Nonlinearity between the n-3 index and PHQ was explored using spline terms, but no evidence of nonlinearity was detected. We also compared individual FA levels (i.e., alpha-linolenic acid, EPA, docosapentaenoic acid (n-3), DHA, LA, gamma-linolenic acid, dihomo-gamma-linolenic acid (DGLA), AA, docosapentaenoic acid (n-6), total n-3, and total n-6) between depressed (PHQ ≥10) and nondepressed patients (PHQ <10) using the t test. All tests for statistical significance were two-tailed with an α level of 0.05. Analyses were conducted using SAS software, release 9.1 (SAS Institute, Cary, North Carolina) and R version 2.1.0. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Participants
From March 2002 to June 2004, 768 patients with confirmed ACS were enrolled in this study. Nine (1.2%) participants did not complete the PHQ, leaving 759 (98.8%) ACS patients for analysis. Their mean age was 61 ± 12.1 years and the majority were male (n = 509, 67%) and Caucasian (n = 704, 91.7%).
Descriptive Data
Among the 759 ACS patients in this study, 118 (15.5%) had depressive symptoms, which were defined as a PHQ score of ≥10. Several differences in comorbidities were found between depressed and nondepressed ACS patients in this study (Table 1). Depressed patients were more likely to be younger, female, of a minority race, and to have a lower level of education as compared with nondepressed patients. In terms of clinical characteristics, depressed patients had more cardiovascular risk factors with higher rates of diabetes mellitus, hypertension, and current tobacco use. Additionally, depressed patients were more likely to have a higher BMI.
Association Between Membrane FA and Depressive Symptoms
In unadjusted analyses, the n-3 index was inversely related the presence of depressive symptoms (β coefficient = −0.49, p < .001, R2 = 0.03). To further explore the relationship between other FA levels and depressive symptoms, we compared individual n-3 and n-6 FA levels between depressed and nondepressed ACS patients (Table 2). Among n-3 FAs, depressed ACS patients had lower levels of n-3 docosapentaenoic acid (p = .006), DHA (p = .008), and total n-3 FA (p = .001) levels compared with nondepressed ACS patients. Several n-6 FAs tended to be lower in depressed patients compared with nondepressed patients; however, none of these differences were statistically significant. The only statistically significant association was found with DGLA (a metabolic precursor to AA), where depressed ACS patients had lower levels than nondepressed patients. DGLA is also the precursor to the 1-series eicosanoids.
Table 2.
Fatty Acid Levels in depressed and non-depressed acute coronary syndrome patients*
| Variable | Depression Status | p-value | |
|---|---|---|---|
| Depressed (PHQ ≥10) n=118 | Not Depressed (PHQ<10) N=641 | ||
| n-3 Fatty Acids | |||
| Alpha-linolenic acid | 0.2% (±0.4) | 0.1% (±0.3) | 0.166 |
| Eicosapentaenoic acid (EPA) | 0.3% (±0.4) | 0.3% (±0.5) | 0.260 |
| Docosapentaenoic acid (n-3) | 1.5% (±0.6) | 1.7% (±0.6) | 0.006 |
| Docosahexaenoic acid (DHA) | 2.6% (±1.2) | 3.0% (±1.5) | 0.008 |
| EPA+DHA | 2.9% (±1.5) | 3.3% (±1.8) | 0.002 |
| Total n-3 | 4.8% (±1.8) | 5.5% (±2.0) | 0.001 |
| n-6† Fatty Acids | |||
| Linoleic acid | 14.1% (±2.6) | 14.3% (±2.5) | 0.477 |
| Gamma-linolenic acid | 0.1% (±0.3) | 0.1% (±0.3) | 0.941 |
| Dihomo-gamma-linolenic acid (DGLA) | 1.6% (±0.6) | 1.8% (±0.5) | 0.002 |
| Arachidonic acid (AA) | 13.9%(±4.4) | 14.2%(±3.6) | 0.415 |
| Docosapentaenoic acid (n-6) | 0.7% (±0.5) | 0.7% (±0.5) | 0.652 |
| Total n-6 | 33.6% (±5.2) | 34.3% (±4.3) | 0.117 |
n-3, omega-3; n-6, omega-6; PHQ, Patient Health Questionnaire.
The 2-sample t-test was used to compare fatty acid levels between depressed and non-depressed patients.
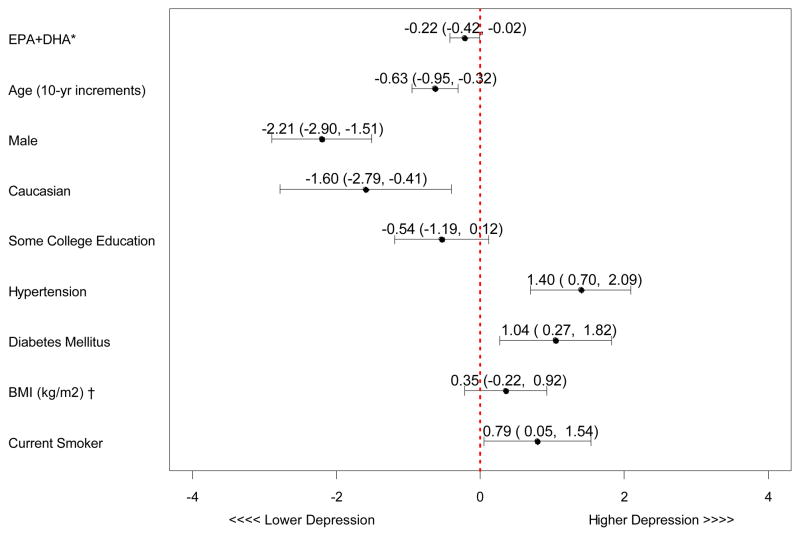
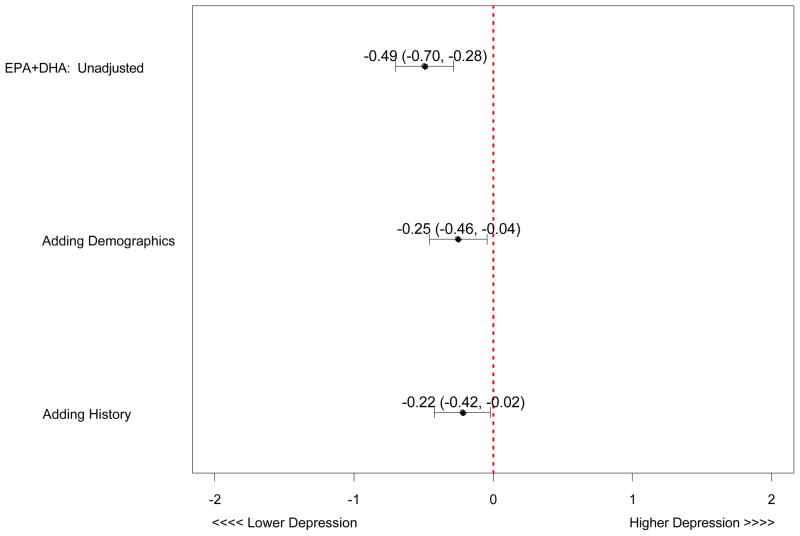
After adjusting for the covariates listed in Table 1, the relationships remained significant (β coefficient = −0.22, p = .034) (Figure 1) such that for every 4.54% point rise in the n-3 index, there was a 1-point decline in depressive symptoms measured by the PHQ. Figure 2 represents the unadjusted and adjusted association between n-3 index and depressive symptoms. Although the relationship between n-3 index and depressive symptoms was attenuated by adjusting for demographic factors, higher n-3 index remained significantly associated with fewer depressive symptoms in the final model.
Figure 1. Adjusted Beta-coefficients of Variables in the Final Multivariable Model Predicting Depressive Symptoms.
*n-3 (omega-3); BMI (Body Mass Index)
Figure 2. Unadjusted and Adjusted Association between EPA+DHA*and Depressive Symptoms.
*n-3 (omega-3)
Demographics (age, gender, race, education level)
History (hypertension, diabetes, Body Mass Index, smoking status)
DISCUSSION
We found that depressed ACS patients had lower blood cell membrane levels of DHA but not EPA compared with nondepressed patients. Because the n-3 index is predominantly driven by DHA, the n-3 index (which includes EPA and DHA) was also significantly reduced in depressed patients. Although there were other significant differences in cardiac risk factors between depressed and nondepressed ACS patients, multivariable adjustment for these factors did not eliminate the independent association between lower n-3 index and greater depressive symptoms. However, only a small portion (3%) of the total variability in PHQ scores was explained by the n-3 index, which suggests that supplementation with fish oil to raise this biomarker may have little impact on PHQ scores. In secondary analyses, low n-3 docosapentaenoic acid (DPA) was also associated with more depressive symptoms, suggesting that either EPA (a precursor to DPA) is not important in this relationship, is subject to higher variability, or serves only to be converted to DPA and DHA (43). There were no relations with the principal n-6 FAs, LA, or AA, but the immediate precursor to AA—DGLA— was lower in depressed patients.
Our study expands the previously described inverse relationship between depressive symptoms and n-3 FA levels in ACS patients by using a larger population and by focusing on cell membrane, rather than plasma, n-3 FA levels. In contrast to plasma FA composition, blood cell membrane n-3 FA composition is not influenced by the patient’s fasting state or by dyslipidemias, and is more likely to reflect biologically relevant n-3 FA status (15,26,27). Furthermore, membrane levels provide a more stable, long-term assessment of n-3 FA status, analogous to the advantage of glycosylated hemoglobin over plasma glucose as an assessment of glycemic control in diabetic patients (27).
Our study is the largest to date to investigate the relationship between n-3 FAs and depression in ACS patients. Frasure-Smith et al. performed a nested case-control study of 108 ACS patients (54 depressed patients and 54 controls) (15). Although the best time to recognize depression in ACS patients is still unclear, cases and controls in this study were identified 2 months after their index hospitalization. It is therefore possible that postdischarge changes in diet or n-3 FA supplementation, which might have been different between groups, could have confounded their findings. Nonetheless, this study found, as we did, a significant relationship between low plasma DHA and total n-3 FA levels and a clinical diagnosis of depression, but no relation with EPA alone. In another investigation of this relationship, Parker et al. enrolled 97 hospitalized ACS patients (seven with major depression and five with dysthymia), but failed to detect a significant correlation between plasma levels of EPA, DHA, or total n-3 FA and depression (26). However, this study was severely limited in its power to detect such an association, with only 12 patients identified as clinically depressed. When depressive symptoms were assessed by the Beck Depression Inventory (as opposed to the dichotomous variable of depression and no depression) in this study, there was a significant and inverse relationship between plasma DHA and depressive symptoms.
Both depression and low n-3 FA levels have been shown in multiple observational studies to be independent risk factors for adverse outcomes in ACS patients (24,44–47), and in this same data set described here, a low n-3 index was associated with increased odds for ACS case status compared with outpatient controls (42). Only recently has there been evidence to suggest that n-3 FA may, in part, explain the adverse outcomes found in depressed MI patients (22). In fact, depression and n-3 FA share multiple overlapping biologic mechanisms that could lead to adverse MI outcomes. Low n-3 FA levels and depression have both been associated with autonomic dysregulation, proinflammatory cytokines, and increased platelet aggregation, all of which are hypothesized to contribute to atherosclerosis and/or plaque rupture (22).
Long-chain n-3 FAs are predominantly obtained from marine sources (e.g., salmon, herring, and mackerel). They can also be synthesized very sparingly in vivo from alpha-linolenic acid which is found in certain plant oils (e.g., flaxseed, canola, soybean, and walnuts). Average intakes of EPA and DHA in the US are 110 to 170 mg/day, and intakes can vary widely depending on location and cultural dietary habits (25). Ecological studies have shown that countries with lower fish intake have higher rates of depression (18,19). Casecontrol and cross-sectional studies have linked depression in general medical populations with low fish intake (48) and with reduced plasma n-3 FA levels (49). The latter is also prospectively associated with higher rates of postpartum depression (20).
To date, six randomized, placebo-controlled trials have been performed with n-3 FA supplements in depressed general medical patients (50 – 55). Although all of these studies have enrolled relatively small numbers of patients, the effects of supplementation on depressive symptoms have been mixed, with four showing improvements in depressive symptoms. These inconsistencies are likely due to methodological issues, such as differences in dose of n-3 FA (which have varied from 0.2 to 4 g/day), and differences in formulation (EPA alone, DHA alone or combined) of n-3 FA. Typically, studies showing a beneficial effect used EPA at a dose of at least 1 g/day with or without DHA. Whereas some studies used n-3 FA supplements alone to treat depression, others used n-3 FA in combination with traditional antidepressants. The studies that showed a beneficial effect of n-3 FA were more likely to have used the latter design. Therefore, the effect of n-3 FA, independent of other more traditional therapeutic options for depression, is difficult to determine and the exact role of n-3 FA in the management of depression warrants further investigation. We found that a 5% point increase in the n-3 index (e.g., from 3% to 8% of total FAs) was associated with a reduction in 1 PHQ point. It would take about 1 additional gram of EPA+DHA per day to accomplish this (27).
In contrast to the uncertainties surrounding the role of n-3 FAs as a treatment for depression, their use in reducing the risk for adverse outcomes after an ACS event is clearer. Multiple observational studies have shown that low levels of n-3 FAs are associated with adverse outcomes, both sudden cardiac death and total coronary heart disease events (3). Randomized trials have also shown that n-3 FA supplementation in post MI patients leads to improved survival (3). Accordingly, the American Heart Association recommended that patients with documented CAD consume around 1 g of EPA+DHA per day. These sentiments are echoed in the American Heart Association/American College of Cardiology guidelines for the management of ST-elevation MI, which recommend that post MI patients be encouraged to increase the amount of n-3 FA in their diet as part of their overall dietary lipid management (Class I Recommendation) (29).
The strengths of this study include a large and well-defined patient population, the assessment of a biomarker of n-3 FA intake (instead of estimates of dietary intake), and the use of a standardized assessment of depressive symptoms. Nonetheless, several limitations should be considered. First, it is a cross-sectional study and, therefore, cannot demonstrate causality between low levels of n-3 FA and depressive symptoms at the time of an ACS. In other words, because those persons with depressive symptoms may have had them before their admission for an ACS, depression itself could have resulted in altered dietary habits with lower fish intake, reducing blood cell n-3 FA levels. Additionally, this study was conducted at only two centers in one city in the central United States, and local dietary habits may have limited the range of available n-3 FA levels from which the association with depressive symptoms could more thoroughly be examined. Multicenter studies are needed to confirm these results and improve their generalizability. Another potential limitation is that the biologic etiologies for depression are complex and not fully known. Therefore, it is possible that our final model adjusted for factors that are along the causal pathway for EPA+DHA and depression. Adjusting for these factors would have biased our adjusted estimate for EPA+DHA and depression toward the null. Finally, because this was an observational study, we cannot exclude the possibility of unmeasured confounding.
CONCLUSION
This study provides additional support for the view that n-3 FA levels are associated with depressive symptoms in ACS patients, and suggests that interventions to raise the n-3 index may help prevent both depressive symptoms and adverse cardiovascular outcomes. The extent to which n-3 FA therapy would be effective in treating depression in ACS patients is still unknown.
Acknowledgments
The authors wish to express their appreciation to Karen Nugent, Amy Shipman and Mary Baston for their important contributions in data collection and sample processing, and to Philip Jones for statistical assistance. Finally, we are indebted to Dr. James Crockett and the Saint Luke’s Hospital Foundation for providing funding for this study.
Financial Sources: This project was principally supported by R-01 HS11282 from the Agency for Healthcare Research and Quality and by a grant from the Saint Luke’s Hospital Foundation.
Glossary
- AA
arachidonic acid
- ACS
acute coronary syndrome
- BMI
body mass index
- CAD
coronary artery disease
- CV
cardiovascular
- DHA
docosahexaenoic acid
- EPA
eicosapentanenoic acid
- FA
fatty acids
- GC
gas chromatography
- MI
myocardial infarction
- n-3
omega-3
- n-6
omega-6
- PHQ
Patient Health Questionnaire-9
- UA
unstable angina
Footnotes
Disclosures: Dr. Harris is a consultant to two companies with interests in omega-3 fatty acids [Monsanto Company and Reliant Pharmaceuticals, Inc.(now GlaxoSmithKline)], has received research grants from both, and is on the speakers’ bureau for Reliant. Dr. Spertus has leadership responsibilities for CV Outcomes, Inc., Health Outcomes Sciences and Outcomes Instruments; is a consultant for Amgen, United Healthcare, and Otsuka; receives research grant support from the National Institutes of Health, Amgen, Lilly, Roche Diagnostics, Atherotech, and the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR); owns the copyrights for the Seattle Angina Questionnaire, the Kansas City Cardiomyopathy Questionnaire, and the Peripheral Artery Questionnaire; and previously received grant support and was a consultant for CV Therapeutics.
References
- 1.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999– 1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 2.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 4.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38:391– 8. doi: 10.1007/s11745-003-1074-0. [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos AP, Leaf A, Salem N., Jr Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43:127–30. doi: 10.1159/000012777. [DOI] [PubMed] [Google Scholar]
- 6.Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 7.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. Am J Clin Nutr. 1995;62:1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Agren JJ, Vaisanen S, Hanninen O, Muller AD, Hornstra G. Hemostatic factors and platelet aggregation after a fish-enriched diet or fish oil or docosahexaenoic acid supplementation. Prostaglandins Leukot Essent Fatty Acids. 1997;57:419– 21. doi: 10.1016/s0952-3278(97)90421-x. [DOI] [PubMed] [Google Scholar]
- 9.Mori TA, Beilin LJ, Burke V, Morris J, Ritchie J. Interactions between dietary fat, fish, and fish oils and their effects on platelet function in men at risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 1997;17:279–86. doi: 10.1161/01.atv.17.2.279. [DOI] [PubMed] [Google Scholar]
- 10.Eritsland J, Arnesen H, Gronseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996;77:31– 6. doi: 10.1016/s0002-9149(97)89130-8. [DOI] [PubMed] [Google Scholar]
- 11.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477– 85. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 12.Uauy R, Mena P, Valenzuela A. Essential fatty acids as determinants of lipid requirements in infants, children and adults. Eur J Clin Nutr. 1999;53(Suppl 1):S66– S77. doi: 10.1038/sj.ejcn.1600745. [DOI] [PubMed] [Google Scholar]
- 13.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 14.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318:549– 57. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- 15.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–6. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Bruinsma KA, Taren DL. Dieting, essential fatty acid intake, and depression. Nutr Rev. 2000;58:98– 108. doi: 10.1111/j.1753-4887.2000.tb07539.x. [DOI] [PubMed] [Google Scholar]
- 17.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam study. Am J Clin Nutr. 2003;78:40–6. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 19.Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 20.Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69:237– 43. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 21.Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–24. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severus WE, Littman AB, Stoll AL. Omega-3 fatty acids, homocysteine, and the increased risk of cardiovascular mortality in major depressive disorder. Harv Rev Psychiatry. 2001;9:280– 93. doi: 10.1080/10673220127910. [DOI] [PubMed] [Google Scholar]
- 23.Sontrop J, Campbell MK. Omega-3 polyunsaturated fatty acids and depression: a review of the evidence and a methodological critique. Prev Med. 2006;42:4– 13. doi: 10.1016/j.ypmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 25.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98:3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Parker GB, Heruc GA, Hilton TM, Olley A, Brotchie H, Hadzi-Pavlovic D, Friend C, Walsh WF, Stocker R. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Res. 2006;141:279–86. doi: 10.1016/j.psychres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Harris WS, Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The prospective registry evaluating myocardial infarction: events and recovery (PREMIER)–evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589– 97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 30.Braunwald E. Unstable angina. A classification Circulation. 1989;80:410–4. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 31.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a selfreport version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737– 44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606– 13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the heart and soul study) Am J Cardiol. 2005;96:1076–81. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–50. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- 36.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the third national health and nutrition examination survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 37.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216– 26. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 38.Leng GC, Smith FB, Fowkes FG, Horrobin DF, Ells K, Morse-Fisher N, Lowe GD. Relationship between plasma essential fatty acids and smoking, serum lipids, blood pressure and haemostatic and rheological factors. Prostaglandins Leukot Essent Fatty Acids. 1994;51:101– 8. doi: 10.1016/0952-3278(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 39.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–7. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 40.Yeh LL, Kuller LH, Bunker CH, Ukoli FA, Huston SL, Terrell DF. The role of socioeconomic status and serum fatty acids in the relationship between intake of animal foods and cardiovascular risk factors. Ann Epidemiol. 1996;6:290–8. doi: 10.1016/s1047-2797(96)00023-3. [DOI] [PubMed] [Google Scholar]
- 41.Gillum RF, Mussolino M, Madans JH. The relation between fish consumption, death from all causes, and incidence of coronary heart disease. The NHANES I epidemiologic follow-up study. J Clin Epidemiol. 2000;53:237– 44. doi: 10.1016/s0895-4356(99)00149-3. [DOI] [PubMed] [Google Scholar]
- 42.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–8. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 43.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257– 65. [PubMed] [Google Scholar]
- 44.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 45.Guallar E, Aro A, Jimenez FJ, Martin-Moreno JM, Salminen I, van’t Veer P, Kardinaal AF, Gomez-Aracena J, Martin BC, Kohlmeier L, Kark JD, Mazaev VP, Ringstad J, Guillen J, Riemersma RA, Huttunen JK, Thamm M, Kok FJ. Omega-3 fatty acids in adipose tissue and risk of myocardial infarction: the EURAMIC study. Arterioscler Thromb Vasc Biol. 1999;19:1111– 8. doi: 10.1161/01.atv.19.4.1111. [DOI] [PubMed] [Google Scholar]
- 46.Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: a prospective study. Am J Epidemiol. 1995;142:166– 75. doi: 10.1093/oxfordjournals.aje.a117615. [DOI] [PubMed] [Google Scholar]
- 47.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon diet heart study. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 48.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–31. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 49.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35– 46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 50.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–9. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 51.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:267–71. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 52.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyleicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–9. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 53.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72:211– 8. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–8. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 55.Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am J Obstet Gynecol. 2003;188:1348–53. doi: 10.1067/mob.2003.275. [DOI] [PubMed] [Google Scholar]