Abstract
Recent studies of the spinal motor system of zebrafish, along with work in other species, are leading to some principles that appear to underlie the organization and recruitment of motor networks in cord: (1) broad neuronal classes defined by a set of transcription factors, key morphological features, and transmitter phenotypes arise in an orderly way from different dorso-ventral zones in spinal cord; (2) motor behaviors and both motoneurons and interneurons differentiate in order from gross, often faster, movements and the neurons driving them to progressively slower movements and their underlying neurons; (3) recruitment order of motoneurons and interneurons is based upon time of differentiation; (4) different locomotor speeds involve some shifts in the set of active interneurons. Here we review these principles and some of their implications for other parts of the brain, other vertebrates, and limbed locomotion.
Keywords: motoneurons, spinal interneurons, transcription factors, locomotion, motor pattern
Introduction
Studies of spinal motor networks have focused in a concerted way on identifying neurons involved in rhythmic motor behaviors with an aim toward unraveling the network responsible for generating the rhythmic movements that underlie much of locomotion.1–5 This admirable, though sometimes elusive, goal has left other, no less important, avenues of study less well populated. Our recent work has been focused on one of these: the patterns of recruitment of motoneurons and interneurons in the spinal cord.6–14 While there is a long-standing body of important studies of how motoneurons are recruited, 15 with a few exceptions, the interneurons have been much less studied, probably because of the difficulties of studying identified populations active in rhythmic locomotion. The zebrafish model has allowed us to attack questions of the functional organization of interneurons (and motoneurons) in ways that are not feasible in other species.
Our results have revealed some simple underlying patterns to the organization of the spinal neurons that link their development and structural organization to their functional roles. This has allowed us to formulate some initial principles of the organization of spinal networks that might extend to networks in the brain. The patterns also allow for predictions about possible features of organization of the mammalian locomotor central pattern generator. Here we articulate the principles and review evidence supporting them from zebrafish, along with other evidence suggesting that they may apply broadly in vertebrate nervous systems. Finally, we also consider their implications for the construction of mammalian central pattern generators.
The generation of cell types
The already classic studies of the transcription factor code in chick and mouse spinal cord have revealed the way in which broad classes/categories of spinal neurons are generated.5,16,17 Different categories of neurons arise from different dorso-ventral zones in spinal cord that express unique combinations of transcription factors. This patterning has been extensively reviewed by others.5 What is important here is that the patterning represents a fundamental underlying organization that, based upon studies of zebrafish, frog tadpoles, chicks, and mice, is shared by all vertebrates. The underlying set of core cell types is therefore much the same in all vertebrates.
This key assertion leads to important broader implications, so a brief account of some of the evidence for it is warranted. The transcription factor expression pattern in tetrapods is one in which the factors are expressed during development in bands across spinal cord at different dorso-ventral locations. These are arranged in a particular order from ventral to dorsal in mice and chicks. While not all of the tetrapod transcription factors have been examined in zebrafish, those that have been studied occupy the same relative positions along the dorso-ventral axis of spinal cord.18–21 The similarity in the order of the bands in the distantly related zebrafish and mice (and some corroborating work in frogs22), suggests a very primitive origin of the patterning.
The parallels among chicks, mice, zebrafish, and frogs extend beyond the expression domains to the morphology, connectivity, and even some aspects of the functional roles of the cells arising from particular domains in these divergent species. The two best documented examples of this are the neurons marked by the transcription factors Engrailed and Chx10 (called Alx in zebrafish). These mark similar neurons in zebrafish and mice. Engrailed neurons are inhibitory neurons (Glycine/GABA) with ipsilateral ascending axons in both species.12,23,24 This is also the case in frog tadpoles and chicks.22,25 In all of these, the neurons not only share the primary axonal trajectory, but also share at least one synaptic output, as they all directly connect to motoneurons. The Chx10 (Alx) neurons, in contrast, are glutamatergic excitatory interneurons with a primary ipsilateral, descending axon in mice and zebrafish.19,26 These neurons directly connect to motoneurons and probably other cell types as well. These are the best examples of the parallels between the different species because they have been examined in some detail, but there is preliminary evidence for a similar overall patterning of other neuronal types as well.
One interesting feature of the code is that neurons arising from particular regions share some phenotypic features, but differ in others. For example, cells with ipsilateral descending axons arising from a domain called V2 diverge into excitatory and inhibitory types, each marked by a different transcription factor.18,26 Recent work shows that these arise via a terminal cell division that produces one excitatory and one inhibitory cell.18 This developmental pattern could provide a mechanism for establishing a rough excitatory and inhibitory balance in spinal cord that might be important for circuit function based upon systems biology work that indicates such a balance enhances control. In addition, a fine tuning of the balance might also occur via switches in transmitter phenotype between the two types, given other evidence that switches can occur after alterations in the level of activity in spinal cord.27 Such switches might allow a fine tuning of the balance of excitation and inhibition without gross morphological changes in the neurons because they represent switches in transmitter phenotype of otherwise morphologically similar cell types.
The implications of the shared transcription factor code are substantial. Until the code was revealed, there was no clear link between cell types in the spinal cord of aquatic animals and those in tetrapods. The code bridges that gap and has immediately allowed for predictions about the likely functional roles of neuronal types in mice based upon their functional role of cells in fish and frogs, where the connectivity and behavioral contributions have historically been easier to study. The engrailed neurons in frogs and fish were implicated in burst termination that might be important for high-speed swimming.12,22 Subsequent studies in mice showed that genetic inactivation of the neurons led to a slowing of locomotor rhythms, at least consistent with what one might expect based upon swimming vertebrates.28 Since not only the spinal cord, but much of the brain as well, is built using some of the same code, explorations of the classes of neuronal types arising from transcription factor domains and their functional organization has implications for structural and functional patterning throughout the nervous system that will be reviewed later in this article.
While the parallels in the code are striking across vertebrates, this does not mean that the cell types and networks in different species are identical. There is good evidence that specialization of neurons arising from within particular transcription factor domains gives rise to different neuronal types with somewhat divergent functions.23 This specialization may not take the same form in different species, just as it might take different forms along the neuraxis in individual animals. Nonetheless, the transcription factor code represents a major unifying principle of organization across vertebrates, which suggests that specialized networks in different species arise by divergence from a common shared neuronal ground plan. Understanding that ground plan and the core wiring and activity patterns at its foundation is therefore an important goal.
Functional order within and across transcription factor domains
While the transcription factor code and its link to major classes of neurons was first defined in studies in mammals and chicks, the functional organization within the classes is more difficult to reveal in those animals because their spinal cords are more complex than in fishes. Evidence that there is clear functional order within and between the transcription factor domains comes from studies of larval zebrafish, where obtaining data about the patterns of activity during behavior is easier because of the ability to image activity with calcium indicators in vivo and to more easily target patch recordings from neurons because of the transparency of the fish. These data have revealed a remarkable order to the recruitment of neurons within and between transcription factor zones that ties together their development, location, and functional properties.
The evidence for a functional organization came not from a directed attack on the question of functional order within neurons sharing transcription factor expression, but rather from studies of the pattern of recruitment of neurons during behaviors of different speeds. While much was known about neurons that contributed to motor behaviors in both swimming and walking vertebrates, questions of how the neurons were recruited during different speeds or strengths of movement were mostly confined to recruitment of motoneurons. We set out to explore how both motoneurons and interneurons of known classes were recruited during changes in the frequency of bending of swimming zebrafish, which is correlated with how fast they move.
Both electrophysiological recordings and calcium imaging of motoneurons in paralyzed fish in which the “fictive” swimming frequency was monitored by recordings from spinal nerves revealed a striking relationship between where a motoneuron was located and the frequency of swimming at which it was recruited.10 Motoneurons were recruited from the bottom of the motor column to its top as the frequency of swimming increased. Measures of input resistance and size showed that, like the recruitment frequency, both were also correlated with position, with the high resistance smaller motoneurons located ventrally and progressively lower resistance and larger motoneurons stacked above them. Earlier work had shown that some of the fastest motoneurons were in the dorsal part of the motor column, with slower ones below them,29–31 but the more recent work showed that there is an overall orderly relationship between location, cellular properties, size, and recruitment.
Remarkably, electrophysiological and optical studies of spinal interneuron recruitment patterns showed that excitatory interneurons followed the same order of recruitment based on location and cellular properties.10,11,32 The most ventral excitatory interneurons were recruited at the lowest swimming frequencies and progressively more dorsal ones were engaged as the frequency increased, just like the motoneurons. This pattern was not related to interneuron size, but rather to location and, to some extent, to properties such as input resistance. The order was evident both within a transcription factor class (the ipsilateral descending excitatory CiD interneurons marked by Alx—the zebrafish version of Chx10 in mammals), and across transcription factor classes, with both ventral Alx neurons and the ventral MCoD interneurons (a commissural excitatory class arising from an Evx domain) recruited at lower swimming frequencies than the more dorsal neurons. Inhibitory interneurons in contrast were recruited in the opposite direction, from dorsal down with increasing swimming frequency.
Importantly, there was no overall relationship between recruitment and soma size for either inhibitory and excitatory neurons, as there was in motoneurons. This may have important implications for principles of organization that extend across neuronal types in spinal cord. The well-known size principle of recruitment of motoneurons applies broadly across all species,15,33,34 including the motoneurons in zebrafish. The evidence based upon soma size, however, indicates that it does not apply to interneurons. Interestingly, there are better predictors of recruitment that do apply to both motoneurons and interneurons.33,34 These include both location and input resistance (which is not always well correlated with soma size among interneurons), and, even more importantly, time of differentiation, which is explored below. Thus, the size principle may be a subset of a broader pattern of organization that links time of differentiation, connectivity, and cellular properties to recruitment.
The best predictor of recruitment patterns is the time of differentiation of the neuron in spinal cord, whether the cell is a motoneuron, or excitatory or inhibitory interneuron. Studies using color change proteins to time stamp neurons that have differentiated at particular times indicate that the neurons differentiating first drive the higher frequency, larger and faster movements in older animals, with neurons driving progressively lower frequency and slower movements added later.6 This age-related order underlies the orderly recruitment of all the neurons based upon location because the neurons are lined up dorsoventrally in cord roughly by when they differentiated. Indeed the pattern of age-related stacking can account for the opposite directions of recruitment of excitatory and inhibitory interneurons because they are stacked in opposite directions based upon age. Thus, there appears to be a very systematic organization that links the time of differentiation of a neuron to its location, cellular properties, and, importantly, the frequency of swimming at which it is recruited. The recruitment based upon age parallels the pattern of development of the animal because the first embryonic movements are large amplitude and fast movements and progressively weaker, and slower movements appear during development as neurons involved in progressively slower movements later in life are added to the differentiating population.6 We know for the motoneurons and some of the interneurons that the link between recruitment and time of differentiation is retained even into adulthood, suggesting that it is not simply an early pattern that is linked to the gradual addition of ion channels as the neurons differentiate.29,35,36
Shifts in interneurons with locomotor speed
There is one major difference in how motoneurons are recruited as compared to excitatory interneurons. As frequency rises, more and more motoneurons are added to the active pool, with those active at low frequencies remaining active at higher ones. Among excitatory interneurons, those active at low frequency are removed from the active pool by inhibition as those involved in higher frequencies of movements are recruited.7 This pattern occurs both within a cell type because ventral CiD interneurons involved in slow swimming are shut off as more dorsal CiD interneurons are recruited, and between cell types because the commissural, excitatory premotor MCoDs, which are active in slow swimming, are turned off as the CiD neurons active in faster swimming are recruited. The implications of this for motor control are significant as they indicate that the populations of neurons driving different speeds of movement change with speed, suggesting possible changes in the networks (maybe even the central pattern generating networks) with speed of locomotion. In the zebrafish, this switch also involves a change in premotor cell type from a commissural excitatory cell type to an ipsilateral descending one. This observation leads to some important potential implications, outlined in a later section, that could shed light on some puzzles in studies of mammalian limb control networks.
Summary of principles of the zebrafish organization, also presented in Figure 1:
Figure 1.
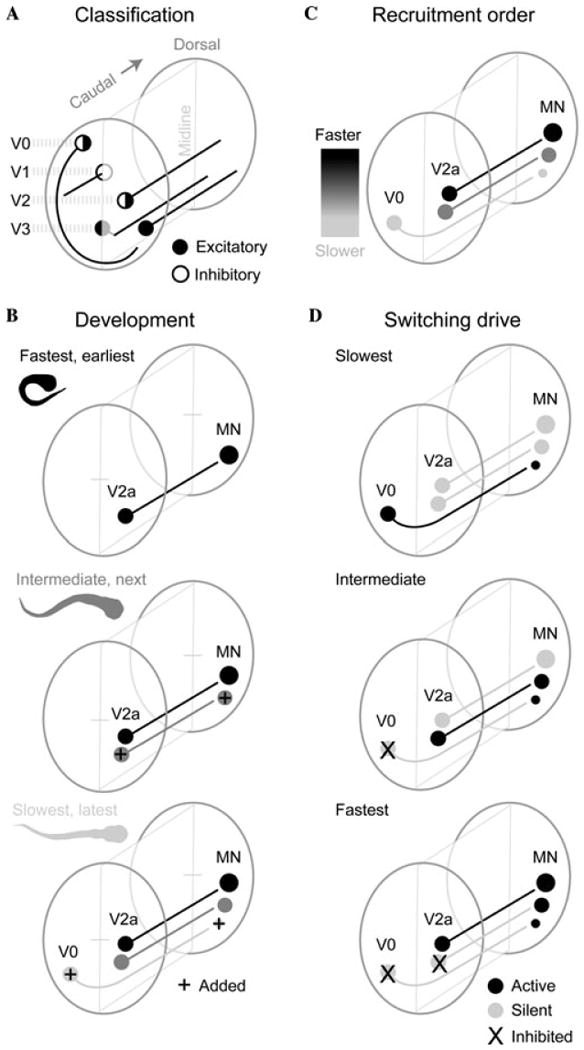
Summary of principles of organization of spinal neurons. (A) Spinal neurons of particular transmitter phenotypes and morphologies are specified in a dorso-ventral fashion by the combination of transcription factors activated by gradients of diffusible morphogens. V0 interneurons are a mixture of excitatory and inhibitory cells, all with commissural axons; V1 cells are inhibitory with a primary ascending ipsilateral axon; V2 interneurons are a mixture of excitatory and inhibitory cells with a primary descending axon; V3 cells are excitatory and have either a commissural or ipsilateral descending axon. (B) A summary schematic of the developmental order of spinal circuitry and the associated movement in larval zebrafish. Neurons responsible for progressively slower movements in larvae are added as zebrafish develop. (C) Schematic summarizing the recruitment order of interneurons and motoneurons, which occurs from the bottom of spinal cord up. (D) Schematic summarizing the switch in premotor interneuron activity responsible for driving the progressive dorso-ventral activation of motoneurons as larvae swim faster and faster.
Principle 1
Broad neuronal classes defined by a set of transcription factors, key morphological features, and transmitter phenotypes arise in an orderly way from different dorso-ventral zones in spinal cord. This core pattern appears to lie at the developmental and phylogenetic base of the construction of spinal networks in all vertebrates. Neurons arising from the zones that define the basic ground plan diversify further in their morphological and functional details to give rise to specialized cell types during development and evolution. For example, neurons arising from the engrailed domain in mammals share a common inhibitory phenotype and ipsilateral axonal projection, but give rise to several cell types including Renshaw cells and Ia inhibitory neurons.23 Distinctions within the engrailed class are less obvious in larval zebrafish (which are free swimming and therefore functional from a motor control perspective), suggesting that they may have diversified during evolution to give rise to several types in mammals. They may, however, even diversify later in the development of zebrafish when the circuits have been more difficult to study (but see Ref. 37).
Principle 2
Motor behaviors and both motoneurons and interneurons differentiate in order from gross, often faster, movements and the neurons driving them to progressively slower movements and their underlying neurons. Gross movements such as escape-like bends and whole body swimming movements and the neurons producing them develop first, with slower movements and the neurons underlying them differentiating later. In zebrafish, much of this differentiation occurs in the egg, or just after hatching, but before the animal is free swimming. Once free swimming, as the speed of swimming gets faster and faster, both motoneurons and interneurons are activated in order from those differentiating last to those that differentiated first. Thus, the fast networks differentiate first, and the slow ones last.
Principle 3
Recruitment order of motoneurons and interneurons is based upon time of differentiation. In zebrafish, one consequence of principle 2 is a topographic map of neuronal recruitment in cord that arises because the neurons remain arranged in the spinal cord largely by the time at which they differentiated. In addition to position, other neuronal features such as input resistance are also correlated with time of differentiation, but are not as good a predictor of recruitment as when the neurons differentiated. Notably, the size principle of motoneurons does not apply in its simplest form to interneurons, as there is no simple relationship between at least soma size and recruitment. The best indicator of when a neuron is recruited as speed increases is when it differentiated, with neurons recruited from youngest to oldest as speed rises.
Principle 4
Different locomotor speeds involve some shifts in the set of active interneurons. Some interneurons active at slow speeds are silenced at faster ones and this pattern occurs both within and between excitatory classes. Thus, the interneurons behave differently from the motoneurons with respect to recruitment because the motoneurons only add neurons to the active pool as speed increases, while the interneurons add new ones while removing others that were active at slower speeds. The networks for slow and fast swimming are not the same and appear to constantly shift as speed increases.
How broad are these principles across species and in the nervous system?
The patterns observed in zebrafish are not unique to this species. As described earlier in this paper, the transcription factor code of cell types in principle 1 was first worked out in other species, but seems present among vertebrates generally. The broad classes of neurons appear to be generated via a similar developmental pattern in every vertebrate studied so far including fish, frogs, chicks, and mice. Other work indicates that development via a transcription factor code like that in spinal cord extends to hindbrain networks in zebrafish (unpublished work) and other species.38,39
The observation in principle 2 that motor behaviors develop from gross and fast to refined and slow is supported by behavioral observations from several species.1,6,40–46 Even data from studies of human movements in utero show that fast, whole body movements such as the startle response are present early and more refined movements of, for example, the limbs come later.41 The behavioral order implies that circuits for gross movements arise before those for refined movements, but there is less information matching cell types to time of differentiation in spinal systems of other species. Old data indicate that hindbrain neurons that drive the fastest movements in zebrafish, such as the Mauthner cell, develop very early, and our more recent studies provide evidence that neurons differentiate from fast to slow in hindbrain as in spinal cord.47 While there is less neuronal-level evidence from mammals, coarse coding neurons with broad effects develop before finer resolution cells in parts of the mammalian nervous system, with, for example, the magnocellular parts of the visual pathways differentiating before the parvocellular ones48–50 and the reticulospinal neurons in the startle response developing early.51,52
Less is known about how age-related order of recruitment of the spinal neurons in zebrafish (Principle 3) applies to other species, but there is increasing evidence that there is considerable age-related functional order in nervous systems. Neurons arising at different times in mammals have different properties, and it may well be that neuronal sub-populations arise at different times during development from individual transcription factor zones in spinal cord.23,53 More direct studies of recruitment and its links to age in mammals and other species will help to resolve the generality of the pattern observed in fish. We do have evidence that there is an orderly recruitment by age in the hindbrain networks, suggesting that such patterns extend beyond spinal cord.54
The details of recruitment within classes of interneurons, and particularly assessment of switches in cell types, as articulated in principle 4, are more difficult to study in other species. Earlier studies of interneuron recruitment in dogfish spinal cord and in Xenopus tadpoles did not find such switches, but they were not done in a way that would be likely to reveal such switches either.55,56 In both cases, the recorded neurons were not identified, so their transmitter phenotype and premotor status is unknown. The relevant premotor neurons might simply have not been studied due to sampling problems. In addition, the Xenopus work was early in the animal's development, at a stage corresponding to one in zebrafish before the slowest networks have even developed, so one would not expect to find such a pattern at that stage. We predict that studies of premotor excitatory interneurons in Xenopus after the slower tail driven swimming movements have developed will show the same pattern as we found in zebrafish.
Mammals are even harder to study than fishes and amphibians. Nonetheless there are hints that may point to speed-related changes in the involvement of neuronal types even in mammals. Genetic destruction of ipsilateral excitatory neurons in the Alx domain leads to mice that move with a normal gait at slow speeds, but that switch at higher speeds to a galloping type gait that they never use normally.57 This speed-related disruption, which also occurs in spinal preparations, is consistent with the possibility of at least partially different networks being engaged at different speed in mammalian spinal cord. Very recent observations from human walking are also consistent with the possibility of speed-related network switches,58 suggesting that it might be a very broad feature of organization.
Total speculation arising from the patterns in fish, but with some predictions
While the breath of the relevance of some features of the simple axial motor patterns in fish remains to be revealed, they nonetheless prompt some more speculative considerations about other aspects of motor organization as well as possible arrangements of networks for limbed locomotion, which are derivatives of primitive axial networks. Of course, limbed locomotion is not identical to axial locomotion, so we cannot expect that everything will be the same.59 Nonetheless, the two are related by evolution and it can be instructive to consider the possible implications of the organization of axial networks for the construction of limb circuits.60,61
Neuromodulation
One striking feature of motor systems is the ability to shape their output by neuromodulation.62–64 The effects of neuromodulators are complex, without an obvious overall pattern in the neuromodulatory changes in features like synaptic strength and excitability.64,65 Perhaps this complexity is in part a consequence of the fact that studies of the neurons are typically done after development is complete, and even after neurons arising from different transcription factor zones and of different ages have migrated to intermingle in the spinal cord or brain. One testable hypothesis is that there will be orderly effects of neuromodulators on neurons and synapses based upon the time of differentiation of the cells. Such an orderly pattern could be obscured later in life as neurons move around. This simple, although untested, idea has some appeal because we know that recruitment patterns are ordered by age. One could imagine that a change in the slope of recruitment (e.g., a more rapid recruitment of more potent interneurons and motoneurons that might be important for a fish in a cold environment) could be achieved by a systematic alteration of the properties of neurons in the population by a neuromodulator. This change could accomplish a more rapid recruitment, while maintaining a smooth recruitment order to allow for a graded movement. If so, we might predict that neuromodulatory effects might be comparable on neurons that differentiated around the same time and change systematically with the time of differentiation of the neuron. It remains to be seen whether there are such age-related patterns in neuromodulatory effects, but they can be explored with relative ease in the zebrafish model.
Limbed locomotion
Fin and limb muscles are derived from somites that primitively gave rise to axial muscles, so we might expect that axial motor organization could inform us about at least some aspects of limb control.66 While there are some likely candidates for neurons in the central pattern generators for axial motor circuits based upon work in lampreys and frog embryos (still not well tested by cell specific perturbations because of their difficulty in these non-genetic models),67,68 the mammalian central pattern generating networks have proved more elusive.17 Surprisingly, genetic perturbations of a variety of known neuronal classes—even large groups of neurons—have failed to disrupt the ability of the networks to produce a rhythm. This has led to speculation that there are other critical neurons that have been missed and, along with observations of motor pattern deletions, to the development of ideas about multilevel CPGs.69 A consideration of evidence from axial networks may shed some light on some of these more mysterious results concerning mammalian central pattern generators.
The interpretation of mammalian perturbation experiments depends heavily on an assumption that there is a network that contains a discrete set of neurons that forms the central pattern generator and that the same neuronal classes (while maybe not the exact same population of neurons) contribute to that CPG over the range of possible locomotor speeds. Our studies of axial motor circuits in zebrafish, however, indicate that there can be switches in cell types at different speeds, raising the possibility that the contributors to the rhythm might change with speed. In particular, there are premotor commissural excitatory interneurons active at slow speeds that are silenced at higher speeds. Most of the mammalian work (and even axial work), while acknowledging the presence of commissural excitatory interneurons, has focused on a conceptual organization in which coordination between the two sides is accomplished largely by crossed inhibitory interneurons (but see Ref. 17). The data from mouse perturbation experiments are typically interpreted in light of assumptions that (1) there are not cell type changes in the CPG with speed and (2) crossed inhibitory neurons are always critical for side to side coordination. Both assumptions may be wrong, and if they are, so might be the interpretations of the genetic perturbation experiments and ideas about the role of cell types in generating the motor output.
For example, work by the Goulding lab concluded that commissural inhibitory neurons are essential for coordination between the two sides of the body.70 This was based upon modern experimental genetic manipulations. In the first, perturbations of Dbx positive neurons, which included all commissural cells, both excitatory and inhibitory, led to disruptions of coordination between the two sides of the body, pointing to an important role for commissural neurons. In the second experiment, only the excitatory commissural neurons were perturbed, and they did not eliminate coordination between the two sides. This led to the inference that commissural inhibitory neurons are critical for normal bilateral coordination. These experiments could be interpreted differently if there are switches in the commissural interneurons used at different speeds. Suppose, for example, that commissural excitatory interactions (between extensor interneurons on one side and flexor interneurons on the other) are more important than commissural inhibitory neurons for coordinating the two sides of the body at low speeds. These could phase flexor and extensors properly on the two sides, with ipsilateral inhibitory neurons being critical for proper phasing between extensors and flexors on the same side at the slow speeds. Suppose further that as speed rises, commissural inhibitory neurons replace the commissural excitatory cells as the primary commissural coordination system, with the interaction changing to one between flexor and flexor pairs (or extensor/extensor) on opposite sides, rather than flexor/extensor pairs (this idea has parallels with that of Crone et al. 2008, but is somewhat different from the change in commissural inhibitory neurons with normal changes in speed that they suggest).57
If this happened, the earlier Dbx perturbations might be interpreted differently. We would expect that the disruption of all commissural neurons would indeed affect bilateral coordination. The switch model would suggest that disrupting excitatory commissural neurons would affect coordination at slow speeds of locomotion. There were no disruptions in the DBx experiments, which on the face of it would seem to refute the switch idea. The absence of effects could, however, be a consequence of the approach used to elicit locomotion. In the Dbx experiments, bath application of drugs was used to activate spinal cord. This massive excitation would not necessarily allow for the normal speed-related changes in the networks and could easily mask the contribution of the excitatory cells by disrupting proper speed-related network shifts.
This is all very speculative, but there is one clear prediction that would at least be consistent with the idea of such speed-related switches. If the commissural inhibitory interneurons were selectively perturbed and the locomotion was assessed in the intact animal, the idea of a switch would predict that bilateral coordination would be normal at slower speeds and disrupted at higher ones. This is akin to the pattern observed for descending excitatory Chx10 neurons, which are involved in higher speeds in fish and whose disruption in mice results in changes in bilateral coordination at higher speeds of locomotion. We are not at all wedded to this hypothesis, but rather want to emphasize that even the interpretation of existing experiments depends on assumptions about the network construction. While the previous discussion focused on side to side coordination rather than rhythm generation itself, it could be that the actual rhythm-generating network changes with speed, which might underlie conclusions that neurons so far identified are not part of the rhythm generator.
In thinking about limb control it is important to keep in mind that in the primitive axial system that gave rise to limbs, the coordination of ipsilateral body segments was no more important than the coordination between the two sides. Without both, swimming is problematic. A focus on single limbs and flexor and extensor coordination between them can to lead to a de-emphasis on the possibility that neurons from one side of the body might, as they were primitively, be critical to normal rhythm generation and patterning of output on the opposite side and that their roles might change with changes in speed. The derivation of limbs from axial muscles increases the possibility that some of rules that underlie axial locomotor circuits in zebrafish presented here may also appear in limb motor networks. This is not to say that there will not be differences (there must be!), but rather that the evolutionary history makes it likely that there may be core similarities as well.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Roberts A, et al. Neural control of swimming in a vertebrate. Science. 1981;213:1032–1034. doi: 10.1126/science.7196599. [DOI] [PubMed] [Google Scholar]
- 2.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Grillner S, Thomas MJ. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlov A, et al. Simple cellular and network control principles govern complex patterns of motor behavior. Proc Natl Acad Sci USA. 2009;106:20027–20032. doi: 10.1073/pnas.0906722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci. 2009;29:13566–13577. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean DL, et al. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol. 2008;68:817–834. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao JC, Fetcho JR. Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci. 2008;28:12982–12992. doi: 10.1523/JNEUROSCI.3330-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLean DL, et al. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DH, et al. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. doi: 10.1016/j.neuron.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Higashijima S, et al. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter DA, Bhatt DH, Fetcho JR. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale ME, Ritter DA, Fetcho JR. A confocal study of spinal interneurons in living larval zebrafish. J Comp Neurol. 2001;437:1–16. doi: 10.1002/cne.1266. [DOI] [PubMed] [Google Scholar]
- 15.Cope TC, Pinter MJ. The size principle: still working after all these years. NIPS. 1995;10:280–286. [Google Scholar]
- 16.Briscoe J, et al. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 17.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Satou C, Higashijima S. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaeron C, et al. Zebrafish evx1 is dynamically expressed during embryogenesis in subsets of interneurones, posterior gut and urogenital system. Mech Dev. 2000;99:167–172. doi: 10.1016/s0925-4773(00)00473-1. [DOI] [PubMed] [Google Scholar]
- 21.Colombo A, et al. Zebrafish BarH-like genes define discrete neural domains in the early embryo. Gene Expr Patterns. 2006;6:347–352. doi: 10.1016/j.modgep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Li WC, et al. Primitive roles for inhibitory interneurons in developing frog spinal cord. J Neurosci. 2004;24:5840–5848. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez FJ, et al. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- 25.Wenner P, O'Donovan MJ, Matise MP. Topographical and physiological characterization of interneurons that express engrailed-1 in the embryonic chick spinal cord. J Neurophysiol. 2000;84:2651–2657. doi: 10.1152/jn.2000.84.5.2651. [DOI] [PubMed] [Google Scholar]
- 26.Lundfald L, et al. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci. 2007;26:2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 27.Borodinsky LN, et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 28.Gosgnach S, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 29.Liu DW, Westerfield M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J Physiol. 1988;403:73–89. doi: 10.1113/jphysiol.1988.sp017239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerfield M, McMurray JV, Eisen JS. Identified motoneurons and their innervation of axial muscles in the zebrafish. J Neurosci. 1986;6:2267–2277. doi: 10.1523/JNEUROSCI.06-08-02267.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fetcho JR. Excitation of motoneurons by the Mauthner axon in goldfish: complexities in a “simple” reticulospinal pathway. J Neurophysiol. 1992;67:1574–1586. doi: 10.1152/jn.1992.67.6.1574. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson B, Pinter MJ. An investigation of threshold properties among cat spinal alpha-motoneurones. J Physiol. 1984;357:453–483. doi: 10.1113/jphysiol.1984.sp015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafsson B, Pinter MJ. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fetcho JR. Morphological variability, segmental relationships, and functional-role of a class of commissural interneurons in the spinal-cord ofgoldfish. J Comp Neurol. 1990;299:283–298. doi: 10.1002/cne.902990303. [DOI] [PubMed] [Google Scholar]
- 36.Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel JP, et al. Locomotor pattern in the adult zebrafish spinal cord in vitro. J Neurophysiol. 2008;99:37–48. doi: 10.1152/jn.00785.2007. [DOI] [PubMed] [Google Scholar]
- 38.Cepeda-Nieto AC, Pfaff SL, Varela-Echavarria A. Homeodomain transcription factors in the development of subsets of hindbrain reticulospinal neurons. Mol Cell Neurosci. 2005;28:30–41. doi: 10.1016/j.mcn.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Moreno N, et al. LIM-homeodomain genes as territory markers in the brainstem of adult and developing Xenopus laevis. J Comp Neurol. 2005;485:240–254. doi: 10.1002/cne.20498. [DOI] [PubMed] [Google Scholar]
- 40.Bradley NS, Bekoff A. Development of coordinated movement in chicks: I. Temporal analysis of hindlimb muscle synergies at embryonic days 9 and 10. Dev Psychobiol. 1990;23:763–782. doi: 10.1002/dev.420230802. [DOI] [PubMed] [Google Scholar]
- 41.de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. Part I. Qualitative aspects. Early Hum Dev. 1982;7:301–322. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 42.Hamburger V, et al. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965;159:1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- 43.Westerga J, Gramsbergen A. The development of locomotion in the rat. Brain Res Dev Brain Res. 1990;57:163–174. doi: 10.1016/0165-3806(90)90042-w. [DOI] [PubMed] [Google Scholar]
- 44.Tunstall MJ, Sillar KT. Physiological and developmental aspects of intersegmental coordination in Xenopus embryos and tadpoles. Sem Neurosci. 1993;5:29–40. [Google Scholar]
- 45.van Mier P, Armstrong J, Roberts A. Development of early swimming in Xenopus laevis embryos: myotomal musculature, its innervation and activation. Neuroscience. 1989;32:113–126. doi: 10.1016/0306-4522(89)90111-5. [DOI] [PubMed] [Google Scholar]
- 46.von Seckendorff-Hoff K, Wassersug RJ. The kinematics of swimming in larvae of the clawed frog, Xenopus laevis. J Exp Biol. 1986;122:1–12. [Google Scholar]
- 47.Mendelson B. Development of reticulospinal neurons of the zebrafish. Part I. Time of origin. J Comp Neurol. 1986;251:160–171. doi: 10.1002/cne.902510203. [DOI] [PubMed] [Google Scholar]
- 48.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 49.Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977;176:23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- 50.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 51.Lingenhohl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altman J, Bayer SA. Development of the brain stem in the rat. Part IV. Thymidine-radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1980;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- 53.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 54.Riley MR, et al. A topographic map of function along the axis of stripes in the hindbrain of zebrafish. Soc Neuro Abstr. 2009;366:591. [Google Scholar]
- 55.Sillar KT, Roberts A. Control of frequency during swimming in Xenopus embryos: a study on interneuronal recruitment in a spinal rhythm generator. J Physiol. 1993;472:557–572. doi: 10.1113/jphysiol.1993.sp019962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mos W, Roberts BL, Williamson R. Interneuronal activity patterns during fictive locomotion of spinal dogfish. Phil Trans R Soc Lond B. 1990;330:341–349. [Google Scholar]
- 57.Crone SA, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Combes D, et al. Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis. J Physiol. 2004;559:17–24. doi: 10.1113/jphysiol.2004.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fetcho JR. A review of the organization and evolution of motoneurons innervating the axial musculature of vertebrates. Brain Res. 1987;434:243–280. doi: 10.1016/0165-0173(87)90001-4. [DOI] [PubMed] [Google Scholar]
- 61.Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain Behav Evol. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- 62.McLean DL, Merrywest SD, Sillar KT. The development of neuromodulatory systems and the maturation of motor patterns in amphibian tadpoles. Brain Res Bull. 2000;53:595–603. doi: 10.1016/s0361-9230(00)00393-2. [DOI] [PubMed] [Google Scholar]
- 63.Sillar KT, et al. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 65.Parker D, Grillner S. Cellular and synaptic modulation underlying substance P-mediated plasticity of the lamprey locomotor network. J Neurosci. 1998;18:8095–8110. doi: 10.1523/JNEUROSCI.18-19-08095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neyt C, et al. Evolutionary origins of vertebrate appendicular muscle. Nature. 2000;408:82–86. doi: 10.1038/35040549. [DOI] [PubMed] [Google Scholar]
- 67.Grillner S, et al. Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog Brain Res. 2007;165:221–234. doi: 10.1016/S0079-6123(06)65014-0. [DOI] [PubMed] [Google Scholar]
- 68.Roberts A, et al. Central circuits controlling locomotion in young frog tadpoles. Ann N Y Acad Sci. 1998;860:19–34. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- 69.Rybak IA, et al. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol. 2006;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanuza GM, et al. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]


