The efficacy of rituximab monotherapy versus splenectomy was compared in a large series of patients with splenic marginal zone lymphoma. Rituximab was very effective and well tolerated and may be substituted for splenectomy as the first-line treatment of choice.
Keywords: Splenic marginal zone lymphoma, Rituximab, Splenectomy
Abstract
Background.
Treatment of splenic marginal zone lymphoma (SMZL) patients is not standardized. Recent data suggest that rituximab is highly effective and could be considered as initial therapy.
Aim.
To assess the efficacy of rituximab monotherapy in a large series of patients with SMZL and compare these results with splenectomy results.
Methods.
The studied population included 85 patients. Fifty-eight received rituximab at a dose of 375 mg/m2 per week for 6 weeks as induction followed by maintenance at the same dose every 2 months for 1–2 years, whereas 27 patients were treated using splenectomy only.
Results.
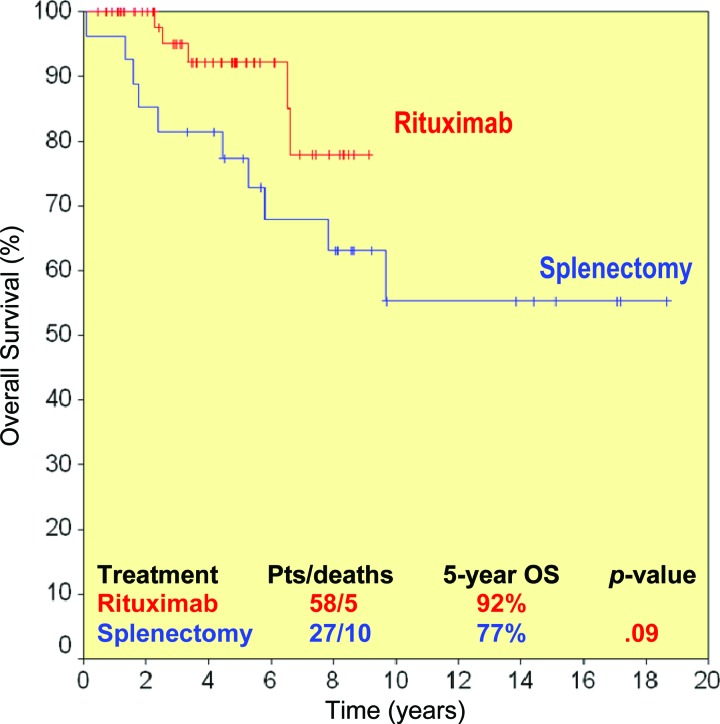
The overall response rate to rituximab 2 months after the end of induction was 95% (complete response [CR], 45%; unconfirmed CR, 26%; partial response, 24%). The median times to hematologic and clinical response were 2 weeks and 3 weeks, respectively. Forty-three of 55 patients already completed the maintenance phase: 28 sustained their initial response, 14 improved their response, and one progressed. Eighty-five percent of splenectomized patients responded, and two were treated with rituximab as consolidation after splenectomy and achieved a CR. The 5-year overall and progression-free survival (PFS) rates for rituximab-treated and splenectomized patients were 92% and 77% (p = .09) and 73% and 58% (p = .06), respectively. Furthermore, maintenance therapy with rituximab resulted in a longer duration of response (at 5 years, PFS was 84% for patients receiving maintenance and 36% for patients without maintenance, p <.0001).
Conclusions.
Rituximab is a very effective and well-tolerated therapy and may be substituted for splenectomy as the first-line treatment of choice for patients with SMZL.
Implications for Practice:
Treatment in splenic marginal zone lymphoma (SMZL) is not standardized. Splenectomy has been considered as the treatment of choice in symptomatic patients. However, this can only result in a partial response. Furthermore, splenectomy is a major surgical procedure with significant morbidity, especially in elderly patients. Recent data, in a relatively small series of patients, suggests that the monoclonal antibody anti-CD20, rituximab, is highly effective with minimal toxicity. In this study, we compare these two treatment modalities in a large number of patients with long follow up. Our data shows that rituximab is associated with better quality of response as almost half of the patients achieved CR in contrast to splenectomized patients, along with better five-year PFS and OS. Furthermore, we stress the importance of maintenance therapy, since it can clearly improve the duration of remission. Based on these findings, we suggest that rituximab should be the treatment of choice in SMZL.
Introduction
Splenic marginal zone lymphoma (SMZL) is a rare form of indolent B-cell lymphoma, accounting for <2% of all lymphoid malignancies [1, 2]. It mainly affects elderly or middle-aged patients, with a median age of ∼65 years. SMZL is characterized by splenomegaly without lymphadenopathy, other than in the splenic hilum, and no other extranodal involvement, except of bone marrow and liver [2]. Cytopenias and lymphocytosis are frequently observed [3]. There is no specific immunophenotypic marker for SMZL. Lymphoma cells express pan-B-cell antigens and surface IgM and/or IgD, whereas they are usually negative for CD5, CD10, and CD23. Less than 3% of cases may coexpress CD5 and CD23 antigens, as observed in chronic B-cell lymphocytic leukemia [3, 4]. Bone marrow is practically always involved, although to a highly variable extent [2, 5–7]. Autoimmune phenomena and monoclonal gammopathy are present in up to one third of patients. SMZL usually runs an indolent clinical course, with a median survival duration >10 years [5–14]. Transformation to diffuse large B-cell lymphoma is observed in ∼10% of patients [13]. The prognosis is variable, and several prognostic markers have been proposed without reproducible results [9–19].
Treatment may be deferred in patients with SMZL until dictated by clinical circumstances. However, there are no well-established criteria for treatment initiation [3, 20]. As with other indolent B-cell lymphomas, treatment should be considered in patients with bulky or symptomatic splenomegaly, B-symptoms, autoimmune phenomena, or significant cytopenias [3, 10, 20]. When treatment is indicated, splenectomy has been considered as the upfront choice [8–13]. Chemotherapy with alkylating agents has been shown to have limited efficacy, whereas purine analogs, although effective in small studies, are associated with considerable toxicity [10, 12, 21, 22]. Recently, we and others documented the remarkable efficacy and minimal toxicity of the chimeric anti-CD20 monoclonal antibody rituximab in patients with SMZL [23–26].
The aim of this study was to further analyze outcomes in a large series of SMZL patients diagnosed and treated in our departments with rituximab as first-line monotherapy in comparison with historical controls, in whom first-line splenectomy was used prior to the introduction of rituximab. Emphasis is now given to the confirmation of the high response rates reported in small series, the long-term follow-up of rituximab-treated patients, and the potential impact of rituximab maintenance therapy.
Patients and Methods
Patients
This is a retrospective study including: (a) 58 patients with SMZL who were diagnosed and treated with rituximab monotherapy in three hematology departments during September 2003 to July 2011 and (b) 27 patients who were diagnosed prior to the rituximab period and were treated with splenectomy only in the same centers. The study was approved by the appropriate institutional review boards. Sixteen of 58 rituximab-treated patients were already reported on [25]. The diagnosis of SMZL was established according to the World Health Organization classification criteria [2]. Demographic features, symptoms, clinical characteristics, laboratory findings, bone marrow histologies, imaging results, treatment modalities, response rates, progression free-survival (PFS) and overall survival (OS) outcomes, and causes of death were analyzed. Anemia was defined as a hemoglobin level <12 g/dL, neutropenia as a neutrophil count <1.5 × 109/L, lymphocytosis as a lymphocyte count ≥4 × 109/L, and thrombocytopenia as a platelet count <100 × 109/L. The International Prognostic Index (IPI) for aggressive lymphomas was also calculated [27].
Treatment
Treatment was administered in patients with bulky or symptomatic splenomegaly, significant cytopenias, B-symptoms, and/or autoimmune manifestations. Splenectomy was the treatment of choice before September 2003 and rituximab was the treatment of choice thereafter. After September 2003, during the rituximab period, a single patient underwent splenectomy elsewhere and was referred immediately to one of the participating centers, where she was simply followed until progression. Thus, there was no selection bias between rituximab and splenectomy during the study period. During the study period, 14 patients received chemotherapy as first-line therapy and were excluded from this analysis. In more detail, during the rituximab period, one patient received chlorambucil monotherapy and another received rituximab plus cyclophosphamide, vincristine, and prednisone (CVP) because of medical decision. In the prerituximab period, 12 patients received chemotherapy as first-line therapy instead of splenectomy; alkylating agent monotherapy was mainly used, including chlorambucil in 11 patients and cyclophosphamide in one patient, whereas one patient received combination chemotherapy with CVP. Chemotherapy was offered to these patients either because of the treating physician's choice or because splenectomy was contraindicated as a result of comorbidities or a poor performance status.
Splenectomy
Splenectomy was performed using standard procedures after appropriate vaccination. Following splenectomy, regular clinical examination and hematologic evaluation were performed. Bone marrow evaluation was not mandatory for response assessment.
Rituximab Monotherapy
The program of rituximab monotherapy included induction and maintenance phases [25]. Rituximab was given at a dose of 375 mg/m2 per week following standard administration instructions. The vast majority of the patients received six weekly infusions (45 of 58, 78%), eight (14%) received eight infusions, three (5%) received four infusions, and the other two patient received three and seven infusions. Most responders (e.g., patients achieving at least a partial response [PR] after the induction phase) received maintenance therapy with rituximab every 2 months at a dose of 375 mg/m2 for 1–2 years, because the optimal duration of such treatment was not standardized during the study period. Similarly, the maintenance strategy was not uniformly adopted and could be followed at the discretion of the treating physician or patient's desire.
Evaluation of Response
Evaluation of response was performed 2 months after the completion of the rituximab induction phase and after the end of maintenance therapy and included a physical examination, whole-body computed tomography, bone marrow aspiration plus biopsy, and, in most cases, blood and bone marrow immunophenotypic analysis. In some patients, the presence of monoclonal IgVH rearrangement was assessed in blood and bone marrow mononuclear cells.
Response assessment was based on the criteria used in our previous report [25] as well as on the consensus criteria proposed by Matutes et al. [3], with minor modifications. Based on these criteria, the following definitions were applied. Complete response (CR) was defined as the resolution of symptoms and organomegaly, normalization of blood counts (hemoglobin ≥12 g/dL, platelet count ≥100 × 109/L, neutrophil count ≥1.5 × 109/L, absolute lymphocyte count <4.0 × 109/L), and no evidence of bone marrow infiltration on immunohistochemistry. Detection of residual monoclonal lymphocytosis with flow cytometry was not necessary for the establishment of a CR; however, most of our patients underwent immunophenotypic analysis of blood and bone marrow mononuclear cells and, in all of them, no evidence of monoclonality was detected in patients with a CR. For the purpose of this study, the category complete response unconfirmed (CRu) was also used in order to describe cases fulfilling the criteria of a CR without bone marrow re-evaluation. PR was defined as the resolution of symptoms and a ≥50% decrease in spleen size and a decrease in the level of lymphoid infiltration in the bone marrow (evaluated by trephine biopsy) along with improvement in blood counts over baseline. A clinical and hematologic response (CHR) was defined as fulfillment of the PR criteria without bone marrow revaluation. No response (NR) or progressive disease (PD) was defined as a <50% improvement in disease manifestations or deterioration of the above, respectively. Molecular response was defined as no detection of IgVH rearrangement in bone marrow mononuclear cells in previously positive patients who had achieved a CR. For this purpose, DNA was isolated from peripheral blood and bone marrow mononuclear cells. Rearranged IgVH was amplified in reactions that contained only one of the 5′ leader region primers for the indicated six VH families and a 3′J primer, as described in detail elsewhere [28].
Statistical Methods
The OS time was calculated as the time between treatment initiation and last follow-up or death resulting from any cause. The cause-specific survival time was calculated as the time between treatment initiation and last follow-up, lymphoma-related death, or treatment-related death. The PFS interval was calculated as the time between treatment initiation and disease progression, relapse, treatment-related death, or last follow-up. Deaths resulting from an unrelated cause without prior relapse or progression were censored. Survival curves were plotted according to the Kaplan–Meier method. Differences between survival curves were evaluated using the log-rank test. Differences regarding baseline patient characteristics were evaluated using the χ2 or Mann-Whitney test, as appropriate. Two-sided p-values < .05 were considered statistically significant.
Results
Patient Characteristics
In the whole population of 85 patients, the median age was 64 years (range, 41–91 years), with a female predominance (47 of 85, 55%). All patients presented with splenomegaly. Bone marrow was involved in all cases, although the extent of infiltration was highly variable, in the range of 10% to >90% (median, 40%). B-symptoms were very rare (4%), whereas cytopenias were common: 47 of 82 patients (57%) had anemia, 13 of 79 patients (18%) had neutropenia, and 20 of 81 patients (25%) had thrombocytopenia. Lymphocytosis was present in half of the patients; the median lymphocyte count was 3.7 × 109/L (range, 0.36–90.0 × 109/L). Lactate dehydrogenase (LDH) was elevated in 33 of 75 patients (44%). Twenty-five (34%) of 73 patients tested had a serum monoclonal M-component. Autoimmune phenomena were recorded in six patients (7%). According to the IPI, most of the patients (58 of 77, 75%) were classified into the low or low–intermediate risk groups. Serologic testing for hepatitis C virus was negative in all patients. Patient characteristics according to treatment group are given in Table 1. Most patient characteristics were well balanced between the two treatment groups. The incidence of thrombocytopenia was higher in splenectomized patients (42% vs. 18%; p = .02). Borderline differences were observed for LDH elevation, which was found more frequently in splenectomized patients (62% vs. 37%; p = .051), and lymphadenopathy, which was more frequent in rituximab-treated patients (30% vs. 12%; p = .07).
Table 1.
Clinical and laboratory findings of splenic marginal zone lymphoma patients at diagnosis according to treatment approach
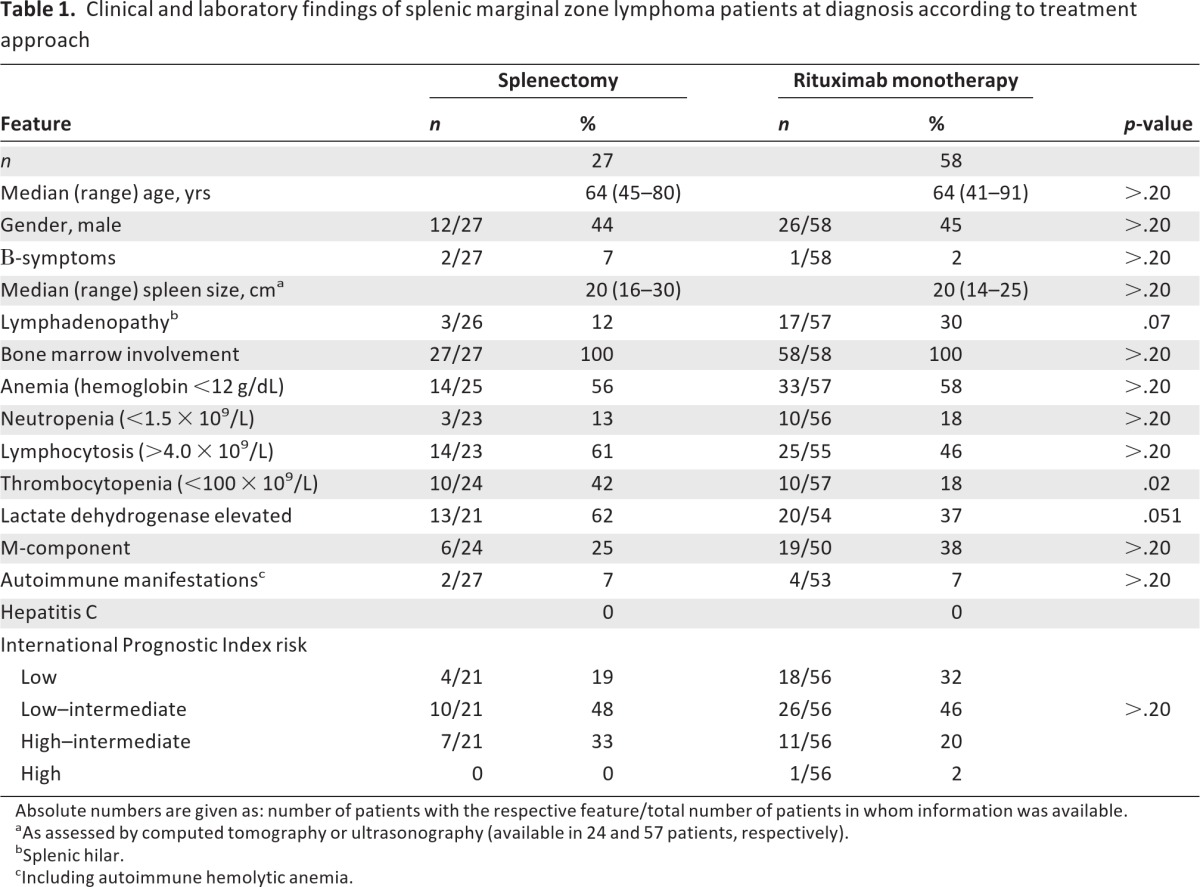
Absolute numbers are given as: number of patients with the respective feature/total number of patients in whom information was available.
aAs assessed by computed tomography or ultrasonography (available in 24 and 57 patients, respectively).
bSplenic hilar.
cIncluding autoimmune hemolytic anemia.
Among the 85 patients analyzed, 20 (25%) were not in need of therapy at diagnosis but progressed during follow-up after a median time of 24 months (range, 7–144 months).
Treatment Outcome
Response according to the therapeutic modality used is described in detail below and is also shown in Table 2.
Table 2.
Response to treatment and survival outcomes of splenic marginal zone lymphoma patients according to therapeutic modality used
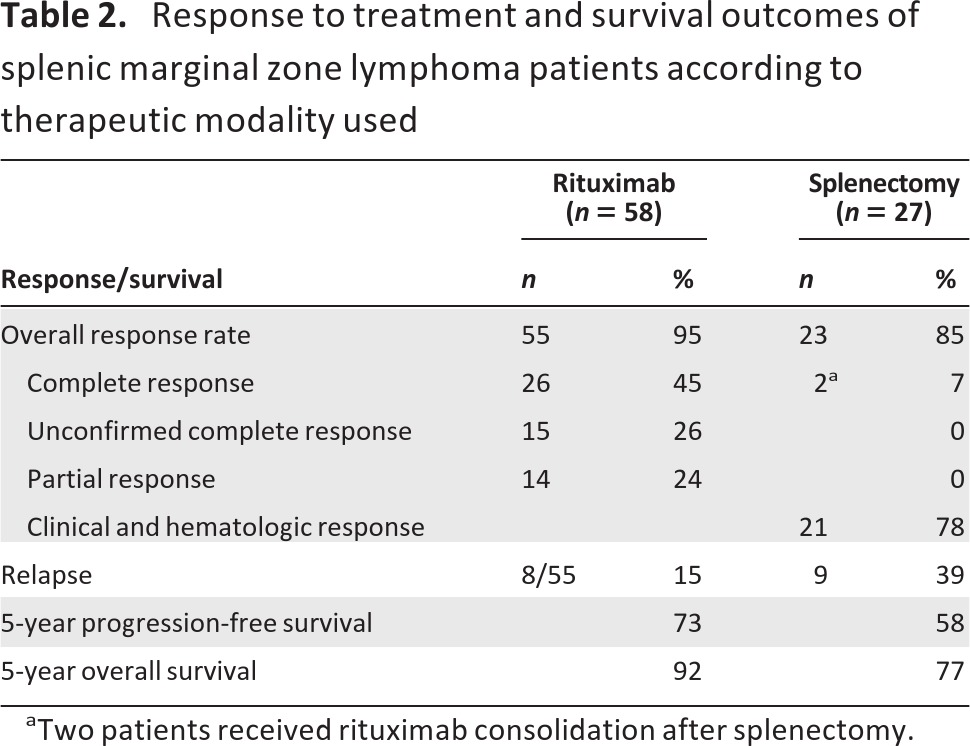
aTwo patients received rituximab consolidation after splenectomy.
Response to Rituximab Monotherapy
After Induction Phase
Evaluation of response 2 months after induction therapy revealed that 55 of 58 patients responded, for an overall response rate (ORR) of 95%. Responses were as follows: 26 (45%) CRs, 15 (26%) CRus, and 14 (24%) PRs (Tables 2 and 3). Two patients (3%) did not respond, and a single patient withdrew early because of rituximab toxicity.
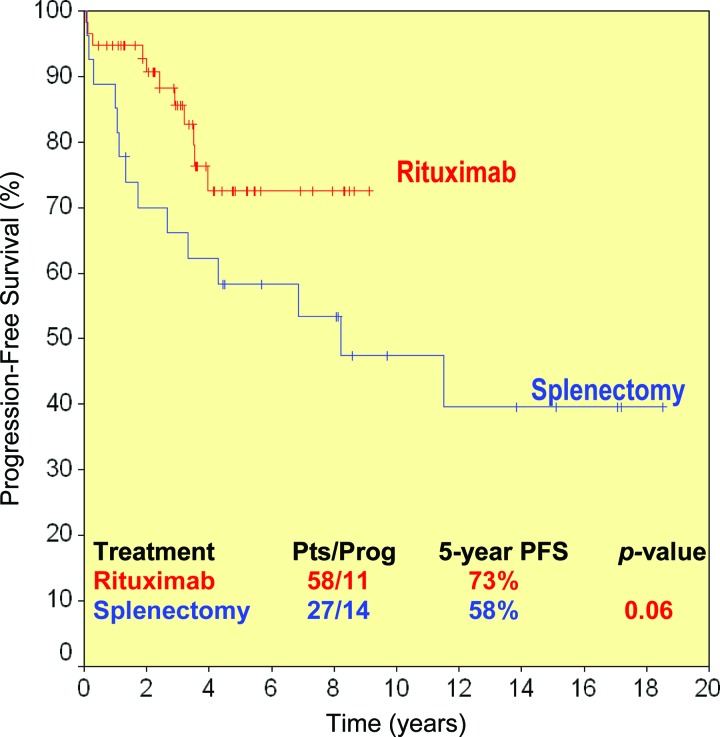
Figure 2.
Progression-free survival (PFS) probability in rituximab-treated patients according to maintenance.
Figure 3.
Overall survival (OS) probability in rituximab-treated (red line) and splenectomized (blue line) patients.
Furthermore, 13 patients who achieved a CR were evaluated for IgVH rearrangement in the blood and bone marrow mononuclear cells, which disclosed molecular remission in nine of them. The median time to normalization of blood counts was 2 weeks (range, 1–8 weeks), whereas the median time to clinical response (resolution of palpable splenomegaly) was 3 weeks (range, 1–8 weeks).
After Maintenance Phase
Among the 55 rituximab responders, 11 did not receive maintenance therapy because of refusal or the decision of the treating physician, one patient had not completed this phase yet, and 43 patients had completed maintenance therapy and were evaluable for response; 29 of them had received maintenance for 2 years and the remaining 14 patients had received maintenance for 1 year.
Response rates at the end of induction and at the end of maintenance are shown in Table 3. Evaluation of response after the end of the maintenance phase revealed that 27 patients (CR, n = 22; CRu, n = 3; PR, n = 2) sustained their initial response whereas 15 patients (CRu, n = 6; PR, n = 9) achieved further improvements, entering a CR after the end of the maintenance phase. However, one patient lost her initial response (CR) during maintenance therapy, with progression of splenomegaly and bone marrow infiltration (Table 3).
Table 3.
Responses after induction phase and at the end of maintenance
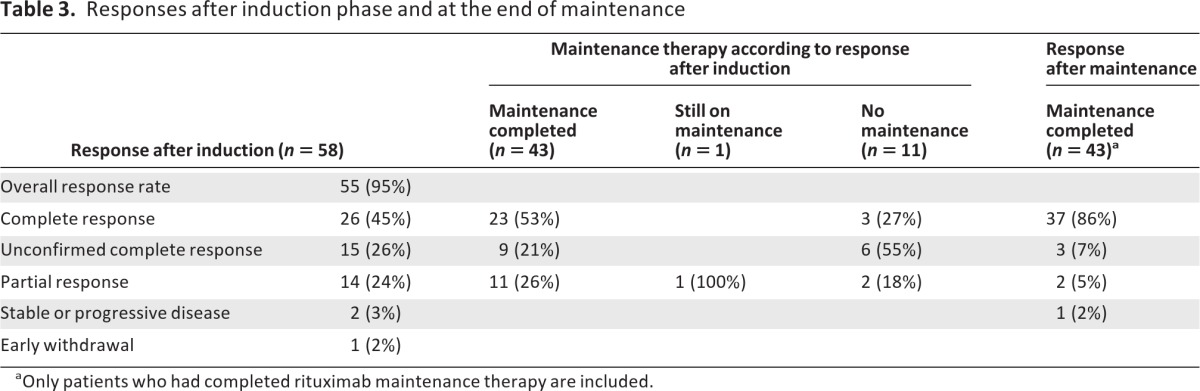
aOnly patients who had completed rituximab maintenance therapy are included.
Follow-Up and Treatment of Relapses After Rituximab Monotherapy
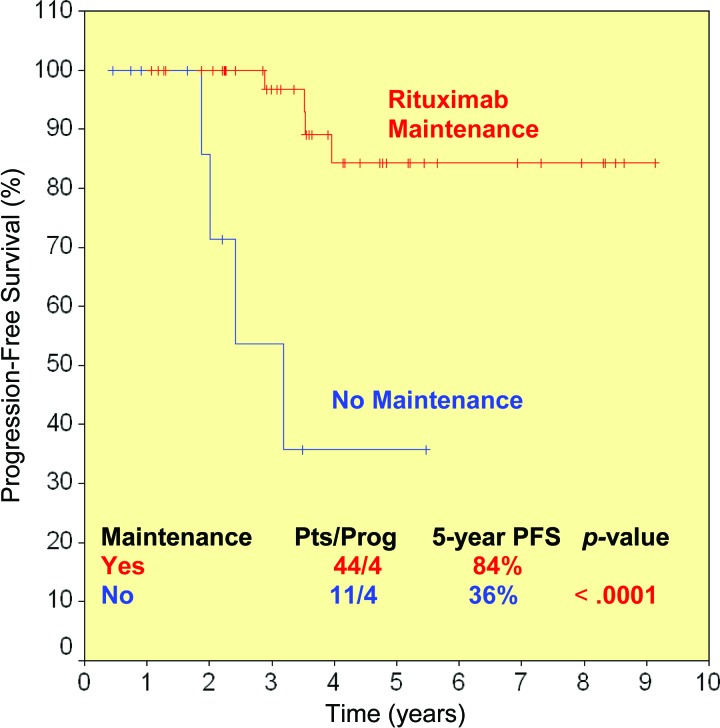
Apart from the three patients with early rituximab failure or intolerance, eight of 55 rituximab responders (15%) relapsed after a median time of 36.5 months (range, 22–48 months). Taking into account these 11 events, the 5-year PFS rate for the 58 rituximab-treated patients was 73% (Table 2 and Fig. 1).
Figure 1.
Progression-free survival (PFS) probability in rituximab-treated (red line) and splenectomized patients (blue line) after 5 years.
Of the 11 patients who did not receive maintenance, four (36%) relapsed at 22, 24, 29, and 38 months. On the other hand, among the 43 patients who had completed maintenance therapy, four relapsed (12%) at a median time of 42 months (range, 35–48 months), whereas the patient remaining on maintenance had not relapsed so far. Maintenance therapy with rituximab was associated with a better PFS outcome. The median PFS interval was 38 months for patients not receiving maintenance, but it was not reached in patients receiving maintenance. At 5 years, the PFS rates were 84% and 36% (p < .0001), for patients who received maintenance and those who did not, respectively (Fig. 2).
As already stated, eight of 55 rituximab responders (15%) relapsed after responding to the induction phase. Six of these eight patients were retreated with rituximab, one patient was splenectomized and achieved a CHR, which was sustained after 29 months, whereas the last patient was not yet in need of treatment (4 months after relapse). Four of the six patients responded to rituximab retreatment, achieving a CR (n = 1), CRu (n = 2), or PR (n = 1), for a response rate of 67%, whereas the other two patients progressed and received salvage therapy with either combination chemotherapy or splenectomy, achieving a CHR in both cases. However, the splenectomized patient progressed 14 months afterward with histologic transformation and died 80 months after the initial diagnosis. One of the four responders to rituximab reinduction experienced a second relapse 30 months later. That patient was retreated with rituximab for 6 weekly cycles and remained in CRu after 26 months.
Five deaths were recorded in the rituximab group, for a 5-year OS rate of 92% Two died of an unrelated cause and three died as a result of lymphoma (Fig. 3).
Response to and Clinical Course After Splenectomy
In total, 27 patients underwent splenectomy as first-line treatment for SMZL. Two of them additionally received 6 weekly infusions of rituximab as consolidation after splenectomy. The ORR to splenectomy was 85% (23 of 27): 21 (78%) patients achieved a CHR, whereas the two patients (7%) who received consolidation with rituximab achieved a CR. Those two patients retained their response at 96 months and 97 months. One patient died 1 month after splenectomy as a result of sepsis, whereas the other three nonresponders were treated with rituximab, chlorambucil, and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The patient who received rituximab monotherapy achieved a CR, which was sustained at 35 months, whereas neither of the other primary refractory patients responded to subsequent treatment and both experienced disease progression with histologic transformation into diffuse large B-cell lymphoma. Both died, at 19 months and 72 months after diagnosis. Among the 23 responders, 10 (43%) progressed at a median time of 27 months (range, 10–137 months). Treatment of relapsing patients included rituximab in four of 10 cases only, either alone (n = 3) or in combination with chemotherapy (n = 1), and chemotherapy alone in five cases (CHOP, n = 4; fludarabine, n = 1), whereas one patient did not receive any therapy. All rituximab-treated patients responded (CR, n = 1; CRu, n = 2, PR, n = 1), in contrast to chemotherapy-treated patients who did not respond. Overall, six of 13 (46%) patients who did not respond or relapsed after splenectomy received rituximab. During the study period, 10 deaths were recorded in the splenectomy group: one toxic death, seven disease-related deaths, one death from a second malignant neoplasm, and one death resulting from an unrelated cause. Furthermore, in four splenectomized patients, histologic transformation into diffuse large B-cell lymphoma was observed, with a median survival time of 46 months (range, 19–72 months).
Bone marrow evaluation 1 year after splenectomy was performed in seven patients. An increase in lymphocytic infiltration was observed in five patients—the median bone marrow infiltration was 30% (range, 10%–60%) prior to splenectomy and 65% (range, 30%–90%) postsplenectomy. A decrease in bone marrow infiltration was noticed in one patient, whereas in another patient bone marrow infiltration remained unchanged. Furthermore, a change in the pattern of bone marrow infiltration was documented in four patients.
Comparison of Efficacy Between Rituximab Monotherapy and Splenectomy
Both rituximab monotherapy and splenectomy were associated with a high response rate, which were comparable (95% vs. 85%; p > .20) (Table 2). By definition, the depth of response was better after rituximab, because at least 45% of the rituximab-treated patients had a true CR. Some patients achieved responses even at the molecular level. Furthermore, all responding patients achieved complete resolution of lymphocytosis with no detection of lymphomatous cells by flow cytometry in most of them, whereas in splenectomized patients, lymphocytosis persisted and an increase in the percentage of bone marrow infiltration was documented in five cases. Both the PFS and OS rates were higher in the rituximab group than in the splenectomy group, although at a borderline level of statistical significance: 5-year PFS rates were 73% and 58% (p = .06) (Fig. 1) and 5-year OS rates were 92% and 77% (p = .09) (Fig. 3).
Treatment Toxicity
Rituximab was well tolerated in most patients. However, two could not continue therapy because of severe adverse events. One patient with known chronic renal failure experienced severe hypotension during the first infusion with deterioration of renal function. A second trial one week later led to the same adverse reaction, so that treatment was withdrawn. In another patient, grade 3 thrombocytopenia developed after 4 weekly cycles of rituximab and further treatment was postponed. That patient remained in CR after 50 months. Grade 2 neutropenia was noticed in three patients. Reactivation of herpes zoster was observed in one patient. Infusion-related side effects, including chills, rigors, fever and/or bronchospasm, and back pain were observed in almost all patients. These events were of mild severity, occurred mainly during the first infusion, and were easily controlled with administration of appropriate support and infusion of rituximab at a lower rate.
Among splenectomized patients, one toxic death resulting from sepsis was recorded 1 month postsplenectomy.
Discussion
In the absence of randomized trials, there is no clearly established standard therapy for patients with SMZL. Based mainly on clinical experience derived from other indolent B-cell lymphomas, treatment initiation is considered appropriate in patients with bulky or symptomatic splenomegaly, cytopenias, B-symptoms, or autoimmune manifestations [3, 20, 29]. In contrast, patients who do not fulfill these criteria may be closely observed. When treatment is indicated, splenectomy has been the most commonly used therapeutic approach so far.
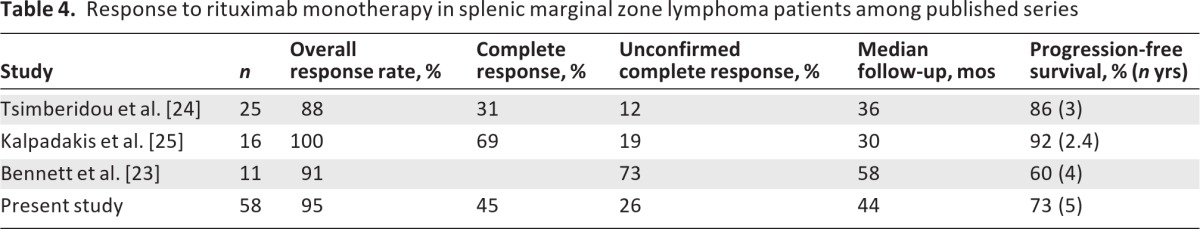
It was recently shown, both by our group and by others, that rituximab monotherapy is highly effective in treating patients with SMZL (Table 4) [23–26, 30–32]. Bennett et al. [23] analyzed 11 patients, some of whom had high-risk features such as older age, prior chemotherapy, bulky splenomegaly, and poor surgical risk. Rituximab therapy resulted in a CHR in 10 of 11 patients (ORR, 91%). The 5-year OS rate was 81% and the 4-year PFS rate was 60%. Furthermore, all relapsed patients responded to a second course of rituximab, and two patients who received a third course because of a second relapse also responded [23]. In the series reported by Tsimberidou et al. [24], the ORR to rituximab monotherapy was 88%, whereas the 3-year PFS rate was 86% in 25 previously untreated patients. Comparison of rituximab (either alone or in combination with chemotherapy) with chemotherapy alone suggested a clear benefit in the rituximab group with respect to the ORR, OS time, and PFS interval. The present study extends our previous observations and confirms the above results in a much larger number of patients with a longer follow-up duration. Among the 58 rituximab-treated patients, 55 responded (95%) and 26 (45%) achieved a CR. The 5-year PFS and OS rates were 73% and 92%, respectively. In accordance with the report of Bennett et al. [23], among six relapsing patients who were retreated with rituximab, four (67%) responded again, and the one patient who relapsed once again 30 months later responded to a third course of rituximab. These data indicate that the efficacy of rituximab is retained in relapsing patients, so it can be used safely in this setting.
Table 4.
Response to rituximab monotherapy in splenic marginal zone lymphoma patients among published series
The role of rituximab maintenance therapy in patients with SMZL has not yet been established. The data presented here suggest, for the first time, that rituximab maintenance further improved responses in 15 of 43 patients (six of nine with a CRu and nine of 11 with a PR) who had not achieved a CR after induction, whereas only one patient lost her CR status during the maintenance phase. More importantly, a comparison between patients who received maintenance and those who did not, revealed that relapses occurred more frequently and more rapidly in patients not receiving maintenance, with 5-year PFS rates of 84% versus 36%, respectively (p < .0001). The above data suggest that maintenance therapy can improve the duration of remission in SMZL patients, similar to what has been shown in patients with follicular and mantle cell lymphoma [33–35]. Although formal restaging was not performed uniformly during maintenance, rituximab-treated patients were followed using clinical examination and laboratory tests every 2 months (i.e., more frequently than those who did not receive maintenance or had simply been splenectomized). Thus, this “clinical progression”-free survival time could not be artificially prolonged as a result of different follow-up strategies in either maintenance versus no maintenance or rituximab versus splenectomy comparisons. However, firm conclusions cannot be drawn yet because the role of maintenance therapy needs further evaluation in a randomized trial. Moreover, it is not yet clear if maintenance treatment is superior to retreatment with rituximab on demand.
Splenectomy provides complete resolution of splenomegaly-related symptoms and improvement of cytopenias. However, it is a major surgical procedure with significant morbidity and potential mortality, especially in elderly patients and in patients with comorbidities. By definition, response to splenectomy cannot be complete because lymphocytosis and bone marrow infiltration persist. Data on splenectomy are relatively limited in the literature, including series of 17–60 patients [9–13, 21, 24]. In general, those series do not include uniformly treated patients but subpopulations who were treated with splenectomy based on the treating physician's choice. ORRs are high, but the early toxic death rate is on the order of 5% [11, 13]. The median time to progression after splenectomy is 4–7 years, whereas almost half the patients do not require further treatment for prolonged periods of time [7, 10–14]. The 5-year OS rate after splenectomy has been reported to be in the range of 65%–90%. The results of the present study are in agreement with those of other published series. However, our study showed a relative high percentage of histologic transformation in splenectomized patients, in contrast to the rituximab-treated group, which may be explained by the hypothesis that splenectomy might not alter the risk for histologic transformation. The effect of splenectomy on bone marrow infiltration still remains undefined because published data are controversial [3, 8, 9, 20, 21, 24, 36]. Franco et al. [36] reported a change in the pattern and increase in the percentage of bone marrow infiltration 1 year after splenectomy in a series of 16 SMZL patients. This observation is in accordance with our findings, because five of seven patients in our study had an increase in bone marrow infiltration along with a change in its pattern [36]. However, other series did not confirm these findings, indicating a reduction in bone marrow infiltration after splenectomy [24]. Sampling variability might provide an explanation for these conflicting data.
The data presented here revealed a trend toward a potential superiority of rituximab over splenectomy in terms of both PFS and OS outcomes, which might be underestimated because two of 27 splenectomized patients also received rituximab after the procedure and achieved durable CRs. The favorable toxicity profile of rituximab makes it an attractive first-line therapy for patients with SMZL that can postpone the need for splenectomy with its morbidity. However, the borderline benefit recorded here should be interpreted with caution. Because this was not a randomized trial, patient characteristics were not absolutely comparable between rituximab-treated and splenectomized patients, although the unbalanced parameters (serum LDH and thrombocytopenia) did not actually affect the PFS and OS rates (data not shown). However, no selection bias was introduced because splenectomy and rituximab were not considered as treatment options during the same time period (see Patients and Methods). More importantly, patients treated with splenectomy prior to the rituximab era might not have had the opportunity to be exposed to rituximab afterward, with a potential adverse effect on OS outcomes. Finally, four of five cases of histologic transformation were recorded in the splenectomy group and might have contributed to the inferior survival rates if this imbalance occurred by chance and splenectomy is not truly associated with a higher risk for transformation.
Chemotherapy as first-line therapy has mainly been used either in patients with adverse risk factors or in those who are not suitable for splenectomy because of limitations related to advanced age or comorbidities. Alkylating agents display limited activity. Among the purine analogs, pentostatin and cladribine present moderate activity with considerable toxicity whereas fludarabine appears to be more effective in a limited number of patients [37–40]. Combination immunochemotherapy has been shown to be very efficacious [24, 41]. However, further studies in a larger number of patients are required in order to decide which patients benefit the most from this combination therapy because the toxicity far exceeds that of rituximab monotherapy.
The present study further supports our previous results on the efficacy of rituximab as first-line treatment for SMZL patients, points out the very favorable toxicity profile of this approach, and suggests a potential superiority over splenectomy. Rituximab maintenance may prolong the duration of remission in responding patients, thus deserving further evaluation.
Author Contributions
Conception/Design: Christina Kalpadakis, Gerassimos A. Pangalis, Theodoros P. Vassilakopoulos
Provision of study material or patients: Christina Kalpadakis, Gerassimos A. Pangalis, Maria K. Angelopoulou, Sotirios Sachanas, Flora N. Kontopidou, Xanthi Yiakoumis, Stella I. Kokoris, Evagelia M. Dimitriadou, Maria N. Dimopoulou, Maria Moschogiannis, Penelope Korkolopoulou, Marie-Christine Kyrtsonis, Marina P. Siakantaris, Theodora Papadaki, Eleni Plata, Panayiotis Tsaftaridis, Helen E. Papadaki, Theodoros P. Vassilakopoulos
Collection and/or assembly of data: Christina Kalpadakis, Gerassimos A. Pangalis, Maria K. Angelopoulou, Sotirios Sachanas, Flora N. Kontopidou, Xanthi Yiakoumis, Stella I. Kokoris, Evagelia M. Dimitriadou, Maria N. Dimopoulou, Maria Moschogiannis, Penelope Korkolopoulou, Marie-Christine Kyrtsonis, Marina P. Siakantaris, Theodora Papadaki, Eleni Plata, Panayiotis Tsaftaridis, Helen E. Papadaki, Theodoros P. Vassilakopoulos
Data analysis and interpretation: Christina Kalpadakis, Gerassimos A. Pangalis, Theodoros P. Vassilakopoulos
Manuscript writing: Christina Kalpadakis, Gerassimos A. Pangalis, Theodoros P. Vassilakopoulos
Final approval of manuscript: Christina Kalpadakis, Gerassimos A. Pangalis, Maria K. Angelopoulou, Sotirios Sachanas, Flora N. Kontopidou, Xanthi Yiakoumis, Stella I. Kokoris, Evagelia M. Dimitriadou, Maria N. Dimopoulou, Maria Moschogiannis, Penelope Korkolopoulou, Marie-Christine Kyrtsonis, Marina P. Siakantaris, Theodora Papadaki, Eleni Plata, Panayiotis Tsaftaridis, Helen E. Papadaki, Theodoros P. Vassilakopoulos
Disclosures
The authors indicated no financial relationships.
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson PG, Piris MA, Berger F, et al. World Health Organization. Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. Splenic B-cell marginal zone lymphoma; pp. 185–187. [Google Scholar]
- 3.Matutes E, Oscier D, Montalban C, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia. 2008;22:487–495. doi: 10.1038/sj.leu.2405068. [DOI] [PubMed] [Google Scholar]
- 4.Matutes E, Morilla R, Owusu-Ankomach K, et al. The immunophenotype of splenic lymphoma with villous lymphocytes and its relevance to the differential diagnosis with other B-cell disorders. Blood. 1994;83:1558–1562. [PubMed] [Google Scholar]
- 5.Franco V, Florena ΑΜ, Iannitto Ε. Splenic marginal zone lymphoma. Blood. 2003;101:2464–2472. doi: 10.1182/blood-2002-07-2216. [DOI] [PubMed] [Google Scholar]
- 6.Oscier D, Owen R, Johnson S. Splenic marginal zone lymphoma. Blood Rev. 2005;19:39–51. doi: 10.1016/j.blre.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Berger F, Felman P, Thieblemont C, et al. Non-MALT marginal zone B-cell lymphomas: A description of clinical presentation and outcome in 124 patients. Blood. 2000;95:1950–1956. [PubMed] [Google Scholar]
- 8.Thieblemont C, Felman P, Callet-Bauchu E, et al. Splenic marginal-zone lymphoma: A distinct clinical and pathological entity. Lancet Oncol. 2003;4:95–103. doi: 10.1016/s1470-2045(03)00981-1. [DOI] [PubMed] [Google Scholar]
- 9.Chacón JI, Mollejo M, Muñoz E, et al. Splenic marginal zone lymphoma: Clinical characteristics and prognostic factors in a series of 60 patients. Blood. 2002;100:1648–1654. [PubMed] [Google Scholar]
- 10.Thieblemont C, Felman P, Berger F, et al. Treatment of splenic marginal zone B-cell lymphoma: An analysis of 81 patients. Clin Lymphoma. 2002;3:41–47. doi: 10.3816/clm.2002.n.010. [DOI] [PubMed] [Google Scholar]
- 11.Troussard X, Valensi F, Duchayne E, et al. Splenic lymphoma with villous lymphocytes: Clinical presentation, biology and prognostic factors in a series of 100 patients. Groupe Frana̧is d'Hématologie Cellulaire (GFHC) Br J Haematol. 1996;93:731–736. doi: 10.1046/j.1365-2141.1996.d01-1711.x. [DOI] [PubMed] [Google Scholar]
- 12.Parry-Jones N, Matutes E, Gruszca-Westwood AM, et al. Prognostic features of splenic lymphoma with villous lymphocytes: A report on 129 patients. Br J Haematol. 2003;120:759–764. doi: 10.1046/j.1365-2141.2003.04165.x. [DOI] [PubMed] [Google Scholar]
- 13.Mulligan SP, Matutes E, Dearden C, et al. Splenic lymphoma with villous lymphocytes: Natural history and response to therapy in 50 cases. Br J Haematol. 1991;78:206–209. doi: 10.1111/j.1365-2141.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 14.Arcaini L, Lazzarino M, Colombo N, et al. Splenic marginal zone lymphoma: A prognostic model for clinical use. Blood. 2006;107:4643–4649. doi: 10.1182/blood-2005-11-4659. [DOI] [PubMed] [Google Scholar]
- 15.Algara P, Mateo MS, Sanchez-Beato M, et al. Analysis of the IgV(H) somatic mutations in splenic marginal zone lymphoma defines a group of unmutated cases with frequent 7q deletion and adverse clinical course. Blood. 2002;99:1299–1304. doi: 10.1182/blood.v99.4.1299. [DOI] [PubMed] [Google Scholar]
- 16.Gruszka-Westwood AM, Hamoudi RA, Matutes E, et al. p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood. 2001;97:3552–3558. doi: 10.1182/blood.v97.11.3552. [DOI] [PubMed] [Google Scholar]
- 17.Camacho FI, Mollejo M, Mateo MS, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: A description of a series of 12 cases. Am J Surg Pathol. 2001;25:1268–1276. doi: 10.1097/00000478-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Ballesteros E, Mollejo M, Rodriguez A, et al. Splenic marginal zone lymphoma: Proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood. 2005;106:1831–1838. doi: 10.1182/blood-2004-10-3898. [DOI] [PubMed] [Google Scholar]
- 19.Salido M, Baró C, Oscier D, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: A multicenter study of the Splenic B-Cell Lymphoma Group. Blood. 2010;116:1479–1488. doi: 10.1182/blood-2010-02-267476. [DOI] [PubMed] [Google Scholar]
- 20.Matutes E. Splenic marginal zone lymphoma with and without villous lymphocytes. Curr Treat Options Oncol. 2007;8:109–116. doi: 10.1007/s11864-007-0026-0. [DOI] [PubMed] [Google Scholar]
- 21.Iannitto E, Ambrosetti A, Ammatuna E, et al. Splenic marginal zone lymphoma with or without villous lymphocytes. Hematologic findings and outcomes in a series of 57 patients. Cancer. 2004;101:2050–2057. doi: 10.1002/cncr.20596. [DOI] [PubMed] [Google Scholar]
- 22.Lefrère F, Hermine O, Belanger C, et al. Fludarabine: An effective treatment in patients with splenic lymphoma with villous lymphocytes. Leukemia. 2000;14:573–575. doi: 10.1038/sj.leu.2401710. [DOI] [PubMed] [Google Scholar]
- 23.Bennett M, Sharma K, Yegena S, et al. Rituximab monotherapy for splenic marginal zone lymphoma. Haematologica. 2005;90:856–858. [PubMed] [Google Scholar]
- 24.Tsimberidou AM, Catovsky D, Schlette E, et al. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer. 2006;107:125–135. doi: 10.1002/cncr.21931. [DOI] [PubMed] [Google Scholar]
- 25.Kalpadakis C, Pangalis GA, Dimopoulou MN, et al. Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol Oncol. 2007;25:127–131. doi: 10.1002/hon.820. [DOI] [PubMed] [Google Scholar]
- 26.Kalpadakis C, Pangalis GA, Vassilakopoulos TP, et al. Rituximab monotherapy is the treatment of choice for splenic marginal zone lymphoma (SMZL) Ann Oncol. 2008;19 Abstract #367. [Google Scholar]
- 27.Shipp MA. Prognostic factors in aggressive non-Hodgkin's lymphoma: Who has “high-risk” disease? Blood. 1994;83:1165–1173. [PubMed] [Google Scholar]
- 28.Kalpadakis C, Pangalis GA, Dimitriadou E, et al. Mutation analysis of IgVH genes in splenic marginal zone lymphomas: Correlation with clinical characteristics and outcome. Anticancer Res. 2009;29:1811–1816. [PubMed] [Google Scholar]
- 29.Catovsky D, Matutes E. Splenic lymphoma with circulating villous lymphocytes/splenic marginal-zone lymphoma. Semin Hematol. 1999;36:148–154. [PubMed] [Google Scholar]
- 30.Bennett M, Schechter GP. Treatment of splenic marginal zone lymphoma: Splenectomy versus rituximab. Semin Hematol. 2010;47:143–147. doi: 10.1053/j.seminhematol.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Fabbri A, Gozzetti A, Lazzi S, et al. Activity of rituximab monotherapy in refractory splenic marginal zone lymphoma complicated with autoimmune hemolytic anemia. Clin Lymphoma Myeloma. 2006;6:496–499. doi: 10.3816/CLM.2006.n.033. [DOI] [PubMed] [Google Scholar]
- 32.Paydas S, Yavuz S, Disel U, et al. Successful rituximab therapy for hemolytic anemia associated with relapsed splenic marginal zone lymphoma with leukemic phase. Leuk Lymphoma. 2003;44:2165–2166. doi: 10.1080/1042819031000123555. [DOI] [PubMed] [Google Scholar]
- 33.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 34.Sachanas S, Pangalis GA, Vassilakopoulos TP, et al. Combination of rituximab with chlorambucil as first line treatment in patients with mantle cell lymphoma: A highly effective regimen. Leuk Lymphoma. 2011;52:387–393. doi: 10.3109/10428194.2010.534518. [DOI] [PubMed] [Google Scholar]
- 35.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 36.Franco V, Florena AM, Stella M, et al. Splenectomy influences marrow infiltration in patients with splenic marginal zone cell lymphoma with or without villous lymphocytes. Cancer. 2001;91:294–301. doi: 10.1002/1097-0142(20010115)91:2<294::aid-cncr1001>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 37.Bolam S, Orchard J, Oscier D. Fludarabine is effective in the treatment of splenic lymphoma with villous lymphocytes. Br J Haematol. 1997;99:158–161. doi: 10.1046/j.1365-2141.1997.3523168.x. [DOI] [PubMed] [Google Scholar]
- 38.Iannitto E, Minardi V, Calvaruso G, et al. Deoxycoformycin (pentostatin) in the treatment of splenic marginal zone lymphoma (SMZL) with or without villous lymphocytes. Eur J Haematol. 2005;75:130–135. doi: 10.1111/j.1600-0609.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- 39.Lefrère F, Hermine O, Frano̧is S, et al. Lack of efficacy of 2-chlorodeoxyadenoside in the treatment of splenic lymphoma with villous lymphocytes. Leuk Lymphoma. 2000;40:113–117. doi: 10.3109/10428190009054887. [DOI] [PubMed] [Google Scholar]
- 40.Riccioni R, Caracciolo F, Galimberti S, et al. Low dose 2-CdA schedule activity in splenic marginal zone lymphomas. Hematol Oncol. 2003;21:163–168. doi: 10.1002/hon.717. [DOI] [PubMed] [Google Scholar]
- 41.Cervetti G, Galimberti S, Sordi E, et al. Significant efficacy of 2-CdA with or without rituximab in the treatment of splenic marginal zone lymphoma (SMZL) Ann Oncol. 2010;21:851–854. doi: 10.1093/annonc/mdp395. [DOI] [PubMed] [Google Scholar]