Abstract
Background
IL-5 activates αMβ2 integrin on blood eosinophils in vitro. Eosinophils in bronchoalveolar lavage (BAL) following segmental antigen challenge have activated β2-integrins.
Objective
To identify roles for IL-5 in regulating human eosinophil integrins in vivo.
Methods
Blood and BAL eosinophils were analyzed by flow cytometry in ten subjects with allergic asthma who underwent a segmental antigen challenge protocol before and after anti-IL-5 administration.
Results
Blood eosinophil reactivity with monoclonal antibody (mAb) KIM-127, which recognizes partially activated β2-integrins, was decreased after anti-IL-5. Before anti-IL-5, surface densities of blood eosinophil β2, αM, and αL integrin subunits increased modestly post-challenge. After anti-IL-5, such increases did not occur. Before or after anti-IL-5, surface densities of β2,αM, αL, and αD and reactivity with KIM-127 and mAb CBRM1/5, which recognizes high-activity αMβ2, were similarly high on BAL eosinophils 48 h post-challenge. Density and activation state of β1-integrins on blood and BAL eosinophils were not impacted by anti-IL-5, even though anti-IL-5 ablated a modest post-challenge increase on blood or BAL eosinophils of P-selectin glycoprotein ligand-1 (PSGL-1), a receptor for P-selectin that causes activation of β1-integrins. Forward scatter of blood eosinophils post-challenge was less heterogeneous and on the average decreased after anti-IL-5; however, anti-IL-5 had no effect on the decreased forward scatter of eosinophils in post-challenge BAL compared to eosinophils in blood. Blood eosinophil KIM-127 reactivity at the time of challenge correlated with the percentage of eosinophils in BAL post-challenge.
Conclusion and Clinical Relevance
IL-5 supports a heterogeneous population of circulating eosinophils with partially activated β2-integrins and is responsible for upregulation of β2-integrins and PSGL-1 on circulating eosinophils following segmental antigen challenge but has minimal effects on properties of eosinophils in BAL. Dampening of β2-integrin function of eosinophils in transit to inflamed airway may contribute to the decrease in lung inflammation caused by anti-IL-5.
Keywords: eosinophils, integrins, PSGL-1, forward scatter, IL-5, blood, bronchoalveolar lavage, segmental lung antigen challenge
Introduction
Eosinophilic airway inflammation is characteristic of asthma [1–7]. Arrest of eosinophils in vessels and their extravasation into the airway wall and through the bronchial tissue and epithelium to the airway lumen are mediated by integrins [8–11]. Integrins are heterodimers of α and β subunits [12, 13]. Eosinophils possess a unique repertoire of seven integrins,α4β1, α6β1, αLβ2, αMβ2, αXβ2, αDβ2, and α4β7, each of which interacts with counter-receptors on other cells or ligands deposited as part of extracellular matrix [10]. Whether a given pair of integrin and ligand participates in adhesion and migration depends on the cell surface density of the integrin, the density of the ligand, and the activation state of the integrin [14–16]. Integrins exist in bent inactive and various extended active conformations, including an intermediate-activity extended conformation with a partially occluded ligand-binding “head piece” and a high-activity extended conformation with a more open “head-piece”, swung-out hybrid domain, and separation of the “legs” of the two subunits [17–19]. Integrin activation states can be monitored by conformation-specific monoclonal antibodies (mAbs)[20, 21].
In vitro, β2-integrins, including αMβ2 (CD11b/CD18), are activated on blood eosinophils in response to IL-5 [10, 22–24]. IL-5 and the IL-5-related cytokines IL-3 and GM-CSF also upregulate surface expression of αM and β2 subunits [25]. αMβ2 mediates adhesion of IL-5-stimulated blood eosinophils or bronchoalveolar lavage (BAL) eosinophils purified after segmental lung antigen challenge (a model of allergic airway inflammation) to diverse ligands, including ICAM-1, fibrinogen, and vitronectin [10, 23, 26]. Whereas adhesion of unstimulated purified blood eosinophils to VCAM-1 is mainly mediated by α4β1, adhesion of BAL eosinophils or IL-5-stimulated blood eosinophils to VCAM-1 also involves αMβ2 [10, 23, 26]. Studies in mice indicate that both β1- and β2-integrins play important roles in directing eosinophils to the lung [8, 11]. Consistent with the in vitro adhesion data, β2-integrins are activated on human BAL eosinophils but not blood eosinophils as assessed by staining with mAb24 [27], which recognizes the high-activity conformation of theβ2 subunit in β2-integrins [17, 18, 28]. MAb24 reactivity and surface expression of β2-integrins on BAL eosinophils correlate with the concentration of IL-5 in BAL fluid post-segmental antigen challenge [27].
To determine the role of IL-5 in humans in regulation of eosinophil integrins in vivo, we used a blocking mAb to IL-5 (mepolizumab)[29–35] as a study reagent. We hypothesized that administration of anti-IL-5 mAb prior to segmental antigen challenge would dampen upregulation and activation of β2-integrins. To test this hypothesis, flow cytometric analyses of integrin surface expression and activation were performed on blood or BAL eosinophils obtained following segmental antigen challenge of asthmatic subjects, comparing results from challenges performed before and after administration of anti-IL-5. We also analyzed eosinophil β1-integrins, which are activated by P-selectin in vitro [24] and for which activation state correlates with eosinophil-bound P-selectin in vivo [24, 36], and P-selectin glycoprotein ligand-1 (PSGL-1), an eosinophil receptor for P-selectin [37]. Finally, because IL-5 has been shown to cause eosinophils in vitro to acquire increased forward scatter [38], we analyzed scattering properties of eosinophils by flow cytometric analysis before and after anti-IL-5 administration.
Methods
Subjects and screening
Ten subjects (Table I) with mild allergic asthma were studied at the University of Wisconsin Asthma, Allergy and Pulmonary Research Unit. Due to safety considerations, and in keeping with the primary objective of the study to use anti-IL-5 not as a therapeutic agent but as a strategy to determine the in vivo effects of IL-5 on eosinophils, only subjects with mild allergic asthma were enrolled. Subjects were screened as described before [27]. Subjects had a history of asthma based on presence of symptoms such as cough, shortness of breath, wheeze, or chest tightness; a positive skin-prick test to at least one aeroallergen; a pre-albuterol (180 μg) forced expiratory volume in 1 s (FEV1) of ≥ 70% of the predicted value (% pred.); a post-albuterol FEV1 of ≥ 80% pred.; current or historical reversibility to albuterol of > 12% or a current or historical provocative concentration of methacholine producing a 20% fall in FEV1 (PC20) of ≤ 8 mg/ml; and an early FEV1 fall following whole-lung inhaled antigen challenge of ≥ 20%. They had not received corticosteroids or leukotriene inhibitors within one month of screening or omalizumab (anti-IgE) within nine months of screening. Other exclusion criteria were concomitant use of any other mAb, respiratory infection within four weeks of study, unstable asthma as indicated by increased symptoms or increased β-agonist use over the previous two weeks, pregnancy, smoking, major health problems other than asthma, previous malignancy, and prior treatment with an anti-IL-5 mAb. At least four weeks before bronchoscopy, subjects underwent a whole-lung inhaled antigen (house dust mite, ragweed, or cat dander) challenge to determine the AgPD20 (the provocative dose of antigen producing a 20% fall in FEV1) and the magnitude of early- and late-phase responses, as described [27]. The study was reviewed and approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Informed written consent was obtained from each subject before participation.
Table 1.
Subject characteristics
| Subject | Age (years) | Sex | Antigen | PC20 (mg/ml) | FEV1 (l) | FEV1 (% pred.) | AgPD20 (CBU) | LPR |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | M | HDM | 0.4 | 3.5 | 75 | 3 | yes |
| 2 | 27 | M | HDM | 0.2 | 4.7 | 101 | 81 | yes |
| 3 | 31 | M | CD | 3.8 | 6.1 | 135 | 44 | no |
| 4 | 36 | M | HDM | 4.9 | 3.9 | 87 | 6 | yes |
| 5 | 22 | M | HDM | 2.9 | 4.3 | 83 | 10 | no |
| 6 | 27 | F | RW | 5.3 | 2.7 | 95 | 3 | no |
| 7 | 27 | M | RW | 1.4 | 3.6 | 71 | 0.08 | yes |
| 8 | 19 | M | HDM | 11 | 4.9 | 103 | 50 | yes |
| 9 | 22 | F | HDM | 0.2 | 3.8 | 108 | 51 | no |
| 10 | 20 | F | HDM | 0.9 | 3.3 | 100 | 13 | yes |
| Mean ± SD or median (25th, 75th percentiles) | 25 ± 5 | 2.1 (0.3,5.1) | 4.1 ± 1.0 | 96 ± 18 |
Group data are presented as mean ± SD if the variable is normally distributed and as median with 25th and 75th percentiles if the variable is non-normally distributed.
FEV1 data are baseline values at screening.
AgPD20, provocative dose of antigen producing a 20% fall in FEV1; CBU, cumulative breath units; CD, cat dander; F, female; FEV1, forced expiratory volume in 1 s; HDM, house dust mite; LPR, late-phase response (FEV1 fall of ≥ 15% 3–8 h from baseline after whole-lung inhaled antigen challenge)[27]; M, male; PC20, provocative concentration of methacholine producing a 20% fall in FEV1; RW, ragweed; % pred., percentage of the predicted value
Segmental bronchoprovocation with allergen and BAL
Blood was obtained immediately pre-challenge (0 h). BAL was performed by instilling 160 ml sterile 0.9% NaCl, warmed to 37°C, into each of two segments, and BAL recovered from these two segments was pooled for analysis [27]. A test dose of 1% of the subject’s AgPD20 was administered into one segment via a wedged bronchoscope. When this dose was well tolerated, as it was in all ten subjects, a dose of 20% of the AgPD20 was instilled in the second segment. Bronchoscopy with BAL was repeated 48 h later with separate sampling of the two segments. Results on BAL cells reported herein are on 48 h-samples from the segment that received 20% AgPD20, except some of the results in Fig. 1, which includes data on BAL cells in the pooled sample from the two segments at 0 h.
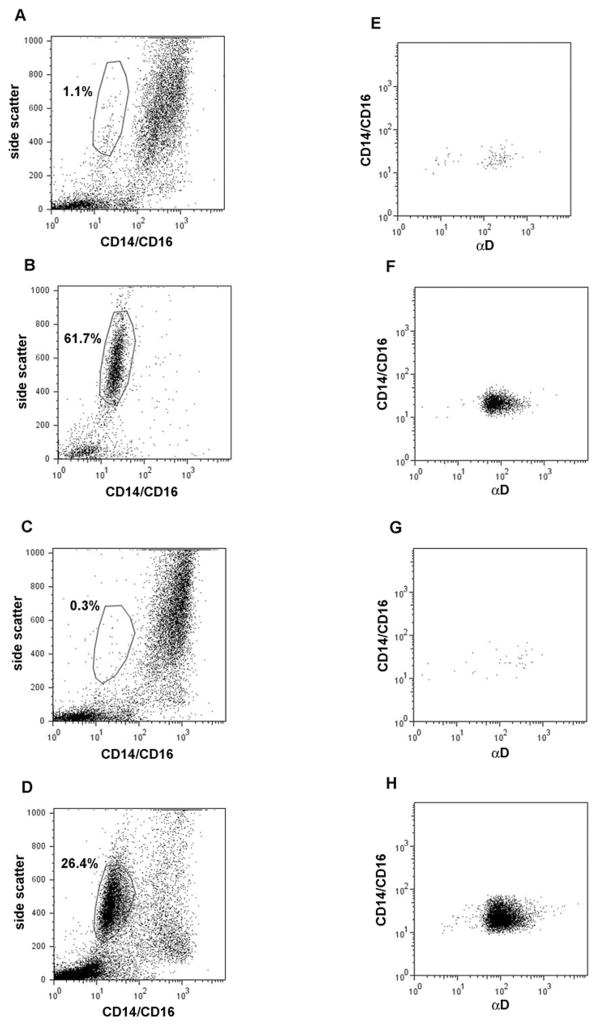
Fig. 1.
BAL cells pre- and post-segmental antigen challenge before and after anti-IL-5 administration. (Left panels, A–D) Dot plots of side scatter versus labeling with FITC-conjugated anti-CD14 and anti-CD16 of unfractionated BAL cells from subject No. 1. (A) pre-segmental antigen challenge before anti-IL-5, (B) 48 h post-segmental antigen challenge before anti-IL-5, (C) pre-segmental antigen challenge after anti-IL-5, (D) 48 h post-segmental antigen challenge after anti-IL-5. Gating (polygon area) was performed in the post-challenge samples (B,D), after which the parameters were applied to the plots in A and C. Proportions of gated eosinophils are indicated in the plots. (Right panels, E–H) Dot plots of labeling of the gated populations in A–D, respectively, with FITC-conjugated anti-CD14 and anti-CD16 versus αD integrin subunit detected with PE-conjugated secondary antibody.
Cell counts
On the days of bronchoscopy, total BAL cell numbers were determined by hematocytometer using Turk’s counting solution containing acetic acid and methylene blue as before [39]. For differential BAL cell counts, two cytospins were prepared from each BAL cell preparation and stained with the Wright-Giemsa-based Hema 3 system (Fisher Diagnostics, Middletown, VA) and 500 cells per slide (1000 cells per sample) were counted. White blood cells were determined in the research laboratory using a Coulter counter, and eosinophils were enumerated by hematocytometer using phyloxin staining.
Anti-IL-5 administration
Four weeks after the first set of bronchoscopies, a single 750 mg dose of anti-IL-5 (mepolizumab, provided by GlaxoSmithKline, study No. MEA110170) was administered intravenously. The peripheral blood eosinophil count was monitored weekly at the University of Wisconsin Hospital and Clinics Core Laboratory as described [36]. If the eosinophil count decreased to ≤ 20% of the baseline value or to < 100 eosinophils/μl one month after anti-IL-5 administration, the subject underwent a second segmental antigen challenge. If not, the subject received a second 750 mg i.v. dose of anti-IL-5, the eosinophil count was again monitored weekly, and the challenge was performed one month after the second dose. Only one of the ten subjects required a second dosing.
Antibodies for flow cytometric analysis of eosinophils
FITC-conjugated anti-CD14 and anti-CD16 were used as previously described [24, 27, 36, 40] to discriminate eosinophils from monocytes and neutrophils, respectively. MAbs for determination of integrin subunit density were anti-β2 (clone L130), anti-αM (LM11), anti-αL (AI111), anti-αD 240I, anti-β1 mAb (MAR4), and PE-conjugated anti-αM (D12). MAbs for detection of active conformations of integrins were anti-β2 KIM-127 and mAb24; anti-αM CBRM1/5; and anti-β1 N29, HUTS-21, and 9EG7. KPL-1 was used to determine P-selectin glycoprotein ligand-1 (PSGL-1) density. PE-conjugated goat anti-mouse and anti-rat IgG Abs were used as secondary Abs, and mouse IgG1 and rat IgG2a as isotype controls. PE-conjugated anti-αM D12 was from BD Biosciences (San Jose, CA). Commercial sources of other antibodies were as described before [23, 24, 26, 27, 36]. KIM-127 [41] was a gift from Nancy Hogg (Cancer Research UK London Research Institute, London, UK).
Flow cytometry
Unfractionated cells were recovered from BAL pre- (at 0 h) and 48 h post-segmental antigen challenge as described [27]. Blood was drawn into vacuum tubes containing CTAD (citrate, theophylline, adenosine, and dipyrimadole) anticoagulant solution (BD Vacutainer Systems, Franklin Lakes, NJ)[36] immediately pre- (at 0 h) and at 48 h post-segmental challenge. Whole unfractionated blood (100 μl) and BAL cells (1 × 105 cells) were processed for flow cytometry, and data were acquired and analyzed and are expressed as described [24, 27, 36]. The sensitivity of the scatter detectors was set in a standardized manner. Blood and BAL cell samples were incubated with primary mAbs as before [24, 27], except that for mAb KIM-127, for which the epitope is temperature-dependent [41], and its isotype control, samples were incubated with primary mAb at 37°C. Forward and side scatter were analyzed from the dataset acquired from second tube run on each sample.
Statistics
The Mann-Whitney U test was used to compare data between two groups. The Spearman rank test was used to analyze correlations. A level of P ≤ 0.05 was considered significant. Analyses were performed using Prism (GraphPad, San Diego, CA).
Results
Subject characteristics at baseline and eosinophil numbers pre- and post-segmental lung antigen challenge before and after anti-IL-5 administration
Flow cytometric analyses of blood and BAL cells were performed on ten subjects with mild allergic asthma (Table 1) who underwent segmental lung antigen challenge before and after administration of anti-IL-5 (mepolizumab). Before anti-IL-5 administration, mean concentration of eosinophils in the circulation 48 h post-segmental antigen challenge was 510/μl (± SEM of 80/μl); after anti-IL-5 it was 40/μl (± 10/μl). Either before or after anti-IL-5, eosinophils were sparse (≤ 1%) in BAL samples obtained at the time of segmental instillation of antigen (Fig. 1). The percentage of eosinophils among BAL cells at 0 h, even in the absence of anti-IL-5, is known to be low; in an earlier study we found a mean of 1.2% BAL eosinophils pre-segmental antigen challenge [39]. Without prior anti-IL-5, the percentage of eosinophils in BAL 48 h post-segmental challenge ranged from 52% to 81% with a mean of 72%, and mean number of eosinophils per volume BAL fluid was 1.37 × 106/ml (± 0.35 × 106/ml). After anti-IL-5, the percentage ranged from 4% to 49% with a mean of 31%, and the mean number of eosinophils was 0.28 × 106/ml (± 0.08 × 106/ml). The percentage of BAL leukocytes that were eosinophils correlated with the concentration of blood eosinophils before and after anti-IL-5 (rs = 0.88, P = 0.002 before anti-IL-5; rs = 0.72, P = 0.02 after anti-IL-5).
Flow cytometric analyses of eosinophils in samples of blood obtained pre- (at 0 h) segmental antigen challenge and in samples of blood and BAL obtained 48 h post-challenge are shown in Table 2. The sets of data on the left and right were obtained before and after administration of anti-IL-5. Data collected on pre-segmental antigen challenge BAL eosinophils are not shown in Table 2, because an adequate number of flow cytometric events was obtained in the before-anti-IL-5 sample of only a single subject (Fig. 1). As exemplified in Figs. 1 and 2, numbers of eosinophils adequate for analysis were gated in blood samples and 48-h post-challenge 48 h BAL samples. Analysis of the data revealed significant differences between some flow cytometric signals of blood eosinophils at 0 and 48 h (*…*), blood and BAL eosinophils obtained at 48 h (†…†), and eosinophils in corresponding samples obtained before and after anti-IL-5 (‡…‡)(Table 2); whereas for other signals no differences were found.
Table 2.
Eosinophil expression and activation of β2- and β1-integrins, expression of PSGL-1, and scatter pre- and post-segmental lung antigen challenge before and after anti-IL-5 administration.
| Measurement | Before anti-IL-5 | After anti-IL-5 | ||||
|---|---|---|---|---|---|---|
| Blood eosinophils | BAL eosinophils | Blood eosinophils | BAL eosinophils | |||
| 0 h (pre-challenge) | 48 h post-challenge | 48 h post-challenge | 0 h (pre-challenge) | 48 h post-challenge | 48 h post-challenge | |
| β2 | 1270 ± 50* | 1450 ± 50*†‡ | 1850 ± 30† | 1170 ± 50 | 1180 ± 60†‡ | 1780 ± 50† |
| KIM-127 (intermediate- and high- activity β2) | 120 ± 20‡ | 170 ± 30† | 920 ± 110† | 70 ± 10‡ | 90 ± 30† | 960 ± 130† |
| mAb24 (high-activity β2) | ND | ND | 730 ± 80 | ND | ND | 800 ± 80 |
| αM | 1360 ± 100 * | 1610 ± 50*†‡ | 2020 ± 30† | 1340 ± 40 | 1370 ± 60 †‡ | 1900 ± 70† |
| CBRM1/5 (high-activity αMβ2) | 60 ± 20 | 80 ± 30 | 230 ± 70 | 30 ± 20 | 40 ± 20† | 280 ± 90† |
| αL | 1060 ± 40* | 1230 ± 50*‡ | 1380 ± 50 | 1010 ± 40 | 1080 ± 60†‡ | 1350 ± 60† |
| αD | 70 ± 10 | 80 ± 20† | 830 ± 110† | 50 ± 10 | 50 ± 10† | 890 ± 90† |
| β1 | 510 ± 40 | 600 ± 40 | 630 ± 30 | 520 ± 40 | 560 ± 60 | 610 ± 30 |
| N29 (intermediate- and high-activity β1 | 210 ± 30 | 250 ± 30 | 250 ± 20 | 200 ± 30 | 220 ± 40 | 270 ± 30 |
| HUTS-21 (high-activity β1) | 50 ± 20 | 20 ± 0† | 70 ± 10† | 30 ± 10 | 20 ± 10† | 70 ± 20† |
| 9EG7 (high-activity β1) | 10 ± 0 | 20 ± 10† | 40 ± 10† | 20 ± 10 | 10 ± 0† | 50 ± 20† |
| PSGL-1 | 1640 ± 40* | 1830 ± 60*‡ | 1800 ± 40‡ | 1560 ± 40 | 1640 ± 50‡ | 1700 ± 30‡ |
| Forward scatter | 360 ± 20 | 370 ± 30†‡ | 270 ± 20† | 330 ± 20 | 320 ± 10†‡ | 270 ± 10† |
| Side scatter | 670 ± 10 | 680 ± 10† | 550 ± 10† | 690 ± 20 | 690 ± 10† | 540 ± 10† |
Flow cytometry was performed on blood eosinophils pre-segmental antigen challenge (0 h) and 48 h post-segmental antigen challenge and on BAL eosinophils 48 h post-segmental antigen challenge before and after administration of anti-IL-5 (mepolizumab). Values shown are specific gMCF (geometric mean channel fluorescence) for expression levels and mean signal intensity for scatter (mean ± SEM), n = 10 subjects.
ND, not determined; PSGL-1, P-selectin glycoprotein ligand-1.
Paired symbols indicate significant (P ≤ 0.05) differences between: *…*blood eosinophils at 0 h and blood eosinophils at 48 h, †…† blood eosinophils at 48 h and BAL eosinophils at 48 h, and ‡…‡ eosinophils in corresponding samples obtained before and after anti-IL-5 administration. Therefore, an interpretation of the symbols requires examination of data in 2 different cells of the table.
Fig. 2.
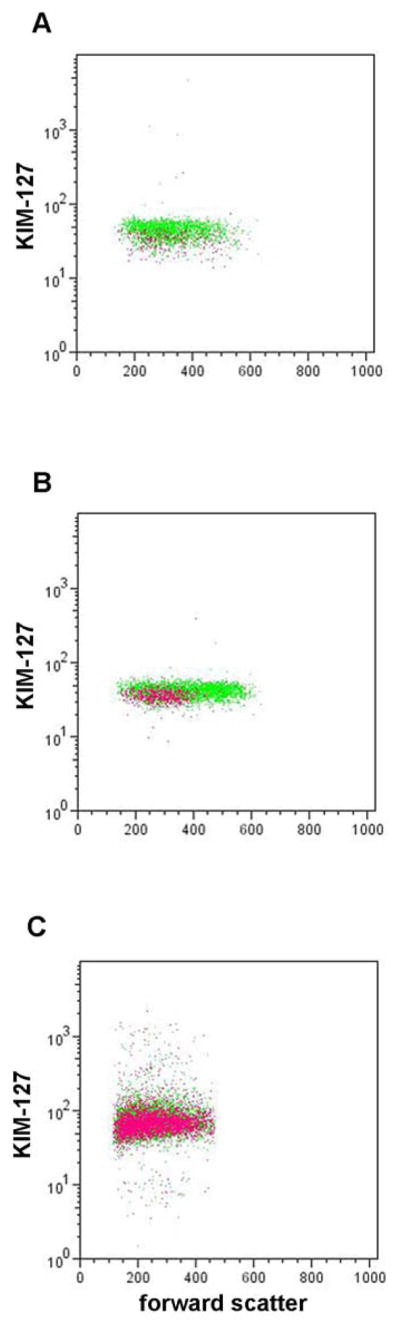
Expression of epitope for mAb KIM-127 in intermediate- and high-activity β2-integrins versus forward scatter of eosinophils before and after anti-IL-5 administration. Representative dot plots of KIM-127 epitope expression versus forward scatter on blood eosinophils pre-segmental antigen challenge (A) and on blood (B) and BAL (C) eosinophils 48 h post-segmental antigen challenge before (green) and after (purple) anti-IL-5. Data acquisition time was 1 min, and thus the number of dots varies according to the numbers of eosinophils. KIM-127 epitope expression on blood eosinophils pre-segmental antigen challenge was significantly lower after anti-IL-5 than before anti-IL-5 when these data were combined with data from the other subjects (Table 2). Forward scatter of blood eosinophils 48 h post-segmental antigen challenge was significantly lower after anti-IL-5 than before anti-IL-5 (Table 2).
Anti-IL-5 administration results in loss of the KIM-127 activation-sensitive β2-integrin epitope on blood eosinophils
Administration of anti-IL-5 was associated with changes in eosinophil surface expression of the epitope for mAb KIM-127 (Table 2), which recognizes β2-integrins with intermediate or high activation [17, 18, 42]. Diminished reactivity, 0.6-fold on the average of the value before anti-IL-5 (Table 2), was statistically significant in analyses of blood samples obtained at 0 h with a trend at 48 h (0.5-fold of the value before anti-IL-5; 0.05 < P > 0.10) (Table 2). No significant differences of the average expression of the epitope for CBRM1/5, which recognizes αM of highly active αMβ2 [43], were found on blood eosinophils pre- or post-antigen challenge either before or after anti-IL-5, although the variability among samples was considerable (Table 2). The effect of anti-IL-5 on KIM-127 reactivity indicates that IL-5 provides a tonic signal to blood eosinophils in vivo that results in intermediate activation of β2-integrins.
Anti-IL-5 blunts upregulation of αMβ2 and αLβ2 on blood eosinophils following segmental antigen challenge
Before anti-IL-5 administration, segmental lung antigen challenge was associatedc with modest increases of surface expression of β2, αM, and αL on blood eosinophils at 48 h compared to pre-challenge; these differences were significant (Table 2). After anti-IL-5, these increases did not occur, and surface β2, αM, and αL on blood eosinophils at 48 h were significantly lower than before anti-IL-5 (0.8-, 0.9-, and 0.9-fold of the 48-h values before anti-IL-5, respectively) (Table 2). Significantly different (0.8-fold of the value before anti-IL-5, P ≤ 0.01) αM expression on blood eosinophils at 48 h after anti-IL-5 compared to before anti-IL-5 was also observed with directly PE-labeled different anti-αM mAb clone D12 (data not shown). There was no effect of segmental antigen challenge or anti-IL-5 on the low blood eosinophil surface expression of αD (Table 2). The fourth β2-integrin α subunit, αX, was not analyzed. These results indicate that in addition to causing steady-state intermediate activation of β2-integrins, IL-5 mediates modest upregulation of surface densities of αMβ2 and αLβ2 in response to segmental antigen challenge.
Activation and expression levels of BAL eosinophil β2-integrins are equivalent before and after anti-IL-5
β2-integrins were upregulated and activated on BAL eosinophils obtained 48 h post-segmental antigen challenge compared to blood eosinophils at the same time-point; these changes were not influenced by anti-IL-5 (Table 2). Thus, KIM-127 and CBRM1/5 reactivities were 5- to 11-fold and 3- to 7-fold higher, respectively, on BAL eosinophils than on blood eosinophils at the same time, and the high signals on BAL eosinophils were the same before and after anti-IL-5 (Table 2). For BAL eosinophils, we were able to test mAb24, which recognizes β2 in highly active β2-integrins [17, 18, 28]. The mAb24 epitope is cation-dependent [44], precluding its use with the blood samples, which were collected in a chelating anticoagulant. MAb24 reactivity of BAL eosinophils was high, as described previously [27], and not significantly changed after anti-IL-5 (Table 2). BAL eosinophils had 1.1- to 1.5-fold higher surface expression of β2, αM, and αL than blood eosinophils in samples obtained 48 h post- challenge (Table 2). Such increases were evident before and after anti-IL-5 with no indication that anti-IL-5 attenuated the levels on BAL eosinophils. Similar 1.6- to 1.8-fold, higher αM expression on BAL eosinophils compared to on blood eosinophils (P ≤ 0.001 both before and after anti-IL-5) was also observed with the PE-labeled anti-αM mAb clone D12, also with no effect of anti-IL-5 (data not shown). The density of αD, which did not change on blood eosinophils after segmental antigen challenge, was 10–18-fold higher on BAL eosinophils than blood eosinophils both before and after anti-IL-5 administration (Table 2).
In the single sample in which there were adequate eosinophils in BAL at 0 h before anti-IL-5 (Fig. 1A), eosinophils had intensity of αD level similar to those at 48 h post-challenge (Fig. 1B,D). The same was true for intensity of CBRM1/5. These limited observations indicate that BAL eosinophils pre-segmental antigen challenge may have β2-integrins that are as upregulated and activated pre-antigen challenge as post-challenge.
Anti-IL-5 has no effect on the activation and expression levels of β1-integrins on blood or BAL eosinophils
In contrast to effects on β2-integrins on blood eosinophils, anti-IL-5 had no effect on eosinophil β1-integrin activation and expression levels on blood or BAL eosinophils (Table 2). Before anti-IL-5, blood eosinophils had detectable reactivity with mAb N29. N29 recognizes the intermediate and high-activity conformation of β1-integrins and is the β1 counterpart of KIM-127. The N29 epitope has been mapped to the PSI domain of theβ1 subunit and the KIM-127 epitope has been mapped to the 2nd EGF-like domain of the β2 subunit; these locations are spatially very close [10, 20, 45–48]. N29 reactivity was similar on blood and airway eosinophils obtained at 48 h (Table 2), as observed before [27]. Reactivities of mAbs HUTS-21 and 9EG7, which recognize the high-activity conformation of β1 integrins [10, 20, 47, 49, 50], were 2- to 5-fold higher on BAL eosinophils than on blood eosinophils, with no attenuating effect of anti-IL-5 on the increases (Table 2).
Anti-IL-5 blunts PSGL-1 upregulation on blood and BAL eosinophils following segmental antigen challenge
In vitro, eosinophil β1-integrins are activated to a N29-positive conformation by P-selectin interacting with eosinophil PSGL-1, whereas P-selectin does not activate eosinophil β2-integrins [24]. Prior to administration of anti-IL-5, there was a modest but significant 1.1-fold increase in PSGL-1 expression on blood eosinophils at 48 h (Table 2) and a similar modest significant 1.1-fold increase in PSGL-1 expression on BAL eosinophils compared to blood eosinophils at 0 h (P ≤ 0.05). After anti-IL-5, expression on both blood and BAL eosinophils at 48 h was 0.9-fold of values before anti-IL-5, differences that were significant (Table 2). Although PSGL-1 was highly expressed in all situations, the minor but significant changes attributable to anti-IL-5 are noteworthy because, unlike changes in β2-integrins, the changes were manifest on both blood and BAL eosinophils.
Forward scatter of blood eosinophils following segmental antigen challenge is decreased by anti-IL-5
As mentioned in the Introduction, IL-5 increases eosinophil forward light scattering in vitro [38]. Such an increase may be indicative of shape change or increased cell size of leukocytes [51, 52] and is possibly associated with migratory capacity [53]. Comparing blood samples before and after anti-IL-5, after anti-IL-5 mean forward scatter at 48 h post-challenge was 0.9-fold the value before anti-IL-5, a difference that was significant (Table 2). In contrast, there were no changes related to anti-IL-5 in side scatter (Table 2), which reports leukocyte granularity [54]. Forward and side scatter of BAL eosinophils were 0.7-to 0.8-fold of the values of blood eosinophils and scattering values were not affected by anti-IL-5 (Table 2).
Because forward scatter and β2-integrin activation of blood eosinophils are both influenced by anti-IL-5, we examined whether there were correlations between KIM-127 reactivity and forward scatter for the group as a whole and for cells in individual samples. Mean reactivity with KIM-127 for individuals did not correlate with mean forward scatter (rs = −0.03, P = 0.93 and rs = 0.11, P = 0.71 for all data points at 0 and 48 h, respectively; rs = −0.18, P = 0.66 and rs = −0.54, P = 0.24 before anti-IL-5; and rs = −0.41, P = 0.35 and rs = 0.22, P = 0.66 after anti-IL-5). In dot plots of KIM-127 reactivity versus forward scatter in individual subjects at 0 h (Fig. 2A) there was a decrease in the KIM-127 signal after anti-IL-5 (purple) compared to before anti-IL-5 (green), as described above, but no evident difference in forward scatter before and after anti-IL-5. At 48 h before anti-IL-5, blood eosinophils were more heterogeneous than at 0 h within an envelope that includes cells with higher forward scatter (Fig. 2B, green). KIM-127 reactivity appeared equal throughout the population, i.e., not higher on the cells with greater forward scattering. This was confirmed by dividing the population in two subpopulations based on forward scatter. The subpopulation with the higher forward scatter values did not have significantly different reactivity with KIM-12, nor with CBRM1/5 or mAbs detecting total β2, αM, or αL, than the subpopulation with lower forward scatter (not shown). After anti-IL-5 at 48 h, the heterogeneity and high forward scattering cells were not found (Fig. 2B, purple). Thus, absence of increased scattering heterogeneity and cells with higher forward scattering account for the significant decrease in mean forward scatter after anti-IL-5 as compared to before anti-IL-5 at 48 h (Table 2). BAL eosinophils had lower forward scatter than blood eosinophils in addition to having higher KIM-127 signal, and there was no difference in forward scatter comparing samples obtained before and after anti-IL-5 (Fig 2C, compare green and purple).
KIM-127 activation-sensitive β2-integrin epitope expression on blood eosinophils correlates with eosinophil numbers in BAL
Because mean KIM-127 epitope expression was decreased on blood eosinophils after administration of anti-IL-5 and there were decreased percentages of BAL eosinophils at 48 h, we examined whether these parameters correlated among subjects. Before anti-IL-5, KIM-127 reactivity on blood eosinophils at 0 h correlated significantly with BAL eosinophil percentage (Table 3). After anti-IL-5, these parameters did not correlate. αM, αL, β2, or β1 levels or N29 reactivity of blood eosinophils at 0 h did not correlate with BAL eosinophils. Signals on blood eosinophils at 48 h, including KIM-127, did not correlate (Table 3). The results indicate that partial activation of β2-integrins on blood eosinophils at baseline, presumably due to exposure to IL-5 in the bone marrow or the circulation, is associated with enhanced eosinophil recruitment to the airway over the 48 h post-challenge.
Table 3.
Correlations between blood eosinophil activation or expression of β2- orβ1-integrins and BAL eosinophil percentage 48 h after segmental antigen challenge before and after anti-IL-5 administration
| Measurement | Before anti-IL-5 | After anti-IL-5 | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 h (pre-challenge) | 48 h post-challenge | 0 h (pre-challenge) | 48 h post-challenge | |||||
| rs | P | rs | P | rs | P | rs | P | |
| KIM-127 (intermediate- and high-activity β2) | 0.74 | 0.05 | 0.32 | 0.50 | −0.31 | 0.50 | −0.18 | 0.71 |
| β2 | 0.62 | 0.09 | 0.05 | 0.91 | −0.27 | 0.45 | 0.02 | 0.96 |
| αM | −0.20 | 0.58 | 0.38 | 0.31 | 0.24 | 0.51 | 0.15 | 0.68 |
| αL | 0.41 | 0.25 | 0.35 | 0.36 | 0.22 | 0.58 | −0.02 | 0.96 |
| N29 (intermediate- and high-activity β1 | 0.30 | 0.41 | 0.33 | 0.39 | 0.28 | 0.43 | 0.12 | 0.76 |
| β1 | 0.25 | 0.49 | −0.03 | 0.95 | 0.28 | 0.42 | −0.12 | 0.75 |
Discussion
We administered IL-5 blocking antibody to subjects with mild allergic asthma to learn the role of IL-5 in regulation of human eosinophil integrins in vivo. Anti-IL-5 significantly decreased circulating eosinophil expression of the intermediate-activity conformation of β2-integrins recognized by mAb KIM-127. However, there was no significant effect of anti-IL-5 on the average reactivity of blood eosinophils with CBRM1/5, which recognizes high-activity conformation of αMβ2 that is induced by treatment of purified blood eosinophils in vitro with IL-5, 5–50 ng/ml [22, 23]. Before anti-IL-5 administration, KIM-127 reactivity of blood eosinophils at baseline correlated significantly with percentage of eosinophils in BAL 48 h post-segmental antigen challenge. The results indicate that IL-5 at the concentration of ~10 pg/ml found in the serum of subjects with atopic asthma [55] supports tonic partial activation or “priming” [56] of β2-integrins on circulating eosinophils and such activation is associated with eosinophil recruitment to the airway.
Further, anti-IL-5 abrogated the modest increases in surface expression of αMβ2 and αLβ2 that occur 48 h post-segmental lung antigen challenge. We also found that anti-IL-5 abrogated the appearance of a more heterogeneous population of circulating eosinophils post-segmental antigen challenge with higher average forward scatter.
We saw no effects of anti-IL-5 on activation of β1-integrins or surface expression of αDβ2 on blood eosinophils. The results indicate that the in vivo roles of IL-5 in supporting integrin steady-state partial integrin activation on circulating eosinophils and upregulation of surface integrin expression in response to antigen challenge are specific for αMβ2 and αLβ2. We did not test the effect of anti-IL-5 on expression of αXβ2 and β7.
In contrast to the effect on β2-integrins on blood eosinophils, anti-IL-5 had no effect on the pronounced activation ofβ1- and β2-integrins and upregulation ofαMβ2 and αLβ2 on airway eosinophils recovered by BAL 48 h post-segmental antigen challenge. In addition, there was strong expression of αDβ2 on BAL eosinophils that was unaffected by anti-IL-5, and, based on a single sample, likely is present in the absence of segmental antigen challenge. We interpret these results as indicating exposure of eosinophils in the inflamed airway to a complex mix of cytokines, chemokines, and other activating agents [27, 57, 58], in which IL-5 plays at most a minor role. However, the possibility that anti-IL-5 does not block IL-5 produced locally in the lung cannot be excluded. Antigen challenge per se and an antigen challenge-induced inflammatory milieu in the airway are not the only possible causes of the BAL eosinophil phenotype. BAL eosinophils may also be activated compared to blood eosinophils because of having undergone arrest, transendothelial migration and encounters with adhesive ligands. Finally, a proportion of the BAL eosinophils may originate from the tissue in addition to from the circulation. CD34+/IL-5 receptor-α mRNA+ cells, described as potential eosinophil precursors, are present in the bronchial mucosa to a greater degree in asthmatic subjects [59]. Although anti-IL-5 caused a decrease in the number of such cells, that decrease was relatively small (30%) [32] compared to the usual decreases in circulating eosinophils. Thus, after anti-IL-5, a greater proportion of the BAL eosinophils may originate from such tissue-dwelling cells that have differentiated and migrated to the airway lumen.
β1-integrins, which were not affected by anti-IL-5, are activated on eosinophils by P-selectin [24] via binding to its receptor, PSGL-1 [37]. We found that PSGL-1 expression on blood eosinophils was modestly increased 48 h post-challenge and that anti-IL-5 caused a decrease in PSGL-1 at 48 h. BAL eosinophils also had upregulated PSGL-1 compared to baseline blood eosinophils at 0 h. In contrast to the lack of effect of anti-IL-5 on BAL eosinophil integrins, anti-IL-5 significantly decreased PSGL-1 on BAL eosinophils. Together, these results indicate that IL-5 is an in vivo stimulator of upregulation of PSGL-1 on both blood and airway eosinophils following segmental antigen challenge.
Post-challenge blood eosinophils following anti-IL-5 administration had lower forward scatter than before anti-IL-5, possibly reflecting a less polarized cell shape or smaller cell size [51–53]. Dot plots demonstrated that segmental antigen challenge elicits a diverse population of circulating eosinophils in the absence of blocking antibody to IL-5. We speculate that the diverse population arises from more dynamic eosinophil kinetics, with greater production and release from the bone marrow coupled to greater movement of circulating eosinophils into the challenged segments. The forward scatter increase and presence of the KIM-127 epitope on circulating eosinophils may both be related to the well-described IL-5 priming of eosinophils for migration and enhanced responsiveness to chemoattractants [56]. Airway eosinophils had both lower forward and side scatter, presumably due to smaller cell size and lower granularity [51, 54] resulting from additional stimuli encountered by eosinophils en route to or in inflamed airway. Decreased granularity of BAL eosinophils has also been observed previously by electron microscopy [60].
An additional possible explanation to the observed effects is redistribution of eosinophils among different pools in the body (e.g., bone marrow, circulating pool, marginated pool, lung, spleen, liver, gastrointestinal tract, and thymus)[61–65]. For instance, decreases on blood eosinophils may also be due to a situation after anti-IL-5 in which cells with higher signals were not released from the bone marrow or marginated or extravasated to different degrees or with different kinetics than in the absence of anti-IL-5.
In another study using mepolizumab, three infusions given with 4 weeks intervals resulted in decreases in expression of tenascin, lumican, and procollagen I in the bronchial basement membrane, number of transforming growth factor (TGF)-β1-expressing airway eosinophils, and TGF-β1 concentration in BAL fluid two weeks after the last infusion [30]. In clinical studies, subjects received mepolizumab monthly over five months or one year and experienced a decreased number of exacerbations during the course of the studies, with differences between the treatment and the control groups becoming apparent only after two months [33, 34]. In our study, effects were observed in nine subjects on eosinophils one month after one dose of mepolizumab administration. Even though one dose was sufficient to cause a change in the eosinophil phenotype, a low number of circulating eosinophils and altered eosinophil phenotype may have to be maintained for a longer time with repeated dosings in order to lead to changes in airway remodeling and beneficial clinical effects.
In Fig. 3, we summarize the results in a model for blood and airway eosinophil αMβ2 and α4β1 integrins and PSGL-1 before and after anti-IL-5 administration. At baseline, blood eosinophils, in the presence of or having been exposed to “tonic” concentrations of IL-5, carry intermediate-activity KIM-127-reactiveαMβ2, but on the average have no or only low levels of high-activity CBRM1/5-reactiveαMβ2. Following challenge, blood eosinophils are subject to modest upregulation ofαMβ2, αLβ2, and PSGL-1, and have high average forward scatter, due to exposure to additional IL-5 in the bone marrow and/or circulation. Blood eosinophils carry intermediate-activityα4β1 that is induced by P-selectin and independent of IL-5. Airway eosinophils have high αMβ2 activation state, presumably triggered by IL-5 family cytokines IL-3 and/or GM-CSF, high IL-5-independent α4β1 activation state, IL-5-dependent upregulated PSGL-1, and IL-5-independent low forward scatter. Finally, even though P-selectin activates β1-integrins [24] and the new data indicate that IL-5 upregulates the P-selectin receptor PSGL-1, the PSGL-1 level present in the absence of IL-5 seems sufficient for theβ1-integrin activation state observed on blood and airway eosinophils, since β1 activation was not affected by anti-IL-5. As blood eosinophil reactivity with KIM-127 at baseline was decreased by anti-IL-5 and correlated with BAL eosinophil percentage, one could speculate that the blood eosinophil KIM-127 signal may be a readout of in vivo IL-5 activity and may predict responsiveness to anti-IL-5.
Fig. 3.
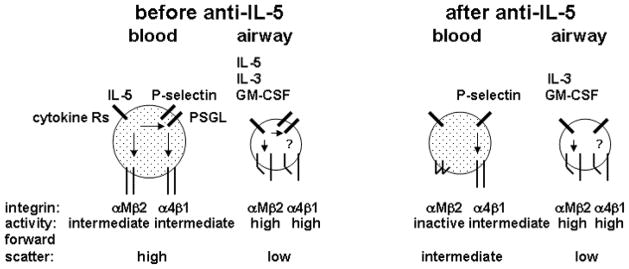
Summary of activation states of αMβ2 and α4β1 integrins, expression of P-selectin glycoprotein ligand-1 (PSGL-1), and forward scatter of blood and airway eosinophils before and after anti-IL-5 administration. The summary is based on present and published [24] data. Before administration of anti-IL-5, blood eosinophils have IL-5-dependent intermediate-activity αMβ2 integrin, have P-selectin-dependent intermediate-activity α4β1, and acquire high PSGL-1 expression and high forward scatter, indicative of cell size and/or shape change, post-segmental antigen challenge. Before anti-IL-5, airway eosinophils have high-activity αMβ2, high-activity α4β1, IL-5-dependent high PSGL-1 expression, and low forward scatter. After administration of anti-IL-5, blood eosinophils have inactive αMβ2, P-selectin-dependent intermediate-activity α4β1 integrin, decreased PSGL-1 expression, and decreased forward scatter. After anti-IL-5, airway eosinophils are the same as airway eosinophils before anti-IL-5 except for decreased PSGL-1 expression. Not shown are the modest increases in total αMβ2 and αLβ2 on blood eosinophils following segmental antigen challenge that are lost after anti-IL-5 and more dramatic increases in αMβ2, αLβ2, and especially αDβ2 on airway eosinophils that are not affected by anti-IL-5.
Acknowledgments
We thank GlaxoSmithKline (GSK) for kindly providing mepolizumab as a gift. GSK did not contribute to the intellectual content of the protocol for integrin analysis. We thank Michele Wolff, Holly Eversoll, Evelyn Falibene, and Gina Crisafi for patient recruitment, screening, and assessments; Richard Cornwell and Keith Meyer for performing the bronchoscopies; Elizabeth Schwantes and Paul Fichtinger for processing blood samples; Larissa De Lain and Lei Shi for processing BAL samples; Nancy Hogg for mAb KIM-127 and mAb24; ICOS for providing anti-αD mAb 240I; Linying Liu for performing staining with PE-conjugated anti-αM mAb D12; Martin Humphries for discussion on activation-sensitive mAbs and integrin conformations; Kathleen Schell for advice on flow cytometry; Michele Wolff, and Elizabeth Schwantes for providing assessment data and advice; Michael Evans for advice on statistics; and Gina Crisafi for administrative help.
This work was supported by Program Project grant P01 HL088594 (N.N.J. and D.F.M.), ARRA supplement grant P01 HL088594-02S1 (N.N.J. and D.F.M.), General Clinical Research Center grant M01 RR03186 (R.N. Golden), and Clinical and Translational Research Center grant UL1 RR025011 (M.K. Drezner) from the National Institutes of Health.
Abbreviations
- AgPD20
provocative dose of antigen producing a 20% fall in FEV1
- BAL
bronchoalveolar lavage
- CBU
cumulative breath units
- CD
cat dander
- F
female
- FEV1
forced expiratory volume in 1 s
- gMCF
geometric mean channel fluorescence
- HDM
house dust mite
- LPR
late-phase response
- M
male
- mAb
monoclonal antibody
- ND
not determined
- PC20
provocative concentration of methacholine producing a 20% fall in FEV1
- PSGL-1
P-selectin glycoprotein ligand-1
- RW
ragweed
- % pred
percentage of the predicted value
Footnotes
Conflict of interest: N.N. Jarjour received honoraria for lectures sponsored by GSK that did not exceed $5000. M.W. Johansson received a fee for consulting from Guidepoint Global. K.A. Gunderson, E.A.B. Kelly, L.C. Denlinger, and D.F. Mosher have no financial conflicts of interest.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25:477–82. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Karp CL. Eosinophils in asthma: remodeling a tangled tale. Science. 2004;305:1726–9. doi: 10.1126/science.1104134. [DOI] [PubMed] [Google Scholar]
- 4.Scott KA, Wardlaw AJ. Eosinophilic airway disorders. Semin Respir Crit Care Med. 2006;27:128–33. doi: 10.1055/s-2006-939515. [DOI] [PubMed] [Google Scholar]
- 5.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 7.Akuthota P, Xenakis JJ, Weller PF. Eosinophils: offenders or general bystanders in allergic airway disease and pulmonary immunity? J Innate Immun. 2011;3:113–9. doi: 10.1159/000323433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee ER, Jiang Y, Henderson WR, Jr, Scott LM. Papayannopoulou T, Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol. 2007;35:605–17. doi: 10.1016/j.exphem.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee ER, Jiang Y, Henderson WR, Jr, Latchman Y, Papayannopoulou T. Absence of alpha 4 but not beta 2 integrins restrains development of chronic allergic asthma using mouse genetic models. Exp Hematol. 2009;37:715–27 e3. doi: 10.1016/j.exphem.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–62. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 16.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–70. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–25. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 18.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–26. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 19.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. 2011;23:607–14. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans. 2004;32:407–11. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- 21.Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–11. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin-dependent adhesion of human eosinophils. J Immunol. 1999;163:3423–9. [PubMed] [Google Scholar]
- 23.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35:378–86. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson MW, Mosher DF. Activation of {beta}1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol. 2011;45:889–97. doi: 10.1165/rcmb.2010-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong CK, Ip WK, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor-induced adhesion molecule expression on eosinophils by p38 mitogen-activated protein kinase and nuclear factor-[kappa] B. Am J Respir Cell Mol Biol. 2003;29:133–47. doi: 10.1165/rcmb.2002-0289OC. [DOI] [PubMed] [Google Scholar]
- 26.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem. 2006;281:32175–87. doi: 10.1074/jbc.M600943200. [DOI] [PubMed] [Google Scholar]
- 27.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–35. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA. Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci U S A. 2001;98:2393–8. doi: 10.1073/pnas.041618598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 30.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 32.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–9. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 33.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 35.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb, in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol. 2010;125:803–13. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 36.Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin, and eosinophil beta1-integrin activation in asthma. Am J Respir Crit Care Med. 2012;185:498–507. doi: 10.1164/rccm.201109-1712OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Symon FA, Lawrence MB, Williamson ML, Walsh GM, Watson SR, Wardlaw AJ. Functional and structural characterization of the eosinophil P-selectin ligand. J Immunol. 1996;157:1711–9. [PubMed] [Google Scholar]
- 38.Choi EN, Choi MK, Park CS, Chung IY. A parallel signal-transduction pathway for eotaxin- and interleukin-5-induced eosinophil shape change. Immunology. 2003;108:245–56. doi: 10.1046/j.1365-2567.2003.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–8. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 40.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta(1) integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol. 2006;117:1502–4. doi: 10.1016/j.jaci.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 41.Robinson MK, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–5. [PubMed] [Google Scholar]
- 42.Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9:282–7. doi: 10.1038/nsb779. [DOI] [PubMed] [Google Scholar]
- 43.Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proc Natl Acad Sci U S A. 1999;96:2215–20. doi: 10.1073/pnas.96.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989;8:3759–65. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–51. [PubMed] [Google Scholar]
- 46.Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- 47.Humphries MJ, Symonds EJ, Mould AP. Mapping functional residues onto integrin crystal structures. Curr Opin Struct Biol. 2003;13:236–43. doi: 10.1016/s0959-440x(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 48.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem. 2005;280:4238–46. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–7. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 50.Luque A, Gomez M, Puzon W, Takada Y. Sanchez-Madrid F, Cabanas C, Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–75. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 51.Terstappen LW, Mickaels RA, Dost R, Loken MR. Increased light scattering resolution facilitates multidimensional flow cytometric analysis. Cytometry. 1990;11:506–12. doi: 10.1002/cyto.990110409. [DOI] [PubMed] [Google Scholar]
- 52.Cole AT, Garlick NM, Galvin AM, Hawkey CJ, Robins RA. A flow cytometric method to measure shape change of human neutrophils. Clin Sci (Lond) 1995;89:549–54. doi: 10.1042/cs0890549. [DOI] [PubMed] [Google Scholar]
- 53.Sabroe I, Hartnell A, Jopling LA, Bel S, Ponath PD, Pease JE, Collins PD, Williams TJ. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J Immunol. 1999;162:2946–55. [PubMed] [Google Scholar]
- 54.Fletcher MP, Seligmann BE. Monitoring human neutrophil granule secretion by flow cytometry: secretion and membrane potential changes assessed by light scatter and a fluorescent probe of membrane potential. J Leukoc Biol. 1985;37:431–47. doi: 10.1002/jlb.37.4.431. [DOI] [PubMed] [Google Scholar]
- 55.Joseph J, Benedict S, Safa W, Joseph M. Serum interleukin-5 levels are elevated in mild and moderate persistent asthma irrespective of regular inhaled glucocorticoid therapy. BMC Pulm Med. 2004;4:2. doi: 10.1186/1471-2466-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koenderman L, van der Bruggen T, Schweizer RC, Warringa RA, Coffer P, Caldenhoven E, Lammers JW, Raaijmakers JA. Eosinophil priming by cytokines: from cellular signal to in vivo modulation. Eur Respir J Suppl. 1996;22:119s–25s. [PubMed] [Google Scholar]
- 57.Liu L, Jarjour NN, Busse WW, Kelly EA. Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma. Am J Respir Crit Care Med. 2004;169:1118–24. doi: 10.1164/rccm.200312-1659OC. [DOI] [PubMed] [Google Scholar]
- 58.Brasier AR, Victor S, Ju H, Busse WW, Curran-Everett D, Bleecker E, Castro M, Chung KF, Gaston B, Israel E, Wenzel SE, Erzurum SC, Jarjour NN, Calhoun WJ. Predicting intermediate phenotypes in asthma using bronchoalveolar lavage-derived cytokines. Clin Transl Sci. 2010;3:147–57. doi: 10.1111/j.1752-8062.2010.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, Kay AB, Hamid Q. CD34(+)/interleukin-5Ralpha messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 60.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–8. [PubMed] [Google Scholar]
- 61.Hogg JC, Doerschuk CM. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 62.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L913–21. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- 63.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5. 4 days. Blood. 2010;116:625–7. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 64.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161–77. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J Leukoc Biol. 2011;89:661–7. doi: 10.1189/jlb.1010567. [DOI] [PMC free article] [PubMed] [Google Scholar]