Abstract
Background & Aims
Patients with cystic fibrosis (CF) have poorly defined defects in biliary function. We evaluated the effects of cystic fibrosis transmembrane conductance regulator (CFTR) deficiency on the enterohepatic disposition of bile acids (BAs).
Methods
Bile secretion and BA homeostasis were investigated in Cftrtm1Unc (Cftr−/−) and CftrΔF508 (ΔF508) mice.
Results
Cftr−/− and ΔF508 mice did not grow to normal size, but did not have liver abnormalities. The gallbladders of Cftr−/− mice were enlarged and had defects in emptying, based on99mtechnetiummebrofenin scintigraphy or post-prandial variationsn gallbladder volume; gallbladder contraction in response to cholecystokinin-8 was normal. Cftr−/− mice had abnormal gallbladder bile and duodenal acidity, and overexpressed the vasoactive intestinal peptide—a myorelaxant factor for the gallbladder. The BA pool was larger in Cftr−/− than wild-type mice, although there were no differences in fecal loss of BAs. Amounts of secondary BAs in portal blood, liver, and bile of Cftr−/− mice were much lower than normal. Expression of genes that are induced by BAs, including fibroblast growth factor-15 and BA transporters, was lower in the ileum but higher in the gallbladders of Cftr−/− mice, compared with wild-type mice, whereas enzymes that synthesize BA were down-regulated in livers of Cftr−/− mice. This indicates that BAs underwent a cholecystohepatic shunt, which was confirmed using cholyl-(Ne-NBD)-lysine as a tracer. In Cftr−/− mice, cholecystectomy reversed most changes in gene expression and partially restored circulating levels of secondary BAs. The ΔF508 mice overexpressed vasoactive intestinal peptide and had defects in gallbladder emptying and in levels of secondary BAs, but these features were less severe than in Cftr−/− mice.
Conclusions
Cftr−/− and CftrΔF508 mice have defects in gallbladder emptying that disrupt enterohepatic circulation of BAs. These defects create a shunt pathway that restricts the amount of toxic secondary BAs that enter the liver.
Keywords: Enterohepatic Circulation, Cholehepatic Shunt, Bicarbonate Secretion, Bile Acid Transporters
Cystic fibrosis (CF) is a disease caused by genetic defects in cystic fibrosis transmembrane conductance regulator (CFTR). CFTR acts as an adenosine 3′,5′-cyclic monophosphate (cAMP)-regulated chloride channel, driving bicarbonate secretion in various epithelia, including those of the intestine, the pancreatic ducts, bile ducts, and gallbladder.1–3 There have been many reports suggesting that CF disrupts bile acid (BA) homeostasis, although conflicting results have been obtained.4–7 Abnormal BA homeostasis may contribute to fat malabsorption and failure to thrive in these subjects. In normal conditions, most BAs cycle between the liver and the intestine. Bile, stored in the gallbladder, is released into the intestine upon feeding. Dietary lipids then are emulsified, and the gut microbiota modifies primary BAs mostly by deconjugation and 7-dehydroxylation, resulting in the formation of secondary BAs. Each cycle of the BA enterohepatic circulation is associated with a small loss of BA in the feces, and with replacement by de novo hepatic synthesis. The mechanisms by which the defects in CFTR affect hepatic biliary function are poorly understood, and the cause of intestinal BA malabsorption, recognized as a common feature in CF, remains unclear.4–6 Furthermore, biliary cirrhosis occurs in approximately 5% of the CF population, but most CF subjects never develop severe liver disease.8 The metabolism of BAs may with other factors condition the development of CF-related liver injury, given the relationship between BA structure and toxicity, and the ability of hydrophilic bile acids to protect cells against the toxicity of hydrophobic (ie, secondary) bile acids.9 In this study, we used CF mouse models, in which liver damage is absent but the mice fail to grow, to determine the impact of cftr deficiency on BA homeostasis.
Materials and Methods
Animals
Cftrtm1Unc (cftr−/−) mice10 in a C57BL/6J (87.5%) and 129SvJ (12.5%) background and ΔF508 mice11 in an FVB;129 background, were provided together with their wild-type littermates by CDTA-TAAM (Orléans, France). All animals were fed a standard AO3/RO3 chow (Safe, Augy, France), and continuously were supplied with a laxative containing polyethylene glycol (Macrogol 4000; Beaufour-Ipsen, Dreux, France), at a concentration of 4.5% in the drinking water, to prevent intestinal obstruction that occurs in CF mice.12 Mice were investigated at the age of 3–5 months, and unless otherwise stated, in the nonfasted state. For stool analyses, mice were housed individually in wirebottomed cages, and stools were collected for 72 hours. All animal procedures were approved by the Institutional Animal Care and Use Department (DSV, Paris, Agreement No. 75-54). Data for males and females, equally distributed in the different experimental groups, are shown separately if different and are combined otherwise.
Hepatobiliary Scintigraphy
99mTechnetium (99mTc)-mebrofenin scintigraphy was performed with a technique used in clinical investigation,13 adapted here to rodents.99mTc-mebrofenin was injected intravenously as a bolus of 250 MBq/kg body weight, in mice under isoflurane anesthesia (see Supplementary Materials and Methods). In some experiments, the animals received a single dose (2 nmol/L/kg body weight) of sulfated cholecystokinin (CCK)-8 (Tocris Cookson, Inc, Ellisville, MO) subcutaneously, immediately after 99mTc-mebrofenin injection. The gallbladder ejection fraction was calculated using the equation: gallbladder radioactivity at 5 minutes – gallbladder radioactivity at 45 or 120 minutes/gallbladder radioactivity at 5 minutes.
Surgical Procedures
Laparotomy was performed under isoflurane anesthesia. For bile collection, the cystic duct was ligated, gallbladder bile was collected by aspiration through a microfine 29G needle, and, in a specific subset of animals, hepatic bile was collected from the common bile duct through a polyethylene catheter every 20 minutes for 1 hour to measure bile flow. Bile volumes were determined gravimetrically, assuming a density of 1 g/mL. Some animals underwent cholecystectomy 1 month before exploration. When the animals, with the exception of those used for bile flow measurements, were killed, blood was withdrawn from the vena cava or the portal vein, and the liver, gallbladder, and small intestine were removed for histology and tissue extractions. Measurements of bile and duodenal pH were performed on fresh samples, with a microprobe (Metrohm, Courtaboeuf France). To assess cholecystohepatic shunting, triple ligation of the cystic duct was performed, gallbladder bile was removed by aspiration, and 40 μL of cholyl-(Ne-NBD)-lysine (10 mmol/L), a fluorescently labeled BA characterized by a high transport rate in the ileum,14 was injected into the gallbladder. At 1, 5, or 20 minutes after injection, the liver was collected and fluorescence was analyzed on frozen tissue sections. In preliminary experiments, the absence of leakage was ascertained by injecting 40 μL of Trypan blue (0.05%; Sigma-Aldrich, Saint-Quentin Fallavier, France) into the gallbladder.
Biochemistry
Serum concentrations of cholesterol, total bilirubin, and liver enzymes were determined with a Beckman Coulter AU640 analyzer. Total and individual BA concentrations were determined by high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) in serum, liver, bile, intestine, and stools, as described15 (Supplementary Materials and Methods).
Reverse-Transcription Polymerase Chain Reaction
After RNA extraction and reverse transcription, real-time polymerase chain reaction was performed with the Sybr Green Master Mix (Applied Biosystems, Courtaboeuf, France) on an Mx3000P (Agilent Technologies, Massy, France) device. Primer sequences are provided in Supplementary Table 1. All reactions were run with 200 nmol/L of each target forward and reverse primer, and 50 nmol/L of each 18S forward and reverse primer. Target gene messenger RNA (mRNA) levels were normalized with respect to 18S ribosomal RNA and expressed as relative levels (2-ΔΔCt).
Immunoblotting
Total membrane fractions were prepared from pooled samples of terminal ileum or gallbladder, and subjected to immunoblot analysis of apical sodium-dependent bile salt transporter (Asbt) and organic solute transporter α/β (Ost α/β ) as described16 (Supplementary Materials and Methods).
Statistics
Mann–Whitney nonparametric tests were used for comparisons, with statistical significance set at a P value of less than .05.
Results
Growth Status and Gut-Liver Phenotype
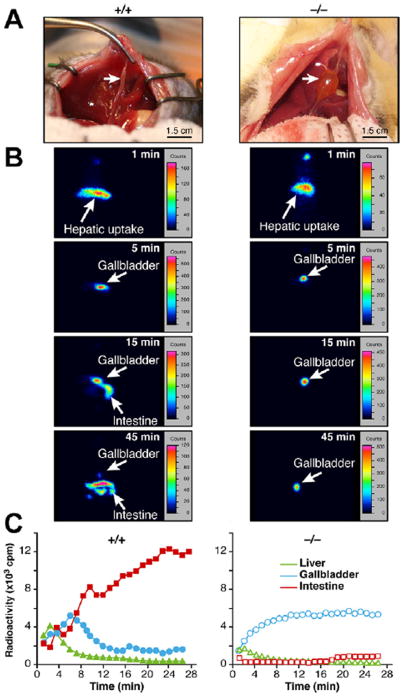
Cftr−/− mice weighed less than their cftr+/+ littermates, and this was the case for both males and females (Supplementary Figure 1A). Liver-to–body weight ratios, histologic data, and serum biochemical data attested to the absence of CF liver disease in this model (Supplementary Figure 1B and Supplementary Table 2). The intestine was also histologically normal (Supplementary Figure 1C). ΔF508 mice displayed a similar phenotype characterized by growth failure and the absence of liver injury (Supplementary Figure 2A and B). Explorations of the abdominal cavity revealed no macroscopic abnormality, with the exception of marked gallbladder enlargement in cftr−/− mice (Figure 1A, right panel).
Figure 1.
Gallbladder emptying defect in cftr−/− mice. Overnight-fed cftr−/− mice and cftr+/+ control littermates were subjected to (A) laparotomy and macroscopic examination of the abdominal cavity, showing enlarged gallbladders in cftr−/− mice in comparison with cftr+/+controls (right and left panels, respectively, arrows). (B and C) Scintigraphic analyses of gallbladder motor function. (B) Typical images recorded at the indicated times after intravenous injection of 99mTc-mebrofenin. (C) Representative time-activity curves generated over regions of interest.
Gallbladder Motor Function
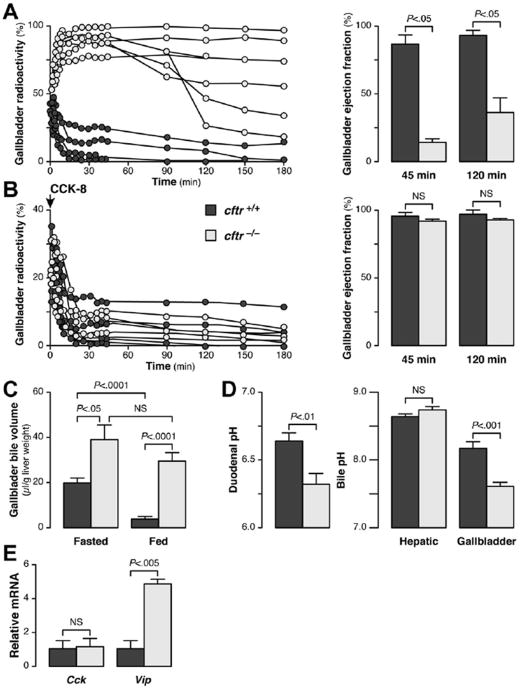
The observed gallbladder enlargement in knockout animals suggested abnormal gallbladder motility in cftr-deficient mice. We performed 99mTc-mebrofenin hepatobiliary scintigraphy, a validated quantitative and reproducible method for the evaluation of gallbladder motor function in human beings,13 using a procedure adapted for small animals. After its injection, 99mTc-mebrofenin rapidly was taken up by the liver and excreted in the bile (Figure 1B, upper panels). In both cftr+/+ and cftr−/− mice, the gallbladder was filled within 5 minutes, but thereafter the dynamics of bile secretion clearly differed between these 2 genotypes. In cftr+/+ mice, radioactivity decreased steadily in the gallbladder and increased in the duodenum (Figure 1B and C, left panels). In these animals, the emptying of the gallbladder into the duodenum was clearly visible at 15 minutes, and by 45 minutes little or no residual radiotracer was detected in the gallbladder. By comparison, gallbladder emptying into the duodenum markedly was delayed in cftr−/− mice (Figure 1B and C, right panels). In these animals, gallbladder filling was prolonged, without release into the intestine, for more than 90–180 minutes, and gallbladder ejection fractions at 45 and 120 minutes were much lower than for controls (Figure 2A). The same analyses were performed in the ΔF508 mice and showed that gallbladder emptying also was altered in these mice, although to a lesser extent than in the knockout mice (Supplementary Figure 3A–C). We repeated these analyses after 1 week in the same knockout animals, which then received a subcutaneous injection of CCK-8 to stimulate gallbladder contraction. CCK-8 triggered gallbladder emptying and the gallbladder ejection fractions measured after CCK-8 injection were similar in knockout and wild-type animals (Figure 2B). Thus, despite abnormal emptying, and in keeping with normal histology of the gallbladder wall (not shown), the gallbladders of cftr-deficient mice were able to contract normally. We concluded that gallbladder emptying was impaired in cftr-deficient mice and that abnormal neurohormonal stimulation, rather than abnormal intrinsic contractility of the gallbladder, probably accounted for this defect.
Figure 2.
Mechanisms of gallbladder emptying defect in cftr−/− mice. cftr−/− mice and cftr+/+ control littermates were subjected to the analysis of (A and B) gallbladder motor function assessed by 99mTc-mebrofenin scintigraphy, (A) in basal fed conditions and (B) 1 week later, in the same animals, after the subcutaneous injection of CCK-8. In each condition, the time-course of gallbladder radioactivity (expressed as a percentage of total injected activity) is shown in individual animals (left panels), and the corresponding gallbladder ejection fractions (right panels) were calculated at 45 and 120 minutes (means ± standard error of the mean [ SEM]). (C) Gallbladder bile volumes after overnight fasting or feeding (means ± SEM of 15 animals). (D) pH (means ± SEM) of the duodenum (n = 15), of hepatic bile (n = 4), and of gallbladder bile (n = 15). (E) Expression of the Vip and Cck genes, analyzed by quantitative reverse-transcription polymerase chain reaction, in the duodenum. The mRNA levels are shown relative to the mean value in cftr+/+ mice (means ± SEM of 6 animals).
Gut-Derived Signaling to the Gallbladder
The regulation of gallbladder motor function is linked closely to digestion and mediated by gut-derived neurohormonal signals. We estimated the global variation of gallbladder volume in response to physiological stimuli by measuring gallbladder bile volumes in fasted and fed animals. These analyses showed that the volume of gallbladder bile was larger in cftr−/− mice than in controls after fasting, and that this difference was even larger after feeding (Figure 2C). Thus, gallbladder bile volume in cftr−/− mice did not differ significantly between overnight feeding or fasting conditions, whereas a marked difference between these two conditions was observed in controls. Based on a model previously proposed to account for changes in the exocrine pancreas in CF,17 we hypothesized that an increase in acidity resulting from the cftr defect might affect the production of factors signaling from the duodenum to the gallbladder. By using a microprobe, we confirmed that duodenal pH was significantly lower in cftr−/− mice than in controls (Figure 2D, left panel). Likewise, the pH of gallbladder bile was significantly lower in cftr−/− mice than in controls, whereas the pH of hepatic bile was similar in cftr−/− mice and controls (Figure 2D, right panel). Duodenal acidity previously was shown to up-regulate the vasoactive intestinal peptide (VIP), an important myorelaxant factor for the gallbladder.18 Vip overexpression was found in the duodenum of both cftr−/− and ΔF508 mice, whereas no change was detected in the levels of mRNA for Cck, the major factor mediating gallbladder contraction (Figure 2E and Supplementary Figure 3D). Thus, in CF, the acidic duodenum generates signals inhibiting gallbladder emptying.
BA Composition
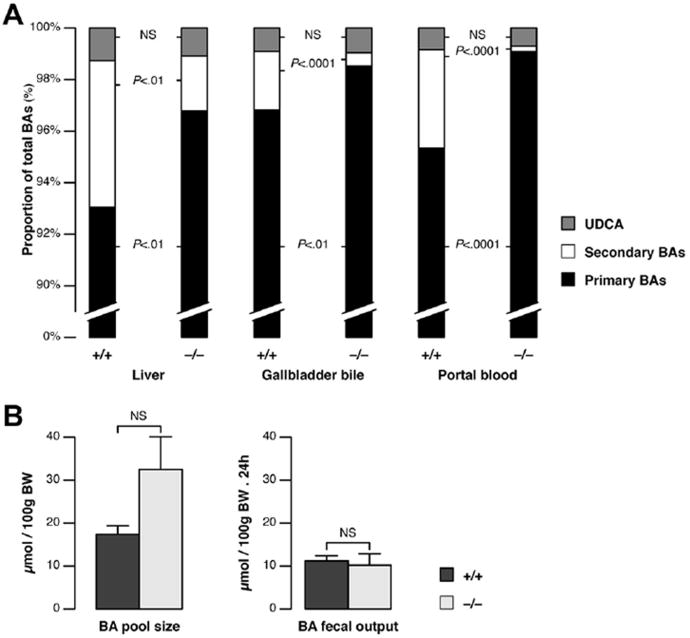
The flow rate of hepatic bile (ie, as produced before entry into the gallbladder) and the output of BAs in bile were similar in cftr−/− and cftr+/+ mice (Supplementary Table 2). However, the failure of the gallbladder to empty would be expected to result in a reduction of BA flux into the intestine. We therefore hypothesized that changes in the metabolism of BAs would occur in cftr-deficient mice. We performed quantitative BA profiling analyses in these mice. The normal composition of BAs in mice is illustrated by the profiles of individual BAs obtained for cftr+/+ mouse liver. Typically, as shown in Supplementary Table 3, the primary trihydroxy BAs, cholic acid, muricholic acid, hyocholic acid, and their conjugates, account for more than 90% of total BAs in mouse. Secondary BAs are far less abundant. The principal secondary BAs are the dihydroxy BAs: deoxycholic acid and hyodeoxycholic acid. As shown in our analyses, most of the BAs in mice are taurine conjugates. In the liver, total BA concentrations in cftr−/− mice were similar to those in controls, but differences were found in the abundance of individual BAs (Supplementary Table 3). Overall, the proportion of secondary BAs was significantly lower in the knockout mice, this decrease being compensated by an increase in primary BA levels, with no change in the amount of ursodeoxycholic acid (Table 1 and Figure 3A). Similarly, the abundance of secondary BAs in the bile and portal circulation was significantly lower in cftr−/− mice than in controls (Table 1 and Figure 3A). The ΔF508 mice also displayed a defect in secondary BAs, as shown by the analyses of their portal blood (Supplementary Figure 2C). Despite a particularly small proportion of secondary BAs in mice of FVB genetic background, the difference between F508 and wild type was significant. C-7 dehydroxylation, leading to the formation of secondary BAs, occurs in the intestine. Our findings therefore are consistent with disruption of the enterohepatic circulation. In addition, the BA pool tended to be larger in cftr−/− mice than in controls, with no difference in fecal BA loss (Figure 3B).
Table 1. Bile Acid Composition in the Liver, Bile, and Portal Blood of cftr−/− Mice and cftr+/+ Littermates.
| cftr+/+ | cftr−/− | P | |
|---|---|---|---|
| Liver tissue (n = 5) | |||
| Total BA concentration, nmol/g liver | 26.2 ± 7.6 | 32.4 ± 3.1 | .3 |
| Primary BAs, % | 93.2 ± 1.17 | 96.6 ± 0.34 | .008 |
| Secondary BAs, % | 5.7 ± 1.16 | 2.13 ± 0.3 | .008 |
| Ursodeoxycholic acid and conjugates, % | 1.27 ± 0.13 | 1.08 ± 0.09 | .55 |
| Gallbladder bile (n = 15) | |||
| Total BA concentration, mmol/L | 91.3 ± 8.1 | 130.4 ± 11.2 | .023 |
| Primary BAs, % | 96.8 ± 0.35 | 98.2 ± 0.19 | .002 |
| Secondary BAs, % | 2.26 ± 0.3 | 0.51 ± 0.12 | <.0001 |
| Ursodeoxycholic acid and conjugates, % | 0.91 ± 0.12 | 0.96 ± 0.06 | .98 |
| Portal blood (n = 15) | |||
| Total BA concentration, μmol/L | 58.6 ± 9.5 | 62.8 ± 11 | .68 |
| Primary BAs, % | 95.3 ± 0.53 | 99.1 ± 0.12 | <.0001 |
| Secondary BAs, % | 3.82 ± 0.46 | 0.21 ± 0.04 | <.0001 |
| Ursodeoxycholic acid and conjugates, % | 0.84 ± 0.19 | 0.71 ± 0.15 | .52 |
NOTE. Total and individual BA concentrations were determined by HPLC-tandem mass spectrometry in liver tissue, gallbladder bile, and portal blood from cftr−/− mice and cftr+/+ littermates. Primary BAs included cholic acid, muricholic acid, chenodeoxycholic acid, hyocholic acid and their conjugates; secondary BAs included deoxycholic acid, hyodeoxycholic acid, lithocholic acid and their conjugates. Values are means ± standard error of the mean.
Figure 3.
BA composition and pool size in cftr−/−mice. BA analyses were performed in cftr−/− mice and cftr+/+ control litter-mates, by HPLC-tandem mass spectrometry, to determine (A) the proportions of primary BAs (cholic acid, muricholic acid, chenodeoxycholic acid, and hyocholic acid), secondary BAs (deoxycholic acid, hyodeoxycholic acid, and lithocholic acid), and ursodeoxycholic acid (UDCA), including their conjugates, in liver tissue (n = 5), gallbladder bile (n = 15), and portal blood (n = 15). Histograms show means (standard error of the mean [ SEM] and total concentrations are shown in Table 1). (B) BA pool size and fecal output (means ± SEM of 8 animals).
Expression of Genes Maintaining BA Homeostasis
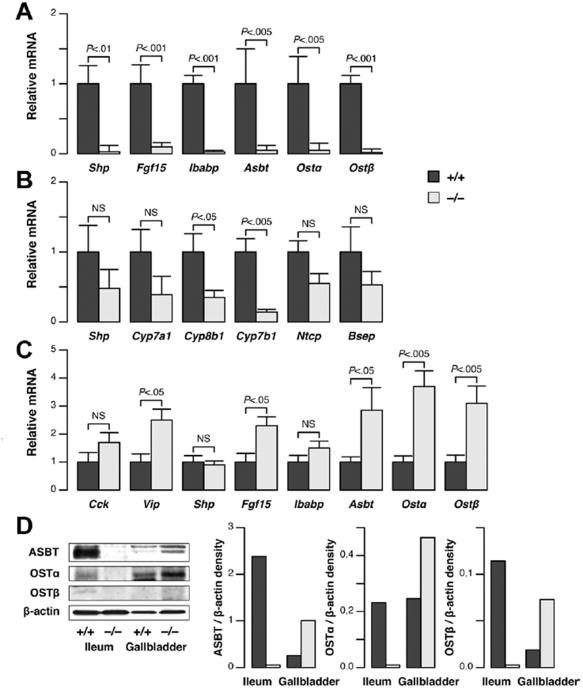
We then evaluated the expression of genes involved in BA synthesis and transport, to define the enterohepatic disposition of BAs in cftr−/− mice. We found that the small heterodimer partner (Shp), fibroblast growth factor 15 (Fgf15), ileal bile acid-binding protein (Ibabp), Asbt, and organic solute transporter α/β (Ost α/β) genes were all down-regulated in the terminal ileum of cftr−/− mice (Figure 4A and D). These genes are induced by BAs. Our findings therefore are consistent with lower rates of BA flux into the intestine. In addition, lower levels of transporter expression imply lower levels of BA absorption in the intestine, potentially accounting for the lack of decrease in BA loss in the feces, in a setting of reduced intestinal flux.
Figure 4.
Changes in gene expression underlying BA homeostasis in cftr−/−mice. Gene expression was analyzed by quantitative reverse-transcription polymerase chain reaction (A) in the terminal ileum, (B) in the liver, and (C) in the gallbladder of cftr−/− mice and cftr+/+ control littermates. The mRNA levels are shown relative to the mean value in cftr+/+ mice (means ± standard error of the mean of 6 animals). (D) Protein levels were analyzed by immunoblot in membrane fractions prepared from pooled samples of terminal ileum (n = 2) or gallbladder (n = 10), and were quantified by densitometry. Two bands for ASBT represent different glycosylated forms, as confirmed by peptide: N-glycosidase F digestion (not shown).
Despite the low levels of BA transit through the intestine in cftr-deficient mice, the concentrations of BAs in the portal vein, which drains blood from the intestine to the liver, were not different from those in controls (Table 1). Moreover, we found no increase in the hepatic expression of genes negatively regulated by BAs, such as the cytochrome P450 (Cyp), Cyp7a1, Cyp8b1, Cyp7b1, and sodium-coupled taurocholate transport protein (Ntcp) genes (Figure 4B). Instead, the expression of these genes was either significantly lower or tended to be lower than that in controls. A nonsignificant trend toward lower Shp and bile salt export pump gene expression also was observed. These findings indicated that BA influx into the liver was maintained in cftr−/− mice, suggesting a cholecystohepatic shunt. Consistent with this hypothesis, we showed that the genes encoding the major transporters responsible for mediating BA absorption in the intestine (ie, Asbt, Ibabp, Ostα/β) were expressed in the gallbladder and that, with the exception of Ibabp, these genes were expressed more strongly in the gallbladders of cftr−/− mice than in those of cftr+/+ mice (Figure 4C and D). The expression of Vip and Fgf15 also was detected in the gallbladder, and was stronger in cftr−/− mice than in controls, whereas Cck was not significantly different (Figure 4C). We postulated that the higher relative expression of BA transporters, and a prolonged contact with BAs in lastingly filled gallbladders, provided optimal conditions for a cholecystohepatic shunt. To determine if BAs in the gallbladder could access the liver directly through a cholecystohepatic shunt, we injected the fluorescent BA, cholyl-(Ne-NBD)-lysine, in the gallbladder after cystic duct ligation. In cftr−/− mice, the fluorescent BA was clearly visible within bile canaliculi 20 minutes after injection (Figure 5A, lower panel). A time-course analysis performed in a limited number of mice showed that fluorescence appeared earlier in cftr−/− mice compared with cftr+/+ mice (Supplementary Figure 4). These results provide direct evidence for a gallbladder shunt of BAs.
Figure 5.
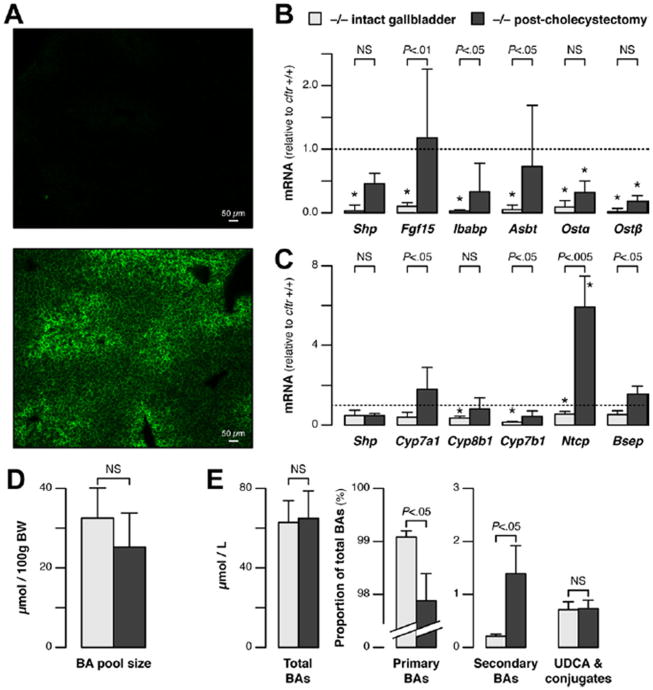
Visualization of the cholecystohepatic shunt and effect of cholecystectomy on BA homeostasis in cftr−/− mice. (A) Cholyl-(Ne-NBD)-lysine was injected into the gallbladder of cftr−/− mice, after cystic duct ligation. Twenty minutes later, the accumulation of cholyl-(Ne-NBD)-lysine was visible within bile canaliculi, predominantly in the periportal area (lower panel, representative of 5 animals); basal fluorescence in the liver from a noninjected animal is shown in comparison (upperpanel). (B-E)cftr−/− mice with intact gallbladders or that had undergone cholecystectomy 1 month earlier were subjected to reverse-transcription polymerase chain reaction analyses of (B) ileal gene expression, (C) hepatic gene expression, and to HPLC-tandem mass spectrometry analyses. The HPLC-tandem mass spectrometry analyses were performed for (D) BA pool size and (E) individual BAs in portal blood to determine total BA concentrations and the proportion of primary BAs (cholic acid, muricholic acid, chenodeoxycholic acid, and hyocholic acid), secondary BAs (deoxycholic acid, hyodeoxycholic acid, and lithocholic acid), and ursodeoxycholic acid [ UDCA], including their conjugates. The mRNA levels are shown relative to the mean value in cftr+/+ mice (means ± standard error of the mean of 6 animals). *P < .05 vs cftr+/+ (as shown in Figure 4A and B).
Effect of Cholecystectomy
To further assess the impact of the gallbladder shunt on CF-induced changes in BA homeostasis, cholecystectomy was performed in cftr−/− mice. One month after cholecystectomy, the ileal expressions of Shp, Fgf15, Ibabp, and Asbt had increased and were no longer significantly different from those in wild-type animals. The expression of Ostα/β also tended to increase, but remained lower than normal (Figure 5B). In the liver, the expression of Cyp7a1, Cyp7b1, Ntcp, and bile salt export pump increased significantly (Figure 5C). The levels of Cyp expression became similar to those in wild type, whereas those of Ntcp exceeded normal, suggesting an important role of the gallbladder in the physiological regulation of this gene. After cholecystectomy, the BA pool was closer in size to that in the wild type (Figure 5D). In addition, in the portal blood, a marked and significant increase in the proportion of circulating secondary BAs occurred, whereas the proportion of primary BAs decreased significantly and that of ursodeoxycholic acid remained constant (Figure 5E). These findings indicate that the BA enterohepatic circulation was restored by cholecystectomy in cftr−/− mice. The observation that cholecystectomy largely reversed both the changes in enterohepatic gene expression and the lack of secondary BAs in CF mice, supported a mechanism of cholecystohepatic shunting in these animals.
Discussion
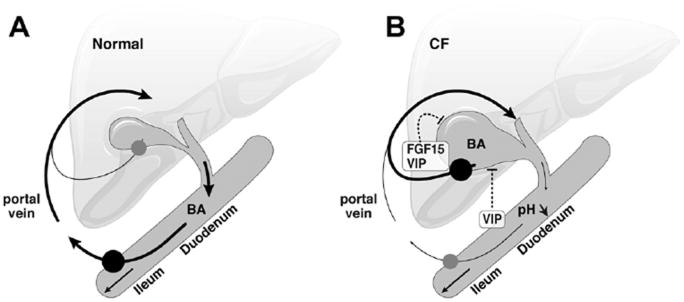
The investigation of biliary dysfunction in CF led to the discovery of a new mechanism of BA recycling. The cftr-knockout mice displayed impaired gallbladder and duodenal alkalization, an acidic duodenum overproducing VIP and a failure of the gallbladder to empty. As a result, the intestinal influx of BAs was impeded and BA transporters were down-regulated in the ileum but overexpressed in the gallbladder. BA synthetic pathways were down-regulated in the liver and the proportion of secondary BAs formed by dehydroxylation in the intestine was markedly lower than in the wild type. Most of these changes were reversed by cholecystectomy. In ΔF508 mice, VIP overexpression defects in gallbladder emptying, and in secondary BAs also were present but less severe than in the knockout, consistent with residual cftr activity in this model. We thus propose a model in which impaired emptying of the gallbladder disrupts the enterohepatic circulation of BAs, and cycling through the liver is maintained, at least partly, by a cholecystohepatic shunt (Figure 6). In CF, this mechanism may, together with pancreatic defects, contribute to fat malabsorption but also may restrict the amount of toxic secondary BAs entering the liver.
Figure 6.
Proposed model of BA recycling in CF. As compared with (A) normal conditions, (B) the duodenum in CF is abnormally acidic and overproduces the relaxing factor VIP, which is also locally produced, together with FGF15, by the gallbladder. Gallbladder emptying is impaired, impeding BA influx into the intestine. BA transporters (closed circles) are down-regulated in the ileum and increased in the gallbladder, providing a cholecystohepatic shunt for BAs. This pathway maintains the level of BA transport back to the liver, and results in a lower proportion of secondary BAs.
Cftr is thought to drive fluid and bicarbonate secretion in the bile ducts and gallbladder. The gallbladder secretes large amounts of fluid in response to hormones acting via cAMP, which facilitates its emptying after food intake. The gallbladders of cftr-deficient mice are unable to secrete fluid in response to cAMP.19 This secretory defect, combined with insufficient stimulation of contraction, may have contributed to impaired gallbladder emptying in these mice. Consistent with previous findings,2,20 we also show that cftr deficiency results in a defect in gallbladder bile alkalization in vivo. Interestingly, the pH of gallbladder bile was significantly lower in the knockout animals than in controls, but the pH of hepatic bile was identical in both groups. We previously showed that expression of genes involved in bile fluid and bicarbonate secretion including CFTR, anion exchanger-2, and VIP receptor-1 was stronger in the gallbladder than in intrahepatic bile ducts.21 The results presented here support the view that the regulation of biliary bicarbonate content takes place predominantly in the gallbladder, which is remarkable, given the importance of bicarbonate secretion for optimal digestion, and, presumably, for the protection of cells against BA-induced injury.22
Duodenal luminal pH is abnormally low in CF, which can be attributed principally to a defect in the ability of the cftr-deficient duodenal surface epithelium to secrete bicarbonate,1 but also to hyposecretion from the gallbladder and pancreatic ducts. Various regulatory gut peptides (eg, VIP) are released in response to duodenal acidification. In agreement with a previous study,17 we showed that Vip mRNA levels were significantly higher in the duodenum of CF mice than in controls. VIP normally triggers relaxation and the secretion of fluid and bicarbonate in the gallbladder.18 De Lisle et al17 suggested that, in CF, the pancreas is stimulated by excessive signaling from the duodenum in an unproductive attempt to neutralize duodenal pH, potentially exacerbating protein plugging in the pancreatic ducts. Our findings indicate that the gallbladder is also a target of abnormal signaling affecting its motor function in CF. In our CF mouse models, the gallbladder emptying defect may result at least partly from excessive VIP-induced relaxation. Moreover, we cannot rule out the involvement of other regulatory peptides, such as somatostatin or neurotensin, also released in response to duodenal acidification and decreasing gallbladder motility.23 The mRNA levels of Fgf15, a hormone responsible for gallbladder filling produced by the distal small intestine in response to BAs,24 were lower in the ileum of cftr−/− mice but, apparently, this decrease was compensated by a local increase in the gallbladder. The mRNA levels of Fgf receptor 3 and Fgf receptor 4, both potential mediators of FGF15 effects on the gallbladder,24 were similar in cftr−/−and cftr+/+mice (Supplementary Figure 5).
The down-regulation of the Fgf15 gene and other BAinducible genes in the ileum of cftr−/− mice was consistent with the reduction of BA influx into the intestine. Fgf15, Shp, Ibabp, and Ostα/β all are up-regulated by BAs. The regulation of Asbt gene expression remains controversial, but there is evidence to indicate that BAs increase ileal Asbt levels.25 We infer that lower levels of BA transporters, such as ASBT and OSTα/β , in enterocytes decreased the intestinal absorption of BAs. Previous reports of very low levels of taurocholic acid uptake by the ileum of CF mice support this possibility.26
Nevertheless, despite the evidence of a decrease in BA absorption in the intestine and in contrast to Asbt-deficient mice,27 the cftr-knockout mice showed no decrease in BA pool size, and no increase in fecal BA loss or in BA synthesis. Instead, the pool size tended to be larger, with lower levels of BA-synthetic enzymes. These findings suggest that BAs followed a cholecystohepatic shunt pathway. Indeed, venous blood from the gallbladder drains into the liver,28 and the gallbladder epithelium expresses BA transporters, able to mediate BA absorption.29,30 Specifically, in the gallbladder of CF mice, the epithelium was in contact with concentrated BAs (Table 1) for prolonged periods of time. Moreover, Asbt and Ostα/β expression levels were higher than normal in the gallbladder and lower in the intestine. Ibabp levels did not increase significantly in the gallbladder, but Ibabp is dispensable for BA transport.31 Importantly, direct evidence for a cholecystohepatic shunt was provided by tracing cholyl-(Ne-NBD)-lysine,14 and by postcholecystectomy normalization of expression levels for genes that were down-regulated in the liver or ileum.
Abnormal enterohepatic circulation and the failure of BAs to gain access to the intestinal lumen, where 7-dehydroxylation by bacteria occurs, can account for the lack of secondary BAs in our CF models. This mechanism is supported by the significant increase in secondary BA levels that occurred 1 month after cholecystectomy, in the portal blood (Figure 5E) and liver (data not shown). BAs have antibacterial properties.32 It is therefore also possible that the decrease in their intestinal flux modified the endogenous microbiota, thereby decreasing the production of secondary BAs. This may explain why the restoration of bile acid metabolism, which takes weeks after the cessation of antibiotherapy,33 was only partially restored 1 month after cholecystectomy. Small-intestinal bacterial overgrowth has been reported in CF mice, but is associated with histopathologic changes, such as excessive mucus accumulation or inflammation, two features absent in our model and that do not occur in animals treated with a laxative.34 In another mouse model of CF in which cholangiopathy developed, the proportion of secondary BAs by contrast was increased,35 consistent with a protective mechanism provided by the lack of secondary BAs in CF.
Previous20 and present results obtained in ΔF508 mice support the relevance of the gallbladder shunt concept in human CF disease, in which a delayed appearance of the BA peak in the duodenum have been reported.36 Enlarged gallbladder volumes has been documented by ultrasonography in CF patients,37 and 99mTc-mebrofenin scintigraphic analyses showed that the gallbladder did not empty after a fatty meal in about half the CF patients tested.38 In CF patients, including those without liver disease, the proportion of the primary BA cholic acid is higher than in controls, whereas those of the secondary BAs, lithocholic acid or deoxycholic acid, are lower.7,39 Our proposed model also explains the low levels of BA uptake by CF ileal mucosa in vitro.5 In the long term, the impairment of gallbladder motility and hyposecretion may contribute to mucin plugging and cholesterol crystal formation, ultimately leading to gallstones or microgallbladders, both of which are common in CF patients.
The fat malabsorption observed in CF is not completely corrected by pancreatic lipase supplementation. Consistently, cftrtm1CAM null mice display low levels of lipolysis, despite normal pancreatic lipase secretion.40 In these mice, lipolytic activity and lipid uptake were restored, however, in part by gastric acid neutralization.40 This observation is in line with our proposed model in which duodenal acidity decreases the release of bile into the intestine by modulating gallbladder motor function, thus contributing to fat malabsorption in CF. By decreasing the levels of circulating secondary hydrophobic BAs, it also may be beneficial for liver cells (ie, cholangiocytes) potentially exposed to other CF-related stresses (eg, bile plugging20 or endotoxin41), and thereby more susceptible to BA. In addition, small changes in the proportions of secondary BAs, which are very low in mice, translate into marked reductions in human beings.7 By restoring secondary BA levels, cholecystectomy may have deleterious effects in CF and other liver disorders associated with gallbladder motor dysfunction, a possibility supported by correlations between cholecystectomy and cirrhosis in epidemiologic studies.42
Supplementary Material
Acknowledgments
The authors thank Yves Menu, Saint-Antoine Hospital, for helpful discussion; Alain Lepape and Julien Sobilo, TAAM UPS44-CNRS, for setting-up scintigraphy; Yves Chrétien, UMR_S 938, for statistical analyses and figure design; Marie Chapoton, Elisabeth Lasnier, Sylvie Dumont and Stéphane Fouquet, IFR65, for technical assistance; Bob Scholte, EMC Rotterdam, for providing the ΔF508 mouse model; and the PHEA and the CDTA-TAAM animal facility teams. The authors also thank Julie Sappa of Alex Edelman & Associates for English use in this manuscript.
Funding: Funded by the Cystic Fibrosis Patients Associations (Vaincre La Mucoviscidose and ABCF2) by Fonds Cholangite Sclérosante Primitive and by Aptalis Pharma, France.
Abbreviations used in this paper
- Asbt
apical sodium-dependent bile salt transporter
- BA
bile acid
- cAMP
adenosine 3′,5′-cyclic monophosphate
- Cck
cholecystokinin
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cyp
cytochrome P450
- FGF
fibroblast growth factor
- HPLC
high-performance liquid chromatography
- Ibabp
ileal bile acid-binding protein
- mRNA
messenger RNA
- Ntcp
sodium-coupled taurocholate transport protein
- Ostα/β
organic solute transporter α/β
- Shp
small heterodimer partner
- Tc
technetium
- VIP
vasoactive intestinal peptide
Footnotes
Conflicts of interest: The authors disclose no conflicts.
supplementary material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2012.02.033.
References
- 1.Hogan DL, Crombie DL, Isenberg JI, et al. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533–541. doi: 10.1053/gast.1997.v113.pm9247473. [DOI] [PubMed] [Google Scholar]
- 2.Chinet T, Fouassier L, Dray-Charier N, et al. Regulation of electrogenic anion secretion in normal and cystic fibrosis gallbladder mucosa. Hepatology. 1999;29:5–13. doi: 10.1002/hep.510290142. [DOI] [PubMed] [Google Scholar]
- 3.Zsembery A, Jessner W, Sitter G, et al. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3-secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95–104. doi: 10.1053/jhep.2002.30423. [DOI] [PubMed] [Google Scholar]
- 4.Weber AM, Roy CC, Chartrand L, et al. Relationship between bile acid malabsorption and pancreatic insufficiency in cystic fibrosis. Gut. 1976;17:295–299. doi: 10.1136/gut.17.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fondacaro JD, Heubi JE, Kellogg FW. Intestinal bile acid malabsorption in cystic fibrosis: a primary mucosal cell defect. Pediatr Res. 1982;16:494–498. doi: 10.1203/00006450-198206000-00019. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien S, Mulcahy H, Fenlon H, et al. Intestinal bile acid malabsorption in cystic fibrosis. Gut. 1993;34:1137–1141. doi: 10.1136/gut.34.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strandvik B, Einarsson K, Lindblad A, et al. Bile acid kinetics and biliary lipid composition in cystic fibrosis. J Hepatol. 1996;25:43–48. doi: 10.1016/s0168-8278(96)80326-6. [DOI] [PubMed] [Google Scholar]
- 8.Colombo C, Battezzati PM, Crosignani A, et al. Liver disease in cystic fibrosis: a prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36:1374–1382. doi: 10.1053/jhep.2002.37136. [DOI] [PubMed] [Google Scholar]
- 9.Miyake H, Tazuma S, Miura H, et al. Partial characterization of mechanisms of cytoprotective action of hydrophilic bile salts against hydrophobic bile salts in rats: relation to canalicular membrane fluidity and packing density. Dig Dis Sci. 1999;44:197–202. doi: 10.1023/a:1026687108185. [DOI] [PubMed] [Google Scholar]
- 10.Snouwaert JN, Brigman KK, Latour AM, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 11.van Doorninck JH, French PJ, Verbeek E, et al. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995;14:4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottart CH, Bonvin E, Rey C, et al. Impact of nutrition on phenotype in CFTR-deficient mice. Pediatr Res. 2007;62:528–532. doi: 10.1203/PDR.0b013e318155a61d. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy GT, Bobba VR, Kingston E. Radionuclide ejection fraction: a technique for quantitative analysis of motor function of the human gallbladder. Gastroenterology. 1981;80:482–490. [PubMed] [Google Scholar]
- 14.Holzinger F, Schteingart CD, Ton-Nu HT, et al. Fluorescent bile acid derivatives: relationship between chemical structure and hepatic and intestinal transport in the rat. Hepatology. 1997;26:1263–1271. doi: 10.1002/hep.510260526. [DOI] [PubMed] [Google Scholar]
- 15.Doignon I, Julien B, Serriere-Lanneau V, et al. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol. 2011;54:481–488. doi: 10.1016/j.jhep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Lisle RC, Isom KS, Ziemer D, et al. Changes in the exocrine pancreas secondary to altered small intestinal function in the CF mouse. Am J Physiol Gastrointest Liver Physiol. 2001;281:G899–G906. doi: 10.1152/ajpgi.2001.281.4.G899. [DOI] [PubMed] [Google Scholar]
- 18.Jansson R, Steen G, Svanvik J. Effects of intravenous vasoactive intestinal peptide (VIP) on gallbladder function in the cat. Gastroenterology. 1978;75:47–50. [PubMed] [Google Scholar]
- 19.Peters RH, van Doorninck JH, French PJ, et al. Cystic fibrosis transmembrane conductance regulator mediates the cyclic adenosine monophosphate-induced fluid secretion but not the inhibition of resorption in mouse gallbladder epithelium. Hepatology. 1997;25:270–277. doi: 10.1002/hep.510250203. [DOI] [PubMed] [Google Scholar]
- 20.Freudenberg F, Leonard MR, Liu SA, et al. Pathophysiological preconditions promoting mixed “black” pigment plus cholesterol gallstones in a DeltaF508 mouse model of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G205–G214. doi: 10.1152/ajpgi.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chignard N, Mergey M, Barbu V, et al. VPAC1 expression is regulated by FXR agonists in the human gallbladder epithelium. Hepatology. 2005;42:549–557. doi: 10.1002/hep.20806. [DOI] [PubMed] [Google Scholar]
- 22.Beuers U, Hohenester S, de Buy Wenniger LJ, et al. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 23.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology. 2008;47:2112–2126. doi: 10.1002/hep.22204. [DOI] [PubMed] [Google Scholar]
- 24.Choi M, Moschetta A, Bookout AL, et al. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 25.Stravitz RT, Sanyal AJ, Pandak WM, et al. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology. 1997;113:1599–1608. doi: 10.1053/gast.1997.v113.pm9352862. [DOI] [PubMed] [Google Scholar]
- 26.Hardcastle J, Harwood MD, Taylor CJ. Absorption of taurocholic acid by the ileum of normal and transgenic DeltaF508 cystic fibrosis mice. J Pharm Pharmacol. 2004;56:445–452. doi: 10.1211/0022357022881. [DOI] [PubMed] [Google Scholar]
- 27.Dawson PA, Haywood J, Craddock AL, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 28.Halvorsen JF, Myking AO. The arterial supply and venous drainage of the gall-bladder. A study of one hundred autopsies. Acta Chir Scand. 1971;137:659–664. [PubMed] [Google Scholar]
- 29.Corradini SG, Elisei W, Giovannelli L, et al. Impaired human gallbladder lipid absorption in cholesterol gallstone disease and its effect on cholesterol solubility in bile. Gastroenterology. 2000;118:912–920. doi: 10.1016/s0016-5085(00)70177-6. [DOI] [PubMed] [Google Scholar]
- 30.Chignard N, Mergey M, Veissiere D, et al. Bile acid transport and regulating functions in the human biliary epithelium. Hepatology. 2001;33:496–503. doi: 10.1053/jhep.2001.22345. [DOI] [PubMed] [Google Scholar]
- 31.Kok T, Hulzebos CV, Wolters H, et al. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto S, Igimi H, Uchida K, et al. Effects of beta-lactam antibiotics on intestinal microflora and bile acid metabolism in rats. Lipids. 1996;31:601–609. doi: 10.1007/BF02523830. [DOI] [PubMed] [Google Scholar]
- 34.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 35.Freudenberg F, Broderick AL, Yu BB, et al. Pathophysiological basis of liver disease in cystic fibrosis employing a DeltaF508 mouse model. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1411–G1420. doi: 10.1152/ajpgi.00181.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weizman Z, Durie PR, Kopelman HR, et al. Bile acid secretion in cystic fibrosis: evidence for a defect unrelated to fat malabsorption. Gut. 1986;27:1043–1048. doi: 10.1136/gut.27.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santamaria F, Vajro P, Oggero V, et al. Volume and emptying of the gallbladder in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 1990;10:303–306. doi: 10.1097/00005176-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor PJ, Southern KW, Bowler IM, et al. The role of hepatobiliary scintigraphy in cystic fibrosis. Hepatology. 1996;23:281–287. doi: 10.1002/hep.510230213. [DOI] [PubMed] [Google Scholar]
- 39.Smith JL, Lewindon PJ, Hoskins AC, et al. Endogenous ursodeoxycholic acid and cholic acid in liver disease due to cystic fibrosis. Hepatology. 2004;39:1673–1682. doi: 10.1002/hep.20238. [DOI] [PubMed] [Google Scholar]
- 40.Bijvelds MJ, Bronsveld I, Havinga R, et al. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol. 2005;288:G646–G653. doi: 10.1152/ajpgi.00295.2004. [DOI] [PubMed] [Google Scholar]
- 41.Fiorotto R, Scirpo R, Trauner M, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou GN. Cholelithiasis, cholecystectomy, and liver disease. Am J Gastroenterol. 2010;105:1364–1373. doi: 10.1038/ajg.2009.737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.