Abstract
Imaging and molecular approaches are perfectly suited to young, transparent zebrafish (Danio rerio), where they have allowed novel functional studies of neural circuits and their links to behavior. Here, we review cutting-edge optical and genetic techniques used to dissect neural circuits in vivo and discuss their application to future studies of developing spinal circuits using living zebrafish. We anticipate that these experiments will reveal general principles governing the assembly of neural circuits that control movements.
Keywords: optical, molecular, neural networks, spinal cord, locomotion, Danio rerio
INTRODUCTION
Technological advances fuel scientific discovery. The recent confluence of optical and genetic approaches heralds a new era in neuroscience (reviewed in: Fetcho and Bhatt, 2004; Miesenbock, 2004; Deisseroth et al., 2006; Miller, 2006; Herlitze and Landmesser, 2007), where networks of neurons will be monitored and manipulated simultaneously in intact behaving animals, rather than a few cells at a time in dissected ones. One model system immediately capable of taking advantage of new “optogenetic” tools is the zebrafish.
Biologists have historically used zebrafish to study basic mechanisms of development because their eggs are fertilized externally, their embryos are optically transparent and they develop quickly. For example, only 4–5 days after egg fertilization, zebrafish are freely swimming with a behavioral repertoire that allows them to pursue prey and avoid predators (Kimmel et al., 1995). The use of zebrafish to examine mechanisms of development has provided a range of molecular tools for physiologists, from mutagenesis to targeted gene expression (reviewed in: Rasooly et al., 2003; Sprague et al., 2006). Thus, the transparency of zebrafish at these early stages, their rapid locomotor development, and their genetic accessibility make them ideal subjects for studying the assembly of motor networks using optogenetic tools.
While zebrafish are established models for development (reviewed in: Granato and Nusslein-Volhard, 1996; Grunwald and Eisen, 2002) and have been used to image and record from motor circuits in vivo (reviewed in: Fetcho and Liu, 1998; Drapeau et al., 2002; O’Malley et al., 2003), they have only recently become the focus of studies combining genetic and optical approaches to examine neural circuits and their links to behavior (Gleason et al., 2003; Higashijima et al., 2003; Hale et al., 2004; Li et al., 2005; Kimura et al., 2006; Smear et al., 2007). Here, we review recent optogenetic techniques and outline future experiments using these approaches to study spinal circuit assembly in zebrafish.
Specifically, we are interested in using the power of this model to address the following questions: How do you define components of a functional circuit in developing spinal cord? How does the structure of identified spinal circuits change during development and what impact does this have on function? What processes orchestrate structural and functional changes to produce mature locomotion? Are there any principles of organization in motor systems that might be revealed more easily early on in life? Providing answers to these developmental questions will undoubtedly yield greater insight into the operation of neural networks in adults. Watching a circuit build itself can help us understand how it fundamentally works.
In considering these issues, it is important to keep in mind the principal advantage of optogenetic approaches, namely, the targeted and minimally invasive delivery of agents designed to image or perturb the structure and function of neural circuits in vivo. So how is this accomplished in zebrafish?
TRANSGENESIS AND IMAGING
Zebrafish formally arrived as a genetic model system with the generation of clones almost 3 decades ago (Streisinger et al., 1981). Since then, the number of approaches designed to perturb or insert genes in developing zebrafish has increased substantially (reviewed in: Kuwada, 1995; Lekven et al., 2000; Westerfield, 2000; Corey and Abrams, 2001; Amsterdam and Hopkins, 2006). Arguably, the most useful for the dissection of neural circuits is the use of transgenesis to label specific cell types. In the past, the generation of DNA constructs with the appropriate promoter and reporter elements required a significant level of expertise. However, recent technological advances, including homologous recombination into bacterial artificial chromosomes (BACs), have obviated the normally labor-intensive cloning approaches used to generate these constructs. More detailed descriptions of BAC-based homologous recombination can be found elsewhere (Jessen et al., 1998; Lee et al., 2001; Thermes et al., 2002; reviewed in: Yang et al., 2006). Suffice to say this approach, as well as transposon-mediated methods (reviewed in: Kawakami, 2005), have greatly simplified the generation of transgenic zebrafish, putting molecular tools in the hands of novices.
The first stable transgenic lines of zebrafish with tissue-specific expression of reporter constructs were created using constitutively active promoters (Higashijima et al., 1997; Long et al., 1997; Higashijima et al., 2000). However, more precise temporal control of expression can be achieved using promoters that rely on heat (Halloran et al., 2000), hormones (de Graaf et al., 1998), or light (Yu et al., 2007) to activate them.
Of course, the selection of the promoter depends entirely on the question you hope to address, as does the selection of the reporter. For in vivo optical studies, a veritable rainbow of fluorescent proteins is available (reviewed in: Miyawaki, 2005; Shaner et al., 2005). Many are man-made derivatives of green fluorescent protein (GFP) originally discovered in jellyfish (Shimomura et al., 1962), while others have been found in corals (Matz et al., 1999). There are also proteins, whose fluorescence can be switched on and off by light, like Dronpa (Habuchi et al., 2005) or converted from green to red, like Kaede (Ando et al., 2002), which adds yet another level of control (reviewed in: Lukyanov et al., 2005).
Genetically encoded fluorescent indicators of neuronal activity and transmitter release are also available (reviewed in: Fetcho and Bhatt, 2004; Miyawaki, 2005; Knopfel et al., 2006), further extending the capability of in vivo imaging to probe neural circuits. Genetic approaches can also be used to silence or activate neurons, as well as to map out circuit connectivity (reviewed in: Kiehn and Kullander, 2004; Callaway, 2005; Miyoshi and Fishell, 2006; Herlitze and Landmesser, 2007). Together, these technologies open up the exciting prospect of identifying, monitoring, and controlling neural circuits in developing zebrafish with light.
Imaging of labeled specimens is typically accomplished in three principal ways, wide-field epifluorescence, confocal microscopy, and two-photon microscopy. Without going into too much detail (see: Yuste and Konnerth, 2005), the salient differences can be summarized according to the modes of excitation and detection. Wide-field epifluorescence uses a light source to excite fluorophores broadly within the specimen, and the fluorescence of a large region is then detected. Confocal microscopy uses lasers and mirrors to scan a spot of light through the specimen and narrow optical sections are extracted at the detection end using an optically conjugate pinhole. For two-photon microscopy, specialized lasers and the nonlinear optical properties of fluorophores provide very localized excitation, so any detected fluorescence must be from the focal point. Nothing so far can match the localized excitation provided by two-photon imaging, which minimizes out-of-focal-plane photobleaching and phototoxicity, and can provide a means to precisely trigger photoactivatable agents (reviewed in: Callaway and Yuste, 2002). For most studies in zebrafish, however, confocal microscopy is more readily available and more versatile than multiphoton imaging.
We have chosen to use these optical and genetic methods to study spinal circuits, because they operate within a very well-defined functional context, movement. This provides a simple setting to ask questions about how neural circuits are designed to produce particular behaviors. So, what have we learned about spinal circuits in zebrafish?
DEFINING DEVELOPING SPINAL NETWORKS
Spinal neurons are traditionally defined using a combination of qualities, including morphology, firing properties, and transmitter phenotype (reviewed in: Jankowska, 2001). Similarly, spinal circuits and networks are assessed by patterns of connectivity (reviewed in: Edgley, 2001). Unfortunately, all these are features that can change during development (reviewed in: Landis, 1990; Bate, 1999), making the reliable identification of functional circuits at different ages more difficult. One notable exception is a local reflex inhibitory circuit in spinal cord that can be identified at different developmental stages using a stimulation paradigm (Xu et al., 2005; Mentis et al., 2006). So-called Renshaw cells are a source of feedback inhibition to motor neurons and are reliably identified by antidromic stimulation of the ventral roots. Nonetheless, it is extremely difficult to assess the contribution of identified neural circuits to developing motor patterns. Ideally, readily identifiable classes of spinal neuron would be labeled and monitored in vivo, as the animal develops and the networks are brought on-line. This is exactly the sort of unprecedented opportunity provided by optogenetics in zebrafish.
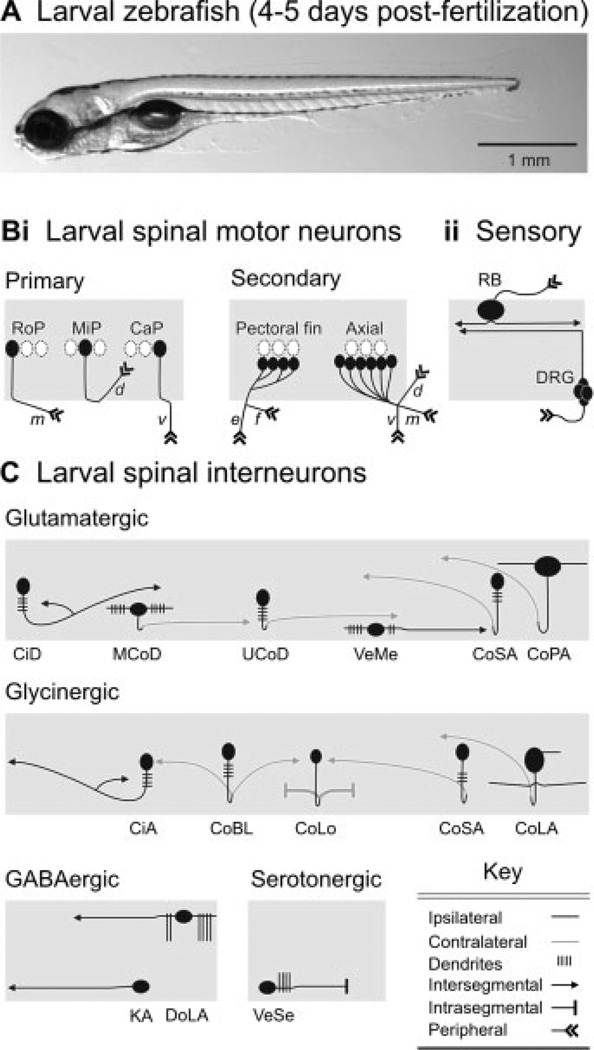
By the time zebrafish have passed 4 days of development [Fig. 1(A)], they are freely swimming and have a diverse array of anatomically distinct spinal neuron classes. For simplicity, the current list has been summarized schematically in Figure 1. There are two classes of motor neurons, primary and secondary [Fig. 1(Bi)], so named due to their respective emergence during development (Myers et al., 1986). Spinal sensory inputs include mechanoreceptive Rohon-Beard cells and the afferents of dorsal root ganglia [Fig. 1(Bii)]. Interestingly from a developmental standpoint, Rohon-Beard cells disappear soon after the fifth day of development, and their function is taken over by the dorsal root ganglia (Bernhardt et al., 1990). From an evolutionary standpoint, this replacement process also occurs in amphibians (Hughes, 1957), but well before changes in spinal network activity associated with metamorphosis (Combes et al., 2004). It is still unclear why this occurs. Finally, there are also a number of glutamatergic, glycinergic, and GABAergic spinal interneurons, in addition to one class of spinal serotonergic interneuron [Fig. 1(C)].
Figure 1.
Identified classes of spinal neuron in larval zebrafish. (A) Photograph of a larval zebrafish taken from the side. The head is to the left, in this and subsequent images. (B) Schematic depictions of identified classes of (i) motor neurons and (ii) sensory neurons. Rostral primary (RoP), middle primary (MiP), and caudal primary (CaP) motor neurons are distinctive based on their location with respect to one another and their nonoverlapping regions of axial muscle innervation (m, middle; d, dorsal; v, ventral). Secondary motor neurons can be divided into classes that innervate either the pectoral fins or axial musculature. Rohon-Beard (RB) sensory neurons and dorsal root ganglion (DRG) cells provide the principle source of mechanosensory input to spinal cord. (C) Schematic depictions of identified classes of spinal interneurons, organized according to transmitter phenotype. Abbreviations: CiD, circumferential descending; MCoD, multipolar commissural descending; UCoD, unipolar commissural descending; VeMe, ventral medial; CoSA, commissural secondary ascending; CoPA, commissural primary ascending; CiA, circumferential ascending; CoBL, commissural bifurcating longitudinal; CoLo, commissural local; CoLA, commissural longitudinal ascending; DoLA, dorsal longitudinal ascending; KA, Kolmer-Adhur; VeSe, ventral serotonergic. List compiled from Myers (1985); Bernhardt et al. (1990); Hale et al. (2001); Higashijima et al. (2004); McLean and Fetcho (2004); Thorsen and Hale (2007). See main text for more details.
While a majority of these classes were originally discovered and described at earlier stages of development, there are some classes of spinal interneuron that were not (e.g., MCoDs, UCoDs, CoLAs, VeMes; see Fig. 1 legend for definitions). It is unclear whether this is merely an artifact of differences in the labeling method or a consequence of their later arrival. Similarly, there are classes of embryonic interneuron, whose presence has not been observed in larvae (e.g., commissural bifurcating or CoB, ipsilateral caudal or IC, ventral longitudinal descending or VeLD). Again, it is unclear whether they disappear, or are simply unrecognizable in their more-differentiated states.
This raises one problem with normal “fix and stain” methods, which stitch together a life history from different snap-shots in time. These methods can lead to mistakes, because they depend on a correct matching between the same neurons at different stages of development. For example, in zebrafish, VeLD interneurons in embryos and MCoD interneurons in larvae have somata located in similar positions, which originally led to the incorrect proposal that VeLDs and MCoDs were one and the same (Bernhardt et al., 1990). However, VeLDs are GABAergic and ipsilaterally projecting (Bernhardt et al., 1992), while MCoDs are glutamatergic and contralaterally projecting (Higashijima et al., 2004). So, unless VeLDs undergo a dramatic change in both axonal trajectory and transmitter phenotype, it is unlikely that they are MCoDs, which raises the question, what happens to VeLDs? This could have important consequences for developing motor patterns, because the function of GABA changes dramatically, as chloride gradients shift during development (reviewed in: Ben-Ari, 2002). Specifically, GABA becomes hyperpolarizing in adults. Therefore, the role inhibitory circuits play in embryonic motor patterns, could differ from their role in more mature ones (Chub and O’Donovan, 2001; Jean-Xavier et al., 2007). Fortunately, the fate of GABAergic VeLD interneurons should be an easy issue to resolve in zebrafish. Labeling VeLDs with vital dyes early and tracking their development in vivo will directly assess whether they are present and unchanged in larvae or transform into MCoDs, or even disappear like Rohon-Beard cells.
Although there are some developmental issues to resolve in the nomenclature, it is nevertheless clear that the classes of spinal neuron present in larvae represent a functioning network. After 4 days of development, zebrafish larvae are fully functioning animals that navigate through their environment using swimming movements that are virtually indistinguishable from adults (McHenry and Lauder, 2006). Furthermore, spinal motor neurons and interneurons studied in adult zebrafish or adult goldfish with identical morphology perform the same function (Westerfield et al., 1986; Fetcho and Faber, 1988; Liu and Westerfield, 1988; Fetcho, 1990). So, how do we get to the larval network from an embryonic one? As the VeLD example illustrates, it would be advantageous to use definitions other than morphology and transmitter phenotype, which could then be used to track circuits through development.
One recent successful strategy is the use of transcription factors. As their name suggests, transcription factors regulate DNA transcription and are thus well placed to control the ultimate identity of neurons. Landmark studies describing the discrete top–down patterning of transcription factor domains have revolutionized studies of spinal circuits (reviewed in: Jessell and Sanes, 2000; Goulding et al., 2002). In zebrafish, genes for the transcription factors Engrailed-1 (En1) and alx (a zebrafish homolog of Chx10) define two discrete classes of spinal interneuron [Fig. 1(C)]. CiAs are labeled by En1 and provide a source of ipsilateral inhibition that gates sensory inputs during locomotor activity (Higashijima et al., 2004). CiDs are labeled by alx and provide a source of ipsilateral excitation that drives locomotor activity (Kimura et al., 2006). The hope is that distinct transcription factors will pick out distinct classes of interneuron, whose function could be inferred from their structural features. This function could then be tested using physiological recording and perturbation methods.
More important perhaps than consistent markers during development in the same species, is the ability to use these same markers to compare circuits between species. Both En1 and alx (Chx10) have been shown to label neurons with similar morphology and transmitter phenotype in mammals (Saueressig et al., 1999; Thaler et al., 1999), but in mammals they are heterogeneous in other respects, indicating functional subdivisions within the populations.
In addition to transcription factors, other markers could be used to selectively label networks of spinal neurons in transgenic animals. Because firing properties and synaptic inputs play an important role in defining neurons and circuits, ion channels (Diez-Garcia et al., 2005), calcium buffers (Meyer et al., 2002), or receptors (Hantman and Perl, 2005; Sato et al., 2005; Prober et al., 2006) could be used as defining features. It is even conceivable that a combination of two or more factors could be used as markers. Fluorescent proteins can be split in two and will only yield fluorescence when reunited in the same cells (Zhang et al., 2004). This means that two different promoters (e.g., a transcription factor and an ion channel) could be used to drive reporter expression, and only neurons where both reporters were expressed, would become fluorescent. Similarly, combinatorial gene expression strategies, like Cremediated site-specific recombination (Thummel et al., 2005), could be used to selectively label neural circuits in vivo.
The key for developmental studies is to use markers that will reliably mark different classes of spinal neuron, so that their anatomy and connectivity can be assessed repeatedly at many consecutive time points [Fig. 2(A)]. This will then set the stage for an examination of the changes in identified spinal circuits during development and how these drive changes in motor behavior.
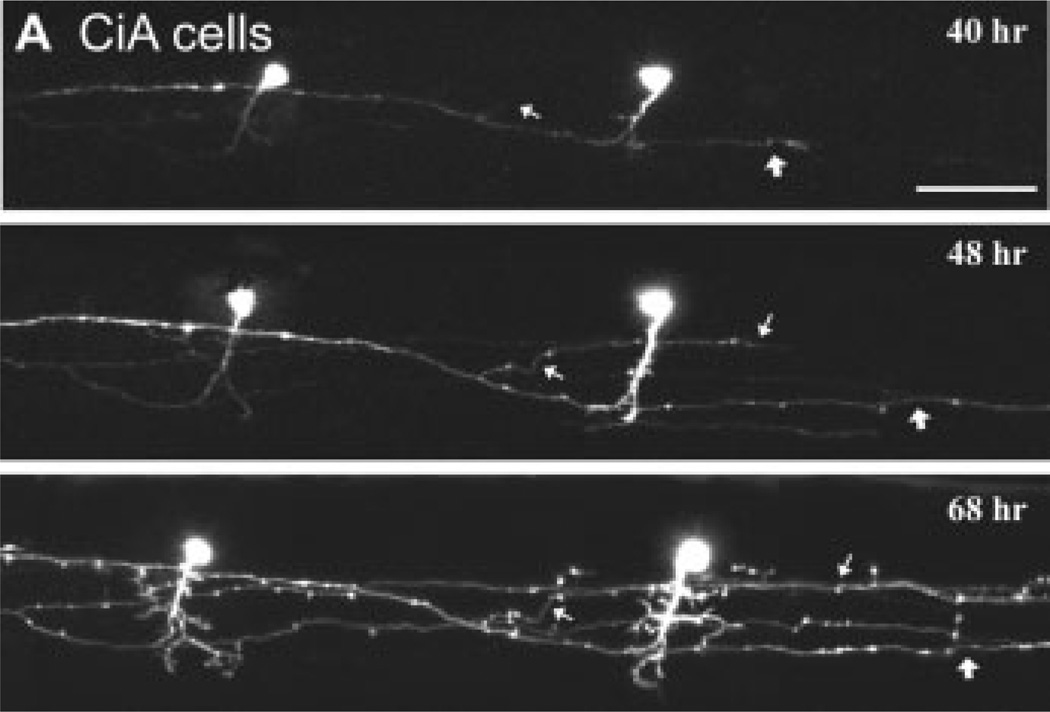
Figure 2.
Tracking the development of identified classes of spinal interneuron in vivo. (A) Confocal time lapse imaging of two inhibitory circumferential ascending (CiA) cells stochastically labeled with GFP using En1 as a promoter. CiAs are so named due to their primary ascending axon, however, they also have a secondary descending one. Successive images at different time points (hours postfertilization noted in respective images) illustrate the growth of the descending axon (at thin and thick white arrows), as well as the elaboration of the dendrites. Scale bar is 50 µm. Image reproduced from Higashijima et al. (2004) with permission (Copyright 2004 by the Society for Neuroscience).
DEVELOPMENTAND SPINAL NETWORK STRUCTURE/FUNCTION IN ZEBRAFISH
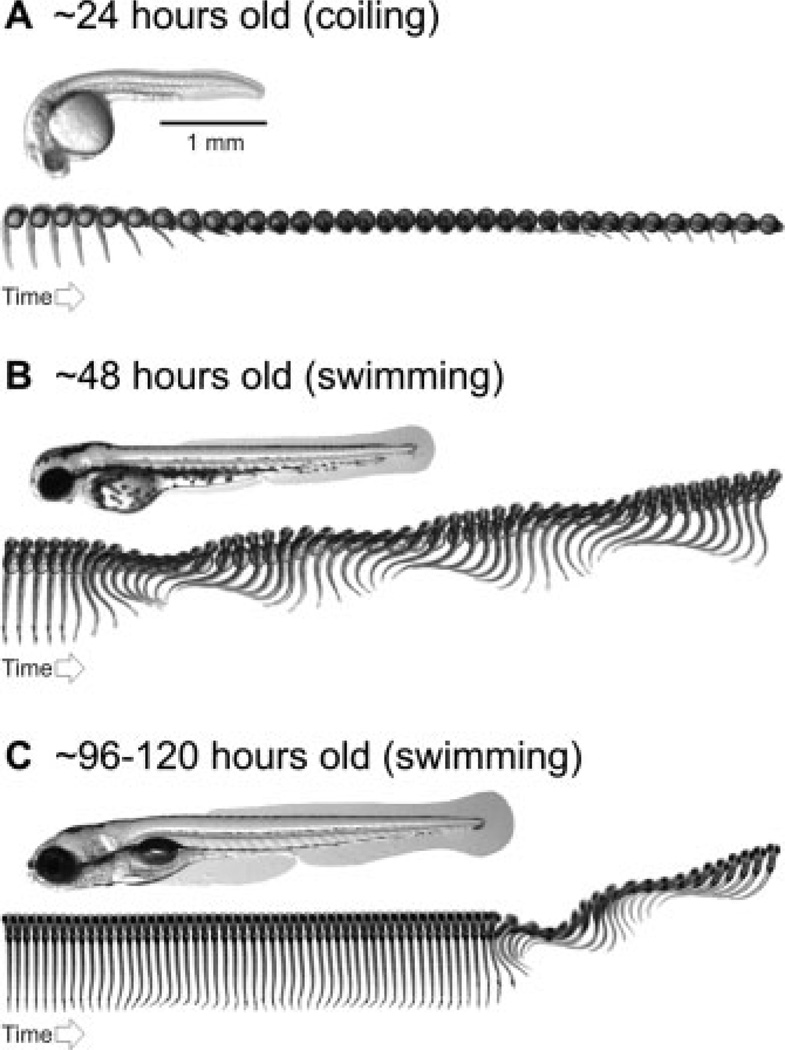
Zebrafish undergo a very stereotyped sequence of locomotor development (Saint-Amant and Drapeau, 1998), as do all animals, including humans (Thelen, 1979; Piek and Carman, 1994; D’Elia et al., 2001). The first movements (>17-hours-old) are spontaneous, powerful muscle contractions that involve coiling of the whole body [Fig. 3(A)]. Next (>27-hours-old) are more coordinated swimming movements with less powerful muscle contractions, which are typically only elicited when the zebrafish is startled [Fig. 3(B)]. Finally (>96-hours-old), spontaneous, goal-directed swimming emerges, with even weaker muscle contractions that involve mostly the tail and the newly functional pectoral fins [Fig. 3(C)]. This sequence of development results in zebrafish larvae that can move smoothly through a range of movements, from fast escapes to slow swimming.
Figure 3.
Stereotyped emergence of locomotor behavior in zebrafish. (A) Photograph of 1-day-old zebrafish embryo taken from the side, below which is a sequence of images captured at a rate of 1000 frames per second. The sequence illustrates spontaneous, embryonic coiling behavior. Each image is 20 ms apart. (B) Photograph of a 2-day-old zebrafish embryo taken from the side, below which is an image sequence captured at a rate of 1000 frames per second. The sequence illustrates swimming behavior elicited by a tactile stimulus to the tail. Each image is 2-ms apart. (C) Photograph of a 4- to 5-day-old zebrafish larva taken from the side, below which is an image sequence captured at a rate of 1000 frames per second. The sequence illustrates spontaneous swimming behavior, shortly followed by swimming evoked by a tactile stimulus to the tail. Each image is 2-ms apart. See Saint-Amant and Drapeau (1998); Budick and O’Malley (2000); Buss and Drapeau (2001); Thorsen et al. (2004); and the main text for more details.
Since spinal motor patterns change both during development and later in life to produce a range of movements, is it possible that these phenomena are linked? For example, are the bending movements produced by embryos and the escape bends produced by larvae generated by the same network? Does the network that produces swimming movements emerge as a refinement of this early network? This is not inconceivable, given that escape and swimming circuits share the need to drive muscle contractions on the same side of the body, while inhibiting them on the other (Fetcho and Faber, 1988). How do we test this? Questions about circuit assembly can be addressed on many levels of organization, from the synapse to the network.
Synaptic Dynamics
Neurons communicate through synapses, which govern the level of excitability and the patterning of activity within a circuit. The process of tagging synaptic markers with fluorescent proteins provides a means of monitoring dynamic wiring processes in vivo. This does not appear to adversely affect function. For example, GFP has been fused to postsynaptic receptors (David-Watine et al., 1999) and ion channels (Marshall et al., 1995; Grabner et al., 1998), with no measurable effect on activity.
Presynaptic and postsynaptic components have been used with great success in vivo to track developing synapses. Synaptophysin is a protein associated with synaptic vesicle recycling (Valtorta et al., 2004). In zebrafish, the ingrowing axons of retinal ganglion cell (RGC) axons have been labeled with a synaptophysin: GFP reporter and monitored as they innervate the optic tectum (Meyer and Smith, 2006). A similar approach using tagged synaptophysin was also performed in vivo in Xenopus to label developing motor neuron axons (Javaherian and Cline, 2005) and developing RGC axons (Ruthazer et al., 2006). These studies clearly demonstrated a direct link between synaptogenesis and axonal elaboration, whereby new axon branches emerge from newly formed synapses. The same process also occurs in dendrites (Niell et al., 2004). In this case, PSD-95, a scaffolding protein associated with the postsynaptic density of excitatory synapses (Sheng, 2001), was fused to GFP and used to label dendritic synapses of tectal neurons in zebrafish.
With respect to presynaptic and postsynaptic communication, in vivo studies of the developing neuromuscular junction in zebrafish suggest that axons are not required for the formation of postsynaptic specializations, but are necessary for their stabilization (Flanagan-Steet et al., 2005). In addition, once these stable postsynaptic specializations are formed, axons tend to compete over the same sites (Walsh and Lichtman, 2003). This arrangement, where functional synapses act as points from which new axonal or dendritic arbors grow, provides an elegant means to construct functional circuits.
Selectively labeling spinal networks with presynaptic and postsynaptic markers will allow tests of the relevance of these mechanisms in central motor networks. It will be interesting to see if spinal interneuron axons similarly compete over dendritic space and how motor neuron (or interneuron) dendrites react to this competition. For instance, when hunting for postsynaptic connections, do emerging premotor axonal branches focus on a single motor neuron, or a homonymous pool of them? Will dominant interneurons emerge or will synaptic connections be distributed evenly within the class? How does the relative distribution of synapses change during development? To what degree is connectivity reflected in motor function?
Cellular Dynamics
The morphology of a neuron has long been suggestive of its function (Ramon y Cajal, 1904). The fact that neuronal form changes so dynamically during development provides an opportunity to study exactly how form contributes to function in the nervous system. Axonal trajectory and dendritic arborizations are major anatomical-defining features of neurons. There is a great deal of work examining the molecular mechanisms underpinning axon guidance and synaptogenesis (reviewed in: Tessier-Lavigne and Goodman, 1996), a field in which zebrafish are beginning to contribute in vivo information (reviewed in: Hutson and Chien, 2002). However, a recent report using optogenetic approaches in zebrafish has revealed a previously underappreciated role for dendritic targeting in shaping neuronal connectivity in vertebrates (Mumm et al., 2006).
In the retina, the innerplexiform layer is comprised of laminar synaptic connections between RGCs and their presynaptic partners, bipolar and amacrine cells (reviewed in: Mumm et al., 2005). In zebrafish, RGCs were individually labeled with GFP and their dendritic arbors tracked during development. Remarkably, they found that dendrites from RGCs selectively grew toward their future synaptic laminae, where axons from amacrine cells were already present. A similar role for dendritic structure dictating the pattern of circuit connectivity (and thus function) has been described in the olfactory (Jefferis et al., 2004) and motor (Landgraf et al., 2003) systems of flies. At least with respect to the olfactory system, it is clear that transcription factors also play a key role in the patterning of dendrites (Komiyama and Luo, 2007), further reinforcing their importance in the formation of neural circuits. Genetic programs that can shape both axonal and dendritic patterning provide some clues as to how the developing nervous system can generate functional networks within such a dynamic environment.
Similar dendritic targeting strategies may be used in central motor circuits. A study in zebrafish embryos imaging the interaction of Mauthner cell axons with primary motor neurons in spinal cord demonstrated that primary motor neurons have highly dynamic dendritic filopodia (Jontes et al., 2000). Like the Mauthner cell, zebrafish spinal interneurons also have a very distinctive morphology, which could also predict their function (Bartelmez, 1915). By individually labeling neurons with either expression of GFP, or the selective activation of Dronpa (Aramaki and Hatta, 2006), driven by the appropriate promoter, it will be possible to track the dendritic and axonal changes in spinal circuits that are associated with changes in motor behavior. What is the relative contribution of axonal versus dendritic targeting to the formation of spinal circuits? Do all spinal circuits follow the same rules and how this might reflect the diversity of cell types? What does this tell us about the limits of circuit design and the function of spinal networks?
Network Dynamics and Activity
Identifying the cells, circuits, and networks that underlie behavior, is a fundamental pursuit of neurobiology. Sensory systems have proven fruitful ground for optogenetics to investigate how the activity of neuronal networks instructs behavior (Wang et al., 2003; Suh et al., 2004; Marella et al., 2006; Verhagen et al., 2007). This is because controlled stimuli can be delivered that elicit known behavioral responses, and the resulting responses in sensory networks monitored. Two areas, where the activity of networks has been imaged during development, are the olfactory and visual systems of zebrafish.
In zebrafish, odors are communicated by the activity patterns of olfactory glomeruli in the olfactory bulb (Friedrich and Korsching, 1997), as they are in the olfactory bulb of mice (Rubin and Katz, 1999) and the antennal lobe of flies (Wang et al., 2003). Using a genetically encoded calcium indicator, inverse pericam, the response of glomeruli to amino acids and bile acids was recently imaged during development (Li et al., 2005). This study found acid-evoked responses in the olfactory bulb that coincided with the emergence of behavioral responses to these odors. Critically, however, a gross pattern of functional topography was established early [Fig. 4(A)]. This pattern was later refined, suggesting that the adult neuronal circuits were a product of local refinements and elaborations, as opposed to large scale reorganizations of the olfactory bulb. This is certainly a parsimonious solution to producing the complexity observed in adults, which would not compromise the function of the network. In other words, mature neural circuits emerge from elaborations of an embryonic ground state, allowing them to continue to function as they develop.
Figure 4.
Genetically encoded calcium imaging of olfactory bulb activity during development. (A) Inverse pericam expression driven by the neuronal promoter HuC labels cells in the olfactory bulb (left). Color-coded response patterns from the area outlined in green captured using wide-field epifluorescence and a cooled CCD camera are to the right and below. The responses to 14 different odor stimuli at 3 and 5 days post fertilization (dpf) from the same larva are illustrated. While signals are certainly stronger in older larvae, the broad spatial organization of odor responses is already apparent early on. Image reproduced from Li et al. (2005) with permission (Copyright 2005 by the Society for Neuroscience). Odorant abbreviations can be found in the original citation.
A study of the visual system of larval zebrafish imaging the emergence of activity patterns also points in a similar direction (Niell and Smith, 2005). As for odors in the olfactory bulb, a topographic map representing the visual field is established early in the optic tectum (the fish correlate of the superior colliculus), at stages when they first begin to respond to visual stimuli. However, while the gross pattern of organization is laid down from the beginning, visual acuity improves substantially as larvae get older. It is likely that this improvement is due to a fine-tuning of the retinotopic map based on experience, since visual acuity did not improve in larvae raised in complete darkness. Like the olfactory bulb, visual circuits are unlikely to be the product of random wiring, followed by the pruning of inefficient synapses, but rather an immediate construction of a functional circuit, upon which refinements and elaborations generate maturity.
The same imaging approaches used in the olfactory bulb and optic tectum could be used to determine the relative contribution of spinal circuits to different locomotor behaviors during development. In addition, to test their time of differentiation, the photoconvertible protein Kaede could be used as a method of in vivo time stamping. Kaede turns from green to red in the presence of UV light (Ando et al., 2002), and has been expressed in the nervous system of zebrafish using neuron specific promoters (Kimura et al., 2006; Sato et al., 2006). Kaede protein that continues to be generated after conversion is green, so the presence of red Kaede protein is an indication that the cells differentiated before the conversion time point [Fig. 5(A,B)]. These approaches would conclusively address whether mature behaviors are also a product of network refinement or the emergence of new circuits, or a mixture of the two.
Figure 5.
Tracking spinal network differentiation in vivo. (A) Schematic illustrating the photoconversion of Kaede protein using ultraviolet (UV) light. In this example, Kaede is restricted to the nervous system. Before conversion Kaede is green, and after conversion it is red. Any neurons containing red Kaede must have been present before conversion. Neurons with no red Kaede differentiated after conversion. (B) An example of the Kaede approach to time-stamp a specific class of spinal excitatory interneuron, circumferential descending (CiD) cells. Kaede expression was driven selectively into CiDs using an alx promoter. The top panel shows a merge of the red and green channels imaged using a confocal microscope, which are individually displayed below. The arrows indicate cells with both red and green fluorescence (yellow), while arrowheads indicate cells with red fluorescence, but marginal levels of green fluorescence. The time of conversion and imaging is as detailed in (A). Scale bar is 20 µm. Image in (B) is reproduced from Kimura et al. (2006) with permission (Copyright 2006 by the Society for Neuroscience).
To test function, it is important to move beyond anatomical description. The beauty of studying motor networks to address issues of network assembly is that there is a clear functional context, from which predictions about perturbation experiments can be made. Specifically, optogenetic perturbations can test with unmatched precision how activity and/or external inputs shape the architecture of nascent networks and their behavioral output.
PERTURBING SPINAL NETWORKS AND LOCOMOTOR DEVELOPMENT
The reason why olfactory and visual centers are such popular subjects for network assembly is their topographic organization. Such regularity provides a powerful means to study how dendrites and axons interact during synaptogenesis to construct functional networks. You can predict where a dendrite should arborize and what axons should contact it. It now appears that the spinal cord may also be topographically organized. It is textbook knowledge that the spinal cord can be divided into 10 laminae, based on broad cytological and functional features. However, it was not clear how interneurons within or between these laminae might be organized to produce various strengths and speeds of movement.
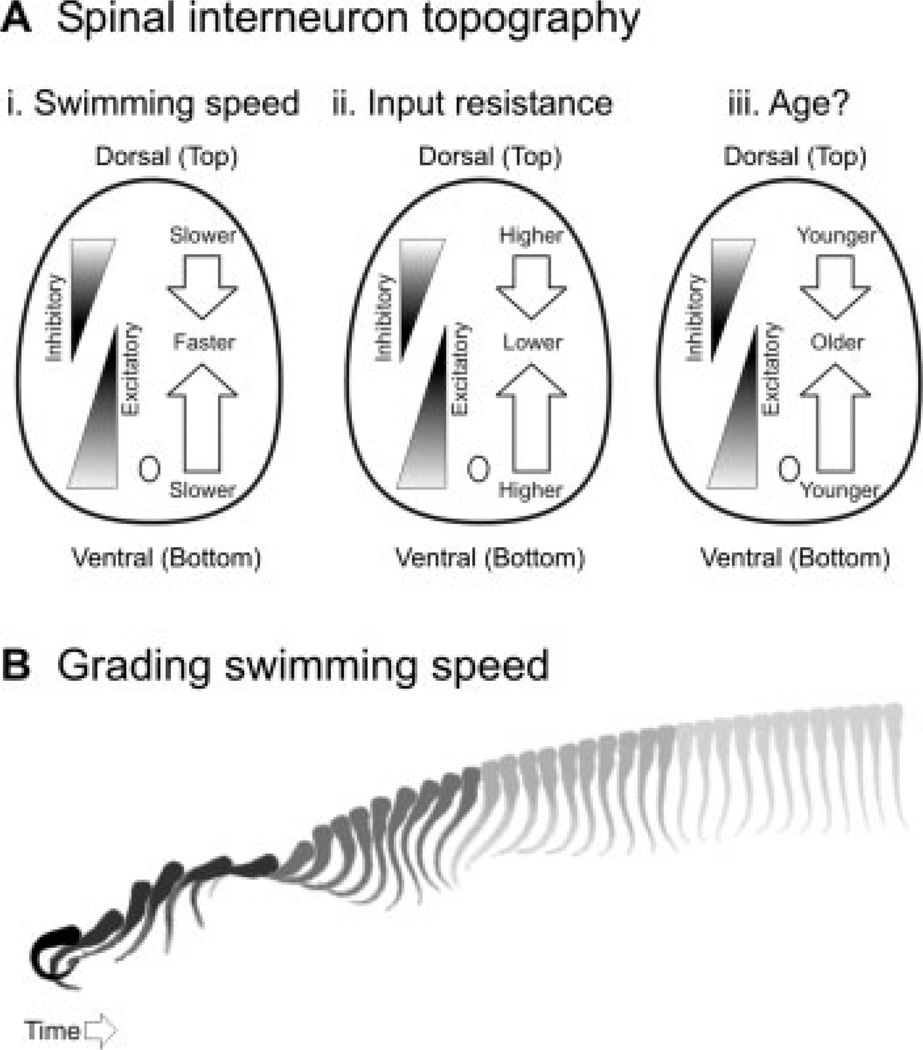
Using a combination of electrophysiological, imaging, genetic, and perturbation approaches in larval zebrafish, we recently demonstrated that the location of a neuron in spinal cord could predict when it was recruited during swimming (McLean et al., 2007). The most ventral motor neurons and excitatory interneurons would fire rhythmically at the slowest swimming frequencies, with progressively more dorsal ones recruited as swimming speed increased [Fig. 6(Ai)]. For inhibitory interneurons, the opposite pattern was apparent; dorsal interneurons would fire at the slowest swimming speeds, with progressively more ventral ones recruited as swimming speed increased [Fig. 6(Ai)]. This topography of recruitment could at least be partly explained by matching gradients in neuronal excitability. The most ventral excitatory and dorsal inhibitory interneurons had the highest input resistances and were therefore the most excitable [Fig. 6(Aii)]. At present, it is unclear what mechanisms in the brain could contribute to the recruitment pattern we observe. We do not know whether or not there are distinct descending inputs that selectively activate slower versus faster spinal circuits. However, given the pattern of excitability, there is not necessarily a need for selective drive, since the proper circuits could be selected based on a general level of drive to spinal cord.
Figure 6.
Gradients of recruitment in spinal cord and the relation to swimming speed. (A) Schematic illustrations of the dorso-ventral gradients of inhibitory and excitatory interneurons with respect to (i) swimming speed, (ii) input resistance, and, more speculatively, (iii) age. (B) Cartoon illustrating a larval zebrafish responding to a tactile stimulus with an escape bend and a bout of swimming. As the speed of the swimming bout decreases, so too does the shade. This is meant to reflect the relative participation of spinal networks driving slow versus faster movements according to the topographic arrangement illustrated in (A). The image sequence was captured at a rate of 1000 Hz and each image is 4-ms apart.
There is also evidence that this topography may be related to age. We already know that primary motor neurons, which are born first, participate in the fastest swimming behaviors. However, a recent study using Kaede driven by the alx-promoter has shown that the oldest, excitatory CiD interneurons are also the dorsal-most, and contribute to the most powerful motor behaviors in hatchling zebrafish embryos [Kimura et al., 2006; see also, Fig. 5(B)]. It is not known whether the same holds true for larvae, but given the pattern of recruitment we observed, it is certainly plausible. Thus, we would predict that the dorsal-most excitatory interneurons and the ventral-most inhibitory interneurons are the oldest [Fig. 6(Aiii)]. This type of organization likely enables larval zebrafish to move efficiently through their speed range [Fig. 6(B)]. The fact that these patterns of recruitment may reflect time of neuronal differentiation raises the possibility that spinal networks emerge in accord with the strength of the movement to which they contribute. The strongest movements and the neurons driving them emerge first during development with weaker movements and neurons added later. In retrospect, such gradients in excitability, recruitment, and age make sense given how important gradients are for setting up spinal patterning (Briscoe et al., 1999).
How do we test the contribution of activity or external inputs to the topographic organization of spinal circuits? Pioneering investigations of developing spinal networks in the past relied on pharmacological perturbations to alter network activity and chemical or physical lesions to remove external inputs (reviewed in: Sillar et al., 1998; O’Donovan, 1999; Nishimaru and Kudo, 2000; Clarac et al., 2004). The arrival of more sophisticated optical and genetic approaches, particularly suited to the zebrafish model, now mean that these perturbations can be extremely precise. For the purposes of this review, we have organized different approaches according to their ability to chronically or acutely perturb network function.
Chronic Perturbations
In mutagenesis, genes are randomly disrupted using either UV light or chemical agents, and their contributions to development are reflected in the phenotype. The genes are then typically identified using a combination of candidate gene and positional cloning approaches. The large-scale Tübingen screen generated a number of zebrafish mutants with severe locomotor deficits (Granato et al., 1996). Unfortunately, from the point of view of central motor circuit development, a majority of the mutant phenotypes studied so far are a consequence of defects in muscle-specific genes (Ono et al., 2001; Ono et al., 2002; Downes and Granato, 2004; Gleason et al., 2004; Hirata et al., 2004; Zhang et al., 2004; Schneider and Granato, 2006). A few, however, have turned out to have central deficits.
For example, zebrafish bandoneon mutants develop normal spontaneous coiling movements, but fail to develop early embryonic swimming movements due to a mutation in a glycine receptor β subunit gene (Hirata et al., 2005). Zebrafish shocked mutants exhibit altered early coiling movements due to a mutation in the glycine transporter-1 gene (Cui et al., 2005), but do eventually develop swimming movements. These mutants could be extremely useful for dissecting the relative contribution of glycinergic signaling during different stages of locomotor development.
The usefulness of a mutation approach relies on the ability to rapidly screen and recognize deficits that would hint at changes in the circuit in which you are interested. This can be tricky with locomotion, because the movement deficits may not be a result of central defects, as illustrated above. One exquisite example of high throughput screening to identify circuit malfunction is the recent identification of a sensorimotor gating mutant, Ophelia (Burgess and Granato, 2007). Using a combination of high-speed imaging and automated movement analysis, the authors isolated mutant fish that failed to attenuate escape responses elicited shortly after a weaker stimulus as normal fish do. Because this attenuation is the zebrafish form of prepulse inhibition (PPI), and impaired PPI is symptomatic of many neuropsychiatric disorders, Ophelia mutants are anticipated to shed light on the genes and circuitry underlying this phenomenon (Burgess and Granato, 2007). Indeed, zebrafish hold great promise for uncovering the molecular and cellular basis of human diseases reviewed in (Amsterdam and Hopkins, 2006; Lieschke and Currie, 2007).
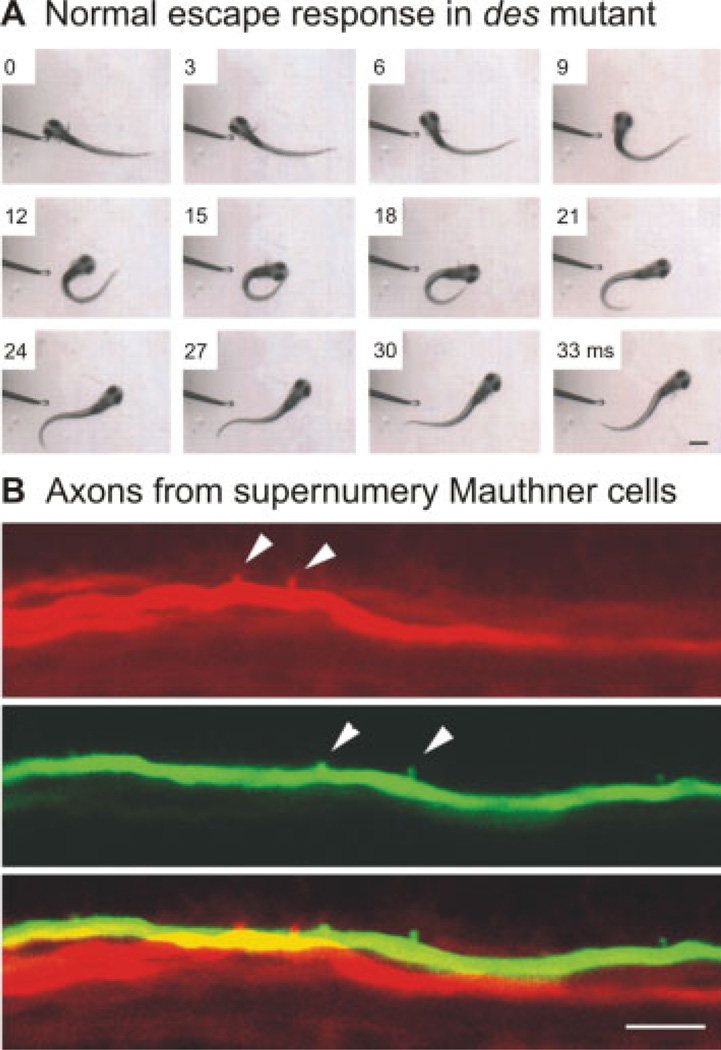
It is important to consider downstream effects that may result from the generation of mutations. The nervous system is, of course, very plastic. This is clearly illustrated by the shocked mutation, where a secondary consequence of disrupted neural activity was a persistence of an embryonic state of electrical coupling in the muscle fibers, which exacerbated the phenotype (compare: Luna et al., 2004; Cui et al., 2005). Sometimes these homeostatic phenomena can also be exploited to investigate neural network plasticity. A mutation in the des/notch1a gene that results in extra Mauthner cells (Gray et al., 2001), was used to test how a functional neural circuit would cope with duplications (Liu et al., 2003). Zebrafish larvae with extra Mauthner cells are not hyperactive, but rather exhibit normal escape movements [Fig. 7(A)]. The escape circuit reconfigures itself, such that each extra Mauthner cell covers a smaller, nonoverlapping territory, dividing the outputs along spinal cord [Fig. 7(B)]. This demonstration of network homeostasis during formation of the escape circuit helps explain how startle circuits in mammals with much larger numbers of neurons might have evolved.
Figure 7.
Deadly seven/notch1a (des) mutants display homeostatic plasticity in the escape circuit. (A) An escape in response to a tactile stimulus to the head appears normal in this des mutant. The larva turns quickly from the stimulus and begins to swim away. Images were captured at 1000 Hz, and every third frame is shown. The time in milliseconds is marked on the frames. Scale bar is 550 µm. (B) A mutation in the des/notch1a gene results in multiple Mauthner cells, two of which have been electroporated here with different colored fluorescent dyes and imaged using a confocal microscope. Arrowheads denote axon collaterals, which do not form in the same location as the merged image at the bottom demonstrates. Scale bar is 10 µm. Images reproduced from Liu et al. (2003) with permission (Copyright 2003 by the Society for Neuroscience).
There are also a number of other chronic methods for perturbing developing circuits in vivo. Morpholino oligonucleotides can be injected into single-cell zebrafish embryos and will bind to messenger RNA to prevent protein synthesis, providing a useful means to “knockdown” gene function (Nasevicius and Ekker, 2000). Morpholinos have been used to reveal the contribution of identified currents to develop spinal circuit excitability and behavior in vivo (Pineda et al., 2005), as well as the role glycinergic signaling plays during spinal interneuron differentiation (McDearmid et al., 2006). Conversely, constructs can be injected into single-cell embryos to chronically enhance gene function. For example, injection of hoxb1b mRNA produces a segmentation duplication in the hindbrain, which results in two rhombomeres that contain Mauthner cells (Hale et al., 2004). This approach provides another elegant means to investigate how ectopic cells integrate into a functional circuit. A similar strategy could be used to probe the consequences of ectopic components within spinal circuits, given the correct promoter.
Cell-specific promoter driven strategies can also be used to functionally silence networks. By expressing tetanus toxin light chain to prevent transmitter release (Sweeney et al., 1995) or an inward rectifying potassium channel (Kir2.1) to hyperpolarize cells (Johns et al., 1999), a relatively recent study demonstrated the importance of spontaneous release and firing activity on the establishment and maintenance of topographic connections in the olfactory bulb (Yu et al., 2004). Kir2.1-mediated silencing has also been used to demonstrate the importance of activity in the formation of RGC axonal contacts in developing zebrafish optic tectum (Hua et al., 2005). Another study in zebrafish has demonstrated that cell-specific expression of tethered peptide toxins can modulate ion channels and receptors in vivo (Ibanez-Tallon et al., 2004). Ideally, however, it is better to have precise spatial and temporal control over a perturbing agent, which is exactly what optogenetic strategies can provide.
Acute Perturbations
Lasers provide a powerful means to target cells and to selectively remove them from the circuit quickly, minimizing any accommodative measures that may take place during development. The earliest studies using lasers in vivo to test the contribution of interneurons to behavior include perturbations of visual orientation in flies (Geiger and Nassel, 1981) and chemotaxis in worms (Bargmann et al., 1993). In zebrafish, laser ablations have been used in vivo to selectively perturb the neural circuits underlying escape behavior (Liu and Fetcho, 1999), visuomotor responses (Roeser and Baier, 2003), prey capture (Gahtan et al., 2005), and swimming (McLean et al., 2007). Lasers have also been used during zebrafish development to assess the contribution of neuroepithelial cells to axon guidance (Kuwada, 1986) and the dynamics of myelination by oligodendrocytes (Kirby et al., 2006). In flies, laser ablation experiments have examined competitive mechanisms underlying the formation of dendritic territories in the peripheral nervous system (Gao et al., 2000). Interestingly, this study found that competitive interactions between neighboring neurons were apparent at larval, but not embryonic stages of development, supporting age-dependent differences in the wiring dynamics.
Laser ablation approaches are limited by the fact that only a handful of cells can be removed at a time. So, while they may be perfect for testing the contribution of relatively small nuclei to development, they are less useful for removing large populations. Here, phototoxic agents could prove more useful. One recent example of a genetically encoded means to ablate cells is called KillerRed (Bulina et al., 2006). KillerRed is a dimeric red fluorescent protein developed from a homolog of GFP that generates reactive oxygen species when illuminated with green light. This is sufficient to kill cells within minutes, but only the ones illuminated. This means a single cell, a subset, or a whole population can be ablated depending on the expression pattern and the degree and localization of illumination.
Clearly, killing cells by an irreversible process is not ideal. Fortunately, there are now acute ways to reversibly activate and silence neurons. A recent example of this uses a combination of channelrhodopsin-2 (ChR2) and Natronomonas pharaonis halorhodopsin (NpHR) to rapidly activate and silence neurons, respectively, using light (Zhang et al., 2007). These proteins have nonoverlapping spectra (meaning they can be used simultaneously) and utilize a nonselective cation channel (ChR2) and a chloride-ion pump (NpHR) to alter neuronal excitability. In embryonic chick spinal cord, ChR2 and a different inhibitor of neuronal activity, vertebrate rat rhodopsin 4 (RO4), have been used to manipulate spontaneous activity (Li et al., 2005). More targeted studies using these approaches will provide the ability to assess the contribution of spontaneous activity within identified classes of interneuron to network assembly. They also open up the exciting possibility of artificially coupling or uncoupling sets of developing neurons to test the effects of coincident activity on wiring.
Light is not the only way to control perturbations. A ligand-based approach was recently used to reversibly perturb spinal rhythmogenesis in developing mammals (Gosgnach et al., 2006). Interneurons arising from a domain called V1 in spinal cord consist of at least two classes; Renshaw cells, which mediate recurrent inhibition to motor neurons and Ia inhibitory interneurons, which mediate feed-forward inhibition to antagonistic motor pools. Both of these classes are labeled by the transcription factor En1, which as mentioned above, labels a single class of spinal interneurons in zebrafish larvae, CiAs. V1 interneurons in mice were selectively silenced by conditionally expressing the Drosophila allatostatin G-protein-coupled receptor and then bath-applying allatostatin. The allatostatin receptor couples to endogenous mammalian inward rectifying potassium channels, which decrease input resistance and thus neuronal excitability (Lechner et al., 2002). The perturbation of the locomotor rhythm that resulted was reversed by simply washing off the allatostatin. Allatostatin produced a slowing of the motor rhythm that corroborated the slow locomotor rhythms observed in mice where V1 interneurons were chronically removed in the same study. The contribution of V1 interneurons to faster locomotor outputs is supported by detailed physiological studies of En1-positive neurons in frog embryos, which are more reliably active during faster swimming movements (Li et al., 2004). En1 labels ascending inhibitory interneurons in frog embryos identical to zebrafish CiAs (Higashijima et al., 2004). Other more recent examples of acute perturbation strategies utilize light-gated glutamate receptors to excite neurons (Szobota et al., 2007) or ligand-gated chloride channels to inhibit them (Lerchner et al., 2007).
Of course, perturbations need to be assessed by some means. In vivo imaging of dendritic fields and axonal arbors will certainly report changes in the location of inputs and outputs, but this is only an inference of network connectivity. It would be useful to have a more direct measure of the degree to which perturbations affect network connectivity. A recent study reports the fabrication of a genetically targetable trans-synaptic tracer that labels only monosynaptically connected partners (Wickersham et al., 2007). This technique improves upon previous ones, where multiple neurons tended to be labeled at different rates, making it difficult to separate direct from indirect connections. It could be imagined that single identified spinal motor neurons or interneurons labeled in such a way would provide in vivo maps of changes in connectivity, not only during development, but also after selective perturbations.
Another means to assess connectivity is through activity. For instance, individual interneurons could be labeled and activated using ChR2 and then responses in likely postsynaptic partners imaged, using either global expression of a genetically encoded calcium indicator or bulk loading with membrane permeable, acetoxymethyl dyes. A similar strategy has been used to assess connectivity in the visual cortex (Peterlin et al., 2000). This would rapidly assess functional outputs. Conversely, the activity of a postsynaptic cell could be recorded using conventional electrophysiology and individual activation of a group of presynaptic neurons labeled with ChR2 could rapidly assess functional inputs (Petreanu et al., 2007). Collectively, these techniques would provide unequaled access to the processes governing spinal network assembly and function.
Perturbation experiments are best when driven by predictions. Thanks to the array of anatomically distinct spinal neurons and the topography we have discovered in zebrafish, we would predict that dendritic arborizations and axonal trajectories also follow topographic profiles according to speed of movement. Thus, the location of dendrites in spinal cord could also reflect a functional field, in the same way the dendritic location of RGCs do. These patterns could easily be perturbed in a predictable way by optogenetically altering network excitability or by removing inputs. The effect on locomotor rhythms could then be assessed.
As a model system, it is important that zebrafish studies reveal principles of organization that allow predictions about similar functional circuits in humans and other animals. Axial circuits likely reflect the primitive condition, from which limb circuits are derived. It is encouraging that shared markers, like the transcription factor En1, reveal morphological and functional similar neurons from species as disparate as fish (Higashijima et al., 2004), frogs (Li et al., 2004), and mice (Gosgnach et al., 2006), because the findings from developing zebrafish may reveal features of organization that apply broadly among vertebrates. It is unclear how the recruitment topography we have described in zebrafish relates to organization of mammalian spinal cord. However, we would predict that if interneurons could be birth-dated in mammals, then the older ones would participate in the more powerful locomotor maneuvers. We would also predict that Chx10-labeled V2 interneurons in mammals, which in zebrafish represent the relatively early born alx-labeled CiDs (Kimura et al., 2006), contribute to faster speeds of mammalian locomotion, since they are specialized for faster swimming speeds in zebrafish (Bhatt et al., 2007; McLean et al., 2007). The power of concrete predictions from a simple model system is that you can test the role of interneurons in the correct context. For instance, the contribution of Chx10-labeled V2 interneurons to spinal rhythmogenesis may not be fully appreciated if perturbation experiments are conducted and the animals are only tested at lower speeds, when these neurons might not be as important.
The bottom line is that zebrafish can reveal principles of organization during development that should apply broadly among vertebrates. These principles need to provide clear predictions to allow for rigorous testing of the breadth of their explanatory power, including their applicability to the motor systems of mammalian species.
CONCLUDING REMARKS
To understand motor systems, it is important to look through development at a tractable behavior within a model system where the circuits can be identified, monitored, and perturbed through time. At present, zebrafish are the best vertebrate system to accomplish this. The sequential emergence of distinct motor behaviors that zebrafish perform later in life generates a number of questions regarding how neural circuits are organized to produce movement. It also provides the opportunity to investigate the strategies used by the nervous system to maintain function while still under construction. All of the optical and genetic tools are now in place to search for general principles of neural circuit development and organization using the zebrafish model.
Acknowledgments
Contract grant sponsors: National Institutes of Health, Human Frontier Science Program Organization.
The authors thank Sandeep Kishore for many helpful discussions, and Jimmy Liao, John Olthoff, Minoru Koyama, and Amina Kinkhabwala for comments on previous drafts of the manuscript.
REFERENCES
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UVinduced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki S, Hatta K. Visualizing neurons one-by-one in vivo: Optical dissection and reconstruction of neural networks with reversible fluorescent proteins. Dev Dyn. 2006;235:2192–2199. doi: 10.1002/dvdy.20826. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorantselective genes and neurons mediate olfaction in Celegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bartelmez GW. Mauthner’s cell and the nucleus motorius tegmenti. J Comp Neurol. 1915;25:87–128. [Google Scholar]
- Bate M. Development of motor behaviour. Curr Opin Neurobiol. 1999;9:670–675. doi: 10.1016/s0959-4388(99)00031-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, Chitnis AB, Lindamer L, Kuwada JY. Identification of spinal neurons in the embryonic and larval zebrafish. J Comp Neurol. 1990;302:603–616. doi: 10.1002/cne.903020315. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, Patel CK, Wilson SW, Kuwada JY. Axonal trajectories and distribution of GABAergic spinal neurons in wildtype and mutant zebrafish lacking floor plate cells. J Comp Neurol. 1992;326:263–272. doi: 10.1002/cne.903260208. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, McLean DL, Hale ME, Fetcho JR. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. doi: 10.1016/j.neuron.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Budick SA, O’Malley DM. Locomotor repertoire of the larval zebrafish: Swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, et al. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. doi: 10.1152/jn.2001.86.1.197. [DOI] [PubMed] [Google Scholar]
- Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Yuste R. Stimulating neurons with light. Curr Opin Neurobiol. 2002;12:587–592. doi: 10.1016/s0959-4388(02)00364-1. [DOI] [PubMed] [Google Scholar]
- Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85:2166–2176. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- Clarac F, Brocard F, Vinay L. The maturation of locomotor networks. Prog Brain Res. 2004;143:57–66. doi: 10.1016/S0079-6123(03)43006-9. [DOI] [PubMed] [Google Scholar]
- Combes D, Merrywest SD, Simmers J, Sillar KT. Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis. J Physiol. 2004;559:17–24. doi: 10.1113/jphysiol.2004.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DR, Abrams JM. Morpholino antisense oligonucleotides: Tools for investigating vertebrate development. Genome Biol. 2001;2:Reviews1015.1011–Reviews1015.1013. doi: 10.1186/gb-2001-2-5-reviews1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WW, Low SE, Hirata H, Saint-Amant L, Geisler R, Hume RI, Kuwada JY. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J Neurosci. 2005;25:6610–6620. doi: 10.1523/JNEUROSCI.5009-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia A, Pighetti M, Moccia G, Santangelo N. Spontaneous motor activity in normal fetuses. Early Hum Dev. 2001;65:139–147. doi: 10.1016/s0378-3782(01)00224-9. [DOI] [PubMed] [Google Scholar]
- David-Watine B, Shorte SL, Fucile S, de Saint Jan D, Korn H, Bregestovski P. Functional integrity of green fluorescent protein conjugated glycine receptor channels. Neuropharmacology. 1999;38:785–792. doi: 10.1016/s0028-3908(99)00015-5. [DOI] [PubMed] [Google Scholar]
- de Graaf M, Zivkovic D, Joore J. Hormone-inducible expression of secreted factors in zebrafish embryos. Dev Growth Differ. 1998;40:577–582. doi: 10.1046/j.1440-169x.1998.t01-3-00001.x. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Feng GP, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Garcia J, Matsushita S, Mutoh H, Nakai J, Ohkura M, Yokoyama J, Dimitrov D, et al. Activation of cerebellar parallel fibers monitored in transgenic mice expressing a fluorescent Ca2+ indicator protein. Eur J Neurosci. 2005;22:627–635. doi: 10.1111/j.1460-9568.2005.04250.x. [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev Biol. 2004;270:232–245. doi: 10.1016/j.ydbio.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Edgley SA. Organisation of inputs to spinal interneurone populations. J Physiol. 2001;533:51–56. doi: 10.1111/j.1469-7793.2001.0051b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR. Morphological variability, segmental relationships, and functional role of a class of commissural interneurons in the spinal cord of goldfish. J Comp Neurol. 1990;299:283–298. doi: 10.1002/cne.902990303. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Bhatt DH. Genes and photons: New avenues into the neuronal basis of behavior. Curr Opin Neurobiol. 2004;14:707–714. doi: 10.1016/j.conb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Faber DS. Identification of motoneurons and interneurons in the spinal network for escapes initiated by the mauthner cell in goldfish. J Neurosci. 1988;8:4192–4213. doi: 10.1523/JNEUROSCI.08-11-04192.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR, Liu KS. Zebrafish as a model system for studying neuronal circuits and behavior. Ann N Y Acad Sci. 1998;860:333–345. doi: 10.1111/j.1749-6632.1998.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Tanger P, Baier H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci. 2005;25:9294–9303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. Control of dendritic field formation in Drosophila: The roles of flamingo and competition between homologous neurons. Neuron. 2000;28:91–101. doi: 10.1016/s0896-6273(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Geiger G, Nassel DR. Visual orientation behaviour of flies after selective laser beam ablation of interneurones. Nature. 1981;293:398–399. doi: 10.1038/293398a0. [DOI] [PubMed] [Google Scholar]
- Gleason MR, Armisen R, Verdecia MA, Sirotkin H, Brehm P, Mandel G. A mutation in serca underlies motility dysfunction in accordion zebrafish. Dev Biol. 2004;276:441–451. doi: 10.1016/j.ydbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gleason MR, Higashijima S, Dallman J, Liu K, Mandel G, Fetcho JR. Translocation of CaM kinase II to synaptic sites in vivo. Nat Neurosci. 2003;6:217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol. 2002;12:508–515. doi: 10.1016/s0959-4388(02)00371-9. [DOI] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA. 1998;95:1903–1908. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Nusslein-Volhard C. Fishing for genes controlling development. Curr Opin Genet Dev. 1996;6:461–468. doi: 10.1016/s0959-437x(96)80068-2. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, et al. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Gray M, Moens CB, Amacher SL, Eisen JS, Beattie CE. Zebrafish deadly seven functions in neurogenesis. Dev Biol. 2001;237:306–323. doi: 10.1006/dbio.2001.0381. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish— Emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Habuchi S, Ando R, Dedecker P, Verheijen W, Mizuno H, Miyawaki A, Hofkens J. Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa. Proc Natl Acad Sci USA. 2005;102:9511–9516. doi: 10.1073/pnas.0500489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale ME, Kheirbek MA, Schriefer JE, Prince VE. Hox gene misexpression and cell-specific lesions reveal functionality of homeotically transformed neurons. J Neurosci. 2004;24:3070–3076. doi: 10.1523/JNEUROSCI.5624-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale ME, Ritter DA, Fetcho JR. A confocal study of spinal interneurons in living larval zebrafish. J Comp Neurol. 2001;437:1–16. doi: 10.1002/cne.1266. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Sato-Maeda M, Warren JT, Su F, Lele Z, Krone PH, Kuwada JY, et al. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Perl ER. Molecular and genetic features of a labeled class of spinal substantia gelatinosa neurons in a transgenic mouse. J Comp Neurol. 2005;492:90–100. doi: 10.1002/cne.20709. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Landmesser LT. New optical tools for controlling neuronal activity. Curr Opin Neurobiol. 2007;17:87–94. doi: 10.1016/j.conb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Masino MA, Mandel G, Fetcho JR. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J Neurophysiol. 2003;90:3986–3997. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol. 1997;192:289–299. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Schaefer M, Fetcho JR. Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:19–37. doi: 10.1002/cne.20279. [DOI] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Downes GB, Cui WW, Zhou W, Granato M, Kuwada JY. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor β-subunit. Proc Natl Acad Sci USA. 2005;102:8345–8350. doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Waterbury J, Cui W, Zhou W, Li Q, Goldman D, et al. Accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development. 2004;131:5457–5468. doi: 10.1242/dev.01410. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Hughes A. The development of the primary sensory system in Xenopus laevis (Daudin) J Anat. 1957;91:323–338. [PMC free article] [PubMed] [Google Scholar]
- Hutson LD, Chien CB. Wiring the zebrafish: Axon guidance and synaptogenesis. Curr Opin Neurobiol. 2002;12:87–92. doi: 10.1016/s0959-4388(02)00294-5. [DOI] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Wen H, Miwa JM, Xing J, Tekinay AB, Ono F, Brehm P, et al. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron. 2004;43:305–311. doi: 10.1016/j.neuron.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: Identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Cline HT. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron. 2005;45:505–512. doi: 10.1016/j.neuron.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Jean-Xavier C, Mentis GZ, O’Donovan MJ, Cattaert D, Vinay L. Dual personality of GABA/glycine-mediated depolarizations in immature spinal cord. Proc Natl Acad Sci USA. 2007;104:11477–11482. doi: 10.1073/pnas.0704832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Meng A, McFarlane RJ, Paw BH, Zon LI, Smith GR, Lin S. Modification of bacterial artificial chromosomes through chi-stimulated homologous recombination and its application in zebrafish transgenesis. Proc Natl Acad Sci USA. 1998;95:5121–5126. doi: 10.1073/pnas.95.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O’Rourke B, Marban E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes JD, Buchanan J, Smith SJ. Growth cone and dendrite dynamics in zebrafish embryos: Early events in synaptogenesis imaged in vivo. Nat Neurosci. 2000;3:231–237. doi: 10.1038/72936. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn. 2005;234:244–254. doi: 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–321. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic-development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. Alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Knopfel T, Diez-Garcia J, Akemann W. Optical probing of neuronal circuit dynamics: Genetically encoded versus classical fluorescent sensors. Trends Neurosci. 2006;29:160–166. doi: 10.1016/j.tins.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Luo L. Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr Biol. 2007;17:278–285. doi: 10.1016/j.cub.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Kuwada JY. Cell recognition by neuronal growth cones in a simple vertebrate embryo. Science. 1986;233:740–746. doi: 10.1126/science.3738507. [DOI] [PubMed] [Google Scholar]
- Kuwada JY. Development of the zebrafish nervous system: Genetic analysis and manipulation. Curr Opin Neurobiol. 1995;5:50–54. doi: 10.1016/0959-4388(95)80086-7. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Jeffrey V, Fujioka M, Jaynes JB, Bate M. Embryonic origins of a motor system: Motor dendrites form a myotopic map in Drosophila. PLoS Biol. 2003;1:E41. doi: 10.1371/journal.pbio.0000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC. Target regulation of neurotransmitter phenotype. Trends Neurosci. 1990;13:344–350. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu DG, de Velasco JM, Tessarollo L, Swing DA, Court DL, Jenkins NA, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Helde KA, Thorpe CJ, Rooke R, Moon RT. Reverse genetics in zebrafish. Physiol Genomics. 2000;2:37–48. doi: 10.1152/physiolgenomics.2000.2.2.37. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Li J, Mack JA, Souren M, Yaksi E, Higashijima S, Mione M, Fetcho JR, et al. Early development of functional spatial maps in the zebrafish olfactory bulb. J Neurosci. 2005;25:5784–5795. doi: 10.1523/JNEUROSCI.0922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Higashijima S, Parry DM, Roberts A, Soffe SR. Primitive roles for inhibitory interneurons in developing frog spinal cord. J Neurosci. 2004;24:5840–5848. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: Zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Liu DW, Westerfield M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J Physiol. 1988;403:73–89. doi: 10.1113/jphysiol.1988.sp017239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Liu KS, Gray M, Otto SJ, Fetcho JR, Beattie CE. Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit. J Neurosci. 2003;23:8159–8166. doi: 10.1523/JNEUROSCI.23-22-08159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV. Innovation: Photoactivatable fluorescent proteins. Nat Rev Mol Cell Biol. 2005;6:885–891. doi: 10.1038/nrm1741. [DOI] [PubMed] [Google Scholar]
- Luna VM, Wang M, Ono F, Gleason MR, Dallman JE, Mandel G, Brehm P. Persistent electrical coupling and locomotory dysfunction in the zebrafish mutant shocked. J Neurophysiol. 2004;92:2003–2009. doi: 10.1152/jn.00454.2004. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marshall J, Molloy R, Moss GW, Howe JR, Hughes TE. The jellyfish green fluorescent protein: A new tool for studying ion channel expression and function. Neuron. 1995;14:211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Liao M, Drapeau P. Glycine receptors regulate interneuron differentiation during spinal network development. Proc Natl Acad Sci USA. 2006;103:9679–9684. doi: 10.1073/pnas.0504871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry MJ, Lauder GV. Ontogeny of form and function: Locomotor morphology and drag in zebrafish (Danio rerio) J Morphol. 2006;267:1099–1109. doi: 10.1002/jmor.10462. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci. 2002;22:7055–7064. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G. Genetic methods for illuminating the function of neural circuits. Curr Opin Neurobiol. 2004;14:395–402. doi: 10.1016/j.conb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Miller G. Optogenetics: Shining new light on neural circuits. Science. 2006;314:1674–1676. doi: 10.1126/science.314.5806.1674. [DOI] [PubMed] [Google Scholar]
- Miyawaki A. Innovations in the imaging of brain functions using fluorescent proteins. Neuron. 2005;48:189–199. doi: 10.1016/j.neuron.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Directing neuron-specific transgene expression in the mouse CNS. Curr Opin Neurobiol. 2006;16:577–584. doi: 10.1016/j.conb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Godinho L, Morgan JL, Oakley DM, Schroeter EH, Wong RO. Laminar circuit formation in the vertebrate retina. Prog Brain Res. 2005;147:155–169. doi: 10.1016/S0079-6123(04)47012-5. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, et al. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers PZ. Spinal motoneurons of the larval zebrafish. J Comp Neurol. 1985;236:555–561. doi: 10.1002/cne.902360411. [DOI] [PubMed] [Google Scholar]
- Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci. 1986;6:2278–2289. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Niell CM, Smith SJ. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain Res Bull. 2000;53:661–669. doi: 10.1016/s0361-9230(00)00399-3. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, Zhou Q, Gahtan E. Probing neural circuits in the zebrafish: A suite of optical techniques. Methods. 2003;30:49–63. doi: 10.1016/s1046-2023(03)00007-0. [DOI] [PubMed] [Google Scholar]
- Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Shcherbatko A, Higashijima S, Mandel G, Brehm P. The Zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J Neurosci. 2002;22:6491–6498. doi: 10.1523/JNEUROSCI.22-15-06491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proc Natl Acad Sci USA. 2000;97:3619–3624. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin- 2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Piek JP, Carman R. Developmental profiles of spontaneous movements in infants. Early Hum Dev. 1994;39:109–126. doi: 10.1016/0378-3782(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Pineda RH, Heiser RA, Ribera AB. Developmental, molecular, and genetic dissection of INa in vivo in embryonic zebrafish sensory neurons. J Neurophysiol. 2005;93:3582–3593. doi: 10.1152/jn.01070.2004. [DOI] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnialike phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Textura del sistema nervioso del hombre y vertebrados. Madrid: Imprenta y Libreria Nicolas Moya; 1904. [Google Scholar]
- Rasooly RS, Henken D, Freeman N, Tompkins L, Badman D, Briggs J, Hewitt AT. Genetic and genomic tools for zebrafish research: The NIH zebrafish initiative. Dev Dyn. 2003;228:490–496. doi: 10.1002/dvdy.10366. [DOI] [PubMed] [Google Scholar]
- Roeser T, Baier H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J Neurosci. 2003;23:3726–3734. doi: 10.1523/JNEUROSCI.23-09-03726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sato T, Takahoko M, Okamoto H. HuC: Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis. 2006;44:136–142. doi: 10.1002/gene.20196. [DOI] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–240. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Reith CA, McDearmid JR. Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in Xenopus larvae. Ann N Y Acad Sci. 1998;860:318–332. doi: 10.1111/j.1749-6632.1998.tb09059.x. [DOI] [PubMed] [Google Scholar]
- Smear MC, Tao HZW, Staub W, Orger MB, Gosse NJ, Liu Y, Takahashi K, et al. Vesicular glutamate transport at a central synapse limits the acuity of visual perception in zebrafish. Neuron. 2007;53:65–77. doi: 10.1016/j.neuron.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, Haendel M, et al. The zebrafish information network: The zebrafish model organism database. Nucleic Acids Res. 2006;34:D581–D585. doi: 10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio) Nature. 1981;291:293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Anim Behav. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mec Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Thorsen DH, Cassidy JJ, Hale ME. Swimming of larval zebrafish: Fin-axis coordination and implications for function and neural control. J Exp Biol. 2004;207:4175–4183. doi: 10.1242/jeb.01285. [DOI] [PubMed] [Google Scholar]
- Thorsen DH, Hale ME. Neural development of the zebrafish (Danio rerio) pectoral fin. J Comp Neurol. 2007;504:168–184. doi: 10.1002/cne.21425. [DOI] [PubMed] [Google Scholar]
- Thummel R, Burket CT, Brewer JL, Sarras MP, Jr, Li L, Perry M, McDermott JP, et al. Cre-mediated sitespecific recombination in zebrafish embryos. Dev Dyn. 2005;233:1366–1377. doi: 10.1002/dvdy.20475. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays. 2004;26:445–453. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene: University of Oregon Press; 2000. [Google Scholar]
- Westerfield M, McMurray JV, Eisen JS. Identified motoneurons and their innervation of axial muscles in the zebrafish. J Neurosci. 1986;6:2267–2277. doi: 10.1523/JNEUROSCI.06-08-02267.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HY, Whelan PJ, Wenner P. Development of an inhibitory interneuronal circuit in the embryonic spinal cord. J Neurophysiol. 2005;93:2922–2933. doi: 10.1152/jn.01091.2004. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang H, Chachainasakul T, Gong S, Yang XW, Heintz N, Lin S. Modified bacterial artificial chromosomes for zebrafish transgenesis. Methods. 2006;39:183–188. doi: 10.1016/j.ymeth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Gao Y, Willis CL, Li P, Tiano JP, Nakamura PA, Hyde DR, et al. Mitogen-associated protein kinaseand protein kinase A-dependent regulation of rhodopsin promoter expression in zebrafish rod photoreceptor cells. J Neurosci Res. 2007;85:488–496. doi: 10.1002/jnr.21157. [DOI] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Yuste R, Konnerth A, editors. Imaging in Neuroscience and Development: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–634. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lefebvre JL, Zhao S, Granato M. Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat Neurosci. 2004;7:1303–1309. doi: 10.1038/nn1350. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ma C, Chalfie M. Combinatorial marking of cells and organelles with reconstituted fluorescent proteins. Cell. 2004;119:137–144. doi: 10.1016/j.cell.2004.09.012. [DOI] [PubMed] [Google Scholar]