The proportion of high-grade glioma patients dying with dignity as perceived by their relatives is assessed and disease- and care-related factors correlated with dying with dignity in high-grade glioma patients are identified.
Keywords: High grade glioma, Dignity, End of life, Quality of life, Quality of care
Abstract
Background.
In the end-of-life (EOL) phase, high-grade glioma (HGG) patients have a high symptom burden and often lose independence because of physical and cognitive dysfunction. This might affect the patient's personal dignity. We aimed to (a) assess the proportion of HGG patients dying with dignity as perceived by their relatives and (b) identify disease and care factors correlated with dying with dignity in HGG patients.
Methods.
We approached relatives of a cohort of 155 deceased HGG patients for the study. Participants completed a questionnaire concerning the EOL phase of the patient, covering several subthemes: (a) symptoms and signs, (b) health-related quality of life, (c) decision making, (d) place and quality of EOL care, and (e) dying with dignity.
Results.
Relatives of 81 patients participated and 75% indicated that the patient died with dignity. These patients had fewer communication deficits, experienced fewer transitions between health care settings in the EOL phase, and more frequently died at their preferred place of death. Relatives were more satisfied with the physician providing EOL care and reported that the physician adequately explained treatment options. Multivariate analysis identified satisfaction with the physician, the ability to communicate, and the absence of transitions between settings as most predictive of a dignified death.
Conclusions.
Physicians caring for HGG patients in the EOL phase should timely focus on explaining possible treatment options, because patients experience communication deficits toward death. Physicians should strive to allow patients to die at their preferred place and avoid transitions during the last month of life.
Implications for Practice:
In our study, we aimed (1) to assess whether high-grade glioma (HGG) patients die with dignity and (2) to identify disease and care-related factors associated with dying with dignity. We found that 25% of HGG patients did not die with dignity. Satisfaction with the physician providing end of life (EOL) care, the absence of transitions in health care settings, and the patient's ability to communicate in the EOL phase are identified as factors important for a dignified death. Our results suggest that physicians caring for HGG patients in the EOL phase should explain possible treatment options in this phase to both patients and their involved relatives. Because the majority of patients experience communication deficits near death, we advocate timely discussion of the patient's preferences regarding treatment in the EOL phase. Furthermore, physicians should strive to let patients die at their preferred place of death and, if possible, should avoid transitions in the last month of life.
Introduction
High-grade glioma (HGG) is an incurable disease with a poor prognosis. Median survival times are in the range of 1–5 years [1]. Thus, all HGG patients will sooner or later be confronted with the end-of-life (EOL) phase resulting from their disease. During this EOL phase, symptom burden becomes high and patients are often troubled by seizures and deficits in cognition, communication, and motor function [2–6]. Furthermore, loss of consciousness, cognitive disturbances, communication deficits, and confusion often hamper the patient's competence to participate in EOL decision making [7, 8]. To date, little is known about quality of life (QOL) in the EOL phase or about quality of death in HGG patients [9].
Preserving dignity is often mentioned as a point of great concern by patients when considering the EOL phase [10], and dying with dignity is emerging as an overarching goal of EOL care [11]. Two types of dignity can be distinguished: basic dignity and personal dignity. Basic dignity is the intrinsic dignity of every human being, which nothing can take away. Personal dignity, on the other hand, is an individual concept. It refers to a personal sense of worth, associated with personal goals and social circumstances. Personal dignity is frequently invoked in reference to death and dying [12]. Chochinov et al. [13] stated that a patient's personal dignity may be influenced by (a) direct illness-related concerns such as level of independence and symptom distress; (b) dignity-conserving repertoire such as autonomy, role preservation, acceptance of disease, and spiritual well-being; and (c) social factors, such as social support and care tenor. Later studies reporting on personal dignity additionally identified communication, care-related factors [14], and the ability to make choices as important issues [15, 16].
Personal dignity in HGG patients has not been reported on so far. It can be hypothesized that personal dignity is often threatened in the EOL phase of HGG patients. High symptom burden combined with communication deficits, loss of independence resulting from physical and cognitive dysfunction, and the inability to participate in EOL decision making are all factors that potentially decrease the patient's perception of dying with dignity. Furthermore, environmental aspects of care and care characteristics might influence dignified dying.
In this study, we aimed to establish the proportion of HGG patients who died with dignity as perceived by their relatives. Furthermore, we aimed to explore whether or not subjective dying with dignity was correlated with (a) disease-related factors, (b) psychological and spiritual well-being, (c) decision making, and (d) quality of care.
Materials and Methods
Participants
In 2009, we surveyed the relatives of deceased HGG patients from a cohort of all adult HGG patients diagnosed in 2005 and 2006 in three tertiary referral centers for brain tumor patients (VU University Medical Center and Academic Medical Center Amsterdam, Amsterdam, The Netherlands and Medical Center Haaglanden, The Hague, The Netherlands). Either the treating physician or information from the medical chart identified the relative closest to the deceased patient. These relatives received a letter explaining the aim of the study and were asked to send back a response form, either allowing the researchers to further inform and contact him or her or declining interest in participation. Relatives who agreed to be further informed received a questionnaire about the EOL phase of the deceased patient. The study protocol was approved by the ethics committees of the three participating hospitals, and informed consent was obtained from all participating relatives.
Data Collection
The questionnaire for relatives was developed in accordance with existing questionnaires in QOL and EOL research [17, 18]. Five relatives (two partners, one parent, and two children of different deceased patients) provided feedback in face-to-face interviews, and the questionnaire was adapted using their comments. In its final version, the questionnaire covered several subthemes: (a) (disease-related) symptoms and signs, (b) health-related QOL (HRQOL), (c) decision making, (d) place and quality of EOL care, and (e) dying with dignity. If applicable, we distinguished, in the questions, the situation in the last 3 months before death (the whole EOL phase) and, specifically, the situation in the last week before death (the actual EOL). The questionnaire consisted of two parts. In the first part, relatives were asked to respond how they thought that the patient would have replied. Most questions in this part of the questionnaire included an option “unknown” to prevent relatives from not answering questions or randomly filling in answers. In the second part, relatives were asked about their own experience with decision making in the particular case and their own opinion on the quality of EOL care that had been provided to their loved one.
Dying with dignity was enquired after in the first part of the questionnaire (i.e., the relative estimated how the patient would have answered). No specific definition was provided for dignity and no specifications or criteria were given on which the respondents could base their rating. Relatives were asked to rate the dignity of the patient's death on a five-point Likert scale (1, very undignified; 2, undignified; 3, not dignified, not undignified; 4, dignified; 5, very dignified).
Furthermore, items suggested to be of potential importance for personal dignity were selected from the questionnaire. Regarding disease-related factors, we included pain, seizures, communication deficits, cognitive functioning, and physical functioning using items and scales derived from prospective HRQOL instruments designed for brain tumor patients [17]. Furthermore, we included general (not disease-specific) domains of HRQOL that might be important in the EOL phase, such as psychological well-being and spiritual well-being [17, 19–21]. With regard to decision making, the following items were addressed: (a) the patient's competence to participate in EOL decision making in the last week, (b) whether or not possible treatment options were discussed, and (c) whether or not decisions were made against the patient's or relative's wishes. Concerning quality of EOL care, we incorporated (a) whether or not the patient deceased at the preferred place of death, (b) whether or not transitions between health care settings took place during the last 3 months of life, (c) whether or not the relative was satisfied with the physician providing EOL care, and (d) the overall quality of care (Likert scale, 1–7).
Data Analysis
SPSS software, version 15.0 (SPSS Inc., Chicago, IL), was used for the statistical analysis.
We divided participants into two subsets: patients who died with dignity (scoring ≥4 on the dignity scale) and patients who did not die with dignity (scoring ≤3 on the dignity scale) as perceived by their relatives.
All disease-related factors and QOL domains derived from prospective HRQOL instruments were converted to 0–100 scales using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 algorithm [22, 23]. On symptom scales, a higher score represents worse QOL, whereas on functioning scales, the overall QOL scale, and the dignity scale, a higher score represents better QOL or dignity. The symptom or functioning score in the last week of life was used for data analysis. If possible, missing data in the last week were imputed using a “last observation carried forward” method, filling in the score of 3 months before death. Not normally distributed scores were dichotomously analyzed. For symptoms and QOL scores, a score >50 was classified as “high” and a score ≤50 represented “low.” Questions regarding decision making and quality of care were dichotomized, if applicable.
We compared data from patients who died with dignity and patients who did not die with dignity as perceived by their relatives using t-tests, χ2 tests, and Fisher's exact tests, as appropriate. All tests were done on a two-tailed basis and a p-value <.05 was considered significant. The predictive value of the individual variables that were significantly (p < .05) associated with dignity was examined in a manual stepwise multiple logistic regression analysis using a backward selection procedure. At each step, we evaluated whether or not the model changed by removing the least significant factor. A p-value <.05 was considered statistically significant.
Results
Participants
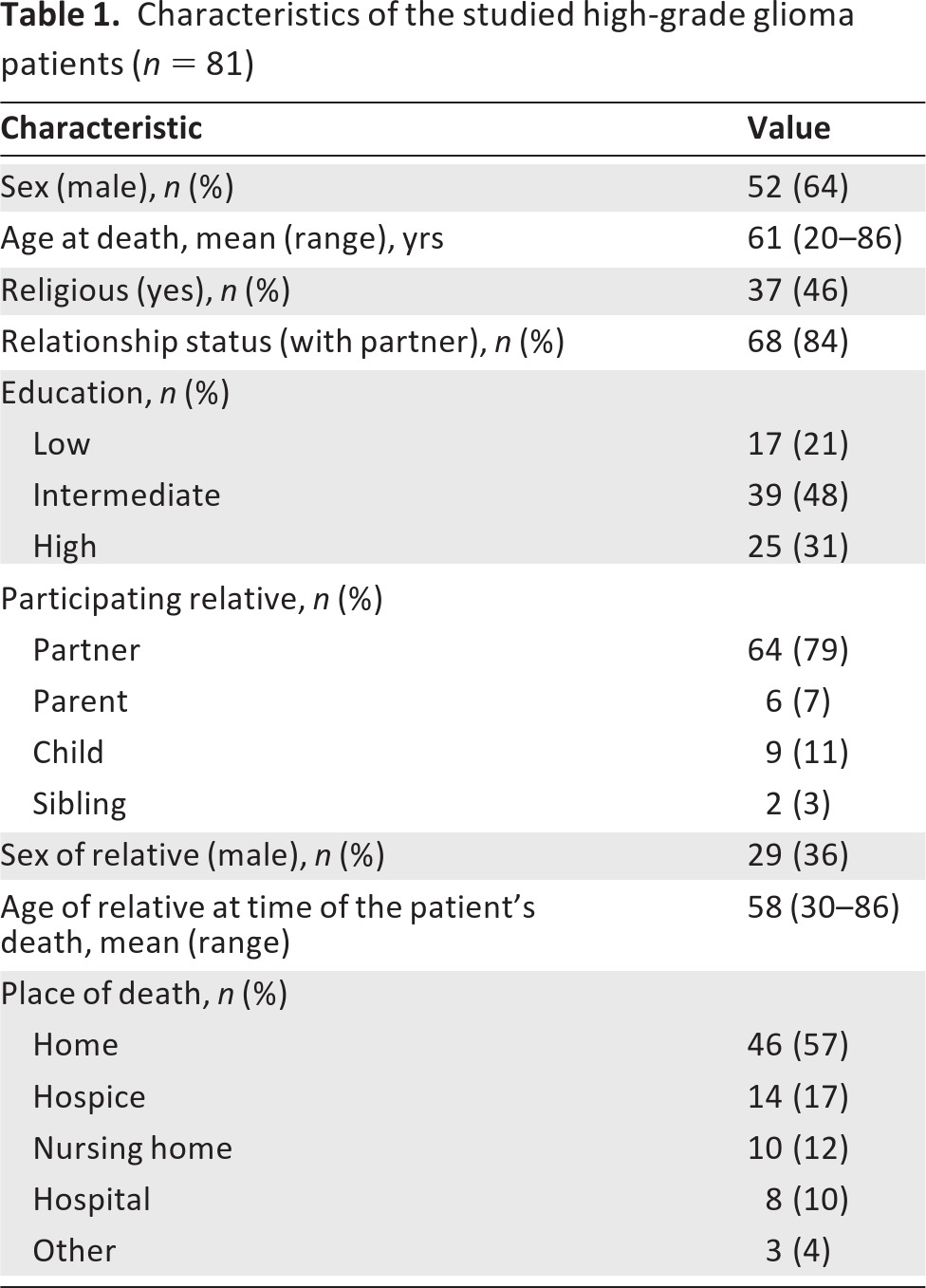
We identified 223 patients diagnosed with HGG in 2005 and 2006 in our three participating hospitals. Of these 223 patients, 39 patients were still alive, four emigrated, and 25 were not traceable. The 155 patients who were known to have died were considered eligible for inclusion in our study. We were able to identify relatives from 131 patients, and these relatives were approached a median of 27 months (interquartile range, 18–34 months) after the death of the patient. Eighty-three relatives participated (response rate, 63%). Two relatives did not fill in the questions concerning dignity, and these cases were thus excluded from the analysis. Characteristics of the 81 patients analyzed in this study are outlined in Table 1. No significant differences in patient characteristics (sex, age at diagnosis, tumor grade) were reported between the 81 patients analyzed in this study and the cohort of 155 patients eligible for inclusion (data not shown).
Table 1.
Characteristics of the studied high-grade glioma patients (n = 81)
Dying With Dignity
Figure 1 shows to what extent a patient's death was dignified according to the relative. The relatives of 61 patients (75%) reported that the patient died with dignity (scoring ≥4 on the dignity scale), whereas the relatives of 20 patients (25%) reported that the patient did not die with dignity (scoring ≤3 on the dignity scale).
Figure 1.
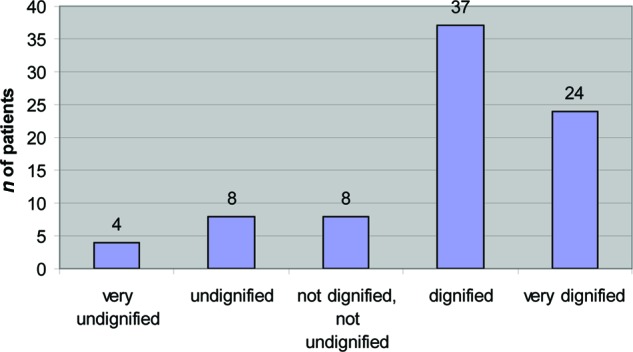
Dignified dying in high-grade glioma patients according to relatives (n = 81).
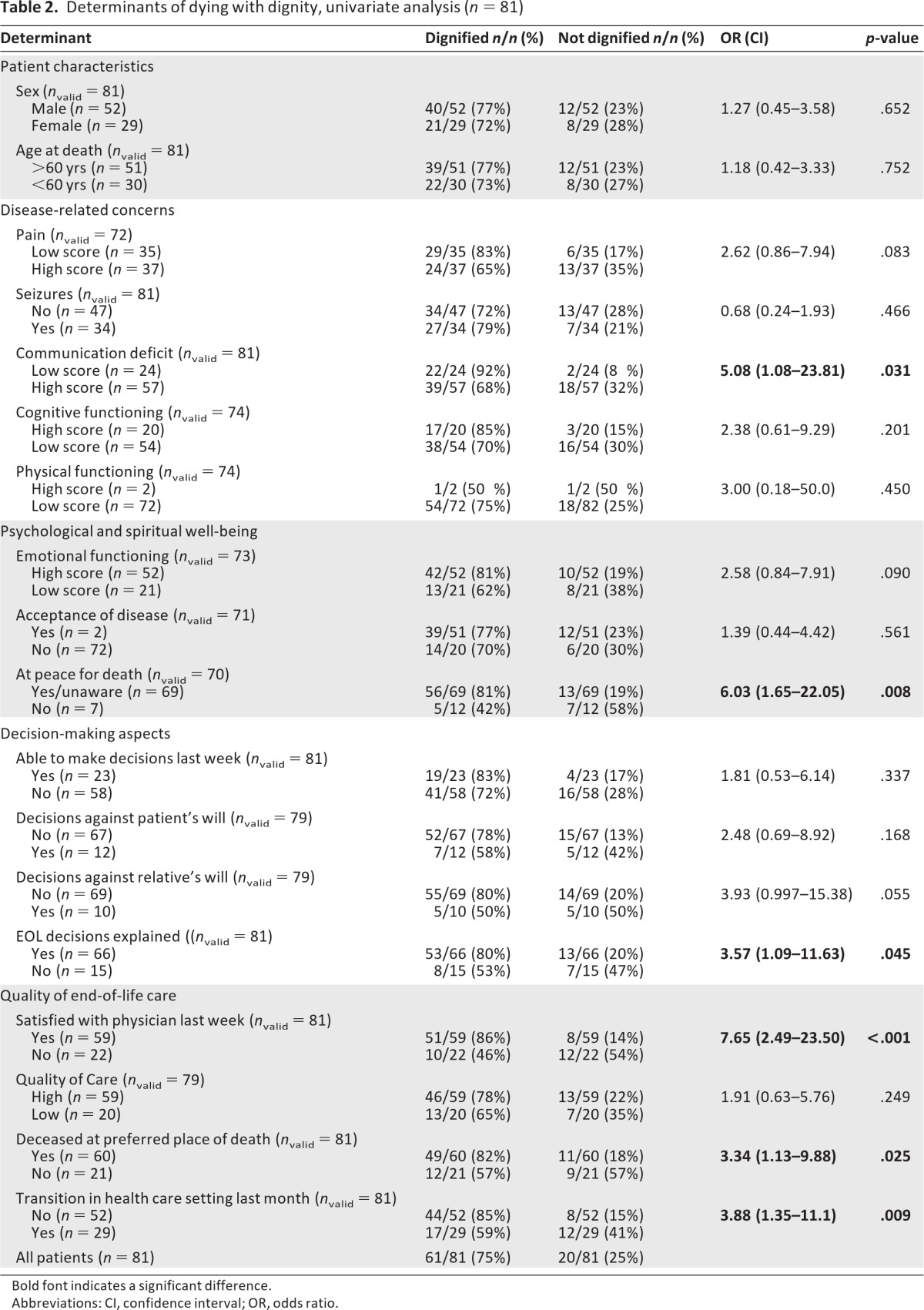
Table 2 shows the correlation between a dignified death and patient characteristics, disease-specific factors, psychological and spiritual well-being, decision-making aspects, and quality of care. We found that patients who died with dignity significantly less often had communication deficits and were more frequently at peace to die. Furthermore, in patients who died with dignity, EOL decisions were more often explicitly discussed and relatives were more satisfied with the physician(s) providing EOL care. Patients who died with dignity more often died at their preferred place of death and experienced fewer transitions between health care settings in the last month. As to place of death, patients who died at home died most often with dignity (83%), followed by hospice (71%), hospital (63%), and nursing home (50%) patients (p = .255).
Table 2.
Determinants of dying with dignity, univariate analysis (n = 81)
Bold font indicates a significant difference.
Abbreviations: CI, confidence interval; OR, odds ratio.
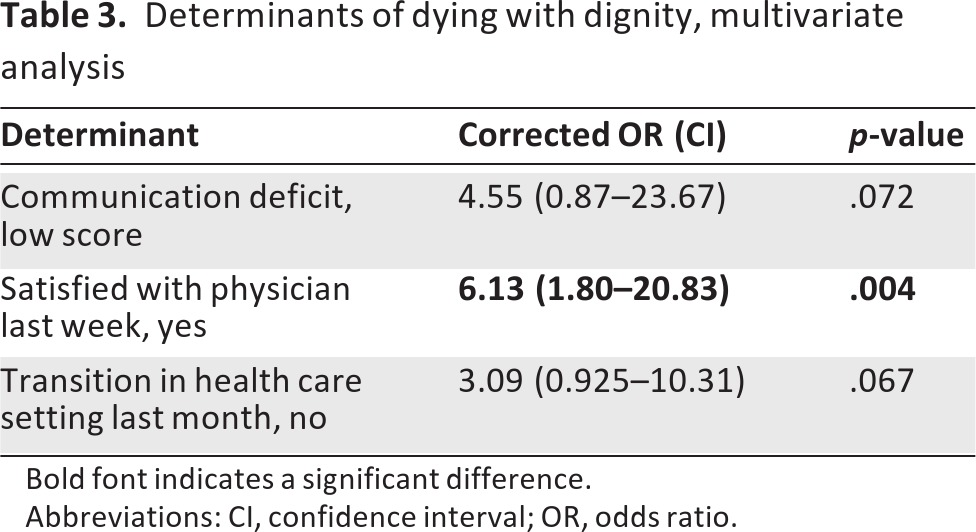
In the univariate analysis, six variables were identified as predictive of a dignified death. Because of our study sample, we were limited in the number of variables we could add to the multivariate model predicting a dignified death. Because the absence of transitions in the last month of life was strongly correlated with dying at the preferred place of death, we decided only to include the most significant factor of these two (i.e., transitions). Furthermore, we included the strongest predictors based on their p-values. We started with a model with four factors (communication deficits, being at peace for death, being satisfied with the physician providing EOL care, transitions). These four factors were evaluated in a stepwise logistic regression analysis using a backward selection procedure. The final model is presented in table 3. The three most important factors predicting dying with dignity in HGG patients are (a) being satisfied with the physician providing EOL care, (b) the absence of transitions between health care settings in the last month of life, and (c) being able to communicate.
Table 3.
Determinants of dying with dignity, multivariate analysis
Bold font indicates a significant difference.
Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
Our study showed that one quarter of HGG patients did not die with dignity, as perceived by their relatives. No previous study has addressed this important issue. The relatives were systematically selected from a well-defined cohort of deceased HGG patients, which adds to the strength of our study.
The percentage of patients who did not die with dignity that we found is relatively high, compared with other cancer cohorts: in a study prospectively examining dignity in incurable general cancer patients in the EOL phase, only 7% of patients had a disturbed sense of dignity [24].
We identified several disease, decision-making, and care-related factors that correlated with dignified dying. With respect to disease-related factors, we found a significant association between the severity of communication deficits and not dying with dignity. This finding is in accordance with Albers et al. [14], who identified that communication is an important issue for dignity at the EOL. Concerning decision making, neither the patient's inability to participate in decision making close before death nor decisions taken against the patient's will appeared to decrease dignity. Previously, both the ability to choose as well as wishes being carried out were identified as important for dignity at the EOL. This contradiction might be explained by the fact that these items were mainly selected as important from the perception of medical staff [25], whereas our study focused on the patient's perspective (as perceived by their relative). According to our results, it proved to be important that the physician explained possible treatment options at the EOL. Although we found no studies relating EOL discussions to dignity, a large, prospective study previously demonstrated that discussing EOL preferences with advanced cancer patients and their proxies reduces distress and improves the QOL of both the patient and the relative at the EOL [26, 27]. EOL care–related aspects appeared to be very important. The strongest independent predictor we identified was being satisfied with the physician providing EOL care. The importance of health care providers was previously demonstrated by Hall et al. [28], who identified the importance of home staff in nursing homes for the patient's sense of dignity. Furthermore, in accordance with previous studies, we found that dying at the preferred place of death was important [14] and that transitions between health care settings in the last month of life decreased dignity.
Our study has several limitations. First, because of its retrospective nature, we were dependent on the relatives' information. Nevertheless, proxy ratings are considered a feasible strategy to gain information if the patient is not able to provide information himself [29], and using proxy ratings is a common and generally acknowledged practice in EOL research [21, 29, 30]. Although the relatives were asked to answer the questions for the patient, relatives may have used their own perception of dignity as a reference to estimate the patient's dignity, and their overall satisfaction with the dying process may also have influenced this perception. Second, a person's sense of dignity and the factors that have impact on dignity change near death [14, 16, 24]. Because dying with dignity was evaluated by applying a single question, these issues were not further explored. Third, our outcome measure has not been validated in previous studies. Fourth, our sample size is relatively small. The final limitation is the fact that relatives answered the questions regarding the patients retrospectively with a relatively long interval since the patient's death, possibly causing recall bias.
In conclusion, our results suggest that, in HGG patients, satisfaction with the physician providing EOL care and the patient's ability to communicate at the EOL are very important for a dignified death. Usually, the latter item cannot be affected by medical intervention in patients with brain tumors, but it is important to realize that physicians caring for HGG patients in the EOL phase should explain possible treatment options at the EOL to patients and their involved relatives. Previous studies suggest that these discussions should be initiated by the physician [31, 32]. Because the majority of patients experience communication deficits near death, we advocate timely discussion of EOL preferences, as we did previously [8]. Physicians should strive to let patients die at their preferred place of death and, if possible, should avoid transitions in the last month of life. If the patient prefers to die at home, specialized palliative home care should be considered because this has been proven to be effective in reducing the number of hospital admissions at the EOL [33]. Future studies should focus on systematically incorporating EOL discussions into clinical practice by active advance care planning.
Acknowledgments
The authors thank Lies Braam, Claudia Nijboer, Alieke Weerdesteijn, and Hanneke Zwinkels for their efforts in identifying patients and tracing relatives.
This work was funded by the St. Jacobusstichting, The Hague.
Author Contributions
Conception/Design: Eefje M. Sizoo, Martin J.B. Taphoorn, Jan J. Heimans, Luc Deliens, Jaap C. Reijneveld, H. Roeline W. Pasman
Provision of study material or patients: Martin J.B. Taphoorn, Jan J. Heimans, Jaap C. Reijneveld
Collection and/or assembly of data: Eefje M. Sizoo, Martin J.B. Taphoorn
Data analysis and interpretation: Eefje M. Sizoo, Bernard Uitdehaag, H. Roeline W. Pasman
Manuscript writing: Eefje M. Sizoo, Martin J.B. Taphoorn, Bernard Uitdehaag, Jan J. Heimans, Luc Deliens, Jaap C. Reijneveld, H. Roeline W. Pasman
Final approval of manuscript: Eefje M. Sizoo, Martin J.B. Taphoorn, Bernard Uitdehaag, Jan J. Heimans, Luc Deliens, Jaap C. Reijneveld, H. Roeline W. Pasman
Disclosures
Martin J.B. Taphoorn: Roche (C/A); Jan J. Heimans: EU Framework Program 7, The Dutch Epilepsy Foundation (NEF), Dutch Ca (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Behin A, Hoang-Xuan K, Carpentier AF, et al. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 2.Faithfull S, Cook K, Lucas C. Palliative care of patients with a primary malignant brain tumour: Case review of service use and support provided. Palliat Med. 2005;19:545–550. doi: 10.1191/0269216305pm1068oa. [DOI] [PubMed] [Google Scholar]
- 3.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, et al. The end-of-life hospital setting in patients with glioblastoma. J Palliat Med. 2008;11:26–30. doi: 10.1089/jpm.2007.0137. [DOI] [PubMed] [Google Scholar]
- 4.Pace A, Di Lorenzo C, Guariglia L, et al. End of life issues in brain tumor patients. J Neurooncol. 2009;91:39–43. doi: 10.1007/s11060-008-9670-x. [DOI] [PubMed] [Google Scholar]
- 5.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12:1162–1166. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostgathe C, Gaertner J, Kotterba M, et al. Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer. 2010;18:1157–1163. doi: 10.1007/s00520-009-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triebel KL, Martin RC, Nabors LB, et al. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73:2086–2092. doi: 10.1212/WNL.0b013e3181c67bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sizoo EM, Pasman HR, Buttolo J, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48:226–232. doi: 10.1016/j.ejca.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ford E, Catt S, Chalmers A, et al. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. 2012;14:392–404. doi: 10.1093/neuonc/nor229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rietjens JA, van der Heide A, Onwuteaka-Philipsen BD, et al. Preferences of the Dutch general public for a good death and associations with attitudes towards end-of-life decision-making. Palliat Med. 2006;20:685–692. doi: 10.1177/0269216306070241. [DOI] [PubMed] [Google Scholar]
- 11.Chochinov HM. Dying, dignity, and new horizons in palliative end-of-life care. CA Cancer J Clin. 2006;56:84–103. doi: 10.3322/canjclin.56.2.84. [DOI] [PubMed] [Google Scholar]
- 12.Pullman D. Human dignity and the ethics and aesthetics of pain and suffering. Theor Med Bioeth. 2002;23:75–94. doi: 10.1023/a:1019521923979. [DOI] [PubMed] [Google Scholar]
- 13.Chochinov HM, Hack T, McClement S, et al. Dignity in the terminally ill: A developing empirical model. Soc Sci Med. 2002;54:433–443. doi: 10.1016/s0277-9536(01)00084-3. [DOI] [PubMed] [Google Scholar]
- 14.Albers G, Pasman HR, Rurup ML, et al. Analysis of the construct of dignity and content validity of the patient dignity inventory. Health Qual Life Outcomes. 2011;9:45. doi: 10.1186/1477-7525-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlug MG, de Vet HC, Pasman HR, et al. The development of an instrument to measure factors that influence self-perceived dignity. J Palliat Med. 2011;14:578–586. doi: 10.1089/jpm.2010.0513. [DOI] [PubMed] [Google Scholar]
- 16.Periyakoil VS, Noda AM, Kraemer HC. Assessment of factors influencing preservation of dignity at life's end: Creation and the cross-cultural validation of the preservation of dignity card-sort tool. J Palliat Med. 2010;13:495–500. doi: 10.1089/jpm.2009.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 18.van Wijmen MP, Rurup ML, Pasman HR, et al. Design of the Advance Directives Cohort: A study of end-of-life decision-making focusing on advance directives. BMC Public Health. 2010;10:166. doi: 10.1186/1471-2458-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albers G, Echteld MA, de Vet HC, et al. Content and spiritual items of quality-of-life instruments appropriate for use in palliative care: A review. J Pain Symptom Manage. 2010;40:290–300. doi: 10.1016/j.jpainsymman.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Teno J, Patrick DL, et al. The concept of quality of life of dying persons in the context of health care. J Pain Symptom Manage. 1999;17:93–108. doi: 10.1016/s0885-3924(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 21.Steinhauser KE, Clipp EC, Tulsky JA. Evolution in measuring the quality of dying. J Palliat Med. 2002;5:407–414. doi: 10.1089/109662102320135298. [DOI] [PubMed] [Google Scholar]
- 22.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 23.Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 Scoring Manual. Third Edition. Brussels, Belgium: European Organisation for Research and Treatment of Cancer Quality of Life Group; 2001. [Google Scholar]
- 24.Chochinov HM, Hack T, Hassard T, et al. Dignity in the terminally ill: A cross-sectional, cohort study. Lancet. 2002;360:2026–2030. doi: 10.1016/S0140-6736(02)12022-8. [DOI] [PubMed] [Google Scholar]
- 25.Periyakoil VS, Kraemer HC, Noda A. Creation and the empirical validation of the dignity card-sort tool to assess factors influencing erosion of dignity at life's end. J Palliat Med. 2009;12:1125–1130. doi: 10.1089/jpm.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall S, Longhurst S, Higginson I. Living and dying with dignity: A qualitative study of the views of older people in nursing homes. Age Ageing. 2009;38:411–416. doi: 10.1093/ageing/afp069. [DOI] [PubMed] [Google Scholar]
- 29.Giesinger JM, Golser M, Erharter A, et al. Do neurooncological patients and their significant others agree on quality of life ratings? Health Qual Life Outcomes. 2009;7:87. doi: 10.1186/1477-7525-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earle CC, Ayanian JZ. Looking back from death: The value of retrospective studies of end-of-life care. J Clin Oncol. 2006;24:838–840. doi: 10.1200/JCO.2005.03.9388. [DOI] [PubMed] [Google Scholar]
- 31.Slort W, Blankenstein AH, Deliens L, et al. Facilitators and barriers for GP-patient communication in palliative care: A qualitative study among GPs, patients, and end-of-life consultants. Br J Gen Pract. 2011;61:167–172. doi: 10.3399/bjgp11X567081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slort W, Schweitzer BP, Blankenstein AH, et al. Perceived barriers and facilitators for general practitioner-patient communication in palliative care: A systematic review. Palliat Med. 2011;25:613–629. doi: 10.1177/0269216310395987. [DOI] [PubMed] [Google Scholar]
- 33.Pace A, Di Lorenzo C, Capon A, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: A cost effectiveness study. J Palliat Med. 2012;15:225–227. doi: 10.1089/jpm.2011.0306. [DOI] [PubMed] [Google Scholar]


