Abstract
Soil has been identified as a significant source of lead (Pb) exposure for both children and adults. Therefore, identifying possibly contaminated soils by soil testing is important to protect public health. Soil Pb test results are usually reported as total Pb (mg kg−1), carried out using a concentrated nitric acid digestion procedure by hot plate (EPA method 3050) or microwave (EPA method 3051) followed by inductively coupled plasma atomic emission spectrometry to determine total Pb in the digest. However, this procedure is both time-consuming and expensive, sometimes costing homeowners and gardeners over $50 per sample. To make soil Pb testing more economically accessible to homeowners and gardeners, several university soil-testing laboratories offer less expensive screening tests designed to estimate total soil Pb. The first objective of this study was to compare three commonly used screening tests, modified Morgan (MM), Mehlich 3 (M3), and 1 M nitric acid (HNO3), to the standard total Pb testing method (EPA method 3051) to find which extractant is the most reliable predictor of total Pb. The second objective was to investigate the effect that different degrees of soil grinding have on the total Pb test and the extracted Pb concentration measured from the 1 M HNO3 test. Results indicate that the strongest predictor of total Pb is 1 M HNO3, followed by M3, and MM, and that thorough grinding is necessary if using less than five grams of soil in a Pb test, in order to adequately homogenize Pb-contaminated samples and achieve acceptable testing reproducibility.
Keywords: heavy metals, soil testing, lead screening test, urban gardens
Introduction
Heavy metal contamination is commonly found in urban areas where people, houses, vehicles, and industry are concentrated. One metal contaminant of major concern in urban soil is lead (Pb). Although the heavy metal Pb is found naturally in soil in concentrations from 1 to 200 mg kg−1 with a mean of 15 mg kg−1 (Zimdahl and Skogerboe, 1977), anthropogenic sources have polluted many soils substantially beyond these levels. In urban areas, Pb is often found in concentrations greater than 1000 mg kg−1 (Langley-Turnbaugh and Belanger, 2007; Langley-Turnbaugh and Belanger, 2010; Mielke, 1999; Wagner and Langley-Turnbaugh, 2008). There are many sources of anthropogenic Pb pollution such as smelting, Pb arsenate pesticides, auto emissions from leaded gasoline, and Pb-based paint.
Exposure to Pb can have negative impacts on human health. The Centers for Disease Control and Prevention cite Pb poisoning as the most common and serious environmental disease affecting children under age five (CDC, 2002). Pb exposure can lead to systemic effects (gastrointestinal effects, anemia, hyper-tension and hearing loss), nervous system effects (behavior and cognition), and effects on the reproductive system. For young children and fetuses, even low levels of Pb exposure can suppress intelligence, neurological and behavioral development (Prüss-Üstün et al., 2004; Shannon, 2003).
Although the direct ingestion of Pb paint fragments and dust has often been thought to be the most important pathway of Pb exposure to children, soil has also been identified as a significant source of exposure for both children and adults (Clark et al., 2006; Mielke and Reagan, 1998; Mielke et al., 1999). Adults can ingest and inhale soil incidentally while engaging in outdoor activities such as gardening. Young children, who commonly engage in hand-to-mouth behavior (unintentional consumption) or pica disorder (intentional consumption), are at the greatest risk of ingesting soil Pb (Mielke, 1999). Additionally, Calabrese and Stanek (1992) estimate that 31.3% of household dust is actually derived from outdoor soil and others have estimated higher percentages (USEPA, 1994).
In 2001, the EPA established two soil Pb hazard standards in order to protect public health. The standards are 400 mg kg−1 total Pb on bare soil of children’s play areas, and 1200 mg kg−1 total Pb on bare soil anywhere else in the yard. The soil standards are intended to identify dangerous levels of Pb, and the property owner should implement measures to reduce or prevent exposure to Pb in soil that exceeds these levels (USEPA, 2001).
Children and adults can be exposed to soil Pb in yards, community gardens and playgrounds particularly in urban areas. Identifying these possibly contaminated soils by soil testing is important to protect public health. Soil Pb concentration can vary greatly over short distances in gardens and yards (Chaney et al., 1982; Langley-Turnbaugh and Belanger, 2007), establishing the need to test more than one soil sample collected from each area. Vegetables grown in lead contaminated soil can contain concentrations of Pb, and consumption of these vegetables can increase Pb exposure and associated health risks (Chaney et al., 1982; Finster et al., 2004; Langley-Turnbaugh and Belanger, 2010; Samsøe-Petersen et al., 2002) again emphasizing the need for more intensive sampling and testing of garden soils.
Soil Pb test results are usually reported as total Pb (mg kg−1), carried out using a concentrated nitric acid digestion procedure by a hot plate (EPA method 3050) or microwave (EPA method 3051) followed by inductively coupled plasma atomic emission spectrometry (ICP-AES) to determine total Pb in the digest (USEPA, 2009). This procedure is both time-consuming and expensive, sometimes costing homeowners and gardeners over $50 per sample. Submitting sufficient soil samples to characterize a site may not be practical for many situations given the high cost of the EPA 3050/3051 total lead test.
To make soil Pb testing more economically accessible to homeowners and gardeners, several university soil-testing laboratories offer less expensive screening tests designed to estimate total soil Pb. Although public health determinations should be based on total metals analysis, screening soils can be useful for initial site characterization, to guide sample collection for confirmatory total metals analysis, and to inform contamination/exposure mitigation efforts (Chaney et al., 1982; Hamel et al., 2003; McBride et al., 2011). The three most common solutions being used to extract soil Pb for screening tests are 1 M nitric acid (HNO3), Mehlich 3 solution (M3), and modified Morgan solution (MM). It is convenient for labs to use M3 or MM because these solutions are already being used to test for plant nutrients in the mid-western and northeastern United States, respectively (University of Delaware, 2011). Although 1 M HNO3 is not a traditional extractant for plant nutrients, it has been found to reliably predict total soil Pb (Chaney et al., 1982; McBride et al., 2011), is inexpensive, and can be implemented easily in the laboratory.
Soil preparation is an important step in any soil testing procedure. Common practice for the total Pb test and for Pb screening tests is to grind the soil to pass a 2-mm screen. Using this grinding size, it has been found that there is often a small coefficient of variation within a sample that has been contaminated with Pb accumulated over a long period of time from sources such as smelting and sludge. However, in Pb paint contaminated soil, particulate paint chips can cause a high coefficient of variation within the sample (Chaney et al., 1982). In these soils a finer grinding size may be necessary to homogenize the soil.
The first objective of this study was to compare the MM, M3 and 1 M HNO3 screening tests to the standard total lead test (EPA method 3051) to find which extractant is the most reliable predictor of total lead. The second objective was to investigate the effect that different degrees of soil grinding have on the total Pb concentration and on the extracted lead concentration measured from the 1 M HNO3 screening test.
Materials and Methods
Screening test comparison
The soil samples used for this study were collected from both an urban and an agricultural setting. Forty-five samples were collected from urban community gardens in New York City, which had possible contamination from sources such as Pb paint, leaded gasoline emissions, incinerators, and coal combustion. Thirty-five samples came from an old apple orchard in Ithaca, NY with a history of Pb arsenate insecticide use. The orchard soil was a silty clay loam, Hudson series (fine illitic, mesic, Glossaquic Hapludalf). Both the urban garden samples and the agricultural (orchard) soils were collected using a trowel from the top 15 cm of the soil.
All soil samples were air-dried, coarsely ground, and sieved to pass a 2-mm plastic screen. A portion of each sample was digested using the EPA 3051 method (USEPA, 2009) and total Pb was determined using ICP-AES at 220.45 nm. For this method, 0.5 g of soil was used. A portion of each sample was also extracted by each of three screening test solutions for soil Pb: MM, M3, and 1 M HNO3.
The MM solution was prepared by combining 1.25 M glacial acetic acid (CH3COOH), 0.62 M ammonium hydroxide (NH4OH), and distilled water (McIntosh, 1969). The solution was stirred and then adjusted to a pH of 4.8 using acetic acid or ammonium hydroxide. Five g of soil was weighed into a 125 mL Erlenmeyer flask to which was added 25 mL of MM solution. Samples were shaken on a rotary shaker for 15 minutes, and then filtered using Whatman No. 42 paper. Flame atomic absorption (FAA, 217 nm) was used to analyze the extracts for Pb, using Pb nitrate standards of 1 to 20 mg L−1 made up in MM solution.
The M3 solution was prepared such that final concentrations of reagents were 0.2 M glacial acetic acid (CH3COOH), 0.25 M ammonium nitrate (NH4NO3), 0.015 M ammonium fluoride (NH4F), 0.013 M nitric acid (HNO3), and 0.001 M EDTA (Mehlich, 1984). The solution was stirred and then adjusted to a pH of 2.4 using either 1 M HCl or 1 M NH4OH. After weighing 5.0 g of soil into a 125 mL Erlenmeyer flask, 50 mL of M3 solution was added and then agitated on a rotary shaker for 5 min. Samples were then paper filtered (Whatman No. 42) and analyzed for Pb using FAA (217 nm) with Pb nitrate standards of 1 to 20 mg L−1 made up in M3 solution.
The 1 M HNO3 was prepared by diluting concentrated trace-metal-grade nitric acid in distilled water. After weighing 5.0 g of soil into a 125 mL Erlenmeyer flask, 50 mL of 1 M HNO3 was added and agitated on a rotary shaker for 1 hr. Samples were then paper filtered (Whatman No. 42) and analyzed for Pb using FAA (217 nm) with Pb nitrate standards of 1 to 20 mg L−1 made in 1 M HNO3.
The amounts of Pb extracted by each method were compared to total Pb (EPA method 3051) using simple linear regression. In order to determine the extraction efficiency of each screening test, the amount of Pb extracted by each method (mg kg−1) was divided by its total Pb concentration (mg kg−1) in the same sample to obtain the percentage of total Pb extracted, as shown by the equation:
Grinding size comparison
This study used twenty soils from NYC urban community gardens that were not included among the 45 samples used to evaluate the screening tests, but had previously been tested for total Pb (EPA 3051 method) and had a concentration in excess of 400 mg kg−1. These soils had been coarsely ground with a mortar and pestle to pass a 2-mm plastic screen. A portion (approximately 8 g) of each coarsely ground sample was more finely ground with a mortar and pestle to a powdery consistency. A sub-portion of the finely ground sample was tested for total Pb (EPA 3051) using ICP-AES and sub-portions of both the coarsely and finely ground portions were tested for 1 M HNO3 extractable Pb by flame atomic absorption. For the EPA 3051 method, 0.5 g of soil was used, while 5.0 g of soil was used for the 1 M HNO3 method (as described above). The concentrations of soil Pb determined by each treatment were compared using simple linear correlation.
Results and Discussion
Screening test comparison
The urban and agricultural soils had a similar range in total Pb concentration of 11 to 1325 mg kg−1 and 24 to 1685 mg kg−1, respectively. The mean and median of total Pb for the urban soils was 218 and 115 mg kg−1, and for the agricultural soils, 312 and 238 mg kg −1, respectively (Table 1.1).
Table 1.1.
Descriptive statistics for soil Pb concentration and extraction efficiency (extraction concentration in relation to total Pb concentration as determined by EPA method 3051) for each Pb testing method.
| Site Location | -------------------------------------------Pb Testing Method------------------------------------------- | |||||||
|---|---|---|---|---|---|---|---|---|
| EPA 3051 | 1 M HNO3 | M3 | MM | HNO3 | M3 | MM | ||
|
| ||||||||
| -------------Pb concentration (mg kg−1)-------------
|
-------------Extraction Efficiency (%)1-------------
|
|||||||
| All samples (n=80) | Min | 11 | 11 | 8 | 1 | 36 | 21 | 2 |
| Max | 1685 | 1992 | 912 | 473 | 256 | 91 | 45 | |
| Med | 186 | 161 | 95 | 16 | 100 | 54 | 11 | |
| Mean | 260 | 273 | 132 | 42 | 101 | 53 | 13 | |
| Urban (n=45) | Min | 11 | 11 | 8 | 1 | 36 | 21 | 2 |
| Max | 1325 | 1102 | 543 | 473 | 256 | 91 | 45 | |
| Med | 115 | 101 | 59 | 7 | 94 | 55 | 8 | |
| Mean | 218 | 206 | 104 | 35 | 99 | 54 | 11 | |
| Agricultural (n=35) | Min | 24 | 17 | 8 | 1 | 50 | 22 | 3 |
| Max | 1685 | 1992 | 912 | 241 | 145 | 78 | 36 | |
| Med | 238 | 269 | 138 | 34 | 108 | 53 | 13 | |
| Mean | 312 | 358 | 168 | 50 | 104 | 52 | 15 | |
Note that extraction efficiency descriptive statistics are based on calculations from individual samples, not on calculations from concentrations reported on the same line on this table.
The least aggressive of the three extractants, MM, had the lowest extraction efficiency, with a range of 2 to 45% and a mean of 13% for all soils. The average extraction efficiency was 11% for urban soils and 15% for agricultural soils (Table 1.1). These extraction efficiencies are similar to those reported by Hamel et al. (2003), who found a median of 15% for all soils tested, although they found that extraction efficiency ranged greatly depending on the origin of the sample.
Of the three screening tests, MM was the least reliable predictor of total Pb. For all soils, the correlation of MM-extractable Pb to total lead had a highly significant R2 value of 0.664. The R2 value for the agricultural soils was 0.730 and for the urban soils it was 0.401. The correlation, however, was highly influenced by the five most contaminated soils, both urban and agricultural, that had a range from 945 to 1685 mg kg−1. Not including these samples, the R2 for all soils (n=75) falls to 0.551 (Figure 1). McBride et al. (2011) found a stronger correlation of MM extractable Pb to total Pb (R2 = 0.84) for soils ranging from 10 to 1182 mg kg−1 total Pb. However, in that case the correlation was highly influenced by four highly contaminated calcareous industrial-site soils. Because the MM solution is effective at dissolving carbonates, it was able to extract a substantial amount of Pb from these samples. When these soils were not included in the correlation, and only soils with less than 500 mg kg−1 soil Pb were considered, the R2 value dropped to 0.303. For samples with less than 720 mg kg−1 total Pb, Hamel et al. (2003) reported an R2 of 0.74 for the correlation of MM extractable Pb to total Pb.
Figure 1.
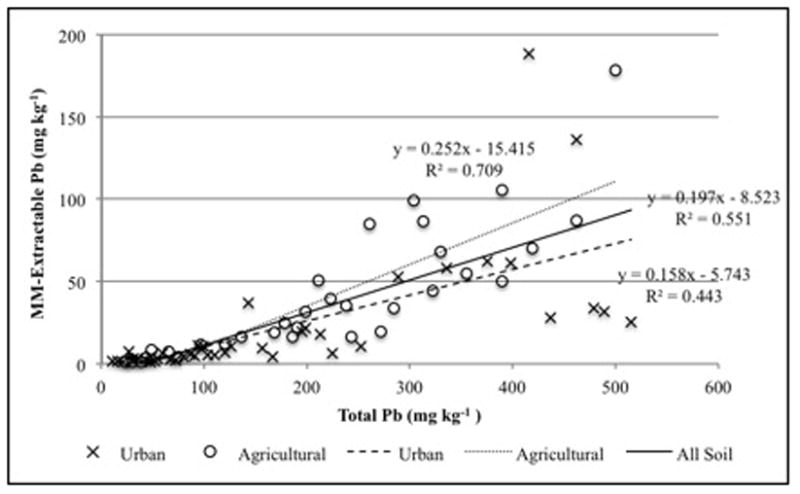
Relationship of Modified Morgan (MM)-extractable Pb to total Pb, excluding soils greater than 945 mg kg−1 total Pb.
The second best predictor of total Pb was M3. Extraction efficiency was very similar for both urban and agricultural soils- 54% and 52%, respectively. The range in extraction efficiency across all soils was 21 to 91%. For all soils, the correlation of M3-extractable Pb to total Pb had an R2 value of 0.869 (Figure 2). The correlation for agricultural soils, with an R2 of 0.894, was better than that for urban soils, which had an R2 value of 0.844. As with MM, the correlation of M3-extractable Pb to total Pb for all soils was influenced by the five soils with soil Pb greater than 945 mg kg−1, though not as strongly. Excluding these five soils, the R2 value for the remaining soils decreased to 0.847. For urban soils (n=43), the R2 decreased to 0.806, and for agricultural soils (n=32), the R2 value actually increased to 0.942 (Figure 2). The correlation of M3-extractable Pb to total Pb determined by this study was relatively stronger than that found by Hamel et al. (2003), who reported an R2 value of 0.60 for soils with less than 720 mg kg−1 soil Pb.
Figure 2.
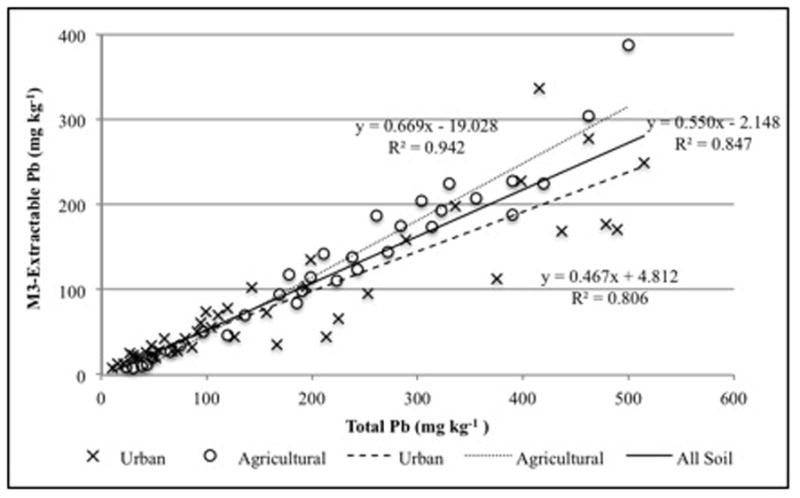
Relationship of Mehlich 3 (M3)-extractable Pb to total Pb, excluding soils greater than 945 mg kg−1 total Pb.
The strongest extractant tested, 1 M HNO3, had an apparent average extraction efficiency of 101% across all soils. For urban and agricultural soils, the apparent average extraction efficiency was 99 and 104%, respectively (Table 1.1). An efficiency so close to 100% is not expected because the 1 M HNO3 test is less chemically aggressive than the total Pb acid digestion method. However, there are other differences between these two soil test procedures, which may account for the higher than expected efficiency of 1 M HNO3. Specifically, the sample size used for the total Pb test is only 1/10 of that used for the 1 M HNO3 screening test (0.5 g vs. 5 g), so that heterogeneity of Pb distribution could influence the measured Pb concentration. For example, as soil sample size for lab testing is reduced, the probability that any relatively large Pb-rich particles (“nuggets”) are excluded from that single sample may increase. Therefore, depending on the number, density and average size of Pb-rich particles in the soil matrix, soil Pb tests based on smaller sample size could more often than not produce lower apparent values for soil Pb than tests based on larger sample size. This concern with soil heterogeneity will be addressed below in the results of the grinding comparison study.
In addition to having the greatest extraction efficiency, 1 M HNO3 was also the strongest predictor of total Pb. For all soils, the R2 value was 0.940. For urban and agricultural soils, it was 0.900 and 0.991, respectively. These values, however, are heavily influenced by the five soils greater than 945 mg kg−1. Nevertheless, not including these samples, the R2 values were still 0.825 and 0.955 for urban and agricultural soils, respectively. For all soils containing less than 945 mg kg−1 total Pb, the R2 value was 0.872 (Figure 3). This correlation is similar to that found by McBride et al. (2011), who reported an R2 value of 0.933 for soils with Pb content of less than 500 mg kg−1.
Figure 3.
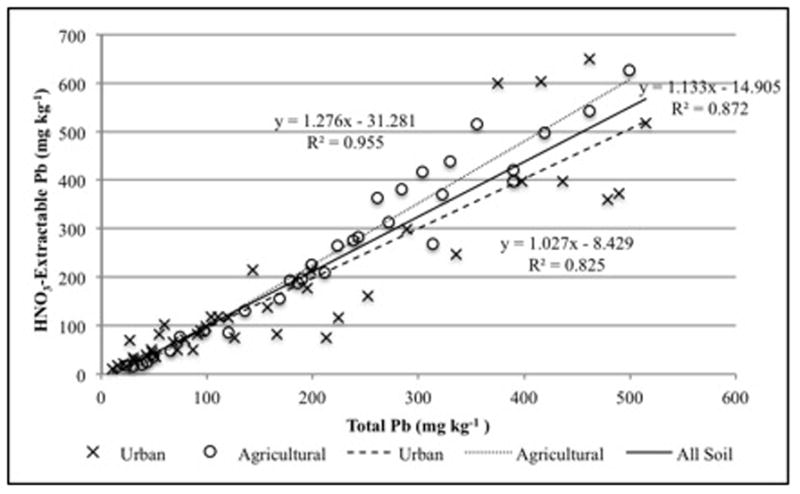
Relationship of 1 M HNO3-extractable Pb to total Pb, excluding soils greater than 945 mg kg−1 total Pb.
The urban garden soil contamination is likely to be a combination of historical aerial deposition from incinerators, coal combustion, and leaded gasoline as well as lead-containing paint from building debris. In contrast, the orchard soil contamination is almost totally due to Pb arsenate pesticide use. There are differences in the ease of extraction of soil Pb from these different sources (McBride et al., 2011), and preliminary investigations have shown evidence of discrete Pb-rich particles (“nuggets”) in both the urban and orchard soil. However, the “nugget” size in many cases is much larger in the urban than in the orchard soils, accounting for more Pb homogeneity in the orchard soil and a very good correlation between HNO3-extractable and total Pb for this soil.
Grinding Size Comparison
There was a poor correlation between 1 M HNO3-extractable Pb and total Pb for coarsely ground soil (< 2 mm) of samples with a concentration greater than 400 mg kg-1 total Pb that were used in this study (Figure 4). While the 1 M HNO3 test extracted a wide range of Pb for these selected soils, all but three of the twenty soils had between 400 and 600 mg kg−1 total Pb as determined by EPA method 3051. However, when portions of the soils were finely ground re-tested for 1 M HNO3-extractable Pb and total Pb, there was an excellent correlation, as shown in Figure 5. The R2 value increased from 0.337 when the samples were coarsely ground to 0.917 after they were finely ground. This suggests that by finely grinding the soil, the Pb was homogenized within the sample so that the 1 M HNO3 test, which uses 5.0 g of soil, correlates to the total Pb test, which uses only 0.5 g of soil. Furthermore, the regression slope in Figure 4 of 0.903 indicates that 1 M HNO3 extracted about 90% of total Pb, suggesting that the near-100% extraction efficiency described earlier was an artifact of soil Pb heterogeneity.
Figure 4.
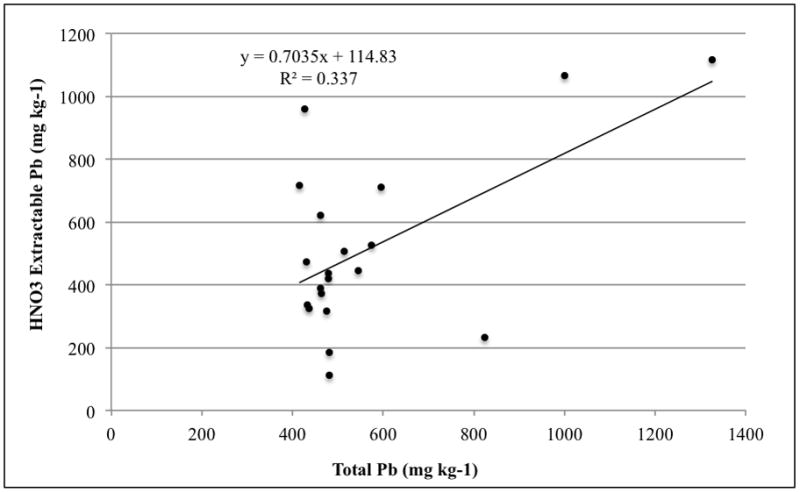
The correlation of 1 M HNO3-extractable Pb to total Pb in coarsely ground soil.
Figure 5.
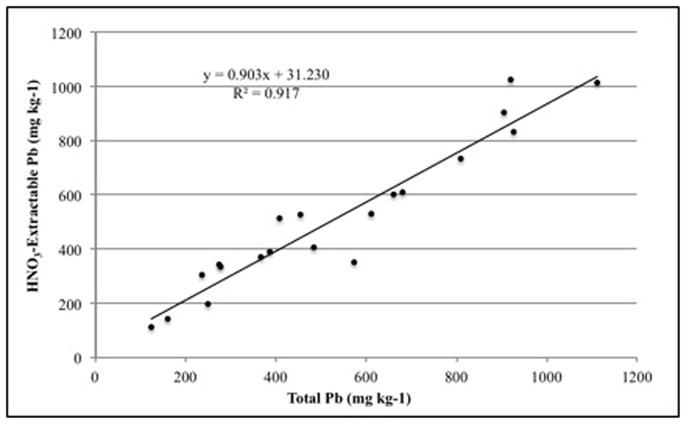
The correlation of 1 M HNO3-extractable Pb to total Pb in finely ground soil.
The correlation of 1 M HNO3-extractable Pb in finely ground soil to 1 M HNO3-extractable Pb in coarsely ground soil had an R2 of 0.881, indicating that these treatments are well correlated (Table 2). This suggests that a high degree of soil Pb homogenization is not as necessary when using the 1 M HNO3 test because the test uses a much larger amount of soil. Specifically, 5 g seems to be sufficient to represent the entire sample without fine grinding, while 0.5 g, as used in the soil digestion for the total Pb test, does not. This is supported by the significant correlation of 1 M HNO3-extractable Pb of coarsely ground to total Pb of finely ground soil (Table 2).
Table 2.
R2 values for correlations of concentrations for various combinations of grinding size (coarse and fine) and Pb test (total and 1 M HNO3).
| Pb Tests | R2 | |
|---|---|---|
| Total, coarse grind | 1 M HNO3, coarse grind | 0.337 |
| Total, fine grind | 1 M HNO3,fine grind | 0.917 |
| 1 M HNO3,fine grind | 1 M HNO3,coarse grind | 0.881 |
| Total, fine grind | 1 M HNO3,coarse grind | 0.749 |
Urban soil samples contaminated with Pb can be highly heterogeneous. Therefore, if only testing a small amount of soil for Pb, as in the EPA 3050/3051 total Pb testing method, it is important to finely grind a larger portion of the soil in order to adequately homogenize it before testing a sub-portion. If the soil is not well ground, but merely put through a 2-mm sieve, then it seems necessary to use more soil for the Pb test. Because the soil samples for the initial screening test comparison were not finely ground (Figures 1–3), it is likely that heterogeneity within Pb contaminated samples had a negative influence on the correlation of extractable Pb to total Pb for all the screening methods. If the soil had indeed been finely ground to reduce within-sample variability, it is probable that there would have been a higher positive correlation between the screening test extractable Pb and total Pb in Figures 1–3. It would then follow that that the average relative extraction of 1 M HNO3 would no longer appear to be greater than 100%, but would reflect the true extraction efficiency.
Conclusions
Of the three screening tests compared in this study, the 1 M HNO3 screening test was the strongest predictor of total Pb. M3-extractable Pb was also highly correlated to total Pb, but because it only extracts an average of 53% of total Pb, it is necessary to use a regression equation in order to determine an estimate of total Pb. Because the 1 M HNO3 screening test extracts such a high proportion of total Pb, it is not necessary to use a regression equation in order to get an estimate of total Pb. The extractant 1 M HNO3 was first proposed for a screening test for total Pb in 1982 by Chaney et al. (1982) who found that in five out of seven samples with different properties, it extracted over 92% of total Pb.
Soil preparation is an important step of soil Pb testing, and can greatly affect the results of a test. It is important to homogenize the soil, due to the inherent heterogeneity of soil Pb in a contaminated sample, especially if measuring total Pb by acid digestion (EPA 3050/3051), which typically only uses 0.5 or 1.0 g of soil. One benefit of using more soil for a test, as is done for the 1 M HNO3 screening test, is the ability to get a more accurate representation of the soil without having to grind as finely.
For future research, it would be useful to develop correlations of extractable Pb to total Pb for soils with different sources of Pb contamination, due to the extractability differences of different species of Pb. This would help to improve the screening tests and increase the accuracy of total Pb estimates.
Acknowledgments
Funding received from the National Institutes of Health and the National Institute of Environmental Health Sciences Grant #15R21ES017921
The authors wish to thank Donna Lopp and Leigh Kalbacker for their time and effort collecting and analyzing many soil samples used for this study. We also thank Edie Stone of the community gardening program GreenThumb for giving us access to the urban community gardens that were soil sampled, in addition to the Dilmun Hill Cornell Student Farm, the origin of the agricultural soils used in this study. Lastly we thank the National Institutes of Health and National Institute of Environmental Health Sciences for financial support.
Contributor Information
Sarah E. Wharton, Email: sew245@cornell.edu, Department of Crop and Soil Sciences, Cornell University, 817 Bradfield Hall, Ithaca, NY 14853, (607) 222-8225
Hannah A. Shayler, Email: has34@cornell.edu, Department of Crop and Soil Sciences, Cornell University, 818 Bradfield Hall, Ithaca, NY 14853, (607) 254-2377
Henry M. Spliethoff, Email: hms01@health.state.ny.us, Center for Environmental Health, New York State Department of Health, 547 River Street, Room 330, Troy, NY 12180-2216, (518) 402-7800, Fax (518) 402-7819
Lydia G. Marquez-Bravo, Email: lgm01@health.state.ny.us, Center for Environmental Health, New York State Department of Health, 547 River Street, Room 330, Troy, NY 12180-2216, (518) 402-7800, Fax (518) 402-7819
Lisa Ribaudo, Email: lnr02@health.state.ny.us, Center for Environmental Health, New York State Department of Health, 547 River Street, Room 330, Troy, NY 12180-2216, (518) 402-7800, Fax (518) 402-7819
Murray B. McBride, Email: mbm7@cornell.edu, Department of Crop and Soil Sciences, Cornell University, 910 Bradfield Hall, Ithaca, NY 14853, (607) 255-1728
References
- Calabrese EJ, Stanek EJ. What proportion of household dust is derived from outdoor soil? Journal of Soil Contamination. 1992;1:253–263. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Managing elevated blood lead levels among young children: recommendations from the advisory committee on childhood lead poisoning prevention. CDC; Atlanta: 2002. [Google Scholar]
- Chaney RL, Sterrett SB, Mielke HW. The potential for heavy metal exposure from urban gardens and soils. In: Preer JR, editor. Proceedings of the symposium on heavy metals in urban gardens. Agricultural Experiment Station of the District of Columbia, University of the District of Columbia; Washington D.C: 1982. pp. 37–84. [Google Scholar]
- Clark HF, Brabander DJ, Erdil RM. Sources, sinks, and exposure pathways of lead in urban garden soil. J Environ Qual. 2006;35:2066–2074. doi: 10.2134/jeq2005.0464. [DOI] [PubMed] [Google Scholar]
- Finster ME, Gray KA, Binns HJ. Lead levels of edibles grown in contaminated residential soils: A field survey. Sci Total Environ. 2004;320:245–257. doi: 10.1016/j.scitotenv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Hamel SC, Heckman JR, Shilke-Gartley KL, Hoskins B. Lead extraction using three soil fertility tests and environmental protection agency method 3050. Commun Soil Sci Plant Anal. 2003;34:2853–2873. [Google Scholar]
- Langley-Turnbaugh SJ, Belanger LG. Phytoremediation of lead in urban residential soils of Portland, Maine. Soil Survey Horizons. 2010;51:95–101. [Google Scholar]
- Langley-Turnbaugh SJ, Belanger LG. Lead distribution in urban residential soils of Portland, Maine. Soil Survey Horizons. 2007;48:18–22. [Google Scholar]
- McBride MB, Mathur RR, Baker L. Chemical extractability of lead in field-contaminated soils: Implications for estimating total lead. Commun Soil Sci Plant Anal. 2011;42:1581–1593. doi: 10.1080/00103624.2011.581729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JL. Bray and Morgan soil extractants modified for testing acid soils from different parent materials. Agronomy Journal. 1969;61:259–265. [Google Scholar]
- Mehlich A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal. 1984;15:1409–1416. [Google Scholar]
- Mielke HW. Lead in the inner cities. Am Sci. 1999;87:62–73. [Google Scholar]
- Mielke HW, Gonzales CR, Smith MK, Mielke PW. The urban environment and children’s health: Soils as an integrator of lead, zinc, and cadmium in New Orleans, Louisiana, U.S.A. Environ Res. 1999;81:117–29. doi: 10.1006/enrs.1999.3966. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Reagan PL. Soil is an important pathway of human lead exposure. Environmental Health Perspectives Supplements. 1998;106:217–229. doi: 10.1289/ehp.98106s1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss-Üstün A, Fewtrell L, Landrigan PJ, Ayuso-Mateos JL. Lead exposure. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks, global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. pp. 1495–1542. [Google Scholar]
- Samsøe-Petersen L, Larsen EH, Larsen PB, Bruun P. Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils. Environ Sci Technol. 2002;36:3057–63. doi: 10.1021/es015691t. [DOI] [PubMed] [Google Scholar]
- Shannon M. Severe lead poisoning in pregnancy. Ambulatory Pediatrics. 2003;3:37–39. doi: 10.1367/1539-4409(2003)003<0037:slpip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA) Guidance manual for the integrated exposure uptake biokinetic model for lead in children. 1994. Washington: US Environmental Protection Agency, Office of Emergency and Remedial Response. EPA/540-R-93-081. [Google Scholar]
- United States Environmental Protection Agency (USEPA) SW-846 on-line. 2009 http://www.epa.gov/osw/hazard/testmethods/sw846/online/. Retrieved May 26, 2011.
- United States Environmental Protection Agency (USEPA) Federal register: January 5, 2001 identification of dangerous levels of lead; final rule. U.S. Office of the Federal Register; Washington, D.C: 2001. pp. 1205–1240. [Google Scholar]
- University of Delaware and United States. Recommended soil testing procedures for the Northeastern United States. Newark, Del: Cooperative Extension, University of Delaware; 2011. [Google Scholar]
- Wagner T, Langley-Turnbaugh S. Case study: Examining the contribution of historical sources of lead in urban soils in Portland, Maine, USA. Journal of Environmental Planning and Management. 2008;51:525–541. [Google Scholar]
- Zimdahl RL, Skogerboe RK. Behavior of lead in soil. Environ Sci Technol. 1977;11:1202–1207. [Google Scholar]


