INTRODUCTION
Five years ago the National Institute of Child Health and Human Development published a workshop report discussing new therapies and preventive approaches for necrotizing enterocolitis (NEC).1 This conference, and a more recent review,2 emphasized the importance of mother’s milk in averting NEC. A number of molecules in human milk may interact and provide a substantial benefit in preventing NEC, but the actual mechanisms remain incomplete.3 Specific prebiotics in human milk likely shape a healthy intestinal microbiota and thus hinder the invasion of epithelia by bacterial pathogens, but a recent review stated prebiotics have no proven effectiveness.2 Lactoferrin (LF), the major whey protein in human milk,4 was not mentioned as a protein that might decrease the occurrence of NEC in recent reviews.1,2 Conversely, reports show that early enteral administration of bovine LF to very low birth weight infants (VLBW, <1500 g birth weight) lowers the incidence of late-onset sepsis (LOS) and NEC.5 This overview will cover a) a historic perspective of LF as a biologic therapy, b) the scientific mechanisms whereby LF prevents NEC, c) pre-clinical and clinical studies of LF in neonatal animals and man that show efficacy, d) the current state of clinical trials involving LF that are designed to prevent LOS and NEC, and e) future directions of research that involve LF and a reduction in NEC.
DISCOVERY OF LACTOFERRIN AS A THERAPEUTIC AGENT
Although identified as a whey protein in 1939, LF was not isolated and purified from human milk until 1960.4 During the past fifty years, investigations have shown how LF acts in the gastrointestinal tract of neonates to enhance the immune system. The pace of LF-associated research accelerated over the past 20 years because biotechnology made bovine and human lactoferrin available for therapeutic applications.6,7 Table 1 summarizes the important scientific reports involving LF that are relevant to neonates and the prevention of NEC. This review cites only investigations that ultimately set in motion a clinical trial that used bovine LF prophylactically to treat preterm infants.
Table 1.
Lactoferrin-related Research Leading to Its Use to Prevent Necrotizing Enterocolitis
| YEAR | DISCOVERY AND MEANING | CITATION |
|---|---|---|
| 1972 | Lactoferrin (LF) with low amounts of bound Fe3+ iron, called apo-lactoferrin, and restricts the growth of Escherichia coli. The study proposed LF in breast milk controls the growth of gut-related bacterial pathogens. |
8 |
| 1987 | Human LF increased thymidine incorporation into rat crypt cells and suggests a role for LF in intestinal growth after birth. |
9 |
| 1991 | LF and lysozyme, anti-bacterial proteins in milk, have an additive effect and kill enteric pathogens. |
10 |
| 1991 | A ‘nicked’ 78 kDa LF that was largely intact was identified in the urine of preterm infants; the modified protein retained iron-binding activity, receptor- binding properties, and the proposed immune cell regulatory functions. The ‘nicked’ protein may represent removal of peptide antibiotics.13 |
11 |
| 1995 | In infant mice, human LF is a maturation factor for B cells enhancing their phenotype and function; this might mediate more secretory IgA into gut lumen. |
12 |
| 1995 | Human LF was expressed in Aspergillus awamori and a fully functional protein could be produced in large quantities using good manufacturing practices. |
6 |
| 1998 | In the stomach, pepsin releases a ‘defensin-like’ peptide from LF that is called lactoferricin, and it disrupts cell membranes of Gram-negative enteric bacteria. |
13 |
| 2001 | Feeding human recombinant LF to neonatal rats before an intestinal infection with Escherichia coli significantly reduces translocation, bacteremia and death. |
14 |
| 2004 | Feeding recombinant human LF (rhLF) + Lactobacillus GG (LLG) had more of an effect than feeding LGG alone in reducing gut-related translocation after an enteral infection with E. coli; rhLF enhanced intestinal colonization with LGG. This research was the basis for the first clinical trial of LF in preterm infants. |
15 |
| 2005 | Feeding LF and vitamin A to calves enhances epithelial cell maturation, villus growth, and size and nature of Peyer’s patches (PP). An accelerated development of PP may result in increased production of secretory IgA. |
16 |
| 2009 | In very preterm infants, oral prophylaxis with bovine LF (bLF) + Lactobacillus GG (LGG) significantly reduced late-onset sepsis and necrotizing enterocolitis compared to bLF only and placebo. Bovine LF + LGG versus bLF alone had no difference when NEC stage ≥2 and death were the outcomes (P = .06). |
5 |
In the 1990s, there was evidence LF had significant in vitro antimicrobial activity when lysozyme was present10 and that the action of pepsin on LF in the stomach released a potent microbicide called lactoferricin (LFcin).13 After the intake of LF in human milk, the aforesaid events in the stomach probably result in a pathogen-free gastric fluid that enters the duodenum. This is the rationale why therapeutic agents that inhibit acid production in neonatal stomach should be used sparingly so pepsin can act on LF to generate LFcin.
In 1998, a well-known formula manufacturer in the USA held the rights to the commercial production of recombinant human lactoferrin (rhLF).6 The company had no proof that feeding rhLF could prevent an enteric infection in newborn infants. A neonatal animal model was sought by the company that showed prophylaxis with rhLF prevented morbidity and death from bacterial enterocolitis. This neonatal model was reported in 2001.14 Additional studies using this model lead to a clinical trial of bovine LF that showed prophylaxis with LF prevented LOS and NEC in preterm infants.5 Before addressing pre-clinical and clinical studies that indicate LF reduces the risk of NEC, it is essential that the multi-functional nature of LF be understood. The next section recaps the actions of LF that result in better outcomes for preterm infants.
BIOCHEMICAL, PHYSIOLOGIC, AND IMMUNOLOGIC CHARACTERISTICS OF LF
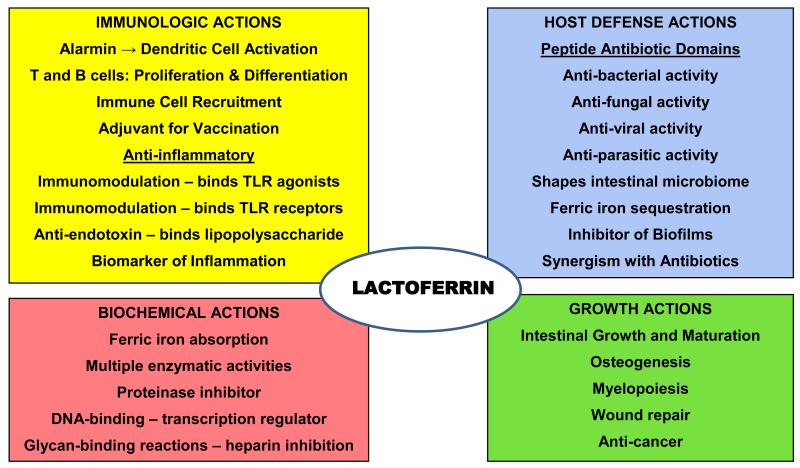
Lactoferrin (LF) is a 78kDa member of the transferrin family and present in human milk, saliva, tears, airway mucus and the secondary granules of neutrophils.4 Figure 1 reviews the wide range of actions attributed to LF. The ways that LF prevent NEC include: a) its role in host defense against pathogens,17,18 b) its immuno-modulatory and anti-inflammatory effects,12,16,19,20 c) its regulation of intestinal cell growth,9,21,22 and d) its biochemical actions that include ferric iron transport,23,24 enzymatic activity,25,26 and nuclear binding and initiation of transcription.18,27,28
Figure 1. Four Major Actions of Lactoferrin That May Act to Prevent Necrotizing Enterocolitis.
The pathophysiology of NEC is complex, but several factors are accepted as effectors of the disease.1,2,29-31 NEC occurs frequently in VLBW infants. Enteral feedings have often been instituted prior to disease onset. A less diverse intestinal microbiota with pathogenic characteristics is associated with microbial invasion or adverse effects of their toxins on intestinal epithelia. Hence, NEC always associated with inflammation of gut-related tissues. Intestinal inflammation reduces blood flow and is associated with coagulation necrosis of the bowel as the endpoint in NEC.
The lone strategy that decreases the risk of NEC was reported twenty years ago.32 To ease the occurrence of NEC, exclusive feeding of milk from a preterm infant’s mother should be the mainstay of neonatal care for VLBW infants.33 Nevertheless, many VLBW infants have a low intake of colostrum or milk in the days following birth, our hypothesis stated “feeding of lactoferrin in sufficient amounts from the first day of life may lessen the prevalence of NEC”. The remainder of this section will present in greater depth, specific actions of LF that are responsible for diminishing the risk of NEC in preterm infants.
-
◆
Breast milk contains LF and lysozyme they can act together in the stomach to destroy Gram-positive and Gram-negative pathogens, and this eliminates their damaging toxins on epithelia or mucosal invasion.10,14,17
-
◆
Whether breast feeding is or is not being used for nourishment, consuming LF can release lactoferricin (LFcin) in the stomach. LFcin kills a wide range of bacterial, fungal, viral, and parasitic pathogens.17,18 Antagonists of acid production in the stomach should be avoided in VLBW infants so pepsin can release LFcin from LF.20
-
◆
The two mechanisms listed above produce a gastric fluid that is free of pathogens. This nearly sterile gastric fluid enters the small intestine. LF is particularly resistant to proteolytic degradation in alimentary tract compared to other milk proteins like casein.11,34 Thus, intact LF is still available in small bowel to act with lysozyme or other peptide antibiotics [e.g., defensins] secreted by Paneth cells; together they can damage or kill microbes.35,36 LFcin also acts within the lumen of the small intestine.17,18
-
◆
LF has several other actions in which it participates within the lumen of small bowel. LF can still block toxicity to or invasion of epithelia by microorganisms. This mechanism avoids epithelia-related injury and involves LF binding to microbial molecules [e.g., endotoxin, CpG, peptidoglycan], bacterial flagellin [disrupts motility], or cellular determinants that pathogens use for adherence [e.g., CD14, Toll-like receptors: −2, −4, −5, and −9]. 20,35,37 These actions are vital to host defense against pathogens, but the effect is also anti-inflammatory (Fig. 2). This latter consequence of LF prophylaxis is fundamental to decreasing the inflammation associated with NEC in preterm infants.
-
◆
LF may provide an initial level of protection via its glycan chains that contain sialic acid which bind proteins of viruses and bacteria.38 Binding of viruses or bacteria to the glycan moiety of LF means pathogens can be carried from the body on LF and eliminated in the feces.
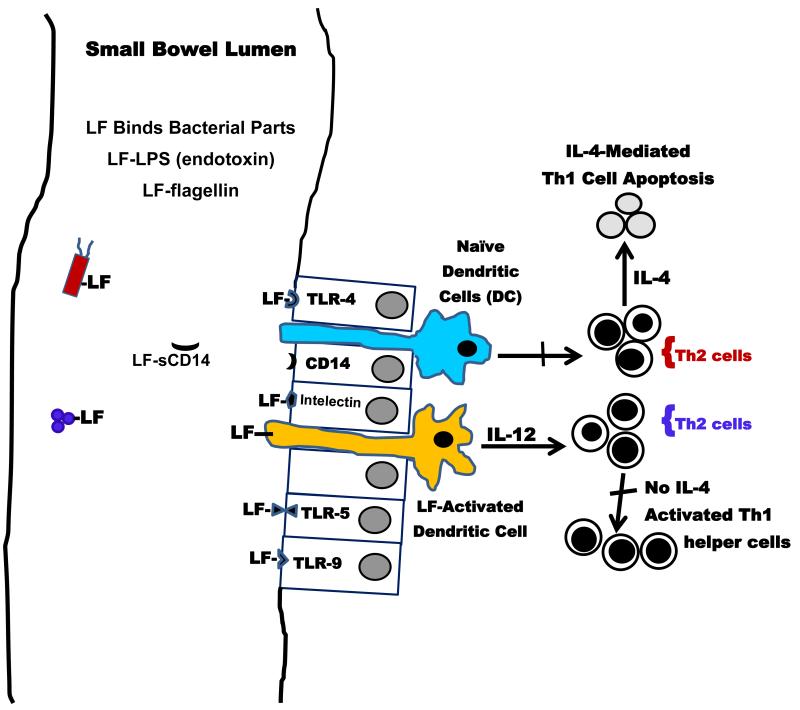
Figure 2. Anti-inflammatory and Immuno-regulatory Effects of Lactoferrin in Small Neonatal Intestine.
Lactoferrin (LF) binds a number of bacterial components including cell wall-associated lipopolysaccharide (LPS), flagellin, and DNA (CpG). This binding inhibits the bacterial components from initiating an inflammatory response. LF also binds to Gram-positive or Gram-negative bacteria either killing the microbe or hindering its invasion of tissue. LF engages pattern recognition receptors (i.e., Toll-like receptors [TLR], CD14 receptor) on gut-related epithelia and thereby restricts transduction of a pro-inflammatory signal or microbial translocation. By adhering to its own receptor, the lipid raft Intelectin, LF further limits infection. Importantly, LF activates dendritic cells (DCs) as they provide surveillance of the gut lumen. This LF-initiated signaling mediates secretion of intereukin-12 by DCs which in turn stops interleukin-4 production by Th2 cells and reverses apoptotic death of Th1 cells. This process creates a competent Th1 helper cell population and restores a proper Th1/Th2 balance that can resist infection.
A second mechanism also eliminates bacteria that invade enterocytes. When neonatal rats were pre-treated with rhLF and then infected with enteral E. coli, a phenomenon called anoikis was observed.39 Apoptotic epithelia were shed from the mucosa surface, and they contained intracellular E. coli. This mechanism is a way to rid the mucosa of infected cells; the apoptotic enterocytes with trapped bacteria inside are released into the lumen and leave the body via peristalsis. This process was not seen in infected neonatal rats that were treated with placebo rather than rhLF.
-
◆
The protease activity of LF is utilized to degrade secreted bacterial proteins that are used by enteric pathogens to form a needle and create pores in host epithelia.40 This mechanism of bacterial invasion applies to several enteric microbes. Additionally, serine protease activity of LF cleaves surface proteins at arginine-rich sites of Haemophilus influenzae and interferes with their viability.41 This microbicidal mechanism is applicable to bacterial pathogens other than Haemophilus.
-
◆
Iron sequestration mediated by LF was reported by Bullen et al. in 1972.8 This aspect of ferric ion metabolism is no longer considered a major anti-bacterial mechanism in the intestinal lumen. However, lactoferrin-mediated iron sequestration in the lung causes twitching motility in Pseudomonas aeruginosa and this prevents the bacterium from forming biofilms.42 Proof exists that biofilm formation occurs on the intestinal mucosa,43,44 but the role of LF in its prevention has not been studied.
-
◆
The proliferation and differentiation of intestinal epithelia are stimulated by LF.9,21,22,45 LF is absorbed from the intestine by means of a specific receptor, Intelectin, that is located on brush border cells.46-48 The binding to LF to intelectin hinders pathogens from adhering to lipid rafts and thereby gaining entry into epithelia.48 LF also plays a role in the absorption of nutrients.7,22,49 The protein can deliver such metal ions as iron, manganese, and zinc and facilitate the absorption of sugars. Whether treatment with LF renders these epithelia less susceptible to toxins or microbial invasion remains to be investigated. Whether tight junctions are more competent after exposure to LF also needs to be studied. Lastly, LF added to formula increased hepatic protein synthesis akin to piglets given colostrum, and this finding has implications for host defense.50
The preceding bullet points addressed the actions of LF related to intestinal host defenses and how these mechanisms reduce gut-related inflammation and infection. The section below outlines how LF acts on a nascent immune system in the intestine.
-
◆
Neonatal studies addressing the influence of LF on T and B cell physiology are limited. Adult studies show that LF induces immature T cell precursors to differentiate via the CD4 antigen.51 When LF binds with monophoshoryl lipid A, an endotoxin component, it acts as an efficient adjuvant of the humoral and cellular immune responses.52 This observation is important because LF may alter the Th2/Th1 bias of neonates that is associated with an increased risk of infections.53
-
◆
Phenotypic changes are induced when splenic B cells of mice are treated with human LF; this treatment also increases surface IgD and complement receptor expression.12 Human LF enabled B cells from normal newborn and adult immuno-deficient CBA/N mice to present antigen to an antigen-specific T-helper type 2 (Th2) cell line.12
-
◆
LF given orally to calves increases the size of Peyer’s patches in the ileum and blood levels of immunoglobulin G.54 Neonatal rats feed rhLF and Lactobacillus GG compared to LGG alone had an accelerated appearance of ‘domed villi’, the precursors of Peyer’s patches.15 This finding may result in increased IgA secretion into the intestinal lumen.55
-
◆
This bullet point is the most important of the review. A human recombinant lactoferrin, designated talactoferrin (TLF), acts as an alarmin and promotes the recruitment and activation of antigen-presenting cells.56 Immunization of mice with ovalbumin in the presence of TLF promoted Th1-polarized antigen-specific immune responses. TLF is also a novel maturation factor for monocyte conversion to dendritic cells.57 Talactoferrin increases the capacity of dendritic cells to trigger proliferation and release IFN-γ in the presence of allogeneic human T cells. It is proposed that LF-mediated maturation of dendritic cells allows secretion of interleukin-12 (IL-12).20 In turn, IL-12 stops Th2 associated production of interleukin-4 (IL-4) saving Th1 cells from IL-4-mediated apoptosis (Fig. 2). This mechanism overcomes a neonatal bias for Th2 cells over Th1 cells; this makes infants susceptible to infections.58 TLF-mediated enhancement of Th1 numbers and functions was likely responsible for the benefit observed during neonatal studies that used TLF prophylaxis to improve outcomes after enteral infection with Escherichia coli.14,15 In the next section, we discuss how these studies lead to a clinical trial that did reduce NEC.
STUDIES OF LACTOFERRIN TO PREVENT NEONATAL INFECTIONS
Pre-clinical Studies
Neonatal animal models of NEC have not been utilized to evaluate whether LF can either mitigate the disease process or reduce it altogether. Two studies have used prophylaxis with rhLF (i.e., talactoferrin) to reduce bacterial translocation after enteral infection with Escherichia coli.
The initial study used neonatal rats and administered rhLF at a dose similar to human breast-fed infants.14 Rat milk has miniscule amounts of LF.59 Treated pups received two days of rhLF before being infected with intra-gastric E. coli on successive days. The prophylaxis had four beneficial effects: a) a 3 log reduction in colony forming units (CFUs) of E. coli in blood (P < 0.001), b) 50% lower CFUs of E. coli in liver cultures (P < 0.02), c) a marked reduction in illness scores in the surviving pups (P < 0.001), and d) a 8% mortality in treated pups versus 57% mortality in rats given placebo (P < 0.001). Histologic studies showed a marked reduction in villus-related pathology in infected pups given prophylactic LF compared to the controls. No gross or microscopic findings of NEC were observed in either treated or control groups.
A second study was designed differently. It was hypothesized that rhLF would facilitate colonization of small bowel with Lactobacillus rhamnosus GG (LGG). It was proposed that rhLF + LGG would prevent bacterial translocation more effectively compared to LGG alone. Bacterial translocation was measured after enteral infection with E. coli by culturing the luminal fluid followed by enumeration of CFUs of E. coli in the homogenized bowel wall. Control pups had lactic acid bacteria in the bowel, but they were not LGG. Pups treated with LGG or rhLF + LGG had significantly higher numbers of LGG in the ileum versus jejunum. Contrary to the hypothesis, rhLF did not augment LGG colonization. After gut infection, E. coli in bowel lumen and E. coli adherent to epithelia and invading the bowel wall were reduced by pre-treatment with rhLF and LGG (P <.05). These two pre-clinical studies resulted in a randomized clinic trial (RCT) that fed bovine LF (bLF) or bLF + LGG to prevent infection in VLBW infants.
Clinical Studies
Only one RCT that has used prophylactic LF in preterm infants has been published to date5,60. The primary outcome was a reduction in LOS caused by bacteria or fungi. The occurrence of stage 2 or 3 NEC was a secondary outcome. The study used bLF or bLF + LGG as a preventive strategy. A single daily dose of 30 to 150 mg of bLF was used in the first two weeks. The infants weighing <1000 g at birth were treated for 6 weeks and infants weighing <1500 g at birth were treated for 4 weeks. The incidence of LOS was 17.3% in the control group (n = 168), while the incidence was LOS was 5.9% in the bLF (n = 153, P = .002) and 4.6% in the bLF + LGG group (n = 151, P < .001), respectively. If infants with birth weights (BW) of 1000 to 1500 g are examined separately, there was not a significant reduction in LOS (bLF, P = .34 and bLF + LGG, P = .07). Death from sepsis was significantly lower in infants with birth weights <1500 g if they received prophylaxis with bLF or bLF + LGG. Infants fed maternal milk exclusively had an additive effect on reducing infection and was a confounding variable.
Stage 2 - 3 NEC was reduced in bLF + LGG group (0/151, P = .002) compared to the bLF group (3/153, P = .09) and control (10/168). This report has been accepted by neonatologist with much enthusiasm, but it must be considered preliminary. Because of the low risk of NEC in very preterm infants, confirmatory studies that use LF to prevent NEC must be performed with a large enough sample size. The next section describes additional research on LF and NEC that is underway.
CURRENT STATE OF NEONATAL CLINICAL TRIALS USING LACTOFERRIN
In reviewing the roster of research projects at ClinicalTrials.gov, there is no single or multi-centered RCT which utilizes LF exclusively to prevent NEC. Table 2 summarizes the completed or ongoing studies that use LF to prevent LOS and are not yet published.
Table 2.
Neonatal Studies of Lactoferrin to Reduce Necrotizing Enterocolitis and Listed in ClinicalTrials.gov
| Title, Institution, and Location | Agent/ Dose | Primary Outcome |
Type of Patients And Number of Subjects |
Status |
|---|---|---|---|---|
|
Pilot Study: Lactoferrin for Prevention of Neonatal Sepsis (NEOLACTO). Universidad Peruana Cayetano Heredia, Lima, Peru |
Bovine LF; 200 mg/Kg/day divided into 3 doses/day for 4 weeks |
Late-onset Sepsis (LOS) |
Infants: <2500 g Birth Weight; N = 190, extended to 414 newborns |
Closed, abstract61 |
|
Study of Talactoferrin Oral Solution for Nosocomial Infection in Preterm Infants. University of Missouri, USA University of Louisville, USA University of Southern California, USA |
Human Recombinant Lactoferrin; 150 mg/dose twice daily, 4 wks weeks |
LOS | Infants: 750 – 1500 g BW; N = 120 (Phase I and II) |
Closed, In data analyses |
|
Oral Lactoferrin Prophylaxis to Prevent Sepsis and Necrotizing Enterocolitis of Very Low Birth Weight Neonates in Neonatal Intensive Care Unit and Effect on T-regulatory Cells. Ankara University, Turkey |
Bovine LF; 200 mg/day, given with either human milk or preterm formula |
LOS and NEC |
Infants: <1500 g BW and <32 wks gestation; N = 60 |
Recruiting |
The Peruvian study was reported its results at the 2012 Pediatric Academic Society meeting.61 Enrollment included 190 infants weighing <2500 g at birth. Bovine LF (Tatua, New Zealand) and placebo (maltodextrin) were given enterally at 200 mg/day in three divided doses over the first 4 weeks of life. Nutrition consisted of maternal milk in 67% of infants and 32% of the preemies received formula. The cumulative incidence of sepsis in the bLF group was 12/95 (12.6%) compared to 22/95 (23.2%) in the placebo group. For infants weighing <1500 g at birth, the occurrence of NEC was 20% in bLF group (8/40) vs. 40% in controls (16/40). The study did not have statistical significance, but the reduction of NEC in the LF group was ~50%. No data was provided about the occurrence of NEC. There were no adverse events in infants that received enteral bLF. This study is now extended to 414 subjects.
Multi-centered studies of LF to prevent NEC are needed and those investigations must be sufficiently powered to achieve an answer about its effectiveness in preterm infants weighing <1000g and 1000 to 1500 g at birth. The next section discusses the future of LF-related research in preterm infants and the challenges ahead.
FUTURE DIRECTIONS INVOLVING LACTOFERRIN AND REDUCTION OF NEC
There are several challenges for investigators that wish to pursue the efficacy of LF in preventing or mitigating NEC. The following bullet points state those concerns.
-
◆
A product that can achieve licensing is needed. Only talactoferrin has Investigational New Drug (IND) status from the Food and Drug Administration (FDA). Bovine LF does not have an IND. Talactoferrin has been extensively tested in vitro and in vivo for genotoxic effects, mutagenicity, dose ranging toxicity studies in animals and man, sterility and stability, pharmacokinetics, presence of endotoxin or infectious agents, and information on formation of antibodies after oral administration. A company applying for drug licensure has to prove it is made using Good Manufacturing Practices (GMP).
-
◆
Two different bovine lactoferrin products have been used in two RCTs. The bLF used in the Italian report5 was a biologic agent made at Dicofarm SpA, Rome, Italy, while the Peruvian study used bLF obtained from Tatua Co-operative Dairy Company, Morrinsville, New Zealand.61 These preparations are food additives and the FDA designates them as Generally Regarded As Safe (GRAS). Thus, the bar is high for licensing of a GRAS product that claims reduction in a major illness. Biologic agents, like humanized monoclonal antibodies that reduce or cure a disease, are usually FDA-approved drugs. For example, probiotic bacteria used to prevent NEC are undergoing substantial scrutiny by the FDA. LF used to prevent LOS or NEC should be a well-characterized biologic agent that passes FDA requirements. An extra criterion that may be required by the FDA is evidence that bLF does not cause Bovine Spongiform Encephalopathy and Variant Creutzfeldt-Jakob Disease. Thus, LF prophylaxis to prevent NEC may be years away because licensing a biologic agent is rigorous.
SUMMARY
Lactoferrin(LF)-related prophylaxis fulfills an immunologic void if a preterm infant is not taking mother’s milk. Scientific evidence is strong that LF enhances immunity in neonates, but only one study suggests bovine lactoferrin can reduce NEC. LF use in the neonatal intensive care unit to prevent NEC remains problematic because a product must be licensed by the FDA. Given the ‘State of the Art’, it is recommended that preterm infants receive colostrum immediately after its collection from the 1st day of life. Colostrum should be continued until mature milk is available. Mature milk should also be used several times per day right after it has been freshly-expressed by the mother. This warning is because freezing or pasteurization significantly lowers the content of LF in the nutrient.62 Caregivers hope that LF prophylaxis becomes available sooner rather than later.
KEY POINTS.
Lactoferrin [LF] is a multi-functional protein and a member of the transferrin family.
Lactoferrin and lysozyme in breast milk kill bacteria. In the stomach, pepsin digests and releases a potent peptide antibiotic called lactoferricin from native LF.
The antimicrobial characteristics of LF may facilitate a healthy intestinal microbiome.
The immuno-modulatory activates of LF activate dendritic cells (DC) and DCs then induce a Th1 helper cell population that resists neonatal infection.
Lactoferrin has anti-inflammatory actions that may mitigate the pro-inflammatory state that is present in the gut before the onset of necrotizing enterocolitis (NEC).
Lactoferrin is the major whey in human milk; its highest concentration is in colostrum. This fact highlights early feeding of colostrum and also fresh mature milk as a way to prevent NEC.
Acknowledgments
Funding: This work was supported in part by NIH grant HD057744 and a Gerber Foundation grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grave GD, Nelson SA, Walker WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62(4):510–4. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman AS. The immune system in human milk and the developing infant. Breastfeed Med. 2007;2(4):195–204. doi: 10.1089/bfm.2007.0024. [DOI] [PubMed] [Google Scholar]
- 4.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80(3):252–67. [PubMed] [Google Scholar]
- 5.Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 6.Ward PP, Piddington CS, Cunningham GA, et al. A system for production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Biotechnology (N Y) 1995;13(5):498–503. doi: 10.1038/nbt0595-498. [DOI] [PubMed] [Google Scholar]
- 7.Lönnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009;12(3):293–7. doi: 10.1097/MCO.0b013e328328d13e. [DOI] [PubMed] [Google Scholar]
- 8.Bullen JJ, Rogers HJ, Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols BL, McKee KS, Henry JF, et al. Human lactoferrin stimulates thymidine incorporation into DNA of rat crypt cells. Pediatr Res. 1987;21(6):563–7. doi: 10.1203/00006450-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ellison RT, 3rd, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88(4):1080–91. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchens TW, Henry JF, Yip TT. Structurally intact (78-kDa) forms of maternal lactoferrin purified from urine of preterm infants fed human milk: identification of a trypsin-like proteolytic cleavage event in vivo that does not result in fragment dissociation. Proc Natl Acad Sci U S A. 1991;88(8):2994–8. doi: 10.1073/pnas.88.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimecki M, Mazurier J, Spik G, et al. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology. 1995;86(1):122–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwata H, Yip TT, Tomita M, et al. Direct evidence of the generation in human stomach of an antimicrobial peptide domain (lactoferricin) from ingested lactoferrin. Biochim Biophys Acta. 1998;1429(1):129–41. doi: 10.1016/s0167-4838(98)00224-6. [DOI] [PubMed] [Google Scholar]
- 14.Edde L, Hipolito RB, Hwang FF, et al. Lactoferrin protects neonatal rats from gut-related systemic infection. Am J Physiol Gastrointest Liver Physiol. 2001;281(5):G1140–50. doi: 10.1152/ajpgi.2001.281.5.G1140. [DOI] [PubMed] [Google Scholar]
- 15.Sherman MP, Bennett SH, Hwang FF, et al. Neonatal small bowel epithelia: enhancing anti-bacterial defense with lactoferrin and Lactobacillus GG. Biometals. 2004;17(3):285–9. doi: 10.1023/b:biom.0000027706.51112.62. [DOI] [PubMed] [Google Scholar]
- 16.Prgomet C, Prenner ML, Schwarz FJ, et al. Effect of lactoferrin on selected immune system parameters and the gastrointestinal morphology in growing calves. J Anim Physiol Anim Nutr (Berl) 2007;91(3-4):109–19. doi: 10.1111/j.1439-0396.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 17.González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301.e1–8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Vogel HJ. Lactoferrin, a bird’s eye view. Biochem Cell Biol. 2012;90(3):233–44. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 19.Legrand D, Mazurier J. A critical review of the roles of host lactoferrin in immunity. Biometals. 2010;23(3):365–76. doi: 10.1007/s10534-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 20.Sherman MP, Adamkin DH, Radmacher PG, et al. Protective Proteins in Mammalian Milks: Lactoferrin Steps Forward. Neoreviews. 2012;13(5):e293–e300. [Google Scholar]
- 21.Buccigrossi V, de Marco G, Bruzzese E, et al. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res. 2007;61(4):410–4. doi: 10.1203/pdr.0b013e3180332c8d. [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, Jiang R, Lönnerdal B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol. 2012;90(3):476–84. doi: 10.1139/o11-075. [DOI] [PubMed] [Google Scholar]
- 23.Lopez V, Suzuki YA, Lönnerdal B. Ontogenic changes in lactoferrin receptor and DMT1 in mouse small intestine: implications for iron absorption during early life. Biochem Cell Biol. 2006;84(3):337–44. doi: 10.1139/o06-059. [DOI] [PubMed] [Google Scholar]
- 24.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012;14(3):207–16. doi: 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furmanski P, Li ZP, Fortuna MB, et al. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J Exp Med. 1989;170(2):415–29. doi: 10.1084/jem.170.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanyshkova TG, Babina SE, Semenov DV, et al. Multiple enzymatic activities of human milk lactoferrin. Eur J Biochem. 2003;270(16):3353–61. doi: 10.1046/j.1432-1033.2003.03715.x. [DOI] [PubMed] [Google Scholar]
- 27.Fleet JC. A new role for lactoferrin: DNA binding and transcription activation. Nutr Rev. 1995;53(8):226–7. doi: 10.1111/j.1753-4887.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 28.Mariller C, Hardivillé S, Hoedt E, et al. Delta-lactoferrin, an intracellular lactoferrin isoform that acts as a transcription factor. Biochem Cell Biol. 2012;90(3):307–19. doi: 10.1139/o11-070. [DOI] [PubMed] [Google Scholar]
- 29.McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. Neoreviews. 2011;12(9):e517–e526. doi: 10.1542/neo.12-9-e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. 2012;39(2):387–401. doi: 10.1016/j.clp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 31.McElroy SJ, Underwood MA, Sherman MP. Paneth Cells and Necrotizing Enterocolitis: A Novel Hypothesis for Disease Pathogenesis. Neonatology. 2012;103(1):10–20. doi: 10.1159/000342340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–7.e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Kuwata H, Yamauchi K, Teraguchi S, et al. Functional fragments of ingested lactoferrin are resistant to proteolytic degradation in the gastrointestinal tract of adult rats. J Nutr. 2001;131(8):2121–7. doi: 10.1093/jn/131.8.2121. [DOI] [PubMed] [Google Scholar]
- 35.Sherman MP. New Concepts of Microbial Translocation in the Neonatal Intestine: Mechanisms and Prevention. Clin Perinatol. 2010;37(3):565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 37.de Araújo AN, Giugliano LG. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 2001;1:25. doi: 10.1186/1471-2180-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker HM, Baker EN. A structural perspective on lactoferrin function. Biochem Cell Biol. 2012;90(3):320–8. doi: 10.1139/o11-071. [DOI] [PubMed] [Google Scholar]
- 39.Sherman MP, Petrak K. Lactoferrin-enhanced anoikis: a defense against neonatal necrotizing enterocolitis. Med Hypotheses. 2005;65(3):478–82. doi: 10.1016/j.mehy.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Ochoa TJ, Noguera-Obenza M, Ebel F, et al. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect Immun. 2003;71(9):5149–55. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrixson DR, Qiu J, Shewry SC, et al. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol Microbiol. 2003;47(3):607–17. doi: 10.1046/j.1365-2958.2003.03327.x. [DOI] [PubMed] [Google Scholar]
- 42.Singh PK, Parsek MR, Greenberg EP, et al. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–5. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Medina M, Naves P, Blanco J, et al. Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC) BMC Microbiol. 2009;9:202. doi: 10.1186/1471-2180-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macfarlane S, Bahrami B, Macfarlane GT. Mucosal biofilm communities in the human intestinal tract. Adv Appl Microbiol. 2011;75:111–43. doi: 10.1016/B978-0-12-387046-9.00005-0. [DOI] [PubMed] [Google Scholar]
- 45.Lönnerdal B, Jiang R, Du X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J Pediatr Gastroenterol Nutr. 2011;53(6):606–14. doi: 10.1097/MPG.0b013e318230a419. [DOI] [PubMed] [Google Scholar]
- 46.Shin K, Wakabayashi H, Yamauchi K, et al. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol Pharm Bull. 2008;31(8):1605–8. doi: 10.1248/bpb.31.1605. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki YA, Wong H, Ashida KY, et al. The N1 domain of human lactoferrin is required for internalization by caco-2 cells and targeting to the nucleus. Biochemistry. 2008;47(41):10915–20. doi: 10.1021/bi8012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danielsen EM, Hansen GH. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem. 2008;64(4):377–82. doi: 10.1007/BF03174093. [DOI] [PubMed] [Google Scholar]
- 49.Lönnerdal B. Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol Rev. 1997;77(3):643–69. doi: 10.1152/physrev.1997.77.3.643. [DOI] [PubMed] [Google Scholar]
- 50.Burrin DG, Wang H, Heath J, et al. Orally administered lactoferrin increases hepatic protein synthesis in formula-fed newborn pigs. Pediatr Res. 1996;40(1):72–6. doi: 10.1203/00006450-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Fischer R, Debbabi H, Dubarry M, et al. Regulation of physiological an pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol. 2006;84(3):303–11. doi: 10.1139/o06-058. [DOI] [PubMed] [Google Scholar]
- 52.Chodaczek G, Zimecki M, Lukasiewicz J, et al. A complex of lactoferrin with monophosphoryl lipid A is an efficient adjuvant of the humoral and cellular immune response in mice. Med Microbiol Immunol. 2006;195:207–216. doi: 10.1007/s00430-006-0020-3. [DOI] [PubMed] [Google Scholar]
- 53.Lee HH, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205(10):2269–80. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prgomet C, Prenner ML, Schwarz FJ, et al. Effect of lactoferrin on selected immune system parameters and the gastrointestinal morphology in growing calves. J Anim Physiol Anim Nutr (Berl) 2007;91(3-4):109–19. doi: 10.1111/j.1439-0396.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 55.de Moreno de LeBlanc A, Dogi CA, et al. Effect of the administration of a fermented milk containing Lactobacillus casei DN-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol. 2008;9:27. doi: 10.1186/1471-2172-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Rosa G, Yang D, Tewary P, et al. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180(10):6868–76. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spadaro M, Caorsi C, Ceruti P, et al. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008;22(8):2747–57. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- 58.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masson PL, Heremans JF. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- 60.Manzoni P, Stolfi I, Messner H, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129(1):116–23. doi: 10.1542/peds.2011-0279. [DOI] [PubMed] [Google Scholar]
- 61.Ochoa TJ, Cam L, Lianos R, et al. Lactoferrin for prevention of sepsis in Peruvian neonates. Pediatric Academic Societies. 2012 doi: 10.1097/INF.0000000000000593. web site. Abstracts2View, E-PAS2012:2170.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans TJ, Ryley HC, Neale LM, et al. Effect of storage and heat on antimicrobial proteins in human milk. Arch Dis Child. 1978;53(3):239–41. doi: 10.1136/adc.53.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]