This study measured the time taken for setting up the different facets of an international phase III study being conducted in 44 participating countries.
Keywords: Activation, Phase III clinical trials, Ethics committee/institutional review board
Learning Objectives
Discuss methods for improving the efficiency of global clinical trials.
Explain the need for national regulatory authorities and collaborative cancer groups to initiate efforts to quicken the activation process in their countries.
Describe the activation process of phase III studies and its complex and heterogeneous regulation across different geographic and economic regions.
Abstract
Purpose.
This study measured the time taken for setting up the different facets of Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO), an international phase III study being conducted in 44 participating countries.
Methods.
Time to regulatory authority (RA) approval, time to ethics committee/institutional review board (EC/IRB) approval, time from study approval by EC/IRB to first randomized patient, and time from first to last randomized patient were prospectively collected in the ALTTO study. Analyses were conducted by grouping countries into either geographic regions or economic classes as per the World Bank's criteria.
Results.
South America had a significantly longer time to RA approval (median: 236 days, range: 21–257 days) than Europe (median: 52 days, range: 0–151 days), North America (median: 26 days, range: 22–30 days), and Asia-Pacific (median: 62 days, range: 37–75 days). Upper-middle economies had longer times to RA approval (median: 123 days, range: 21–257 days) than high-income (median: 47 days, range: 0–112 days) and lower-middle income economies (median: 57 days, range: 37–62 days). No significant difference was observed for time to EC/IRB approval across the studied regions (median: 59 days, range 0–174 days). Overall, the median time from EC/IRB approval to first recruited patient was 169 days (range: 26–412 days).
Conclusion.
This study highlights the long time intervals required to activate a global phase III trial. Collaborative research groups, pharmaceutical industry sponsors, and regulatory authorities should analyze the current system and enter into dialogue for optimizing local policies. This would enable faster access of patients to innovative therapies and enhance the efficiency of clinical research.
Implications for Practice:
To our knowledge, this represents the first study evaluating different prospectively collected timelines in the activation process of an international phase III study conducted across different geographic and economic regions. We acknowledge that using activation timelines from a single phase III study limits definitive conclusions regarding the “proficiency” of specific geographic or economic regions to deal with clinical trials activation. Nevertheless, the performed comparisons demonstrated significant delays and bottlenecks across all economic and geographical regions. This study serves as a reference point in drawing attention to the need for improving the efficiency of global clinical trials. It should encourage similar analyses in upcoming clinical studies and motivate national regulatory authorities and collaborative cancer groups to initiate efforts to quicken the activation process in their countries.
Introduction
Clinical trials represent the main bridge linking biomedical discoveries to patient benefit. Participation in phase III clinical trials is beneficial for both patients and health care providers. It allows patients to be treated in controlled conditions with interventions likely to be superior to the current standard of care, or safe enough to provide the same magnitude of benefit when superiority is not achieved. Health care providers also benefit from clinical trials by improving their therapeutic armamentarium, gaining experience with novel therapeutic interventions, and directly contributing to the development of science. Importantly, health care systems may also benefit from patients' treatment being reimbursed by clinical trial sponsors instead of the government.
Nonetheless, the participation of physicians and patients in clinical studies is often hindered by bureaucratic impediments, particularly in some areas of the world. The launch of a clinical study is time consuming and influenced by a complex network of multiple oversight bodies with varying objectives and responsibilities [1, 2]. For clinical trials involving several countries, such complexity is likely to be exponentially increased but not well understood. Time intervals from study concept to actual activation have been reported to be as long as 800 days for recently conducted clinical studies [3]. Such delays may directly compromise the validity of a clinical study because new discoveries may make the study's main clinical question outdated. Also, in countries where delays occur, the number of patients accrued will definitely be inferior to other countries where processes are faster, potentially compounding inequities to care.
Efforts to quicken the activation process of clinical studies have been undertaken by institutions such as the National Cancer Institute (NCI) in the United States, with the specific aim of doubling the speed of trial activation [3]. European institutions have also proposed modifications aiming to standardize and optimize the process [4]. In contrast, there is a relative lack of information about how geographical regions outside North America and Europe launch international studies; in particular, there seems to be a lack of concerted effort to speed up study activation.
Clinical studies used to be mainly conducted in North America and Europe. However, over the past decade, they have rapidly expanded to include populations from several regions [5]. For example, Asia and South America together accounted for 9% of the patient population in clinical studies in 2003, but this increased to 18% by 2007 [6]. An analysis of how emerging geographical regions regulate and activate large international phase III studies, as well as a direct comparison to economies or regions with a long tradition and extensive expertise in clinical studies, is a prerequisite for standardizing regulatory processes globally.
The Breast International Group (BIG), in collaboration with pharmaceutical companies, has played a major role in the globalization of breast cancer clinical trials [7]. BIG is a network of 50 breast cancer research groups from around the world. National representatives of these groups have the opportunity to participate in the discussions related to new studies and express local needs, which in turn contribute to the design and launch of studies considered to be of importance to the participating country.
Under the academic umbrella of BIG, the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO; ClinicalTrials.gov identifier NCT00490139) phase III study represents an important opportunity to evaluate how different geographical regions deal with the process of activating clinical studies. ALTTO is a study testing adjuvant chemotherapy with one year of anti-HER2 therapy (lapatinib alone, trastuzumab alone, their sequence or their combination) [8]; it has recruited over 8,300 patients with early-stage breast cancer across 44 participating countries. In this study, we evaluate different aspects of the activation of ALTTO across different geographic and economic regions.
Methods
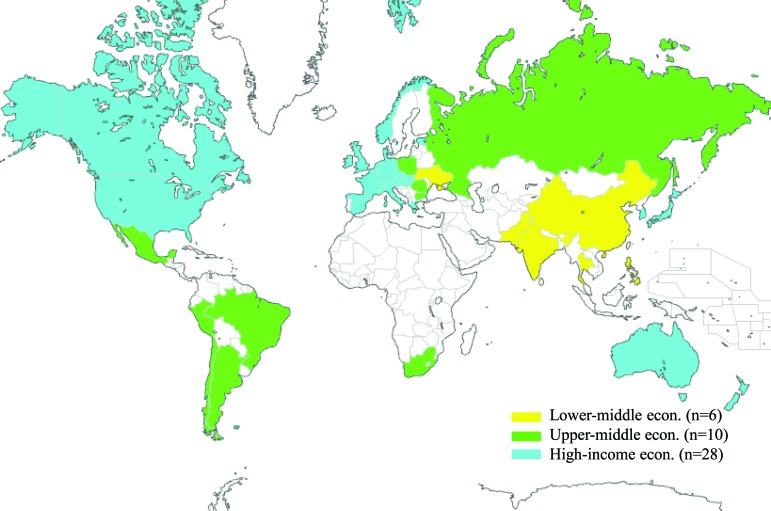
The ALTTO activation timelines in each participating country were recorded by the pharmaceutical industry sponsor of the study and transferred to the Breast European Adjuvant Study Team Data Centre, coordinating the study under the BIG umbrella, in Brussels, Belgium. Missing or conflicting information was retrospectively checked and updated or corrected by the participating centers. Data were compared across geographical regions (Europe, North America, Asia-Pacific, South America, and Africa) and economic regions as defined by the World Bank classification of economies (high, upper-middle, and lower-middle income) where ALTTO was conducted (Fig. 1) [9] .
Figure 1.
Participating countries in the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization study (color-coded according to the World Bank classification of economies).
Timelines
The following time intervals were evaluated for each participating country:
Time to regulatory authority (RA) approval, defined as the time interval between protocol submission by the sponsor to the RA and its approval
Time to ethics committee or institutional review board (EC/IRB) approval, defined as the interval from submission by the study sponsor to EC/IRB and its approval (for participating countries with multiple EC/IRB approval intervals, the average of time intervals was used for comparisons)
Time from RA to first patient, defined as the interval between RA approval and first patient randomized into the study in that particular country
Time from EC/IRB to first patient, defined as the interval from first EC/IRB approval to the first patient included in the study in that particular country
Time for a protocol amendment approval (i.e., changes in study design), defined as the time intervals from amendment submission to EC/IRB and RA to its approval
Time from first-to-last patient, defined as the interval from the first patient randomized in the study in a particular country to the last patient randomized in the study
Note that because of changes in the study design, ALTTO in North America recruited for almost 1 year longer than in the rest of the world. The date of last randomized patient outside of North America was selected for the present analysis.
Statistical Analysis
Descriptive statistics including means, medians, and ranges were calculated for the different timelines evaluated. Differences between studied groups were calculated using one-way analysis of variance (ANOVA) following data normalization on square roots of the different timelines obtained. Geographical regions represented by only one participating country (i.e., Africa) were not included in the ANOVA calculations. Differences were considered statistically significant if the p value was <.05.
Results
Protocol Demographics
Of the countries participating in ALTTO, 24 (55%) are located in Europe, 12 (27%) are in the Asia-Pacific region, 4 (9%) are in South America, 3 (7%) are in North America, and 1 (2%) is in Africa. Twenty-eight (64%) of the participating countries have high-income economies, 10 (23%) have upper-middle income economies, and 6 (13%) have lower-middle income economies.
Timeline Analysis
Time to Study Activation Across Geographical Regions
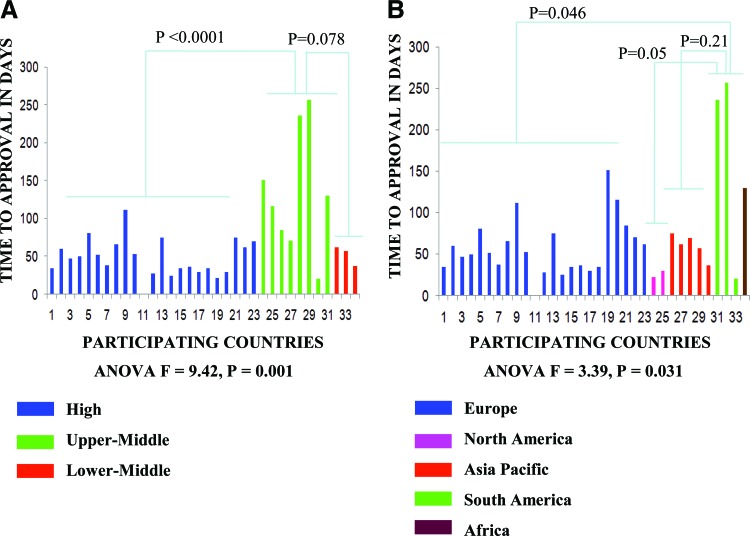
Table 1 summarizes the timelines for study activation across geographical regions. Time to RA approval varied significantly across regions (Fig. 2). South America had an absolute longer time to RA approval (median: 236 days, range: 21–257 days) compared with Europe (median: 52 days, range: 0–151 days), North America (median: 26 days, range: 22–30 days), Asia-Pacific (median: 62 days, range: 37–75 days), and Africa (130 days). The observed difference was statistically significant between group means (F = 3.4; p = .031). Post-hoc analyses showed significance for South America compared to Europe (p = .046) but not when compared to North America (p = .05) or Asia Pacific (p = .21).
Table 1.
Timelines in the activation process of Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization across geographic regions
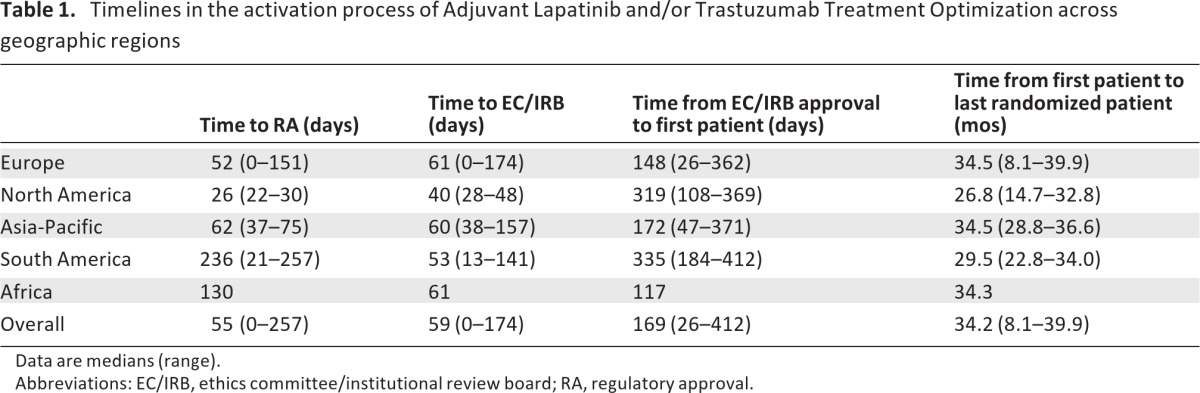
Data are medians (range).
Abbreviations: EC/IRB, ethics committee/institutional review board; RA, regulatory approval.
Figure 2.
Time to regulatory authority approval according to geographical (A) and economic (B) regions.
Time to EC/IRB approval was collected across 698 participating centers. Because of the large number of centers participating through the NCI collaborative group network in the U.S., EC/IRB time intervals were collected only from the 20 highest recruiters among these centers. No statistically significant difference between group means was observed for time to EC/IRB approval (F = 0.86; p = .47). Overall, EC/IRB approval times occurred within a median of 59 days (range: 0–174 days) across geographical regions. No statistically significant difference between groups was observed for the mean time interval of RA approval to first randomized patient across the studied regions (F = 2.5; p = .08).
However, in absolute terms, North America had a longer time interval from RA approval to first patient randomization (316 days) when compared to other regions. This may have occurred because of additional regulatory processes in North America. ALTTO was activated in North America through the North Central Cancer Treatment Group (NCCTG), one of cooperative groups funded by the NCI. NCCTG was required to obtain NCI approval of ALTTO before launching the study, a process that lasted 18.3 months. Of importance, ALTTO activation in North America preceded NCI efforts to make the process of activation of clinical studies quicker and more efficient [8].
Regarding the time interval between first EC/IRB approval and first randomized patient, South America had a statistically significantly longer time interval (median: 335 days, range: 184–412 days) when compared to Europe (median: 148 days, range: 26–362 days; p = .014).
Timelines to Study Activation Across Economic Regions
Table 2 outlines the time intervals for study activation across economic regions. Upper-middle economies required a significantly longer time to RA approval (median: 123 days, range: 21–257 days) than high-income (median: 47 days, range: 0–112 days) or lower-middle income economies (median: 57 days, range: 37–62; Fig. 2. The observed difference was statistically significant between group means (F = 9.4; p = .001). Post-hoc analyses showed significance for upper-middle compared to high-income (p < .0001), but not for upper-middle compared to lower-middle income economies (p = .08).
Table 2.
Timelines in the activation process of Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization across economic regions
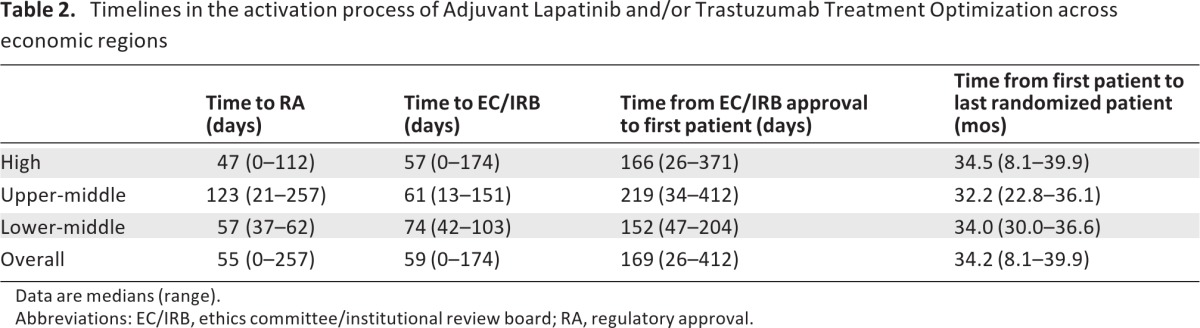
Data are medians (range).
Abbreviations: EC/IRB, ethics committee/institutional review board; RA, regulatory approval.
In line with what was observed for geographical regions, no statistically significant difference between group means was observed for time to EC/IRB approval (F = 0.22; p = .80). The median time interval from RA approval to first randomized patient was 160 days (range: 35–405 days) for high-income, 77 days (range: 34–216 days) for upper-middle income, and 193 days (range: 132–245 days) for lower-middle income economies. The observed difference was statistically significant across group means (F = 3.4; p = .048), and a trend towards longer time interval was observed for high-income in comparison to upper-middle income economies (p = .07). No statistically significant difference between group means was observed for the time interval from first EC/IRB approval to first randomized patient across the studied economic regions (F = 1.16; p = .33).
Timelines to Approve a Study Protocol Amendment
Differences in timelines to approve a study protocol amendment by RA and EC/IRBs were not statistically significant across economic and geographic regions. Overall, the median time spent by RA to approve an amendment was 34 days (range: 8–140 days). No statistically significant difference between group means was observed for time to RA approval across geographical regions (F = 0.55; p = .6) or economic regions (F = 0.26; p = .9). Overall, the median time spent by EC/IRB to approve an amendment was 35 days (range: 13–668 days). Again, no statistically significant difference between group means was observed for time to EC/IRB approval across geographical regions (F = 0.8; p = .5) or economic regions (F = 0.6; p = .6).
Impact of Long Intervals to Study Start on Recruitment Period
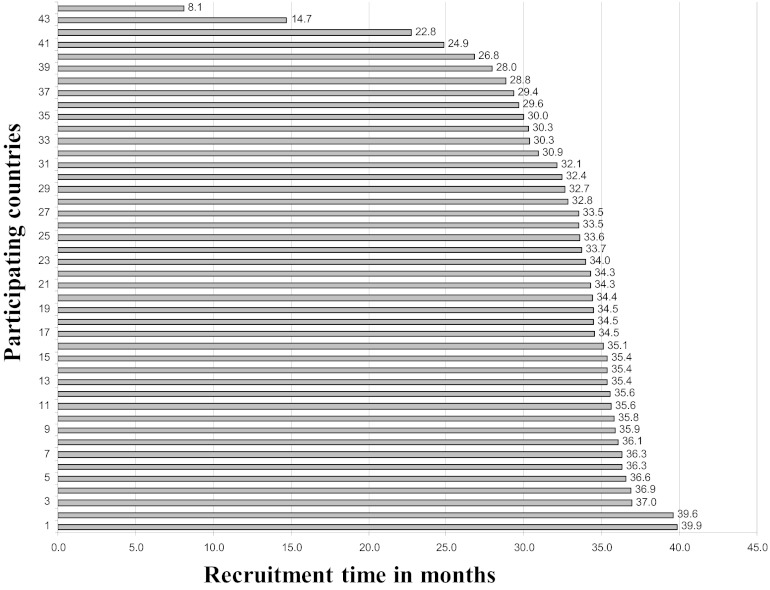
The time intervals from the first randomized patient in each participating country to the last randomized patient in the study (study enrolment closure) were measured for all participating countries and across the defined economic and geographic regions. No statistically significant differences between group means were observed across geographical regions (F = 2.2; p = .1) or economic regions (F = 0.4; p = .7). Figure 3 shows that the time interval from the first randomized patient in each participating country to study enrollment closure varied between 8.1 months and 39.9 months.
Figure 3.
Time interval from the first randomized patient in each country to study enrollment closure.
Discussion
This study reveals long timelines to activate a global phase III study across 44 participating countries. Cross-region comparisons highlighted not only significant variation but, more importantly, homogeneously long time intervals between submission and receipt of approval from RAs, as well as long time intervals between EC/IRB study approval and actual enrollment of patients. By contrast, EC/IRB approval and processing of protocol amendments occurred within reasonable timelines, regardless of geographic and economic region studied.
The major limitation of this study is the attribution of timeline differences to different global regions for a single phase III study without a contemporary study as direct comparator. ALTTO represents one of the largest intervention phase III studies ever conducted in the field of breast cancer (over 8,300 patients), and finding an appropriate study comparator with available activation timelines data proved to be unfeasible.
In consonance with our findings, previous reports have highlighted the need to make study activation more time efficient. In North America, the NCI has created an Operational Efficiency Work Group charged with identifying barriers to speedy protocol timelines [3]. Moreover, previous publications have highlighted the need to optimize the timelines required to activate studies conducted in South America [10]. In the present analysis, similar timelines were observed for EC/IRB activation, regardless of geographical or economic regions. In North America and Europe, attempts to improve EC/IRB efficiency have been previously implemented [4, 11, 12] and may have positively influenced the observed results across these regions.
Unexpectedly, extremely long time intervals from study approval by EC/IRBs to the first randomized patient were observed across all economic and geographical regions. This may have occurred because of the time required to negotiate contracts between the study's sponsor and participating centers, delays in importing the study drug, and/or interest of investigators to refer patients to the study. Previous reports have emphasized the need for standardized language for clinical research contracts between industry sponsors and academic centers, but to our knowledge, there is a lack of progress in this direction [5, 13]. Recently, a study reported on the profile of 1,500 specialty physicians who recruited patients onto clinical studies [14]. Longer periods of time spent with new patients and the presence of students, residents, and multidisciplinary tumor boards to discuss cases were associated with a higher likelihood of referring patients to studies [14]. In ALTTO, however, data about these variables were not prospectively collected, limiting our ability to draw conclusions about their importance.
In addition to being complex to launch, phase III studies are commonly modified by protocol amendments generating new waves of RA and EC/IRB evaluation and approval. In the current analysis, amendment approval timelines were managed homogeneously and without significant delays across study regions. This suggests that barriers other than RA and EC/IRB exist and should be properly addressed. Theoretically, delayed timelines to approve a study may compromise the ability of a participating region to enroll patients into studies. In ALTTO, recruitment times as short as 8 months were observed in certain countries, whereas the average recruitment time was four times higher.
Although this single snapshot of a global phase III study may not reflect the reality of regulatory processes worldwide, it represents a first effort to promote a global discussion about clinical study activation. We hope that this study will help investigators in different regions to identify the bottlenecks in activating multinational trials and to address the problem of clinical trial participation. Improving the efficiency of the activation process would speed up the ability to gather scientific knowledge and evaluate its applicability. Most importantly, it would ultimately benefit the many patients who volunteer to participate in clinical trials. This is the only way to improve treatments for patients with cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
The ALTTO trial is sponsored by GlaxoSmithKline. Collection, analysis, and interpretation of the current data were done independently by an academic group of statisticians (Frontier Science), I.B., and O.M.-F.
We thank the women who participated in the study; the Breast European Adjuvant Study Team Data Centre; the Frontier Science team; the Breast International Group headquarters; the ALTTO steering committee; the Independent Data Monitoring Committee; GlaxoSmithKline; and all the investigators who participated in ALTTO.
Author Contributions
Conception/Design: Otto Metzger-Filho, Evandro de Azambuja, Ian Bradbury, Kamal Saini, José Bines, Sérgio D Simon, Veerle van Dooren, Gursel Aktan, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Jose Baselga, Edith Perez, Martine Piccart
Provision of study materials or patients: Otto Metzger-Filho, Evandro de Azambuja, José Bines, Sérgio D Simon, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Binghe Xu, Jose Baselga, Edith Perez, Martine Piccart
Collection and/or assembly of data: Otto Metzger-Filho, Evandro de Azambuja, Kamal Saini, José Bines, Veerle van Dooren, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Jose Baselga, Edith Perez, Martine Piccart
Data analysis and interpretation: Otto Metzger-Filho, Evandro de Azambuja, Ian Bradbury, Kamal Saini, José Bines, Sérgio D Simon, Veerle van Dooren, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Jose Baselga, Edith Perez, Martine Piccart
Manuscript writing: Otto Metzger-Filho, Evandro de Azambuja, Ian Bradbury, Kamal Saini, José Bines, Sérgio D Simon, Veerle van Dooren, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Binghe Xu, Jose Baselga, Edith Perez, Martine Piccart
Final approval of manuscript: Otto Metzger-Filho, Evandro de Azambuja, Ian Bradbury, Kamal Saini, José Bines, Sérgio D Simon, Veerle van Dooren, Gursel Aktan, Kathleen Pritchard, Antonio Wolff, Ian Smith, Christian Jackisch, Istvan Lang, Michael Untch, Frances Boyle, Binghe Xu, Jose Baselga, Edith Perez, Martine Piccart
Disclosures
Gursel Aktan: GlaxoSmithKline (E, OI); Kathleen I. Pritchard: GlaxoSmithKline, Amgen, Roche, Novartis, Pfizer, Boehringer Ingelheim, AstraZeneca (C/A); Christian Jackisch: GlaxoSmithKline, Roche (C/A, H); Frances Boyle: GlaxoSmithKline (H); Jose Baselga: GlaxoSmithKline (C/A). The other authors indicated no financial relationships.
Section Editor: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex, (O); founder and member of the board of directors for Citizen's Oncology Foundation.
Reviewer “A”: None
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Dilts DM, Sandler AB. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24:4545–4552. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 2.Dilts DM, Sandler AB, Baker M, et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: The Case of Cancer and Leukemia Group B. J Clin Oncol. 2006;24:4553–4557. doi: 10.1200/JCO.2006.06.7819. [DOI] [PubMed] [Google Scholar]
- 3.DeVita VT., Jr The clinical trials system is broken. Nat Clin Pract Oncol. 2008;5:683. doi: 10.1038/ncponc1263. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzbauer AA, Lamby PE, Mutzbauer I, et al. Procedures for ethical review for clinical trials within the EU. BMJ. 2009;338:b1893. doi: 10.1136/bmj.b1893. [DOI] [PubMed] [Google Scholar]
- 5.Glickman SW, McHutchison JG, Peterson ED, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–823. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 6.Malakoff D. Clinical trials and tribulations. Spiraling costs threaten gridlock. Science. 2008;322:210–213. doi: 10.1126/science.322.5899.210. [DOI] [PubMed] [Google Scholar]
- 7.Gnant M, Piccart M, Goldhirsch A, et al. Developing an international network for breast cancer research: The BIG experience. Clin Invest. 2011;1:623–628. [Google Scholar]
- 8.Tomasello G, de Azambuja E, Dinh P, et al. Jumping higher: Is it still possible? The ALTTO trial challenge. Expert Rev Anticancer Ther. 2008;8:1883–1890. doi: 10.1586/14737140.8.12.1883. [DOI] [PubMed] [Google Scholar]
- 9.The World Bank. How we classify countries. [Accessed September 1, 2011]. http://data.worldbank.org/about/country-classifications.
- 10.Lee BL, Liedke PE, Barrios CH, et al. Breast cancer in Brazil: Present status and future goals. Lancet Oncol. 2012;13:e95–e102. doi: 10.1016/S1470-2045(11)70323-0. [DOI] [PubMed] [Google Scholar]
- 11.Klingmann I. EU Directive 2001/20/EC: Implementation of GCP in the conduct of clinical trials. Official Journal of the European Communities. [Accessed September 1, 2011]; http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:121:0034:0044:en:PDF. [Google Scholar]
- 12.The Central Institution Review Board Initiative. [Accessed September 1, 2011]. Available at http://www.ncicirb.org.
- 13.Drazen JM. Institutions, contracts, and academic freedom. N Engl J Med. 2002;347:1362–1363. doi: 10.1056/NEJMe020122. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Keating NL, Potosky AL, et al. A population-based assessment of specialty physician involvement in cancer clinical trials. J Natl Cancer Inst. 2011;103:384–397. doi: 10.1093/jnci/djq549. [DOI] [PMC free article] [PubMed] [Google Scholar]