Abstract
Objective
Pleiotrophin (PTN) is a cytokine that is expressed by monocytes/macrophages in ischemic tissues and that promotes neovascularization, presumably by stimulating proliferation of local endothelial cells. However, the effect of PTN on monocytes/macrophages remains unknown. We investigated the role of PTN in regulating the phenotype of monocytes/macrophages.
Methods and Results
RT-PCR, real-time PCR, and fluorescence-activated cell sorter analysis revealed that the expression of PTN by monocytic cells led to a downregulation of CD68, c-fms, and CD14 monocytic cell markers and an upregulation of FLK-1, Tie-2, vascular endothelial-cadherin, platelet endothelial cell adhesion molecule-1, endothelial NO synthase, von Willebrand factor, CD34, GATA-2, and GATA-3 endothelial cell markers. Fibrin gel assays showed that the treatment of mouse and human monocytic cells with PTN led to the formation of tube-like structures. In vivo studies showed that PTN-expressing monocytic cells incorporated into the blood vessels of the quail chorioallantoic membrane. The intracardial injection of PTN-expressing monocytic cells into chicken embryos showed that cells integrated only into the developing vasculature. Finally, the injection of PTN-expressing monocytes into a murine ischemic hindlimb model significantly improved perfusion of the ischemic tissue.
Conclusions
PTN expression by monocytes/macrophages led to a downregulation of their monocytic cell markers and an upregulation of endothelial cell characteristics, thus inducing the transdifferentiation of monocytes into functional endothelial cells.
Keywords: transdifferentiation, pleiotrophin, macrophage, endothelial cell
Neovascularization is a hallmark of chronic inflammatory diseases. By releasing a wide array of cytokines such as pleiotrophin (PTN), monocytes/macrophages play a key role in this neovascularization.
PTN is a developmentally regulated 136-aa (15.3 kDa) secreted growth/differentiation cytokine that is expressed during embryogenesis but rarely in adults (eg, few sites in the brain). PTN is differentiation or growth factor for various cell types (thus named PTN); it has mitogenic, antiapoptotic, transforming, angiogeneic, and chemotactic biological activities that can differ between its target cells.1 Cells transformed by PTN develop into highly vascularized, aggressive tumors when implanted into nude mice (Jackson Laboratories, Bar Harbor, Me). In ischemic tissues, PTN is expressed by macrophages within an area of exuberant neovasculature that is formed at the margins of the infarct and in endothelial cells of the newly formed vessels,2,3 suggesting a role for PTN in the neovascularization of ischemic tissue. Nothing is known about the effect of PTN on monocytes/macrophages.
Monocytes/macrophages display a high degree of plasticity, as demonstrated by their ability to transdifferentiate into endothelial cells in vitro and in vivo.4–14 Because PTN stimulates different progenitor cells to enter lineage-specific differentiation pathway, we hypothesize that the expression of PTN by activated monocytes/macrophages in ischemic tissue may affect fate of the cells in an autocrine fashion by altering their phenotype into endothelial cells. The data presented here support this concept.
Materials and Methods
Cloning of full-length PTN, preparation of bicistronic retrovirus, primer sequences, reaction conditions, RT-PCR, real-time PCR, histology, cell culture, and fibrin gel assay are described in detail in the online supplement (available at http://atvb.ahajournals.org).
Quail Chorioallantoic Membrane Assay
The quail chorioallantoic membrane (CAM) assay used fertilized Japanese quail eggs, which were cultured ex-ovo essentially as described.15 Fertilized Japanese quail eggs (Coturnix coturnix japonica) were obtained primarily from Boyd’s Bird Co. They were maintained at 37°C under ambient atmosphere, cracked in a sterile laminar flow hood at embryonic stage 3 (E3), transferred into 10-cm2 wells of polystyrene tissue culture dishes, and cultured further at 37°C. Cells expressing PTN/green fluorescence protein (GFP) were placed on the surface of each E7 CAM; the CAMs were incubated for 3 days and then fixed. A total of 60 CAM specimens were used, 12 CAMs per group. The CAM of a fixed specimen was dissected and mounted between a glass slide and a cover slip. Fluorescent and confocal images of terminal arterial vessels from the middle region of the CAM were acquired in gray scale.
Results
Activated Monocytes/Macrophages Express PTN
PTN is expressed by activated macrophages in the ischemic rat brain. To investigate the expression of PTN by macrophages in vitro, mouse peritoneal macrophages and the mouse RAW monocytic cell line were treated with tumor necrosis factor-α, and the expression of PTN was measured by Northern blot. We found that although resting monocytes/macrophages did not express PTN, their activation with tumor necrosis factor-α markedly upregulated PTN expression (see online supplement), suggesting that activated macrophages express PTN, and, in turn, this cytokine can affect activity of these cells in an autocrine fashion.
PTN Downregulates the Expression of Monocytic Cell Markers
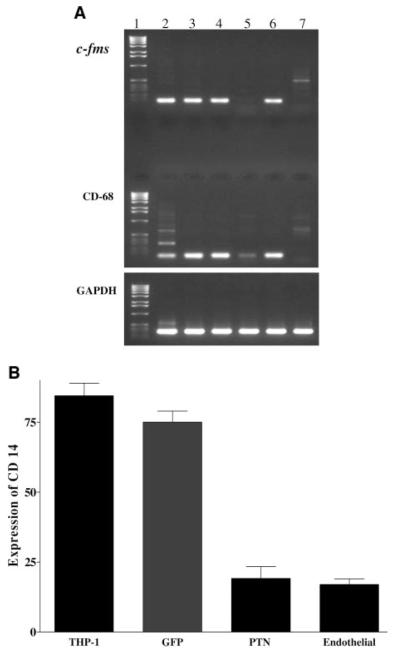
To investigate the autocrine impact of PTN expression on monocytes, human THP-1 and mouse RAW monocytic cell lines were transduced with a bicistronic retroviral vector expressing PTN and GFP (see online supplement). The presence of GFP allowed us to track the fate of the cells in vivo. The transduced cells were analyzed for the expression of monocytic cell markers by RT-PCR. Uninfected THP-1 cells (Figure 1A, lane 2), cells treated with phorbol 12-myristate 13-acetate (Figure 1A, lane 3), cells infected with GFP retrovirus (Figure 1A, lane 4), or PTN antisense strand (Figure 1A, lane 6) expressed monocytic cell markers c-fms and CD68. Retroviral transduction of cells with the PTN sense strand markedly downregulated expression of c-fms and CD68 (Figure 1A, lane 5). These markers were not detected in the negative control human coronary artery endothelial cells (Figure 1A, lane 7). GAPDH amplification showed that the RT-PCRs proceeded efficiently for all tested samples. Real-time PCR analysis confirmed the RT-PCR analysis and showed that the expression of CD68 was downregulated by ≈6.4 to 7.6-fold when compared with uninfected THP-1 cells, cells transduced with antisense PTN, or GFP (see online supplement). In addition, fluorescence-activated cell sorter (FACS) analysis revealed that PTN downregulated the expression of CD14 by ≈76%, similar to the level found in the negative control endothelial cells when compared with untransduced THP-1 cells or cells transduced with control GFP or PTN sense strand (Figure 1B).
Figure 1.
PTN downregulates expression of monocytic cell markers. A, Total RNA was extracted from THP-1 cells grown in 10% serum (lane 2), induced to differentiate into macrophage-like cells by addition of 25 ng/mL phorbol 12-myristate 13-acetate (PMA; lane 3), transduced with retroviral bicistronic vector expressing: GFP (lane 4), PTN sense strand (lane 5), PTN antisense strand (lane 6) followed by treatment with PMA. The exponentially growing human coronary artery endothelial cells (lane 7) were used as a negative control. Analyzed monocytic cell markers were c-fms and CD-68 with primers predicted to amplify 97- and 132-bp DNA fragments, respectively. GAPDH primers were used as control a for the RT-PCR. Lane 1 is a DNA ladder marker. B, Flow cytometry analysis was performed by incubating 5×105 THP-1 cells expressing PTN or GFP with phycoerythrin-labeled anti-CD14 antibody from PharMingen. Human coronary artery endothelial cells were used as a negative control. Uninfected THP-1 cells were used as positive control, and human coronary endothelial cells (endothelial) were used as a negative control. FACS analysis was performed at the Cedars-Sinai Research Institute Core Facility. Each experiment was repeated 3 times, and each bar graph represents mean±SEM of 3 experiments.
PTN Coaxes Monocytic Cells to Acquire an Endothelial Cell Phenotype
We then asked whether PTN affects the endothelial commitment of monocytes. To explore this, the expression of endothelial cell markers in human and mouse monocytic cells that had been transduced with PTN sense, PTN antisense, or GFP were investigated by RT-PCR. The untransduced human monocytic THP-1 cells (Figure 2A, lane 1), mouse monocytic RAW cells (Figure 2A, lane 2), and human promonocytic U937 cells (Figure 2A, lane 3) did not express endothelial cell markers. However, cells that were transduced with the PTN sense strand (Figure 2A, lane 9) expressed vascular endothelial growth factor receptor-2 (FLK-1), Tie-2, vascular endothelial-cadherin (VE-cadherin), platelet endothelial cell adhesion molecule-1, endothelial NO synthase, von Willebrand factor (vWF), and CD34, similar to that of positive control human coronary artery endothelial cells (Figure 2A, lane 6). In contrast, these markers were not detected in THP-1 cells transduced with the PTN antisense strand (Figure 2A, lane 10) or the GFP control vector (Figure 2A, lane 11). Likewise, endothelial cell markers were not detected in nonmonocytic cells, such as NIH 3T3 cells (Figure 2A, lane 4), human coronary artery smooth muscle cells (Figure 2A, lane 5), RPMI 8226 B lymphocyte plasmacytoma cell line (Figure 2A, lane 7), and human skin fibroblasts (Figure 2A, lane 8). The weak expression of FLK-1 in smooth muscle cells (Figure 2A, lane 5) is consistent with the expression of this endothelial cell marker in human smooth muscle cells.16
Figure 2.
PTN upregulates expression of endothelial cell markers in the monocytic cells. A, RT-PCR analysis of endothelial cell markers. Total RNA isolated from untransduced and transduced cells were used for RT-PCR analysis, using specific primers for endothelial cell markers (see online supplement). To ensure semiquantitative results of the RT-PCR analysis, the number of PCR cycles for each set of primers was checked to be in the linear range of the amplification. In addition, all RNA samples were adjusted to yield equal amplification of GAPDH as an internal standard. The amplified products were separated on 1.2% agarose gels and stained with ethidium bromide. B, A representative flow cytometry analysis of αvβ3 integrin expression by the monocytic cells. GFP (top left panel) or PTN-expressing THP-1 cells (bottom left panel) were incubated with a 1:100 dilution of anti-human αvβ3 mouse antibody (Chemicon Co). After washing, cells were incubated with 1:500 dilution of phycoerythrin-labeled anti-mouse antibody (Sigma), fixed, and analyzed by FACS, as described above. In addition, human coronary artery endothelial cells in the absence (top right panel) or presence of anti-αvβ3 antibody (bottom right panel) were used as a positive control. C, RT-PCR analysis of transcription factors. The PCR was performed as described in A, and the primers and PCR condition are described in the online supplement.
Real-time PCR analysis was used to compare the expression level of the VE-cadherin, vWF, and platelet endothelial cell adhesion molecule-1 genes in the PTN-transduced THP-1 cells with those of positive control human endothelial cells. The expression levels of these genes were 0.8×105, 2.9×105, and 1.3×105 copies/100 ng of RNA, respectively (P value all <0.001). These levels of expression are similar to those of the positive control human endothelial cells (0.6×105, 3.2×105, and 1.4×105 copies/100 ng endothelial cell RNA; P value all <0.001, respectively).
This phenotypic modulation of monocytic cells by PTN was further substantiated by investigating the expression of endothelial cell-specific αvβ3 integrin by flow cytometry. FACS analysis revealed that 80±4% of THP-1 cells expressing PTN are positive for αvβ3 (Figure 2B, bottom left panel) compared with 1±3% of THP-1 cells expressing GFP (Figure 2B, top left panel). The level of αvβ3 integrin in the PTN-expressing THP-1 cells (80±4%) is similar to those of human coronary artery endothelial cells (Figure 2B, bottom right panel). The omission of the anti-αvβ3 antibody reduced positivity to 4±3% (Figure 2B, top right panel). Further, FACS analysis revealed that 72±5% of THP-1 cells transduced with the PTN sense strand expressed Tie-2 compared with 7±3% of cells transduced with GFP retroviral vector.
PTN Mediates the Phenotypic Modulation of Monocytes at the Transcriptional Level
Next, we asked whether the phenotypic modulation of monocytic cells by PTN is regulated at the transcriptional level. To accomplish this, the expression of the transcription factors GATA-2 and GATA-3 that affects endothelial cell commitment17–20 was measured. RT-PCR analysis showed that untransduced THP-1 (Figure 2C, lane 1), monocytic RAW (Figure 2C, lane 2), and U937 cells (Figure 2C, lane 3) as well as THP-1 cells transduced with either the PTN antisense strand (Figure 2C, lane 10) or the GFP control vector (Figure 2C, lane 11) did not express these transcription factors. In contrast, THP-1 cells infected with the PTN sense strand (Figure 2C, lane 9) expressed both GATA-2 and GATA-3 similar to the control human coronary artery endothelial cells (Figure 2C, lane 6). Nonmonocytic cells such as mouse NIH 3T3 cells (Figure 2C, lane 4), smooth muscle cells (Figure 2C, lane 5), RPMI 8226 B lymphocyte plasmacytoma cells (Figure 2C, lane 7), and human dermal fibroblasts (Figure 2C, lane 8) did not express GATA-2 and GATA-3. Figure 2 of the supplement further demonstrates the colocalization of GATA-2 expression and VE-cadherin in the transdifferentiated cells.
The monocytic cell lines that we used (THP-1 and RAW cells) are established cell lines with known monocytic cell characteristics that do not exhibit characteristics of multipotent cells. The expression of differentiated cell markers such as CD68, c-fms, and CD14 in THP-1 cells support this concept. However, to investigate the maturity/immaturity of THP-1 cells and RAW cells in more detail the expression of AC133 and Oct-4, 2 well-known markers of stem/progenitor cells21–23 were studied (Figure 2C). Among the examined cells, AC133 was expressed only in human U937 cells (Figure 2C, lane 3), and Oct-4 was expressed in mouse NIH 3T3 cells (Figure 2C, lane 4) and human RPMI 8226 B lymphocyte plasmacytoma cells (Figure 2C, lane 7).
PTN Induces Functional Transdifferentiation of Monocytic Cells Into Endothelial Cells
In Vitro Studies
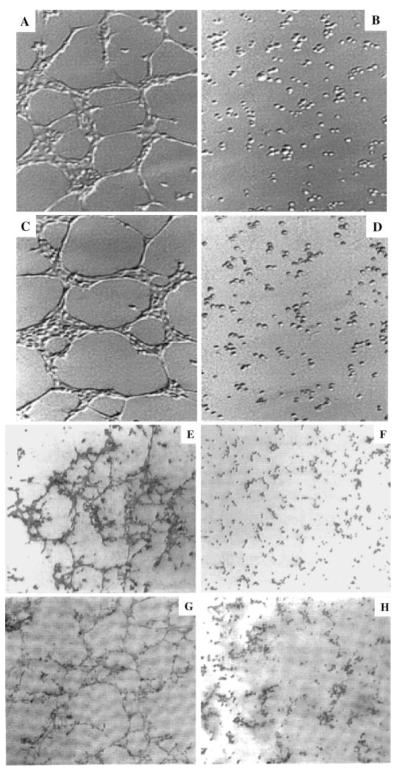
To determine whether the transdifferentiated cells function like endothelial cells, they were cultured in a fibrin gel assay and tube formation was investigated. This showed that cultured THP-1 cells expressing PTN (Figure 3A) or RAW cells expressing PTN (Figure 3C) formed capillary-like structures. In contrast, the morphology of THP-1 cells (Figure 3B) or RAW cells (Figure 3D) that expressed GFP did not change.
Figure 3.
PTN coax monocytic cells to form tubular structures in vitro. Fibrin gels were prepared essentially as described.26 THP-1 cells (5×105) expressing PTN (A) or GFP (B) or RAW cells expressing PTN (C) or GFP (D) were seeded on the fibrin matrix, incubated at 37°C, and the formation of tubular structures was analyzed by phase-contrast microscopy. Primary mouse peritoneal cells were isolated by standard methods using 10- to 12-week-old BALB/C mice. The nonadherent cells were removed after 3 and 7 days of culture, and the cells were further cultured for 14 days, at which time conditioned media (100 μL) derived from RAW cells expressing PTN/GFP (E) or GFP (F) were added to cells for 48 hours. For the adsorption experiment, the conditioned media were preincubated with 20 μg of a goat anti-human PTN polyclonal antibody (Calbiochem; H) or goat IgG (G) at 37°C for 30 minutes. The media were then incubated with agarose–anti-goat antibody for 30 minutes at room temperature to remove the complex before addition to the cells.
To determine whether PTN affects the phenotype of primary monocytes, peritoneal macrophages were isolated from mice and cultured in a fibrin gel in the presence of conditioned media derived from either RAW cells expressing PTN/GFP or GFP. PTN induced the formation of capillary-like structures in the primary cells (Figure 3E), whereas cells exposed to the GFP-derived media did not form such structures (Figure 3F). To assess whether the activity found in the conditioned media is specifically related to PTN, the media were preabsorbed with an anti-PTN neutralizing antibody or an isotype-matched nonspecific antibody before its addition to cells. The nonspecific antibody had no effect on capillary formation (Figure 3G), whereas the anti-PTN neutralizing antibody markedly downregulated capillary formation (Figure 3H).
In Vivo Studies
The quail CAM was used to determine whether the transdifferentiated cells incorporate into blood vessels. Fertilized Japanese quail eggs were cultured ex-ovo at E3, and then RAW cells expressing PTN were transplanted onto the surface of the CAM at E7 (see online supplement). RAW cells or 293 cells expressing GFP were used as controls in addition to PBS. After 3 days, at E10 (see online supplement), CAMs were analyzed by fluorescence and confocal microscopy.
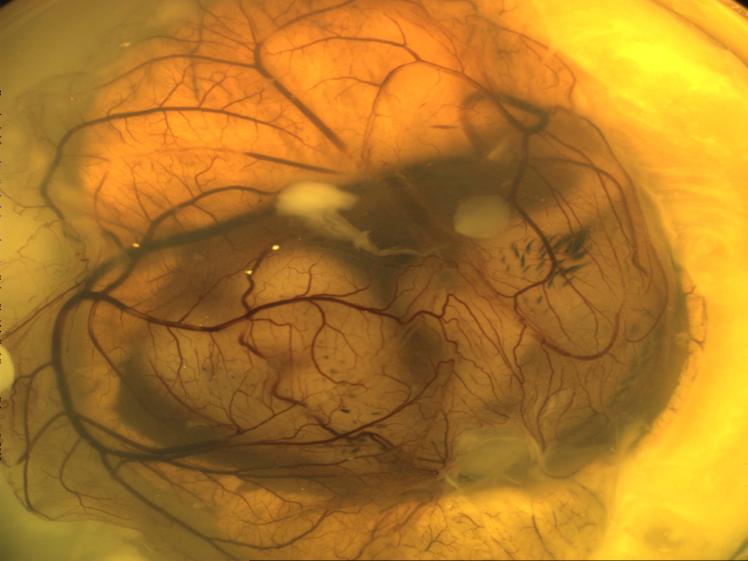
RAW cells expressing PTN/GFP integrated into large and small CAM blood vessels at various branches in 9 of 12 quail embryos (Figure 4A; see online supplement). In contrast, RAW cells expressing GFP did not get incorporated into any blood vessels of the CAM (Figure 4B). To further demonstrate the specificity of the incorporation of RAW cells, the confocal image of the newly formed fluorescent-labeled blood vessels was overlapped with a differential interference contrast image. This improved the contrast, and the incorporation of cells into the blood vessels was examined in the context of a full view of the CAM vasculature. Although the expression of GFP per se was insufficient for the incorporation of RAW cells into the CAM (Figure 4C), expression of PTN was sufficient for the integration of cells into CAM vasculature (Figure 4D). This integration generated chimeric quail–mouse blood vessels.
Figure 4.
PTN-expressing monocytic cells specifically incorporate into blood vessels. RAW cells (1×106) expressing PTN/GFP (A and D) or GFP (B and C) were implanted onto the surface of E7 quail CAM and then were incubated further at 37°C for 72 hours, at which time they were fixed in 4% paraformaldehyde/2% glutaraldehyde/PBS. Fluorescent and confocal images of terminal arterial vessels from the middle region of the CAM were acquired in gray scale at a resolution of 13 μm per pixel. The fixed CAMs with fluorescence images overlapped with differential interference contrast (C and D) images at ×4 magnification.
However, the CAM assay did not reveal whether the cells incorporated into already established or developing blood vessels. To determine this, RAW cells expressing either PTN/GFP or GFP were injected intracardially into stage 16-17 chicken embryos. At this stage, the chicken brain and ocular system are being developed, whereas their cardiovascular system is already established. The embryos were collected 2 to 3 days after injection, fixed, and sectioned (n=10 embryos/cell types). The sections were stained with either anti-GFP or anti–Tie-2 antibodies. In embryos injected with RAW cells expressing PTN/GFP, most of the positive staining appeared along the developing vessels in the head, eyes, and intersomitic regions (Figure 5, PTN panel). In contrast, embryos injected with GFP-expressing RAW cells did not stain (Figure 5, GFP panel). This demonstrates that only the RAW cells expressing PTN had the ability to incorporate into developing blood vessels.
Figure 5.

PTN-expressing cells specifically integrate into developing vasculature. PTN/GFP- or GFP-expressing RAW cells (1 to 2×105 cells in 2 to 4 μL PBS) were injected into the hearts of stage 16-17 chick embryos (10 embryos/cell types). Embryos were killed 2 to 3 days after injection, fixed, embedded in OTC, and frozen sections were cut and stained with anti-GFP polyclonal antibodies (Santa Cruz Biotechnology) or anti–Tie-2 antibodies. The immunopositivity (brown color) was observed in embryos injected with RAW cells expressing PTN/GFP. The positivity localized along the developing blood vessels in the head, eyes, and intersomitic region (right panel). In contrast, immunostaining of embryo injected with RAW cells expressing the GFP gene show no staining (left panel). In some cases, faint staining was detected around the amniotic cavity.
Finally, the ability of transdifferentiated cells to improve blood flow into ischemic tissue was measured. Hindlimb ischemia was induced in BALB/C mice followed by the injection of RAW cells expressing PTN 1 day after surgery. Laser Doppler perfusion imaging monitored blood flow at days 7, 14, and 21 after surgery. BALB/C background mice were selected for these experiments because RAW cells are congenic to this mouse strain, thus avoiding a potential graft-versus-host complication. Figure 6A shows that blood flow recovery at 7 days after surgery was significantly higher in mice injected with PTN-expressing RAW cells when compared with control mice injected with GFP-expressing RAW cells. Fluorescence images of frozen sections from the mice injected with the PTN/GFP-RAW cells show that the cells were incorporated into blood vessels of ischemic hindlimb (Figure 6B), whereas such incorporation was not detected in the control mice (data not shown). Cumulative laser Doppler perfusion imaging data show that mice injected with cells expressing PTN had 60±3% increase in blood flow at 7 days, 50±4% at 14 days, and 30±3% at 21 days compared with control animals injected with PBS at similar time points (Figure 6C).
Figure 6.
PTN-expressing monocytic cells improve blood flow into ischemic murine hindlimb. BALB/C male mice (Jackson Laboratories; 20 to 25 g) were sedated, and the left femoral artery was ligated with 8-0 silk sutures at its proximal origin from the iliac artery and distally at the bifurcation into the popliteal and saphenous arteries to induce mild ischemia. RAW cells expressing PTN/GFP (1×106 cells in 200 μL PBS per mouse) were injected intravenously (tail vein) 24 hours after surgery (8 mice per group). Mice injected with 200 μL of PBS were used as controls (8 mice per group). Repeated hindlimb blood flow measurements over the region of interest (from the patella to the midfoot) were obtained at baseline, immediately after surgery, and serially over 3 weeks by laser Doppler perfusion imaging. Perfusion is expressed as a ratio of right (ischemic) to left (normal) limb. Representative color-coded images (red is highest velocity, green intermediate, and blue, lowest velocity), which reflect red blood cell velocity at day 7 after surgery, are shown (A). The top 4 panels show BALB/C mice injected with PBS, and the bottom 4 panels show animals injected with RAW cells expressing PTN/GFP. B, The immunofluorescence image of the frozen section from the ischemic hindlimb 7 days after injection of PTN/GFP cells shows that the cells are incorporated into the artery and veins. C, Cumulative results for the groups of mice monitored for 21 days after surgery are shown graphically as a ratio of blood flow in ischemic limb to that in the nonischemic limb at each time point. The Student t test analysis showed that the increase in the blood flow is statistically significant in the mice group injected with PTN/GFP compared with control groups at all the timer points examined. Values are mean±SE (P<0.05 are considered statistically significant).
Discussion
PTN is produced by activated monocytes in the highly vascularized region of ischemic brain and is thought to promote angiogenesis by stimulating sprouting/proliferation of endothelial cells. However, nothing is known about the autocrine effect of PTN in ischemic tissues (ie, its effect on monocytes/macrophages). These cells exhibit a high degree of plasticity, as demonstrated by their ability to alter their phenotype into functional endothelial cells. We found that activated monocytes/macrophages express PTN, and, in turn, this cytokine interacts with cells leading to the downregulation of monocytic cells markers and upregulation of fully differentiated endothelial cell markers. Real-time PCR analysis showed that the level of expression of these markers is similar to those found in the human coronary artery endothelial cells, suggesting that the PTN-mediated upregulation of endothelial markers is functionally relevant. This idea is further underscored by the expression of αvβ3, an integrin that is required for the interaction of endothelial cells with the matrix and the formation of tube-like structures. The fibrin gel assay confirmed this and further supported the notion that the transdifferentiated cells exhibit the characteristics of functional endothelial cells in vitro. In vivo studies bore out our in vitro findings and show that the transdifferentiated mouse cells incorporate into blood vessels. In the CAM assay, the RAW cells were implanted onto the CAM, which allowed them to distribute throughout the CAM, meaning that the cells had the opportunity to randomly incorporate into any part of the CAM structure. However, they specifically integrated into the CAM vasculature, suggesting that the cells had all the necessary information required for homing into the vascular tree. The differential interference contrast image further supports this notion and further showed that the homing and integration of the implanted cells was specific to the CAM blood vessels. Chicken embryo experiments further supported this specificity of integration and further demonstrated that although the intracardially injected cells distributed throughout the developing embryo, PTN-expressing cells incorporated only into the developing blood vessels of the brain and eye but not into the already established cardiovascular system of the embryo. This ability of transdifferentiated mouse cells to incorporate into developing blood vessels also appears to be a conserved phenomenon because similar results have been reported with human peripheral blood monocytes in which ex vivo–expanded purified CD14 cells exhibited endothelial cell characteristics in vitro and were incorporated into newly formed blood vessels in vivo.6
This PTN-induced transdifferentiation appears to be regulated at the transcriptional level because both the GATA-2 and GATA-3 transcription factors that are known to regulate the endothelial cell markers are also upregulated by PTN in monocytic cells, suggesting that this phenotypic alteration may be related to the nuclear reprogramming of the monocytic cells. This transcriptional regulation of the endothelial commitment of monocytic cells by PTN may not be related to the pluripotency characteristics of cells because both THP-1 and RAW cells did not express CD133 and Oct-4, 2 well-known markers of stem/progenitor cells. In addition, primary mouse peritoneal macrophages acquired endothelial cell characteristics when exposed to PTN. Together, these data suggest that PTN has the ability to alter the phenotype of fully differentiated monocytes/macrophages into fully differentiated endothelial cells. This phenotypic modulation is consistent with the classical definition of transdifferentiation (ie, an alteration of the phenotype of 1 fully differentiated cell type into another fully differentiated phenotype).24
The ability of monocytes/macrophages to transdifferentiate into endothelial cells does not seem to be related to their proliferative state. Resting primary human peripheral blood monocytes are known to transdifferentiate into endothelial cells.4–14,25 Similarly, we found that PTN induces transdifferentiation of mouse peritoneal cells, a nonproliferating cell type. The proliferating human THP-1 cells and mouse RAW cells also transdifferentiated into endothelial cells. Together, these data suggest that the ability of monocytes to transdifferentiate into endothelial cells is independent of their proliferative activity, suggesting that tissue macrophages found in chronically inflamed tissues may be able to transdifferentiate into endothelial cells in the presence of PTN.
In addition to primary cells, we used THP-1 and RAW clonal cells to investigate the transdifferentiation activity of PTN for 2 reasons. First, these cells are replicating, and therefore they allowed us to label them with GFP and track their fate in vivo. Second, these cells are a homogeneous population of differentiated cells, and they are not contaminated with other cell types. This allowed us to exclude the potential contribution of contaminating cells to the PTN-induced transdifferentiation of monocytes, a possibility that cannot be excluded when primary monocytes are used. In addition, the use of clonal cells excludes cell fusion between 2 cell types as a potential mechanism for PTN-mediated transdifferentiation.
Monocytes/macrophages are known to transdifferentiate into endothelial cells. However, factor(s) that regulate this event remain unknown. PTN is expressed by activated monocytes/macrophages in the highly vascularized regions of ischemic brain. We offered evidence that PTN produced by macrophages interacts with them in an autocrine fashion, coaxing the cells to transdifferentiate into functional endothelial cells. These transdifferentiated cells have the ability to increase blood flow into ischemic tissue. Thus, in addition to previously reported angiogenic activity, our data identified a novel activity for PTN that could be partly responsible for the neovascularization of inflamed tissues.
Methods
Cell culture
All the cell lines including human THP-1 and mouse RAW 264.7 monocytic cell lines were obtained from American Type Culture Collection and grown according to their instruction. Mouse peritoneal macrophages were isolated from BALB/C mice and cultured according to standard protocols. Human aortic endothelial cells and smooth muscle cells were obtained from Cell Applications, INC. and cultured according to their recommendation.
Cloning of PTN
We used the full-length human PTN open reading frame (accession # NM_002825) to clone this gene. The PTN cDNA was generated by reverse transcription of human brain polyadenylated mRNA (Clontech) and amplification via polymerase chain reaction with specific primers for PTN (5′AAAATGCAGGCTCAACAGT and 5′TGTTTGCTGATGTCCTTT). The PCR product was cloned into the pCRII-TOPO vector (Invitrogen) and five clones were selected for further analysis. The nucleotide sequence analysis of these clones revealed that they contained full-length PTN cDNA (3 clones of sense and 2 clones of anti-sense orientations). To further validate the sequence veracity, it was electronically translated into a protein using the ExPASY Translate tool program and its molecular weight was determined by the ExPASY Compute pI/Mw tool program. The theoretically translated product was composed of 136 amino acids with a molecular weight of 15.3 kDa and a pI=10.3. We concluded that the cloned cDNA sequence matched the full-length PTN nucleotide and amino acid sequences found in the GenBank database. We subcloned this gene into a retroviral vector in order to transduce monocytic cells.
Generation of retroviral vectors, retrovirus production, and the infection of monocytic cells
We constructed a bicistronic retroviral vector for our in vitro experiments. The bicistronic retroviral vector was constructed using the pLP-EGFP-C1 plasmid (Clontech) containing the enhanced green fluorescent protein (GFP) gene under the control of the CMV promoter. The PTN cDNA was positioned downstream to the CMV promoter. Next, we cloned an internal ribosomal entry site (IRES) sequence downstream of PTN and upstream of GFP in order to generate the bicistronic retroviral vector. The IRES in this vector permits simultaneous expression of PTN and GFP from one mRNA. This bicistronic retroviral vector has several advantages over monocistronic vectors: 1] it allows us to follow PTN gene expression in infected cells in vitro and in vivo by monitoring for GFP expression, 2] PTN translation occurs independently of GFP translation allowing for the secretion of PTN from cells while GFP remains in the cells, and 3] transduced cells can be isolated by FACS.
All retroviral expression plasmids were constructed using the pLP-C1-IRES-GFP (Clontech) retroviral vector and standard molecular biology techniques. In these cassettes, transcription is initiated by promoter sequences within the viral 5′ long terminal repeat (LTR) and terminated by polyadenylation sequences within the 3′ LTR. Translation of the first (PTN) and second (GFP) cistrons from a single mRNA proceeds by ribosome binding to the 5′ Cap and IRES sequences, respectively. The full-length cDNA of human PTN was cloned into the BamH/Notl sites of pLP-C1-IRES-GFP. The retrovirus was packaged using a 293 packaging cell line provided by Clontech. After transfection of the packaging cells, the medium was changed at 10 hrs. and 24 hrs. after transfection. Virus collected between 24 and 48 hrs. after transfection was used for infection. Retroviral titers between 1×106 and 2×107 cfu/ml were determined by limiting dilution with NIH 3T3 cells. For infection, 4×105 human THP-1 or mouse RAW monocytic cells were seeded in 25-cm2 flasks 24 hrs. before infection in normal growth medium (DMEM/10%FBS) to obtain exponentially growing cultures. The medium was replaced with 4 mls. of retroviral supernatant (approximate MOI 2.5-25 cfu/cell) supplemented with 4 pg/ml polybrene. After 12 hrs, retroviral supernatants were removed and replaced with fresh normal medium for 48 hrs. Under these conditions, no apparent toxicity was observed in cultured cells after a 12 hr. exposure to retroviral supernatants containing polybrene.
Next, we asked whether the GFP reporter gene expression could be used to isolate distinct populations of PTN-expressing cells and if gene expression is maintained over multiple passages of cultured cells. Cultured THP-1 or RAW monocytic cells were infected with either the PTN-IRES-GFP virus or the control IRES-GFP virus. Polyclonal populations of cells (3×105 each) expressing low or high levels of the GFP reporter gene were then isolated by flow cytometry. Immediate “post-sorting” analysis confirmed the isolation of distinct populations of cells based on their GFP expression (not shown). Cells were subcultured for an additional passage and analyzed for the expression of the GFP reporter gene by flow cytometry and for the expression of PTN by Western blot analysis. The sorted cells maintained their relative expression levels of GFP, and there was an excellent correlation between GFP and PTN expression. We concluded that GFP reporter gene expression could be used to isolate distinct populations of cells expressing different levels of PTN by flow cytometry.
We confirmed the expression of PTN sense and anti-sense strands in the infected THP-1 and RAW monocytic cells by Northern blot (not shown). We found that unactivated monocytic cells do not express PTN mRNA; however, cells infected with PTN sense or anti-sense strand expressed PTN mRNA (not shown). PTN mRNA was not detected in the cells infected with the GFP control vector. Western blot analysis of cultured media collected from THP-1 and RAW cells infected with the PTN sense strand revealed that the PTN protein is secreted into the media, whereas cells infected with PTN anti-sense strand or control GFP vector did not express the protein (not shown). After confirming the stable expression of PTN in monocytic cells, we used these cells to determine the effect of PTN on the biology of monocytes/macrophages.
In vitro cell culture
All cell lines were obtained from American Type Culture and propagated according to their instructions. Human coronary artery endothelial cells and smooth muscle cells were obtained from (Cell Applications, Inc., San Diego, CA) and cultured according to their recommendations.
RT-PCR and real-time PCR analysis
Total RNA was extracted from cells using QIAGEN Inc. (Valencia, California, USA) RNeasy kits. Extracted RNA was treated with DNase I (Ambion) in the presence of RNasin (Promega) to remove DNA contamination before cDNA synthesis. cDNA was synthesized with oligodeoxythymidylic acid primers (Boehringer Mannheim) and Superscript 11 reverse transcriptase (Life Technologies, Inc.). The cDNA primers and PCR conditions for the amplification of the endothelial cell markers are described in this supplement. Real-time PCR (TaqMan) analysis was performed on a Perkin-Elmer/Applied Biosystems Prism 7700 Sequence Detector. Matching primers and fluorescence probes were designed for each of the genes according to the Primer Express program provided by Perkin-Elmer/Applied Biosystems.
To quantitate the amount of specific mRNA in the samples, a standard curve was generated for each run using the plasmid containing the gene of interest (dilutions ranging from 20 to 2 × 106 copies). In addition, a standard curve was generated for GAPDH ranging from 200 fg to 2 ng. This enabled the standardization of the initial RNA content of a tissue relative to the amount of GAPDH.
Histology
The immunostaining of human atherosclerotic plaques and cultured cells was performed essentially as we have described before 2. For the immunostaining of cultured cells, cells were cultured onto poly-L-lysine coated cover slips using the culture media described previously. The cells were fixed in 4% paraformaldehyde and permealized with 0.1% Tween-20 in PBS. The non-specific binding sites were blocked by 5% BSA in PBS. The primary and secondary antibodies were diluted in Dako dilution buffer (Dako, Carpinteria, CA) and added to the cells. Dako Liquid DAB Substrate-Chromogen System was used for color development. For fluorescent immunostaining the Alexa Fluor 633 goat anti-rabbit antibody and Alexa Fluor 568 goat anti-mouse antibody were obtained from Molecular Probes Inc. (Eugene, Oregon).
Fibrin gel assay
Fibrin gels were prepared essentially as described 3. Briefly, human fibrin matrices were prepared by the addition of 0.1 U/ml thrombin to a mixture of 5 U/ml factor XIII (final concentrations), 2 mg fibrinogen, 2 mg Na-citrate, 0.8 mg NaCl and 3 pg plasminogen per ml. RPMI 1640 medium then 600 μl of this mixture was added to the wells of 24-well plates. After clotting, the fibrin matrices were soaked with media supplemented with serum to inactivate the thrombin. THP-1 or RAW cells or primary monocytes were seeded on the fibrin matrix for 24-48 hrs. The formation of tubular structures was analyzed by phase-contrast microscopy.
Chicken chimera assay
PTN/GFP or GFP transduced RAW cells (in 2 μl PBS) were injected into the heart of stage 16-17 chick embryos with glass needles 4. Embryos were harvested 2-3 days after injection and fixed with 2% paraformaldehyde at 4 °C for 10 min. The embryos were frozen in OCT, sections were cut at 6-7 μm and stained with anti-GFP and anti-Tie-2 antibodies as described above. A total of 10 embryos were used per infected cell type.
Mouse hind limb ischemia model
BALB/C mice (Jackson Labs) were sedated and a longitudinal incision was made on the medial side of the thigh inferior to the inguinal ligament to a point proximal to the patella. The left femoral artery was ligated at its proximal origin from the iliac artery and distally at the bifurcation into the popliteal and saphenous arteries. The unligated right contralateral artery was used as a control. RAW cells expressing PTN/GFP or GFP were injected into seven sites (10 μg/site) of each hind limb (for all treatment groups). After the indicated times, measurements of the ischemic (left): normal (right) limb blood flow ratio were performed using a Laser Doppler Perfusion Image (LDPI) analyzer (Perimed Inc, Stockholm, Sweden). To minimize data variables due to ambient light and temperature, the LDPI index was expressed as the ratio of left (ischemic) to right (nonischemic) limb blood flow. Differences between treatment groups were determined by ANOVA, followed by the Duncan test when the main effect was significant (P < 0.05). Animal experiments were approved by the Cedars-Sinai’s Institutional Animal Care and Use Committee.
RT-PCR analysis
Reverse Transcription (RT)-PCR Conditions
RT and activation: 30°C for 30 min, 95°C for 15 min.
Amplification: 94°C for 1 min, 55°C for 1 min, 72°C for 1 min.
Final extension: 72°C for 10 min.
Optimal Cycle Numbers
Optimal cycle numbers were determined for each gene to ensure that conditions were in the linear range of PCR amplification:
PECAM1: 28 cycles
VE-cad: 32 cycles
CD34: 30 cycles
Flk-1: 31 cycles
Tie-2: 31 cycles
eNOS: 37cycles
VWF: 32cycles
GATA-2: 33 cycles
GATA-3: 29 cycles
OCT-4: 31 cycles
GAPDH: 24 cycles
Primer Sequences
PECAM1:
GCTGTTGGTGGAAGGAGTGC/GAAGTTGGCTGGAGGTGCTC
VE-Cad:
CCGGCGCCAAAAGAGAGA/CTGGTTTTCCTTCAGCTGGAAGTGGT
CD34:
TGAAGCCTAGCCTGTCACCT/CGCACAGCTGGAGGTCTTAT
Flk-1:
CAACAAAGCGGAGAGGAG/ATGACGATGGACAAGTACCC
Tie-2:
CCTTAGTGACATTCTTCC/GCAAAAATGTCCACCTGG
eNOS:
ACTTCTGCGCCTTTGCTC/TGTCCAGGAAGAAGGGGTGAGA
VWF:
GGAAGACCCAGTGCTGTGAT/GTCTTCCTGCACTCCAGCTT
GATA-2:
CCCTAAGCAGCGCAGCAAGAC/TGACTTCTCCTGCATGCACT
GATA-3:
ACCCCACTGTGGCGGCGAGAT/CACAGCACTAGAGACC
OCT-4: GAGAACAATGAGAACCTTCAGGAGA/TTCTGGCGCCGGTTACAGAACCA
GAPDH:
AGCCACATCGCTCAGACACC/GTACTCAGCGGCCAGCATCG
Results
Past studies have shown that PTN is expressed by activated macrophages in the highly vascularized region of ischemic rat brain 5,6, suggesting that activated monocytes/macrophages express PTN in ischemic tissue. To determine whether activated cultured monocytes/macrophages express PTN, mouse peritoneal macrophages and the RAW monocytic cell line were activated by TNF-α and then PTN expression was investigated by Northern blot 7. The untreated peritoneal cells (Fig. I, lane 1) and RAW cells (lane 4) did not express PTN mRNA; however, addition of 25 ng/ml TNF-α markedly up-regulated PTN expression in the peritoneal (lane 2) and RAW (lane 3) cells. In sharp contrast, addition of the cytokines to mouse smooth muscle cells (lanes 5 and 6) had no effect. These data demonstrate that PTN is not expressed by resting monocytes/macrophages; however activated cells express PTN. The released PTN can affect surrounding cells, a paracrine effect, and/or affect monocytes/macrophages, an autocrine effect. We examined the autocrine effect of PTN.
Fig. I.
Expression of PTN by activated monocytes/macrophages. Untreated mouse peritoneal macrophages (lane 1 and PTN-transduced mouse RAW cells (lane 4) were cultured in RPMI+10% fetal bovine serum. In some studies, the media was supplemented with or 25 ng/ml TNF-α (lane 2, peritoneal cells; lane 3, RAW cells) for 6 hrs. For comparison, mouse aortic artery smooth muscle cells, passage 4 (lane 5) or these cells treated with TNF-α (lane 6) were used. The blot was probed with the PTN cDNA probe (top panel). To control for loading, the blot was re-probed with β-actin (lower panel).
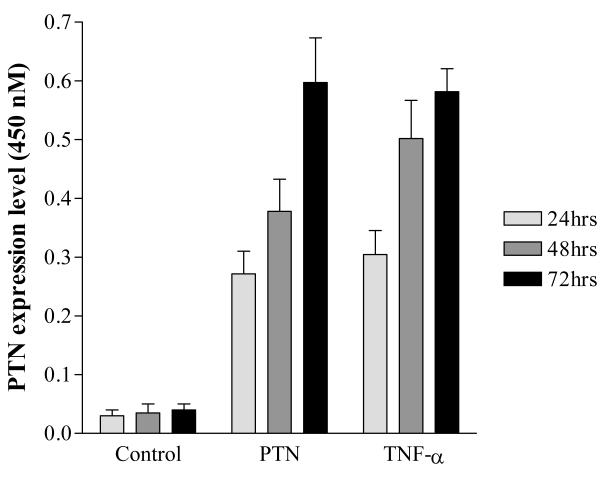
Monocytes/macrophages display a high degree of plasticity as demonstrated by their ability to transdifferentiate into endothelial cells8-24. Therefore, we hypothesized that PTN may affect monocytes/macrophages by altering their phenotype. To investigate this, we had two options: adding exogenous recombinant PTN to cells and investigate its impact on cell phenotype or to generate monocytes that stably express PTN and then determine cell phenotype. We did not adopt the first approach for two reasons: 1), the exogenous PTN may not accurately replicate the full spectrum of activity of the endogenously produced cytokine, and 2) the recombinant PTN does not retain the full range of activities of native protein 1. Therefore, we elected to clone PTN into a retroviral vector and generate stably transduced monocytic cells expressing the cytokine. To determine the biological relevance of retrovirally-produced PTN, we compared the expression level of PTN in activated peritoneal macrophages with those of retrovirally-transduced RAW monocytic cells. The mouse peritoneal macrophages were left untreated or treated with TNF-α and the media were collected 24, 48, and 72 hrs after treatment. The transduced RAW cells were cultured in RPMI defined media and induced to differentiate into macrophages by the addition of 25 nM PMA for 24 hrs. The media were then replaced with fresh media and then collected 24, 48, and 72 hrs later. The PTN levels in the collected media were measured by ELISA. Consistent with the Northern blot data, we found that resting peritoneal cells (Fig. II, ControlP) and untreated RAW cells expressed little, if any, PTN (Fig.II, ControlR). However, transduced RAW cells (Fig. II, PTN) or activated peritoneal cells (Fig. II, TNF-α) secreted similar levels of PTN in a time-dependent fashion. These results confirmed the Northern blot data and in addition showed that the level of secreted PTN by the transduced cells is similar to that of activated peritoneal macrophages, suggesting that the transduced cells produce biologically relevant levels of PTN. As shown in the paper, this level of PTN can alter phenotype of monocytic cells.
Fig. II.
Representative expression levels of secreted PTN. PTN-infected RAW cells (1×105) were cultured for 24, 48, and 72 hrs. and media were collected (PTN). The mouse peritoneal macrophages (1×105) were exposed to 25 ng/ml of TNF-α for 24, 48, and 72 hr, and the media were collected. The collected media from both groups were analyzed by standard ELISA assay. Media collected from uninfected, unstimulated RAW cells (1×105), 24, 48, and 72 hrs. after plating were used as control (Control). The data represent averages of triplicate determinations.
To validate the semi quantitative RT-PCR data, quantitative real-time PCR analysis was performed using primer sets specific for CD68. The data was obtained by comparing fold-induction normalized to the same gene from uninfected THP-1 RNA and with GAPDH. The expression of CD68 was down-regulated approximately 6.4- to 7.6-fold in the PTN expressing THP-1 cells compared to THP-1 cells expressing anti-sense PTN or GFP, or uninfected THP-1 cells treated or untreated with PMA (Fig. III). The level of CD68 expression in PTN-expressing THP-1 is close to that of negative control human endothelial cells.
Fig. III.
Amplification plot for the expression of CD68 expression in the monocytic cells. Oligonucleotide primer pairs for CD68 gene and an oligonucleotide probe labeled with a reporter fluorescent dye at the 5′ end and quencher dye at the 3′ end were designed using Oligo 4.0 software (National Bioscience, Plymouth, MN). Total RNA with DNase I treatment was used to synthesize first-stand cDNA with RT (GIBCOBRL) and oligo(dT) 15 Primer (Promega). Total RNA (50 ng) was added to a 50 μl reverse transcriptase-polymerase chain reaction (RT-PCR) reaction mixture according to the manufacturer’s protocol (Roche Molecular Systems). The products of the RT reactions was used to seed real-time PCR by using an ABI Prism 7700 Sequence Detector by comparing with GAPDH (internal control) and individual standard curve performed in triplicate. The thermal cycling conditions included one cycle at 48°C for 30 minutes, one cycle at 95°C for 10 minutes, 40 cycles at 95°C for 15 s, annealing at 60°C for 1 minute, and a final hold at 25°C for 2 minutes. Standard curves for the expression of each gene were generated by serial dilution of a standard preparation of total RNA isolated from cultured monocytic or endothelial cells.
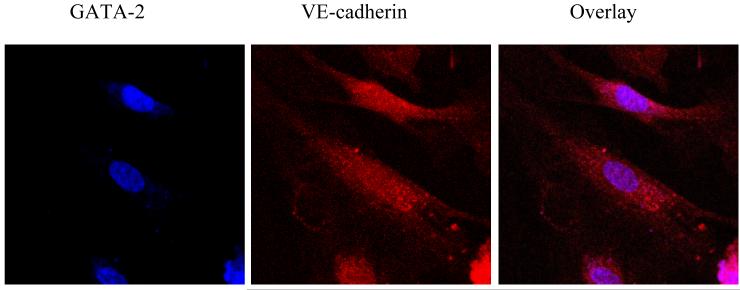
Additional analysis was performed to determine the relationship between the distribution of transcription factors and endothelial cell markers by immunostaining and confocal microscopy in the transdifferentiated cells. VE-cadherin expression was found to be localized on the surface of PTN-expressing RAW cells (Fig. IV, red color) while GATA-2 expression was concentrated in the nucleus (blue color). The overlay showed the co-expression of the two endothelial cell markers. Such an expression pattern was not detected in cells infected with the GFP control vector (data not shown). Taken together, the PCR results, immunostaining data, and FACS analysis demonstrate that PTN regulates the expression of endothelial cell markers in monocytic cells, possibly at the transcriptional level.
Fig. IV.
For confocal microscopy, the secondary antibodies (Alexa Fluor 633 goat anti-rabbit antibody (blue) and Alexa Fluor 568 goat anti-mouse antibody (red)) were used at 1:500 dilutions, as recommended by Molecular Probes. After the indirect immunolabeling, cells were mounted in Fluoromount GTM (SouthernBiotech) and examined with either a conventional fluorescence microscope (Nikon) or a Zeiss LSM 510 confocal microscope.
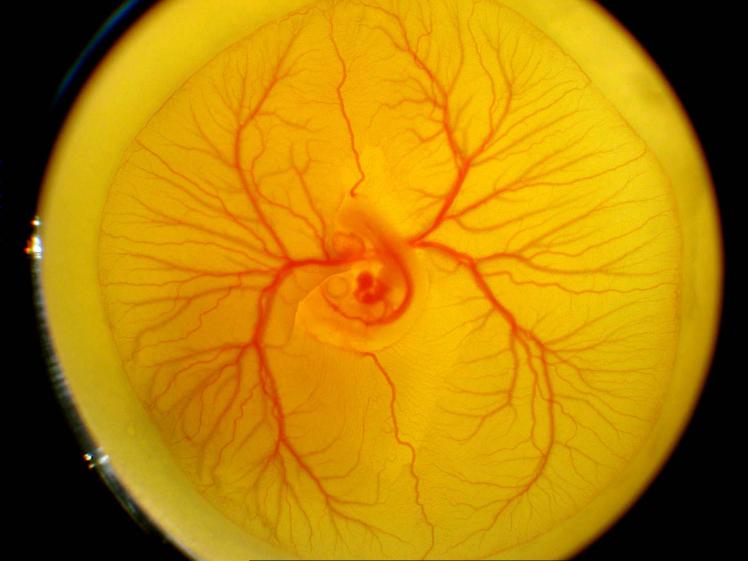
The ability of PTN-expressing cells to incorporate into blood vessels was determined by implanting transfected cells onto the Quail chorioallantoic membrane. The quail eggs were cultured (Fig. V, panel A), and then at E7 RAW (panel B) cells expressing PTN/GFP or GFP were implanted onto the surface of the CAM and then the CAMs were incubated. After 56 hrs. (panel C), CAMs were harvested fixed and examined by fluorescence microscopy. Implantation of PTN/GFP-RAW cells onto the CAM led to incorporation of cells into CAM vasculature (panel D). The implantation of mouse endothelial cells expressing GFP onto the CAM was used as a positive control (panel E).
Fig. V.
Quail chorioallantotic membrane (CAM) assay. Fertilized eggs of Japanese quail were cultured at 37 °C under ambient atmosphere, for 56 hours, and then opened at embryonic day 3 (panel A) after incubation of the eggs and cultured further at 37°C in 6-well plates. At E7 (Panel B), 1×106 RAW cells in prewarmed PBS (200 μl) were applied in drops to the surface of each CAM. The embryos were incubated further at 37°C for 72 hours, at which time they were harvested (panel C), fixed in 4% paraformaldehyde/2% glutaraldehyde/PBS. Panel D and E show fluorescent Image of CAM implanted with RAW cells expressing PTN/GFP or mouse endothelial cells expressing GFP, respectively.
Acknowledgments
This work was supported by grants from the National Institutes of Health (ROI HL50566), Eisner Foundation, Heart Funds, and Women’s Cancer Funds of Entertainment Industry Fund. B.G.S. is an established investigator of American Heart Association.
Contributor Information
Behrooz G. Sharifi, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif.
Zhaohui Zeng, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Lai Wang, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Lei Song, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Haiming Chen, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Minghui Qin, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
M. Rocio Sierra-Honigmann, Department of Surgery, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Sebastian Wachsmann-Hogiu, Department of Surgery, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif..
Prediman K. Shah, Atherosclerosis Research Center, Division of Cardiology, Burns and Allen Research Institute, Cedars-Sinai Medical Center, Los Angeles, Calif.
References
- 1.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem (Tokyo) 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 2.Takeda A, Onodera H, Sugimoto A, Itoyama Y, Kogure K, Rauvala H, Shibahara S. Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience. 1995;68:57–64. doi: 10.1016/0306-4522(95)00110-5. [DOI] [PubMed] [Google Scholar]
- 3.Yeh HJ, He YY, Xu J, Hsu CY, Deuel TF. Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J Neurosci. 1998;18:3699–3707. doi: 10.1523/JNEUROSCI.18-10-03699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 5.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 7.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 8.Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 9.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 10.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 11.Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K, Sumitran-Holgersson S. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation. 2004;110:3699–3707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003;100:2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005. 2005;115:2316–2319. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons-Wingerter P, Lwai B, Yang MC, Elliott KE, Milaninia A, Redlitz A, Clark JI, Sage EH. A novel assay of angiogenesis in the quail chorioallantoic membrane: stimulation by bFGF and inhibition by angiostatin according to fractal dimension and grid intersection. Microvasc Res. 1998;55:201–214. doi: 10.1006/mvre.1998.2073. [DOI] [PubMed] [Google Scholar]
- 16.Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol. 2001;188:359–368. doi: 10.1002/jcp.1121. [DOI] [PubMed] [Google Scholar]
- 17.Lee ME, Temizer DH, Clifford JA, Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991;266:16188–16192. [PubMed] [Google Scholar]
- 18.Gumina RJ, Kirschbaum NE, Piotrowski K, Newman PJ. Characterization of the human platelet/endothelial cell adhesion molecule-1 promoter: identification of a GATA-2 binding element required for optimal transcriptional activity. Blood. 1997;89:1260–1269. [PubMed] [Google Scholar]
- 19.Jahroudi N, Lynch DC. Endothelial-cell-specific regulation of von Willebrand factor gene expression. Mol Cell Biol. 1994;14:999–1008. doi: 10.1128/mcb.14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan PJ, Tsang D, Pedic CM, Abbott LR, Shinkel TA, d’Apice AJ, Pearse MJ. The human ICAM-2 promoter is endothelial cell-specific in vitro and in vivo and contains critical Sp1 and GATA binding sites. J Biol Chem. 1998;273:11737–11744. doi: 10.1074/jbc.273.19.11737. [DOI] [PubMed] [Google Scholar]
- 21.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 22.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 23.Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 24.Slack JM, Tosh D. Transdifferentiation and metaplasia—switching cell types. Curr Opin Genet Dev. 2001;11:581–586. doi: 10.1016/s0959-437x(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 25.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, Imada T, Iwasaka T. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol. 2002;22:1804–1810. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 26.Koolwijk P, van Erck MG, de Vree WJ, Vermeer MA, Weich HA, Hanemaaijer R, van Hinsbergh VW. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J Cell Biol. 1996;132:1177–1188. doi: 10.1083/jcb.132.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Fang W, Hartmann N, Chow DT, et al. Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer. J Biol Chem. 1992;267(36):25889–25897. [PubMed] [Google Scholar]
- 2.Wallner K, Li C, Shah PK, et al. Tenascin-C Is Expressed in Macrophage-Rich Human Coronary Atherosclerotic Plaque. Circulation. 1999;99(10):1284–1289. doi: 10.1161/01.cir.99.10.1284. [DOI] [PubMed] [Google Scholar]
- 3.Koolwijk P, van Erck MG, de Vree WJ, et al. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J Cell Biol. Mar. 1996;132(6):1177–1188. doi: 10.1083/jcb.132.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000 Nov 2;408(6808):92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 5.Takeda A, Onodera H, Sugimoto A, et al. Induction of heparin-binding growth-associated molecule expression in reactive astrocytes following hippocampal neuronal injury. Neuroscience. 1995 Sep;68(1):57–64. doi: 10.1016/0306-4522(95)00110-5. [DOI] [PubMed] [Google Scholar]
- 6.Yeh HJ, He YY, Xu J, et al. Upregulation of pleiotrophin gene expression in developing microvasculature, macrophages, and astrocytes after acute ischemic brain injury. J Neurosci. 1998 May 15;18(10):3699–3707. doi: 10.1523/JNEUROSCI.18-10-03699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaFleur DW, Fagin JA, Forrester JS, et al. Cloning and characterization of alternatively spliced isoforms of rat tenascin. Platelet-derived growth factor-BB markedly stimulates expression of spliced variants of tenascin mRNA in arterial smooth muscle cells. J Biol Chem. 1994;269(32):20757–20763. [PubMed] [Google Scholar]
- 8.Fernandez Pujol B, Lucibello FC, Gehling UM, et al. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000 May;65(5):287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 9.Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003 Mar 4;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 10.Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003 Nov 18;108(20):2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 11.Schmeisser A, Garlichs CD, Zhang H, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001 Feb 16;49(3):671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 12.Elsheikh E, Uzunel M, He Z, et al. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005 Oct 1;106(7):2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 13.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003 Nov 14;93(10):980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 14.Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002 Oct 8;106(15):2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 15.Kamihata H, Matsubara H, Nishiue T, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol. 2002 Nov 1;22(11):1804–1810. doi: 10.1161/01.atv.0000039168.95670.b9. [DOI] [PubMed] [Google Scholar]
- 16.Nowak G, Karrar A, Holmen C, et al. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation. 2004 Dec 14;110(24):3699–3707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 17.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003 Nov 28;93(11):1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 18.Gulati R, Jevremovic D, Peterson TE, et al. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003 Sep 23;108(12):1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest. 2005 Sep 1;115(9):2316–2319. doi: 10.1172/JCI26354. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005 Sep 1;115(9):2363–2372. doi: 10.1172/JCI23874. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moldovan NI, Havemann K. Transdifferentiation, a Potential Mechanism for Covering Vascular Grafts Grown Within Recipient’s Peritoneal Cavity With Endothelial-Like Cells. Circ Res. 2002 Aug 9;91(3):1e. doi: 10.1161/01.res.0000029424.94320.f4. 2002. [DOI] [PubMed] [Google Scholar]
- 23.Anghelina M, Krishnan P, Moldovan L, et al. Monocytes/Macrophages Cooperate with Progenitor Cells during Neovascularization and Tissue Repair: Conversion of Cell Columns into Fibrovascular Bundles. Am J Pathol. 2006 Feb 1;168(2):529–541. doi: 10.2353/ajpath.2006.050255. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glod J, Kobiler D, Noel M, et al. Monocytes form a vascular barrier and participate in vessel repair after brain injury. Blood. 2006 Feb 1;107(3):940–946. doi: 10.1182/blood-2004-11-4403. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]