Abstract
Mammalian chitinases belong to the glycosyl hydrolase 18 family based on structural homology and the family includes a large number of bacterial and eukaryotic chitinases. Among the mammalian chitinases, chitotriosidase (CHIT1) and acidic mammalian chitinase (AMCase) are capable of hydrolyzing the β-(1, 4)-linkage between the adjacent N-acetyl glucosamine residues of chitin. CHIT1 is one of the most abundantly secreted proteins, being mainly produced by activated macrophages and epithelial cells. CHIT1 plays a pivotal role in the context of infectious disease including malaria and fungi infections as a host defense towards chitin in pathogen's cell structure and as a diagnostic marker of disease. In contrast, CHI1 released by activated Kupffer cells in liver could induce hepatic fibrosis and cirrhosis. Increased serum levels of CHIT1 were observed in patients with many disorders, including Gaucher's disease, bronchial asthma, and atherosclerosis. Therefore, CHIT1 seems to have dual (regulatory and pathogenic) roles depending on the disease and producing cell types during the inflammatory conditions.
Keywords: chitinase, inflammation, receptor, autoimmunity, infection
Introduction
Chitinase in man was initially detected by its capability to hydrolyze chitotrioside substrates and was named chitotriosidase [1]. Chitotriosidase (CHIT1 or chitinase-1) is mainly produced by activated macrophages both in normal and inflammatory conditions [1, 2]. CHIT1 activity is significantly increased in plasma and tissues of patients with Gaucher's disease, a genetic disorder in which lipids accumulate in macrophages of certain organs including spleen, lungs, liver, kidneys, brain and bone marrow, as compared to normal individuals [3]. Therefore, the serum levels of CHIT1 have been used as a valuable diagnostic biomarker for monitoring the therapeutic efficacy of treatments for Gaucher's disease or β-glucocerebrosidase deficiency [1].
The CHIT1 gene is localized in the chromosome 1q31-q32 [4], and is composed of 12 exons spanning about 20 kb of genomic DNA [5]. A recessive inherited mutation of CHIT1 gene, which consists of 24-bp duplication in exon 10 that subsequently activates a cryptic 39-splice site in the same exon, generates a spliced form of mRNA [2]. The spliced mRNA encodes an enzymatically inactive CHIT1 protein that completely lacks the 29 amino acids in an internal stretch and this particular mutation is the predominant cause of CHIT1 deficiency [6]. Of note, the individuals bearing the mutant allele exhibit an increased susceptibility to chitin-containing pathogens including Wuchereria bancrofti filarial, Plasmodium falciparum malaria, Cryptococcus neoformans and Candida albicans [7–10]. These results strongly suggest that CHIT1 is a very critical enzyme to regulate the susceptibility to infection with the above organisms, which include chitin-as structural components.
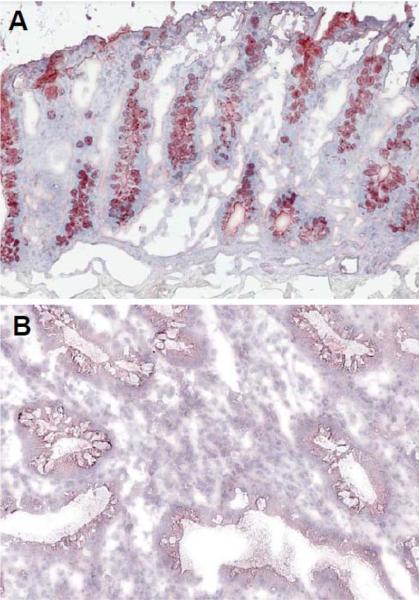
It has been reported that CHIT1 is involved in the modulation of the tissue remodeling processes in fibroblastic hepatic tissue [11]. CHIT1 is actively produced by activated Kupffer cells, resident macrophages of the liver, which activate hepatic stellate cells to synthesize collagen, and the overproduction of collagen subsequently induces hepatic fibrosis and liver cirrhosis [2]. In addition, the CHIT1 produced by macrophages enhances atherosclerotic plaques formation and subsequent thrombosis [12, 13]. In contrast, our group discovered that the production of CHIT1, which we also found to be produced by colonic epithelial cells, is markedly down regulated during the active phase of inflammatory bowel disease such as ulcerative colitis [Figure 1]. Therefore, CHIT1 may have organ- as well as cell-specific effects in the context of infectious diseases and inflammatory disorders.
Figure 1. Decreased CHIT1 expression in human IBD.
Immunohistochemical analysis of the expression of CHIT1 is shown. Colonic tissue obtained from normal individual (A) and ulcerative colitis patient were stained with rabbit anti-CHIT1 polyclonal antibody. Objective, 10×.
1. Definition/classification of chitinases (glycohydrolase 18 family)
Chitin, one of the most abundant biopolymers in nature, is an essential structural component of arthropods; including crustaceans, insects, mollusks, nematodes and worms, but mammals do not possess chitin as a structural component [14, 15]. It is a linear β (1 → 4) linked polymer of N-acetyl-D-glucosamine units. Chitinases are a family of proteins that specifically bind and degrade chitin, and are highly evolutionarily conserved. Mammalian chitinases belong to the family of glycoside hydrolase 18, which also encompasses enzymatically inactive chitinases [Table 1] [16–18]. Enzymatically active mammalian chitinases cleave chitin polymers into oligosaccharides of varying sizes (endochitinase activity) and release glucosamine monosaccharides from the end of a chitin polymer (exochitinase activity) [19]. Chitinases function in both innate and adaptive immunity and are necessary for the following three different functions: Chitinases are expressed in certain organisms during development to aid in the remodeling of their biological matrices to accommodate changes in body size and shape. Secondly, chitinases help some organisms digest chitin containing food. Chitinases are also expressed in mammals, including humans and mice, that are prone to cause some reactions with chitin-containing pathogens (e.g. house dust mites, fungi, parasite) to degrade the chitin protective covering on the infectious microorganisms. This way the inner core of chitin is susceptible to attacks by chitinases that ultimately lead to death and expulsion from the body of the chitin-containing organisms [20, 21].
Table 1.
Summary of mammalian chitinases and CLPs
| Name of chitinases | MW (KDa) | Chitinase Producing cells |
|---|---|---|
| Human | ||
| Chitotriosidase (chitinase-1, CHIT1) | 51 | Monocytes, epithelial cells |
| Acidic mammalian chitinase (AMCase) | 50 | Monocytes/macrophages, lung epithelial cells, NK cells |
| Chitinase 3-like 1 (CHI3L1, YKL-40, HC-go39) | 42 | Macrophages, CECs, neutrophils, fibroblasts, articular chondrocytes |
| Chitinase 3-like 2 (CHI3L2, YKL-39) | 43 | articular chondrocytes |
| Oviductal glycoprotein | 75 | NK cells |
| TSA 1902L | 40 | NK cells |
| Stabilin-1 interacting chitinase-like protein (SI-CLP) | 45 | Macrophages, sinusoidal endothelial cells |
| Mouse | ||
| Chitinase 1 (chitotriosidase) | 51 | Monocytes |
| AMCase | 50 | Monocytes/macrophages, lung epithelial cells, NK cells |
| Chitinase 3-like-1 (CHI3L1, Brp39) | 40 | Macrophages, CECs, neutrophils, articular chondrocytes |
| Chitinase 3-like-3 (CHI3L3, ECF-L, Ym-1) | 44 | Neutrophils, gastrict epithelial cells |
| Chitinase 3-like-4 (CHI3L4) | 44 | Monocytes/macrophages |
| Oviductin | 78 | NK cells |
| SI-CLP | 44 | Macrophages, sinusoidal endothelial cells |
Abbreviations: CECs, colonic epithelial cells; NK, natural killer
2. Mammalian chitinases and chitinase-like proteins (CLPs)
Although chitin is not expressed in mammals, chitinases are still produced in order to protect mammals from exogenous pathogens, which contain chitin as their structure [1, 22]. Mammalian chitinases are divided into chitinases and chitinase-like proteins depending on their enzymatic activity. The true chitinases include acidic mammalian chitinase (AMCase) and the chitotrisidase (CHIT1), which was the first mammalian chitinase discovered and studied. The chitinase-like proteins include chitinase 3-like 2 (YKL39) and chitinase 3-like 1 (YKL40), among others [Table 1] [17, 18]. The structure of chitinases is such that N-terminal catalytic domain of the glycohydrolase 18 family of proteins adopts the triose-phosphate isomerase fold, which is characterized by the (β/α)Υ barrel structure. In this barrel, the β4 strand contains a conserved sequence of motif (DXXDXDXE), where D is aspartic acid, E is glutamic acid, and X is any amino acid. This forms the active site of the enzyme, with glutamic acid being the key residue that donates the proton that is required for hydrolyzing the β(1→4) glycosidic bond in chitin. In contrast, this necessary glutamic acid has been substituted by leucine (in case of CHI3L1), isoleucine, or glutamine (in case of CHI3L1 or eosinophil chemotactic factor) and this accounts for the lack of chitinolytic activity [23]. However, these proteins without the enzymatic activity are still capable of binding to chitin with high affinity, as the conserved chitin-binding aromatic residues on triose-phosphate isomerase barrel remains intact [24, 25].
Human mammalian chitinase family members are located on the chromosome adjacent to the major histocompatibility complex paralogue on genes, implying a role for chitinases in innate and adaptive immunity, as stated previously [17]. While the enzymatically active chitinases, including AMCase and CHIT1, are able to degrade chitin and actively participate in destroying pathogens, CLPs also have an important biological role by binding chitin with a high affinity [26, 27]. They may function in the recognition of pathogen-associated molecular patterns encoded in chitin, thereby signaling to the host immune system to mount an appropriate pathogen directed attack. In addition, in case of CHI3L1, it significantly enhances the adhesion and invasion of potentially pathogenic bacteria on/into colonic epithelial cells, presumably by interacting with chitin/chitin-binding protein complex expressed on the bacteria [28, 29].
While CHIT1 is made most actively in macrophages and neutrophils, AMCase is made most actively in macrophages and epithelial cells, being especially active in the lungs [30, 31]. It has been reported that the productions of chitinases and CLPs are upregulated in Type 2 helper T cell (Th2) related inflammation (e.g., bronchial asthma, rhinitis, allergen-induced inflammation) [31]. CHIT1 has been shown to be a marker for Gaucher's disease, atherosclerosis, nonalcoholic fatty liver disease, and juvenile idiopathic arthritis, among others [32–34]. CHI3L1 is mainly upregulated in inflammatory bowel disease, liver fibrosis, rheumatoid arthritis, and cancers. All of this indicates that chitinases and CLPs are upregulated highly under acute and/or chronic inflammatory conditions [35, 36].
3. Biological roles of CHIT1 in normal conditions
CHIT1 is the first mammalian chitinase to be discovered and characterized [37]. It is an enzymatically active chitinase that shows transglycosylation activity toward chitin [38]. It is the major chitinase measured in both healthy and disease states [39]. CHIT1 is produced, stored, and secreted by macrophages and neutrophils, which play the prime roles in innate immune responses, suggesting an active role in maintaining the homeostasis in innate immune system [20]. However, the exact mechanism of function has not been fully defined yet [21]. As mentioned above, CHIT1 is one of the most abundant proteins produced by activated and differentiated macrophages; the production of CHIT1 is positively associated with the activation status of macrophage linage [40]. Since the peripheral (e.g. serum, tissue) macrophages are highly active during the development of acute/chronic inflammatory conditions, the enzymatic activity of CHIT1 has been increased in those abnormal conditions [1, 22]. CHIT1 plays a pivotal role in the defense against chitin-containing human pathogens, including fungi and insects [41]. Interestingly, CHIT1 deficiency and carrier frequency occur in 6% and 30–40% of the general population due to mutations, respectively [48, 61]. Recombinant CHIT1 inhibits hyphal growth of fungi, suggesting a physiological role in the host defense mechanism against the invasion/attack of chitin-containing pathogens [20, 41]. There has recently been evidence that the enzymatic role of CHIT1 extends to bacteria [9]. In addition, research shows that humans deficient in chitotriosidase activity have an increased rate of microfilarial infection because of suppressed chitinolytic activity that allows the pathogen to reproduce in the host [7].
Chitotriosidase is synthesized as a 50 kD protein containing a 39 kD N-terminal triose phosphate isomerase (TIM) barrel structure containing the catalytic groove and a C-terminal chitin-binding domain connected by a short hinge region [5]. The 39 kD catalytic region resembles the catalytic regions found in chitinases of lower organisms [42, 43]. This enzyme is predominantly secreted after cleavage as the 50 kD form. However the 39 kD form, which also has chitinolytic activity, accumulates in lysosomes of the secreting cells including macrophages and neutrophils. The 39 kD form of chitotriosidase is predominantly found in the tissues, while the 50 kD form is found secreted in the bloodstream [40]. The chitotriosidase gene, highly conserved throughout evolution, encompasses 12 exons, spanning around 20 exons and encoding multiple splice forms. This gene has variations that significantly affect its glycohydrolase enzymatic activity [39]. Evidence shows that this gene is regulated by protein tyrosine kinase (PTK), phospho inositide-3 -kinase (PI3K), and mitogen-activated protein kinases (MAPK) signaling transduction components, giving evidence that there are different signaling pathways activated that have a cumulative effect on regulating the expression and activity of chitotriosidase [38].
4. Biological roles of CHIT1 in pathogenic conditions
Gaucher's Disease
Gaucher's disease is a form of sphingolipidosis which results in liver and spleen enlargements and severe bone complications [44]. It is the most common lysosomal storage disease caused by a lack of the enzyme glucocerebrosidase (also known as acid β-glucosidase), due to autosomal recessive inheritance [3, 45]. Lysosomal hydrolase is the enzyme that is responsible for the breakdown of glucocerebroside, which is an important component of cell membranes. Gaucher's disease is classified into three types based on central nervous system symptoms. Type I Type II, and Type III show non-neuropathic, neuronopathic/acute, and neuropathic/chronic symptoms, respectively. Among the three types of symptoms, Type I is the most prevalent one. Generally, Gaucher's disease is diagnosed by measurement of β-glucosidase activity in leukocytes and fibroblasts or in chorionic villi and cultured amniocytes (lamb cells) for prenatal diagnosis [46–49]. A genetic test for a defect in the β-Gaucher's disease gene is confirmatory of this disease [50].
This disease is characterized by glucocerebroside laden macrophages, known as Gaucher cells, which are surrounded by inflammatory phagocytes [44]. The storage of glucocerebroside results in proinflammatory activation of macrophages, with consecutive ballooning, decreased phagocytic activity, and destruction of viscera (e.g. spleen, liver, kidneys, lungs, brain, bone, and bone marrow). Gaucher cells secrete biomarkers into the blood that are valuable in diagnosing Gaucher's disease, clinical severity assessment, and monitoring the efficacy of enzyme replacement therapy [51, 52]. CHIT1 has been included as one of the secreted biomarkers for Gaucher's disease [53]. The elevation of CHIT1 in these patients may reflect a particular state of activation of macrophages, which subsequently leads to an excessive production of CHIT1 [54, 55]. In a healthy population, CHIT1 activity is very low and originates in the circulating polymorphonuclear cells. In patients with Gaucher's disease, the activity of this enzyme is elevated 10 – 1000 fold in blood [1, 2, 56–60]. However, CHIT1 deficiency and carrier frequency occur in 6% and 30–40% of the general population due to mutations, respectively [48, 61]. This is also a good parameter for monitoring the enzyme replacement therapy (ERT) in Gaucher's disease. The decrease in CHIT1 activity after ERT may cause an alteration in tissue macrophage activation rather than a reduction of Gaucher cells in this disease. CHIT1 activity has the sharpest decline during the first year of ERT. In general, ERT had the highest efficacy during the first six months and after the first year, during which CHIT1 had the highest reduction in its enzymatic activity. This suggests that the rapid initial reduction in CHIT1 activity during ERT may be due to the alteration of either activation or differentiation of macrophages and their precursors, rather than a decrease in Gaucher cell burden [62, 63]. The serum levels of CHIT1 activity generally reach a plateau approximately two years after starting ERT. However, the serum levels of CHIT1 were still 10 times normal levels, even after 5 years of ERT [64]. The persistent high levels of CHIT1 could indicate that ERT is unable to treat and exclude some Gaucher cells in affected regions. In addition, after ERT cessation, macrophages became laden with glucocerebrosides again, indicating increased synthesis of CHIT1 and recurrence of Gaucher's disease. It is still unclear the association between the molecular mechanisms of macrophage activation/ infiltration and CHIT1 activity during the course of Gaucher's disease development [64]
Niemann-Pick Disease
Another lysosomal strorage disease, Niemann-Pick Disease (NPD) is inherited in an autosomal recessive pattern, and can be divided into type C1 (NPC1) and type C2 (NPC2) based on the genetic mutations in NPC1- or NPC2-gene. NPC1 gene encodes a 1278 amino acid glycoprotein with 13 transmembrane domains, and over 130 mutations have been identified in this gene. NPC1 is mainly involved in lipid trafficking in lysosomes, but its exact function is still largely unknown [65]. Loss of function in this gene leads to an accumulation of a broad range of lipids, including sphingomyelin, cholesterol, glycosphingolipids, and sphingosine. The loss of function in this gene results in the deficiency of exiting lipoprotein-derived cholesterol from lysosomes [65]. This particular alteration in cholesterol and glycolipid homeostasis leads to multiple and characteristic symptoms that include hepatosplenomegaly, liver dysfunction, and neurological abnormalities (e.g. progressive ataxia), cognitive decline, dystonia, cataplexy, vertical supranuclear gaze palsy, seizures, and impairment of swallowing reflexes [66], which originates from an abnormal intracellular accumulation of lipids, predominantly un-esterified cholesterol, in peripheral tissues. In the central nervous system, the accumulation of glycosphingolipids is proportionately more significant and play key roles in the pathogenesis associated with the disease. Sphingosine is the primary accumulating lipid in NP type C.
Glycosphingolipids, in particular, glucocerebroside, will accumulate in cells deficient in NPC1. There is an accumulation of glucocerebroside in the endosomallysosomal pathway. One therapy that has been designed for NP Disorders is N-butyldeoxynojrimycin (NB-DNJ) or misglustat, an inhibitor of glucocerebroside. The inhibitor stops the specific lipids from accumulating to pathological levels in the patients with NP, and delays the onset of the disease, reverses manifestations, and prolongs survival [66]. Niemann-Pick Disease is also characterized by foam cells, known as Niemann-Pick cells, because of their foamy or soapsuds appearance. These cells actively secrete CHIT1 into the circulation [67].
Same as Gaucher's disease, the serum levels of CHIT1 are highly elevated in NPD. CHIT1 activity above >4000 nmol/h per mL are predictive of NPD. The CHIT1 activity does not overlap but is distinct between patients and controls, which increases its ability to act as a therapeutic monitoring tool. The elevated CHIT1 activity can be explained by the increased tissue macrophage infiltration, which could have contributed to the increased, but variable, CHIT1 levels, depending on severity of infiltration [68] in this disease. However, its use a disease marker is limited by the fact that about 5% of NPD patients are homozygous for a common 24-base mutation and have no CHIT1 activity as a result [67].
Infectious Disease
Malaria is an example of an infectious disease in which CHIT1 levels are elevated [69]. A characteristic clinical feature in patients infected by Plasmodium falciparum is anemia, which is related to red blood cell destruction, phagocytosis, and hypersplenism [70, 71]. The red blood cell destruction triggers CHIT1 overproduction by macrophages, due to the accumulation of iron and erythrocyte membrane degradation products within those cells. The plasma levels of CHIT1 were found to be positively associated with the hematological parameters including thrombocytopenia degree and serum ferritin levels [69, 72]. This result strongly suggests that the increased plasma CHIT1 levels in malaria reflect an activation of the reticulo-endothelial system. The mechanisms of activation of CHIT1 in malaria seem to be the same as those in patients with Gaucher's disease [2].
Diabetes Mellitus
Type 2 Diabetes Mellitus (DM), which occupies the 90–95% of all cases of diabetes worldwide, is the leading cause of cardiovascular morbidity and mortality worldwide [73]. DM is characterized by hyperglycemia due to a combination of insulin resistance and inadequate compensatory insulin secretion. Chronic hyperglycemia is associated with long-term structural damage, dysfunction, and failure in several organs and tissues, which lead to the development of chronic and multiple complications in the affected organs. These complications are often characterized as the ischemic change of microvascular, which include retinopathy, nephropathy, and/or neuropathy. There are genetic factors that play an important role in the development of type 2 DM and the genetic susceptibilities make it more likely that when exposed to certain environmental factors including chemicals, diet, and infection, type 2 DM will develop [74]. Serum CHIT1 activity is increased in patients with newly diagnosed, untreated, and uncomplicated type 2 DM. This elevation is positively associated with the age, plasma glucose, and asymmetric dimethylarginine (ADMA) levels of those patients [76]. ADMA is naturally occurring component of human blood plasma as an analogue of Larginine, and is formed as a metabolic byproduct of protein turnover in any cell types of the human body [75]. Elevated levels of ADMA inhibit nitric oxide synthesis and impair endothelial function; these levels are increased in patients with DM [75]. The correlation between elevated CHIT1 and ADMA levels could be a sharp biomarker of predicting endothelial dysfunction, which contributes to progression of atherosclerotic lesions, in type 2 DM patients [76].
Sarcoidosis
Sarcoidosis is a multisystemic, noncaseating granulomatous disease of unknown origin characterized by the accumulation of activated proliferating T cells and mononuclear phagocytes in the affected resulting in granuloma formation [77]. The most commonly affected areas are the lungs, including hilar and mediastinal lymph nodes, but the disease can also extend to eyes, skin, liver, peripheral lymph nodes, kidneys, joints, muscles, and the central nervous system. It is thought that these granulomatous formations represent a deficiency in cellular immune processing after exposure to a chemical or infectious agent [78]. Generally, it has a good prognosis, although some patients may develop progressive interstitial lung disease leading to end stage fibrosis [79].
Most patients with active sarcoidosis have highly increased activity of CHIT1 in serum and bronchoalveolar lavage. Levels are significantly higher in the advanced stages (stages 3 and 4) of sarcoidosis than those of early stages (stages 0 and 1) [80]. The spread of the disease to organs other than the lungs appears to be reflected by higher serum levels of CHIT1. However, treatment with corticosteroids and remission of disease causes a decrease in the CHIT1 activity in the majority of patients [81]. CHIT1 is selectively expressed and released by human polymorphonuclear leukocytes, including macrophages after specifically stimulated with granulocyte macrophage colony stimulating factors (GM-CSF) [10]. The role of CHIT1 in the innate immune system is likely to be defense against chitin containing organisms, such as insects and fungi. The higher activity of chitotriosidase among subjects with sarcoidosis could reflect a specific reaction to chitin, a major component of fungi [67]. It has been suggested that fungi may be the causative agent in sarcoidosis [82, 83]. However, some patients with sarcoidosis had low values of CHIT1 in the range of the controls, which could reflect the presence of an inherited CHIT1 deficiency in these individuals [6]. In summary, CHIT1 seems to be not an ideal biomarker of sarcoidosis due to the fact that the higher levels of CHIT1 overlap with many other diseases, including fibrosis, asbestosis and other diseases involving macrophage activation, and is only useful if the patient′s serum values of CHIT1 are much higher. [84].
Atherosclerosis
Atherosclerosis is an inflammatory disease that is characterized by progressive deposition of lipids and fibrous matrix in the arterial wall. The initiation of atherosclerosis involves activation of endothelial cells, which facilitates monocyte infiltration of the vessel wall. After monocytes are differentiated into macrophages, these cells accumulate lipids from the circulation, remain in the vessel wall, and become lipidladen foam cells. These special types of macrophages are activated by growth factors and cytokines secreted by activated endothelial cells as well as macrophages. The activated macrophages inside the atherosclerotic lesion play a central role in atherosclerosis and the formation of foam cells from macrophages represents a landmark for atherosclerosis. In general, chronic arterial disease has two life-threatening complications, myocardial and cerebral infarction. The long-lasting process of atherogenesis involves important modifications in the cellular and extracellular matrix and lipid components of the arterial wall, resulting in intimal thickening, vessel lumen narrowing, and increased susceptibility to thrombosis [85, 86]. The development of foam cells is mainly attributed to overloading of lipids, particularly cholesterol and cholesterol ester, into the cells through a scavenger-receptor mediated process [87], and therefore lipid laden foam cells represents a landmark of atherosclerosis. Lipid accumulation promotes the expression of genes in the macrophage that influence the inflammatory process that occurs in atherogenesis [12].
In patients with atherosclerosis, serum levels of CHIT1 are elevated up to 55 fold as compared to normal individuals, and there is a clear association between CHIT1 expression and lipid-laden macrophages inside human atherosclerotic vessel walls [88]. Serum CHIT1 activity was shown to be related to the severity of the atherosclerotic lesions, suggesting a possible role as a marker of atherosclerotic extension. High serum CHIT1 activity in patients with atherosclerosis demonstrates the presence of activated macrophages in these subjects. Both patients with atherothrombotic stroke (ATS) and ischemic heart disease (IHD) were reported having significantly higher CHIT1 activities than the healthy control individuals did. Of note, the ATS subjects had higher CHIT1 activity than IHD subjects, indicating that subjects with ATS had an extended atherosclerotic process than those with IHD whose process is localized/restricted to the coronary vessels [89]. In those patients who were homozygous for the defective allele, a 24-bp duplication in exon 10 resulting in a splicing modification and generation of an mRNA with an in- frame deletion of 87 nucleotides, the CHIT1 activity was undetectable. The increase in CHIT1 activity was significantly higher in homozygous subjects for the major allele than in heterozygous subjects for the defective allele [90]. The increase in serum CHIT1 activity was also found to be age dependent. This can be explained by the age dependent accumulation of lipid-filled macrophages during the progression of atherosclerosis [75]. Macrophage accumulation localized in the supra aortic and coronary vessels is associated with increased serum CHIT1 activity, which reflects the state of activation of macrophages [91]. Since macrophages are present in all phases of atherosclerosis and increase the number and activity depending on the severity of those phases, CHIT1 activity in macrophages has been accepted as one of the biomarkers for atherosclerotic plaque formation [92]. The average CHIT1 activity in serum remained constant after 6 months of cholesterol and triglyceride-lowering treatment with either atorvastatin or bezfibrte, suggesting that low-density lipoprotein (LDL)-cholesterol and triglyceride reduction obtained with both drugs did not modify the macrophage CHIT1 expression/activity in these subjects. The plasma lipid correction does not seem to interfere in the CHIT1 expression level in vivo, supporting the idea that CHIT1 activity cannot be used to monitor progression of atherosclerosis [89].
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn′s disease, are a group of chronic inflammatory disorders that affect individuals throughout life. Dysregulated host-microbial interactions play an important role in the development of IBD [93–96]. In humans, the large intestine is colonized by a huge amount of commensal bacteria, which are predominantly anaerobic bacteria, and many of them are unable to be cultured using standard microbiological techniques [96]. In general, the composition of the commensal bacterial flora is altered in IBD patients, with increased populations of aggressive and detrimental bacteria, including Bacteroides, adherentinvasive Escherichia coli (AIEC), Eubacterium and Peptostreptococcus species, Fusobacterium varium and intestinal Helicobacter species [96, 97]. In fact, some potentially pathogenic bacteria in normal microflora, including AIEC and Bacteroides species, are likely to be strongly associated with the initiation of intestinal inflammation, particularly in Crohn′s disease [96–98].
Recently, our group has identified that CHI3L1 is actively produced by colonic epithelial cells and lamina proprial macrophages under inflammatory conditions [28, 29]. We have demonstrated an unexpected role for CHI3L1 in enhancing bacterial adhesion and invasion on/into colonic epithelial cells. As we described in the above section, CHI3L1 is characterized by a strong binding affinity to chitin without enzymatic activity to degrade it [99]. Healthy individuals do not synthesize CHI3L1, but a significantly increased levels of CHI3L1 is observed in the serum and colonic tissues of patients with IBD as well as colon cancer. Therefore, CHI3L1 is a very reliable biomarker and indicator for these diseases.
Enzymatically active AMCase is detectable in the gastrointestinal tract of both mouse and man [100]. It has been previously reported that CHIT1 is distinctly expressed in the gastrointestinal tract in mice, while the expression is observed almost exclusively in phagocytes in man [100]. However, both CHI3L1 and AMCase are strongly expressed in both macrophages and epithelial cells [28, 101], and therefore we hypothesized that CHIT1 might be also expressed in not only phagocytes but also colonic epithelial cells. We found that CHIT1 is actively produced by mucin-producing cells in the colon of healthy individuals as detected by immunohistochemical analysis using rabbit anti-human CHIT1 polyclonal antibody (Epitomics, Burlingame, CA) [Figure 1A]. In contrast, the expression was significantly downregulated in the colon of patients with active ulcerative colitis [Figure 1B] as compared to normal individuals. Therefore, here we predict that CHIT1 production must be a very important factor in regulating the intestinal homeostasis in normal conditions by digesting the chitin-including organisms (pathogens), and the reduction of this enzymatically active chitinase may be closely associated with the development and/or exacerbation of colonic inflammation. Further intensive investigations will be necessary to give proof of the possibility.
5. CHIT1 in cancer
Golab et al recently reported that the CHIT1 activity in the serum of patients with lung cancer and with inflammatory exudate was a useful parameter in differentiation between lung cancer and inflammation [102]. They found that there were slightly elevated CHIT1 activities in patients with lung cancer as compared to patients with lung inflammation or normal individuals. However, treatment with corticosteroid decreased these levels significantly. This result indicates that CHIT1 activity can be used to differentiate between inflammation and cancer in the lung, as well as disease progression [102, 103]. Prostate cancer is the most frequently diagnosed non-skin cancer and the second highest cause of cancer death in men. Therefore, screening for prostate cancer at an early stage is important for disease treatment. When CHIT1 activity was compared in both benign prostatic hyperplasia and primary prostate cancer to determine whether this could be a marker of disease activity and for screening purposes, it was found that CHIT1 activity was significantly increased in benign prostatic cancer patients. In primary prostatic cancer patients, high CHIT1 activities were observed only in patients with high Gleason scores, which has been most widely used diagnostic method of prostate cancer tissue. The correlation between Gleason scores and CHIT1 activity indicates the importance of macrophage involvement in cancer progression. This indicates that CHIT1 may have a role in the progression of cancer to the malignant state. Macrophage produced factors play a critical role in cancer progression through paracrine signaling pathways and/or through destruction of extracellular matrix, which enhances invasion and metastasis [104]. In another study, CHIT1 expression was seen in some primary carcinomas; however, the levels were lower than those observed in inflammation-associated pre-neoplastic tissue were. As a result, it can be seen that induction of CHIT1 occurs because of inflammatory processes, and mammalian chitinases may be important therapeutic targets to limit the development of inflammation-associated carcinogenesis as well as autoimmune disorders [105].
6. Conclusion
Both enzymatically active and inactive mammalian chitinases play highly important roles in the pathogenesis of chronic inflammation as well as allergic reactions. Enzymatically active CHIT1 is also associated with several diseases, which are associated with macrophage activation. Some human diseases discussed in this review strongly support the dual and conflict roles (regulation and progression) of CHIT1 during the development of inflammatory and/or infectious diseases. The overall effects of CHIT1 seem to be dependent on many factors, including the stage of inflammation and the specific cell types and organs involved. In addition, distributions and expressions of CHIT1 are also highly distinct. Further studies will enable us to understand the importance of this novel chitinase during the development of inflammatory disorders, infectious disease and solid tumors.
Acknowledgements
We would like to thank Ms. Cindy W. Lau for her excellent assistance in preparing this manuscript. This work has been supported by the National Institute of Health (DK 80070, DK74454, DK64289 and DK43351), and grants from the Eli and Edythe L. Broad Medical Foundation and American Gastroenterological Association Foundation to E. Mizoguchi.
Abbreviations used
- AMCase
acidic mammalian chitinase
- ATS
atherothrombotic stroke
- CHIT1
chitotriosidase (chitinase-1)
- CLPs
chitinase-like proteins
- DM
diabetes mellitus
- ERT
enzyme replacement therapy
- IBD
inflammatory bowel disease
- IECs
intestinal epithelial cells
- IHD
ischemic heart disease
- NPD
Niemann-Pick Disease
- WT
wild-type
Footnotes
Conflict of interest: The authors have no financial conflicts of interest.
References
- 1.Hollak CE, Weely SV, Oers MHV, et al. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–92. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malaguarnera L. Chitotriosidase: the yin and yang. Cell Mol Life Sci. 2006-1;63:3018–3029. doi: 10.1007/s00018-006-6269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aerts JM, Hollak CE. Plasma and metabolic abnormalities in Gaucher's disease. Baillieres Clin Haematol. 1997;10:691–709. doi: 10.1016/s0950-3536(97)80034-0. [DOI] [PubMed] [Google Scholar]
- 4.Eiberg H, Den Tandt WR. Assignment of human plasma methylumbelliferyltetra-N-acetylchitotetraside hydrolase or chitinase to chromosome 1q by a linkage study. Human Genet. 1997;101:205–207. doi: 10.1007/s004390050615. [DOI] [PubMed] [Google Scholar]
- 5.Fusetti F, von Moeller H, Huuston D, et al. Structure of human chitotriosidase. Implications for specific inhibitor design and function of mammalian chitinase-like lectins. J Biol Chem. 2002;277:25537–25544. doi: 10.1074/jbc.M201636200. [DOI] [PubMed] [Google Scholar]
- 6.Boot RG, Renkema GH, Verhock M, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 7.Choi EH, Zimmerman PA, Foster CB, Zhu S, et al. Genetic polymorphisms in molecules of innate immunity and susceptibility to infection with Wuchereria bacrofti in South India. Genes Immun. 2001;2:248–253. doi: 10.1038/sj.gene.6363767. [DOI] [PubMed] [Google Scholar]
- 8.Malaguarnera L, Simpore J, Prodi DA, et al. A 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Sharan to the Mediterranean area: role of parastic diseases and environmental conditions. Genes Immun. 2003;4:570–574. doi: 10.1038/sj.gene.6364025. [DOI] [PubMed] [Google Scholar]
- 9.Labadaridis I, Dimitriou E, Theodorakis M, et al. Chitotriosidase in neonates with fungal and bacterial infections. Arch Dis Child Fetal Neonatal Ed. 2005;90:F531–532. doi: 10.1136/adc.2004.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Eijk M, van Roomen CP, Renkema GH, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 11.Malaguarnera L, Di Rosa M, Zambito AM, et al. Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am J Gastroenterol. 2006;101:2060–2069. doi: 10.1111/j.1572-0241.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis: an inflammatory disease. N Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Moreno PR, Falk E, Palacios IF, et al. Macrophages infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 14.Tharanathan RN, Kittur FS. Chitin - the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 15.Goodday GW. The ecology of chitin degratiotic. Advance in microbial ecology. Vol. 11. New York Plenum Press; New York: 1990. pp. 387–430. [Google Scholar]
- 16.Vandevenne M, Campisi V, Freichels A, et al. Comparative functional analysis of the human macrophage chitotriosidase. Protein Sci. 2011 doi: 10.1002/pro.676. 10.1002/pro.676. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funkhouser JD, Aronson NN., Jr. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96–111. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bussink AP, Speijer D, Aerts JM, et al. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177:959–970. doi: 10.1534/genetics.107.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Z, Lambert NC, Guthrie KA, et al. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Elias JA, Homer RJ, Hamid Q, et al. Chitinases and chitinase-like proteins in TH2 inflammation and asthma. J Allergy Clin Immunol. 2005;116:497–500. doi: 10.1016/j.jaci.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Park SK, Cho HW, Heo KW, et al. Role of acidic mammalian chitinase and chitotriosidase in nasal polyps. Otolaryngol Head Neck Surg. 2009;141:462–6. doi: 10.1016/j.otohns.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Kucur M, Isman FK, Balci C, et al. Serum YKL-40 levels and chitotriosidase activity as potential biomarkers in primary prostate cancer and benign prostatic hyperplasia. Urol Oncol. 2008;26:47–52. doi: 10.1016/j.urolonc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Chou YT, Yao S, Czerwinski R, et al. Kinetic characterization of recombinant human acidic mammalian chitinase. Biochemistry. 2006;45:4444–4454. doi: 10.1021/bi0525977. [DOI] [PubMed] [Google Scholar]
- 24.Fusetti F, Pijning T, Kalk KH, et al. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–37760. doi: 10.1074/jbc.M303137200. [DOI] [PubMed] [Google Scholar]
- 25.Webb Dc, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem. 2001;276:41969–41976. doi: 10.1074/jbc.M106223200. [DOI] [PubMed] [Google Scholar]
- 26.Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149:369–77. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 27.Dickey BF. Exoskeletons and exhalation. N Engl J Med. 2007;357:2082–2084. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 28.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130:398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Kawada M, Chen CC, Arihiro A, et al. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88:883–895. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 30.Hall AJ, Morroll S, Tighe P. Human chitotriosidase is expressed in the eye and lacrimal gland and has an antimicrobial spectrum different from lysozyme. Microbes Infect. 2008;10:69–78. doi: 10.1016/j.micinf.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland TE, Maizels RM, Allen JE. Chitinases and chitinase-like proteins: potential therapeutic targets for the treatment of T-helper type 2 allergies. Clin Exp Allergy. 2009;7:943–55. doi: 10.1111/j.1365-2222.2009.03243.x. [DOI] [PubMed] [Google Scholar]
- 32.Artieda M, Cenarro A, Ganan A, et al. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003;23:1645–52. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- 33.Malaguarnera L, Di Rosa M, Zambito AM, et al. Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut. 2008;55:1313–1320. doi: 10.1136/gut.2005.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner JK, Scholl-Bürgi S, Hössinger D, et al. Chitotriosidase activity in juvenile idiopathic arthritis. Rheumatol Int. 2008;28:949–50. doi: 10.1007/s00296-008-0558-z. [DOI] [PubMed] [Google Scholar]
- 35.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodeling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 36.Kawada M, Hachiya Y, Arihiro A, et al. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007;56:21–27. doi: 10.2302/kjm.56.21. [DOI] [PubMed] [Google Scholar]
- 37.Sumarac Z, Suvajdžić N, Ignjatović S, et al. Biomarkers in Serbian patients with Gaucher disease. Clin Biochem. 2011 doi: 10.1016/j.clinbiochem.2011.05.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Di Rosa M, Zambito AM, Marsullo AR, et al. Prolactin induces chitotriosidase expression in human macrophages through PTK, PI3-K, and MAPK pathways. J Cell Biochem. 2009;107:881–9. doi: 10.1002/jcb.22186. [DOI] [PubMed] [Google Scholar]
- 39.Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9:401–8. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boot RG, Renkema GH, Strijland A. Cloning of cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem. 1995;270:26252–6. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- 41.Boot RG, Blommaart EF, Swart E, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276:6770–8. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 42.Fusetti F, von Moeller H, Houston D, et al. Structure of human chitotriosidase. Implications for specific inhibitor design and function of mammalian chitinase-like lectins. J Biol Chem. 2002;277:25537–44. doi: 10.1074/jbc.M201636200. [DOI] [PubMed] [Google Scholar]
- 43.Van Aalten DM, Synstad B, Brurberg MB, et al. Structure of a two-domain chitotriosidase from Serratia marcescens at 1.9-A resolution. Proc Natl Acad Sci USA. 2000;97:5842–47. doi: 10.1073/pnas.97.11.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beutler E, Grabowsky GA, Scrivner CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. McGraw Hill Inc; New York: 1995. [Google Scholar]
- 45.Cooper DN, Schmidtke J. Molecular genetic approaches to the analysis and diagnosis of human inherited disease: an overview. Ann Med. 1992;24:29–42. doi: 10.3109/07853899209164142. [DOI] [PubMed] [Google Scholar]
- 46.Daniels LB, Glew RH, Diven WF, et al. An improved fluorimetric leukocyte beta-glucosidase assay for Gaucher disease. Clin Chim Acta. 1981;115:369–375. doi: 10.1016/0009-8981(81)90251-5. [DOI] [PubMed] [Google Scholar]
- 47.Wenger DA, Clark C, Sattler M, et al. Synthetic substrate β-glycosidase activity in leukocytes: a reproducible method for the identification of patients and carriers of Gaucher's disease. Clin Genet. 1978;13:145–153. doi: 10.1111/j.1399-0004.1978.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 48.Staretz-Chacham O, Lang TC, LaMarca ME, et al. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123:1191–1207. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsden D, Levy H. Newborn screening of lysosomal storage disorders. Clin Chem. 2010;56:1071–1079. doi: 10.1373/clinchem.2009.141622. [DOI] [PubMed] [Google Scholar]
- 50.Rice EO, Barranger JA. Laboratory diagnosis of and genetic counseling for Gaucher disease. Gaucher Clin Perspect. 1996;4:1–15. [Google Scholar]
- 51.Vellodi A, Foo Y, Cole TJ. Evaluation of three biochemical markers in the monitoring of Gaucher disease. J Inherit Metab Dis. 2005;28:585–592. doi: 10.1007/s10545-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 52.Deegan PB, Cox TM. Clinical evaluation of biomarkers in Gaucher disease. Acta Paediatr. 2005;94:47–50. doi: 10.1111/j.1651-2227.2005.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 53.Aerts JMFG, Hollak CEM, Van Breemen M, et al. Identification and use of biomarkers in Gaucher disease and other lysosomal storage diseases. Acta Paediatr. 2005;94:43–46. doi: 10.1111/j.1651-2227.2005.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 54.Vedder AC, Cox Brinkman J, Hollak CE, et al. Plasma chitotriosidase in male Fabry patients: a marker for monitoring lipid-laden macrophages and correction by enzyme replacement therapy. Mol Genet Metab. 2006;89:239–244. doi: 10.1016/j.ymgme.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Guffon N. Gaucher disease and chitotriosidase. Rev Med Interne. 2006;27:S26–S29. doi: 10.1016/s0248-8663(06)80008-1. [DOI] [PubMed] [Google Scholar]
- 56.Aerts JMFG, Boot RG, Blammaart EFC, et al. Chitotriosidase: applications and features of the enzyme. Gaucher Clin Perspect. 1999;7:4–8. [Google Scholar]
- 57.Guo Y, He W, Boer AM, et al. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J Inherit Metab Dis. 1995;18:717–722. doi: 10.1007/BF02436762. [DOI] [PubMed] [Google Scholar]
- 58.Bouzas L, Guinarte JC, Tutor JC. Chitotriosidase activity in plasma and mononuclear and polymononuclear leukocyte populations. J Clin Lab Anal. 2003;17:271–275. doi: 10.1002/jcla.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baroe R, Sotgiu S, Musumeci S. Plasma chitotriosidase in health and pathology. Clin Lab. 2007;53:321–333. [PubMed] [Google Scholar]
- 60.Wajner A, Michelin K, Burin MG, et al. Comparison between the biochemical properties of plasma chitotriosidase from normal individuals and from patients with Gaucher disease, GM1-gangliosidosis, Krabbe disease and heterozygotes for Gaucher disease. Clin Biochem. 2007;40:365–369. doi: 10.1016/j.clinbiochem.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Novakovic I, Maksimovic N, Cvetkovic S. Gene polymorphisms as marker of disease susceptibility. J Med Biochem. 2010;29:135–138. [Google Scholar]
- 62.Harmanci O, Bayraktar Y. Gaucher disease: new developments in treatment and etiology. World J Gastroenterol. 2008;14:3968–3973. doi: 10.3748/wjg.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brady RO, Murray GJ, Barton NW. Modifying exogenous glucocerebrosidase for effective replacement therapy in Gaucher disease. J Inherit Metab Dis. 1994;17:510–519. doi: 10.1007/BF00711365. [DOI] [PubMed] [Google Scholar]
- 64.Sumarac Z, Suvajdžić N, Iggjatović S, et al. Biomarkers in Serbian patients with Gaucher disease. Clin Biochem. 2011;44:950–4. doi: 10.1016/j.clinbiochem.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Zervas M, Somers KL, Thrall MA, et al. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Bio. 2001;11:1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 66.Lo SM, McNamara J, Seashore MR. Misdiagnosis of Niemann-Pick disease type C as Gaucher disease. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9214-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brinkman J, Wijburg FA, Hollak CE. Plasma chitotriosidase and CCL18: early biochemical surrogate markers in type B Niemann-Pick disease. J Inherit Metab Dis. 2005;28:13–20. doi: 10.1007/s10545-005-4416-9. [DOI] [PubMed] [Google Scholar]
- 68.Sheth JJ, Sheth FJ, Oza NJ, et al. Plasma chitotriosidase activity in children with lysosomal storage disorders. Indian J Pediatr. 2010;77:203–205. doi: 10.1007/s12098-009-0249-0. [DOI] [PubMed] [Google Scholar]
- 69.Barone R, Simporé J, Malaguarnera L, et al. Plasma chitotriosidase activity in acute Plasmodium falciparum malaria. Clin Chim Acta. 2003;331:79–85. doi: 10.1016/s0009-8981(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 70.Weatherall DJ, Abdalla S. The anaemia of Plasmodium falciparum malaria. Br Med Bull. 1982;38:147–151. doi: 10.1093/oxfordjournals.bmb.a071751. [DOI] [PubMed] [Google Scholar]
- 71.Weiss L. The spleen in malaria: the role of barrier cells. Immunol Lett. 1990;25:165–172. doi: 10.1016/0165-2478(90)90109-4. [DOI] [PubMed] [Google Scholar]
- 72.Kwiatkowski D. The molecular genetic approach to malarial pathogenesis and immunity. Parassitologia. 1999;41:233–240. [PubMed] [Google Scholar]
- 73.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Eng J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 74.Souza BM, Assmann TS, Kliemann LM, et al. The role of uncoupling protein 2 (UCP2) on the development of type 2 diabetes mellitus and its chronic complications. Arg Bras Endocrinol Metabol. 2011;55:239–248. doi: 10.1590/s0004-27302011000400001. [DOI] [PubMed] [Google Scholar]
- 75.Sibal L, Agarwal SC, Home PD, et al. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sonmez A, Haymana C, Tapan S, et al. Chitotriosidase activity predicts endothelial dysfunction in type-2 diabetes mellitus. Endocrine. 2010;37:455–459. doi: 10.1007/s12020-010-9334-4. [DOI] [PubMed] [Google Scholar]
- 77.Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003;361:1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 78.Rossman MD, Kreider ME. Lesson learned from ACCESS (A case controlled etiologic study of sarcoidosis) Proc Am Thorac Soc. 2007;4:453–456. doi: 10.1513/pats.200607-138MS. [DOI] [PubMed] [Google Scholar]
- 79.Reich JM. Mortality of intrathoracic sarcoidosis in referral vs population-based settings: influence of stage, ethnicity and corticosteroid therapy. Chest. 2002;121:32–39. doi: 10.1378/chest.121.1.32. [DOI] [PubMed] [Google Scholar]
- 80.Bargagli E, Bianchi N, Margollicci M, et al. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Invest. 2008;68:479–483. doi: 10.1080/00365510701854975. [DOI] [PubMed] [Google Scholar]
- 81.Boot RG, Hollak CEM, Verhoek M, et al. Plasma chitotriosidase and CCL18 as surrogate markers for granulomatous macrophages in sarcoidosis. Clin Chim Acta. 2010;411:31–36. doi: 10.1016/j.cca.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 82.Teřcelj M, Rott T, Rylander R. Antifungal treatment in sarcoidosis - a pilot intervention trial. Resp Med. 2007;101:774–778. doi: 10.1016/j.rmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Teřcelj M, Salobir B, Rylander R. Microbial antigen treatment in sarcoidosis – a new paradigm? Medical Hypotheses. 2008;70:831–834. doi: 10.1016/j.mehy.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 84.Tercelj M, Salobir B, Simcic S, et al. Chitotriosidase activity in sarcoidosis and some other pulmonary diseases. Scand J Clin Lab Invest. 2009;69:575–578. doi: 10.1080/00365510902829362. [DOI] [PubMed] [Google Scholar]
- 85.Lusis AJ, Mar R, Pajukanta P. Genetics of Atherosclerosis. Annu Rev Genomics Hum Genet. 2004;5:189–218. doi: 10.1146/annurev.genom.5.061903.175930. [DOI] [PubMed] [Google Scholar]
- 86.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:19–32. [PubMed] [Google Scholar]
- 87.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karadag B, Kucur M, Isman FK, et al. Serum chitotriosidase activity in patients with coronary artery disease. Circ J. 2008;72:71–75. doi: 10.1253/circj.72.71. [DOI] [PubMed] [Google Scholar]
- 89.Artieda M, Cenarro A, Ganan A, et al. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003;23:1645–1652. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- 90.Canudas J, Cenarro A, Civeira F, et al. Chitotriosidase genotype and serum activity in subjects with combined hyperlipidemia: effect of the lipid-lowering agents, atorvastatin and bezafibrate. Metabolism. 2001;50:447–450. doi: 10.1053/meta.2001.21696. [DOI] [PubMed] [Google Scholar]
- 91.Boot RG, van Achterberg TA, van Aken BE, et al. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 19:687–694. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 92.Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 93.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter billis infection accelerates and H. hepaticus infection delays the development of colitis in multi drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;160:739–751. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflammatory Bowel Disease. 2003;9:246–259. doi: 10.1097/00054725-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Sartor SB. Role of commensal enteric bacteria in the pathogenesis of immune-mediated intestinal inflammation: lessons from animal models and implications for translational research. J Pediatr Gastroenterol Nutr. 2005;40:S30–S31. doi: 10.1097/00005176-200504001-00018. [DOI] [PubMed] [Google Scholar]
- 97.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 98.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;22:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 99.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signaling pathways. J Biochem. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boot RG, Bussink AP, Verhoek M, de Boer PAJ, Moorman AFM, Aerts JFMG Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005;53:1283–1292. doi: 10.1369/jhc.4A6547.2005. [DOI] [PubMed] [Google Scholar]
- 101.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chhitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2003;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 102.Golab K, Passowicz-Muszynska E, Jankowska R. Serum activity of chitotriosidase, lysozyme and cathepsin H in patients with lung cancer and patients with inflammatory exudate (preliminary report) Pol Merkur Lekarski. 2009;26:194–197. [PubMed] [Google Scholar]
- 103.Tercelj M, Salobir B, Simcic S, Wraber B, Zupancic M, Rylander R. Chitotriosidase activity in sarcoidosis and some other pulmonary diseases. Scand J Clin Lab Invest. 2009;69:575–578. doi: 10.1080/00365510902829362. [DOI] [PubMed] [Google Scholar]
- 104.Kucur M, Isman FK, Balci C, Onal B, Hacibekiroglu M, Ozkan F, Ozkan A. Serum YKL-40 levels and chitotriosidase activity as potential biomarkers in primary prostate cancer and benign prostatic hyperplasia. Urol Oncol. 2008;26:47–52. doi: 10.1016/j.urolonc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 105.Qureshi AM, Hannigan A, Campbell D, Nixon C, Wilson JB. Chitinase-like proteins are autoantigens in a model of inflammation-promoted incipient neoplasia. Genes Cancer. 2011;2:74–87. doi: 10.1177/1947601911402681. [DOI] [PMC free article] [PubMed] [Google Scholar]