This review examines evidence to support various strategies to protect pediatric oncology patients from influenza-related morbidity. Influenza vaccination should be considered standard. Additional evidence-supported measures include antiviral treatment, antiviral prophylaxis, cohorting of patients, and hospital infection control measures.
Keywords: Influenza, Vaccine, Chemotherapy, Chemoprophylaxis
Learning Objectives
Identify optimal vaccination strategies and define the vaccine response rates among pediatric chemotherapy patients.
Explain the advantage of beginning empiric antiviral therapy.
Describe the need for family member vaccination, hygiene measures, and social distancing.
Abstract
Influenza is a common respiratory pathogen. Its severity can be unpredictable, but people with chronic illness are at increased risk of severe infection, complications, and death from influenza. This review examines evidence to support various strategies to protect pediatric oncology patients from influenza-related morbidity. Influenza vaccination should be considered standard. Additional evidence-supported measures include antiviral treatment, antiviral prophylaxis, cohorting of patients, and hospital infection control measures. Data from other high-risk populations support the vaccination of family members, double-dose or high-dose vaccination, and the use of barrier methods. These measures have the potential to optimize patient outcomes because there will be fewer treatment interruptions for acute illness. These strategies can also protect patients from prolonged hospitalizations and morbidity related to influenza.
Implications for Practice:
Pediatric oncology patients can have very severe infections with the influenza virus. Their vulnerability is due to chronic illness and immune suppression. Simple strategies, such as ensuring vaccination, infection control measures, and education regarding personal protection can protect this population that is susceptible to severe influenza.
Introduction
Influenza is an acute respiratory infection affecting 10%–20% of the population each year in all age groups [1]. Most people with influenza exhibit an uncomplicated febrile respiratory illness and recover within a week; however, several high-risk groups have been identified in which severe complications such as pneumonia, encephalitis, and cytokine storm occur at higher rates [2]. The high-risk groups broadly include infants, the elderly, and people with chronic illness. Influenza-related deaths range from <10 per 100,000 for healthy people up to >600 per 100,000 for chronically ill adults [3].
All patients with hematologic or solid cancers undergoing chemotherapy are considered to be at high risk of influenza-related complications; children with malignancies are particularly vulnerable. A nationwide sentinel disease surveillance system in Taiwan estimated that between the years 1999 and 2005, the incidence of influenza-related hospitalizations was 82.4 per 100,000 patients <20 years old with malignancies [4]. Active surveillance for influenza-like illness conducted on pediatric and adult oncology units during the 2009 influenza pandemic revealed that 20% of patients hospitalized with fever were infected with influenza [5]. Other studies have suggested that as many as two-thirds of pediatric oncology patients who were diagnosed with influenza during that 2009 pandemic required hospitalization [6]; complications occurred in 10%–20% of those hospitalized [7–10]. This review includes recent information on the risks associated with specific pediatric oncology patient populations, preventive strategies, and management options.
Methods
A systematic review of the literature was performed. Additional studies that were known to the authors were included in some cases as substantive foundation material. PubMed was searched for the timeframe of 1990 to August 2012. The search terms included chemotherapy, influenza, vaccine, oseltamivir, oncology, neoplasm, child cancer, immunosuppression, vaccination barriers, and parental compliance with vaccination. Review articles were included where synthetic information was pertinent. In addition, the Cochrane database was examined to identify additional studies. For each article included in this review, the complete publication was examined.
Epidemiology of Influenza in Oncology Patients
Transmission
Prevention is the cornerstone of all efforts to control influenza and understanding the source of infection is critical. Influenza is easily transmitted within the health care setting. Investigations of outbreaks that occurred in pediatric oncology units have identified infected visitors [11] and infected health care workers [12, 13] as common sources of infection. These observations highlight the importance of universal influenza vaccination of health care workers as well as enforcement of appropriate use of sick leave. Multiple reports have also described the role that group activities have played in propagation of influenza outbreaks on pediatric oncology units [11, 12]. Providing protection for patients is complex because infected individuals may be difficult to identify and transmission can occur from asymptomatic individuals. The usual incubation period for influenza ranges from 1 to 4 days. The virus is typically shed in respiratory secretions 1 day before the onset of symptoms and young children can continue to shed virus for 10 or more days [14]. The duration of viral shedding in immunocompromised patients can be even more prolonged, allowing for sustained transmission in hospitals [7, 15].
Risk Factors for Severe Influenza
Lymphopenia, not neutropenia, was commonly detected in children with cancer who were hospitalized with influenza [8]. An analysis of a series of 27 patients with laboratory-confirmed influenza and malignancy demonstrated that chronic steroid use and delayed initiation of oseltamivir were associated with influenza-related lower, as compared with upper, respiratory tract infection [16]. Additional risk factors include recent administration of chemotherapy and a low absolute lymphocyte count (usually <500 cells/μL) [7, 8, 17, 18]. Studies performed in adult oncology patients have demonstrated that influenza A and hematologic malignancies are both independent risk factors for severe infection [19].
Several case series of children with malignancy who were infected with 2009 pandemic influenza reported that up to 20% of children with malignancy developed severe disease when infected with this strain [19–21]. Elbahlawan et al. reported that 5 of 28 patients hospitalized with pandemic influenza developed acute respiratory failure [22]. Due to the unique epidemiology of this strain of influenza, it is difficult to generalize these findings to years in which other strains of influenza are in circulation. Although vaccination is effective, severe disease has occurred in vaccinated patients, suggesting that the most immunologically vulnerable patients may not respond optimally to vaccines [17].
Clinical Features
Hospitalization
Respiratory viral infections in children undergoing cancer therapy are seen more frequently and are more severe than in healthy children [18]. Studies done prior to implementation of routine yearly influenza vaccinations showed that children with cancer are more likely to contract influenza than healthy children in similar environments [23].
Children receiving cancer therapy are more likely to be hospitalized than healthy children when they contract influenza. Up to two thirds of such children are admitted to the hospital, prompted primarily by occurrence of fever [7–9]. These hospitalizations last from 2 to 7 days on average [8]. Most patients are febrile at presentation, and fevers can last 1–2 weeks [8].
In addition to frequent hospitalization, influenza in children with cancer causes significant clinical morbidity. Severe respiratory complications, including respiratory failure, pneumonia, and need for ventilatory support, occur in 10%–20% of these hospitalized children [8, 10, 17]. Less severe respiratory complications, such as hypoxia and need for bronchodilators, are common [8, 17]. A small number of children require prolonged oxygen therapy after the acute illness has subsided [8].
Complications
Intensive care admission occurs in up to 10% of influenza-infected children with cancer, and death occurs in up to 5% [17]. Bacteremia as a complication of influenza infection in these children occurs in about one in six children. Pathogens as Pseudomonas spp., Enterobacter spp., Streptococcus spp. and coagulase-negative staphylococci [6, 8] have been described. Secondary bacterial infections may also occur as clinical infections, such as pneumonia or otitis media [10]. Children infected with influenza during the 2009 pandemic were reported to be more likely to develop pneumonia when they were neutropenic [7]. Less common complications of influenza infection include fungal pneumonias (e.g., Aspergillus pneumonia), other invasive fungal infections, and hemophagocytic lymphohistiocytosis [24–26].
For children with cancer, influenza infection may last up to twice as long as in healthy children [10, 23]. In addition, viral shedding can occur for up to 6 weeks. Canadian children who were infected with the 2009 pandemic influenza continued to shed virus for a median of 46 days [7].
Impact on Chemotherapy
Illness due to influenza in children with cancer causes significant delay in chemotherapy or other cancer-specific therapy in 20%–80% of patients [7, 8, 10, 23]. In one population of children with 2009 pandemic influenza infection, 55% of the children experienced treatment delays averaging 3 weeks; the longest delay in this group was 43 days. Others have also reported average treatment interruptions of 3 weeks or more [6, 8]. The impact of treatment interruptions due to influenza specifically is unknown; however, emerging evidence suggests that relapse rates are higher among children with acute lymphoblastic leukemia who do not receive full maintenance chemotherapy [27]. Studies of other malignancies, primarily in adults, suggest that interruptions are deleterious but the specific outcomes related to treatment delays have not been defined for most types of malignancy [28–32].
In one population of children with 2009 pandemic influenza infection, 55% of the children experienced treatment delays averaging 3 weeks; the longest delay in this group was 43 days. Others have also reported average treatment interruptions of 3 weeks or more. The impact of treatment interruptions due to influenza specifically is unknown; however, emerging evidence suggests that relapse rates are higher among children with acute lymphoblastic leukemia who do not receive full maintenance chemotherapy.
Treatment of Influenza
There are two antiviral medications that are approved by the U.S. Food and Drug Administration and recommended for treatment or chemoprophylaxis of influenza [33–36]. Oseltamivir and zanamivir have activity against influenza A, influenza B, and 2009 pandemic influenza virus. Oseltamivir has been approved in patients older than 2 weeks. Zanamivir has been approved for chemoprophylaxis in children older than 5 years and for treatment in children older than 7 years. Treatment with antiviral medications has been shown to decrease fever duration, reduce the risk of complications from influenza, and shorten the length of hospital stay in the general population. Because of resistance, treatment with amantadine or rimantadine is not recommended.
Early treatment (ideally within 48 hours) is recommended for patients with confirmed or suspected influenza who are hospitalized, have severe or progressive illness, or are at high risk for complications. Treatment should not wait for laboratory confirmation of disease in patients who are at high risk of complications. Patients can have multiple risk factors, which may further increase the risk of morbidity: age younger than 2 years, residence in a chronic care facility, immunosuppressive medications, human immunodeficiency virus, and chronic disease including pulmonary, cardiovascular, renal, hepatic, hematological, neurologic, and metabolic conditions; diabetes; mental retardation or severe developmental delay; muscular dystrophy; and spinal cord injury.
Few studies have evaluated the impact of antiviral therapy specifically for the treatment of influenza in patients undergoing cancer treatment or after bone marrow transplantation (BMT). One study showed BMT patients with 2009 pandemic influenza had prolonged viral shedding (median: 46 days) after symptom resolution; longer treatment was correlated with shortened duration of viral shedding [7]. In another cohort of 62 patients over 12 consecutive influenza seasons, early antiviral therapy was suggested to contribute to decreased progression to pneumonia and decreased viral shedding [37]. Patients were more likely to develop pneumonia if infected earlier after transplantation. In another cohort of BMT patients receiving oseltamivir within 2 days of symptoms, only 5% developed pneumonia and none died from influenza [38].
For non-BMT patients, advantages of early antiviral treatment are similar to those in healthy children [39]. Patients treated with oseltamivir had significant reductions in the risk for respiratory illnesses (other than pneumonia), otitis media, and hospitalization. Delays in treatment were associated with increased progression to lower respiratory tract infection and mortality in this population [40, 41].
Almost all strains of influenza are susceptible to oseltamivir and zanamivir [42]; however, there are a few case reports of resistant influenza isolated from immunocompromised patients. Ison et al. reported three cases of immunocompromised patients with influenza with molecular markers of resistance to anti-influenza drugs [43]. Another study found that half of patients receiving chemotherapy had oseltamivir-resistant influenza viruses with resistant strains identified both prior and during therapy [44].
Influenza Vaccination for Children on Chemotherapy
Although the Advisory Committee on Immunization Practices and the American Academy of Pediatrics have consistently recommended influenza vaccination for children undergoing treatment for malignancy, there has traditionally been little acceptance of this recommendation by oncologists [45–47]. Although overall humoral immunity is compromised, vaccine responses still occur and vaccination is a critical strategy [48–50]. Many hospitals now include influenza vaccination rates among high-risk populations as a quality measure, which would be expected to improve the overall vaccination rates among children on chemotherapy.
Beginning with two landmark studies in the 1970s, all the studies to date have defined serologic responses to the influenza vaccine as the outcome metric [51–58]. Although serologic responses are a critical measure, there is no consensus on what level of antibody should be considered protective in an immunocompromised population [59–61]. Hemagglutination inhibition titers of 1:40 confer protection in the majority of healthy young adults [62–64]. However, in a study of vaccine efficacy in pediatric oncology patients, there was evidence of breakthrough infection in individuals with pre-existing titers of 1:320 [65]. In spite of universal vaccination in the study population, a breakthrough infection rate of 15% using serologic conversion was found. Using positive polymerase chain reaction testing as a second ascertainment strategy, a breakthrough infection rate of approximately 8% was found [65]. These studies suggest that a high rate of protection is conferred by vaccination but also reveal that there is still a need to further optimize management.
A total of 10 studies were identified that included primary data on influenza vaccine efficacy in children undergoing chemotherapy since 1990 [51, 52, 54, 56, 57, 66–70]. These studies generally included patient populations that were heterogeneous in terms of types of malignancy, patient age, and phase or time on chemotherapy; however, they all generally reached the same conclusions. Nearly all the studies found that the patients were generally capable of mounting a response, although the magnitude of the response was generally lower than in healthy controls. A summary of the recent studies is given in Table 1. Two reviews of primary data led to the common recommendation to vaccinate while on maintenance chemotherapy. In these studies, patients were found to have protective anti-influenza antibody rates of 30%–70% (titers of at least 1:32 or 1:40) [52, 70]. Seroconversion rates of 30%–80% (a fourfold increase) were seen in patients [51, 54, 56–58, 66–70].
Table 1.
Recent pediatric oncology vaccine studies
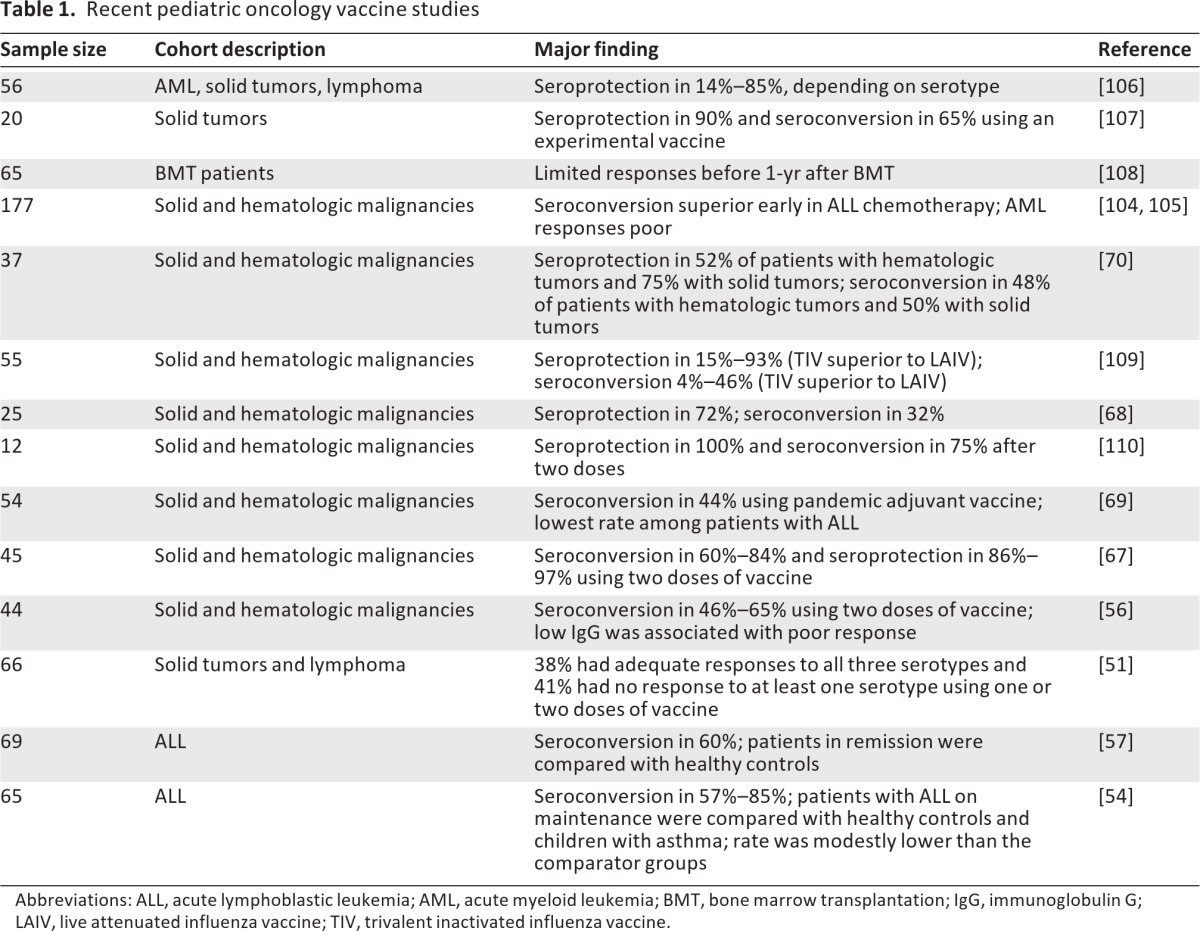
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BMT, bone marrow transplantation; IgG, immunoglobulin G; LAIV, live attenuated influenza vaccine; TIV, trivalent inactivated influenza vaccine.
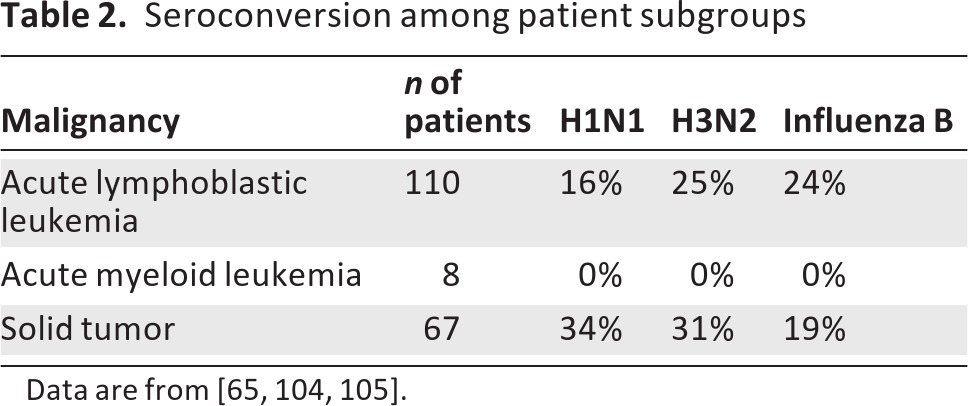
It has been presumed that the type and phase of chemotherapy could impact vaccine response [69]. One recent study directly compared responses across tumor types. This study found that subjects with acute myelogenous leukemia had extremely limited serologic responses to the vaccine (Table 2). Surprisingly, patients with acute lymphocytic leukemia had the best serologic response early in the treatment protocol [65]. In children with solid tumors, responses were limited but did not appear to vary with the aggregate time on chemotherapy. A reasonable strategy is to vaccinate children with cancer as soon as the vaccine is available. Evidence also supports giving multiple doses of the vaccine. Although trials are limited in children, this has been shown in other settings to boost vaccine serologic responses [71, 72].
Table 2.
Seroconversion among patient subgroups
Vaccine Refusal
Parents of healthy children who are not interested in vaccination for their children consistently describe several reasons. Many parents are concerned about side effects, feel that their child has a low risk of disease, or feel that the vaccine causes disease [73]. Other parents are not interested after soliciting advice from their social network [74]. For patients with chronic illness, one of the strongest predictors for vaccination is physician recommendation [75–78]. Other reasons for vaccinating chronically ill children include relative recommendation, easy access to physician office or reminder, belief in vaccine efficacy, and higher parental educational level [79]. Not surprisingly, parents are more interested if they feel that the underlying illness is severe or the vaccine will lessen symptoms or exacerbations from the chronic disease [77, 79].
Kersun et al. published their experience in surveying 100 consecutive parents in an outpatient oncology clinic [80]. The children had a range of oncologic diagnoses and almost all were currently receiving chemotherapy. In all, 94% were current with their immunizations prior to diagnosis and 85% planned to receive the influenza vaccine during the upcoming season. However, 30% were worried their child would get influenza from vaccine, 23% worried their child was too ill, and 56% were concerned about side effects. Of those surveyed, 11 parents stated their child would not receive vaccine, although almost all were current on immunizations prior to diagnosis. More than half of these parents were not confident in the vaccine and felt it was unnecessary. These data highlight the importance of education in the comprehensive approach to influenza control.
Alternative Prevention Strategies
Additional strategies can help to reduce the risk of influenza infection in children with malignancies, including chemoprophylaxis and other behavioral interventions. These measures should be considered adjuncts to vaccination and should not be used in lieu of vaccination.
Primary chemoprophylaxis can be an effective alternative to vaccination in patients who cannot receive influenza vaccination or who cannot respond to influenza vaccine (e.g., a severe primary or secondary immunosuppressing condition). A recent Cochrane review demonstrated that chemoprophylaxis was approximately 90% effective in preventing influenza A in otherwise healthy children. However, it is estimated that 17 children would need to receive chemoprophylaxis to prevent a single case of influenza.
Primary Chemoprophylaxis
Primary chemoprophylaxis refers to the use of antiviral medications to prevent influenza infection. Various chemoprophylaxis strategies have been shown to be effective and are recommended for high-risk patient populations who cannot be vaccinated, cannot respond to vaccination, or have not yet received the vaccine [81]. Seasonal chemoprophylaxis refers to the daily administration of an anti-influenza medication for the 14- to 18-week period when local influenza virus circulation is at its peak. Because of relatively high rates of resistance in circulating strains of influenza A, amantadine and rimantadine are not currently recommended for chemoprophylaxis against influenza [42]. Primary chemoprophylaxis can be an effective alternative to vaccination in patients who cannot receive influenza vaccination or who cannot respond to influenza vaccine (e.g., a severe primary or secondary immunosuppressing condition). A recent Cochrane review demonstrated that chemoprophylaxis was approximately 90% effective in preventing influenza A in otherwise healthy children [82]. However, it is estimated that 17 children would need to receive chemoprophylaxis to prevent a single case of influenza.
There are two reports of chemoprophylaxis in pediatric oncology patients. One study described an influenza outbreak (n = 6) in a pediatric oncology unit with successful chemoprophylaxis of all exposed hospitalized patients [11]. Oseltamivir treatment was instituted in addition to isolation of infected patients, use of protective equipment, and visitor restriction. No new cases developed after these measures were instituted. Another outbreak of four cases of influenza A occurred in an outpatient residential facility for BMT patients [83]. Oseltamivir prophylaxis was given to 45 patients. No new cases of influenza A developed after initiation of oseltamivir. There is one study evaluating oseltamivir prophylaxis during influenza season for pediatric patients with cancer and BMT [84]. Patients receiving chemotherapy or BMT (n = 32) were given oseltamivir prophylaxis for 8 weeks. None of the patients developed influenza.
Secondary Chemoprophylaxis
Secondary chemoprophylaxis refers to the use of antiviral medications for a relatively short period after a known or suspected exposure. Studies performed in households in which a person with a documented influenza infection resides have revealed that antiviral medications can prevent secondary cases of influenza [85, 86]. These studies have not been performed in households with immunocompromised children. Oseltamivir prophylaxis has been recommended in the setting of a nosocomial outbreak of influenza on an oncology unit to interrupt further transmission (as described above) [87]. When postexposure prophylaxis is used in the household setting, it is typically administered for 2 weeks. When oseltamivir is used to control an outbreak of influenza in a health care setting, the drug is typically continued for 1 week after the last documented case of nosocomial influenza.
Barrier Protections
Appropriate application of standard and transmission-based precautions is important to prevent patient-to-patient transmission of influenza. In the hospital setting, transmission-based precautions are recommended for any patient with known or suspected influenza. For most institutions, droplet precautions are used, although some institutions use droplet and contact precautions for respiratory viral infections due to the multiple modes through which these viruses can be transmitted. Cohorting or segregating infected patients to limit their contact with uninfected patients is also an important component of influenza control and can be applied in either an inpatient or outpatient setting [88]. Although sometimes used by families during outings to group activities (e.g., parties, shopping), there have not been any systematic studies of mask use in public to protect oncology patients from infection with influenza.
Social Distancing and Other Interventions
The Centers for Disease Control and Prevention [89, 90] recommends that oncology patients follow “good health habits” as one component of preventing seasonal influenza. These practices include such measures as avoiding close contact with people who are sick, frequent hand cleaning, and avoiding self-inoculation by not touching the mucus membranes of the eyes, nose, and mouth. Additional precautions include the early identification and separation of patients with symptoms of influenza-like illness from other patients.
Conclusions
Pediatric oncology patients are at increased risk of severe influenza. A unified, global strategy can minimize the impact of influenza on treatment and limit patient morbidity and mortality. Vaccination is underused and is probably the single most important intervention [45]. Vaccination is generally effective and should be the cornerstone of all prevention strategies. Vaccination of family members is an adjunct strategy that could be more widely used [91].
To protect oncology outpatients during influenza season, the following outpatient practices are recommended:
Prescreen all scheduled patients for influenza-like illness symptoms.
Instruct patients with influenza-like illness symptoms to don a mask upon arrival to the clinic.
If possible, place patients with influenza-like illness into a private examination room upon arrival.
If immediate placement in a private examination room is not possible, segregate patients with influenza-like illness symptoms within the waiting room to avoid mingling of infected and uninfected patients [89].
In the inpatient setting, droplet precautions and chemoprophylaxis for at-risk patients is warranted. The other component of optimal treatment is the approach to infection control, including specific hospital guidelines, social distancing, and hygiene measures in the home and a concerted effort at the early recognition of influenza in the patient and in others in the hospital setting. Table 3 outlines evidence-supported measures to reduce influenza in pediatric oncology populations.
Table 3.
Evidence-based interventions to minimize influenza morbidity and mortality
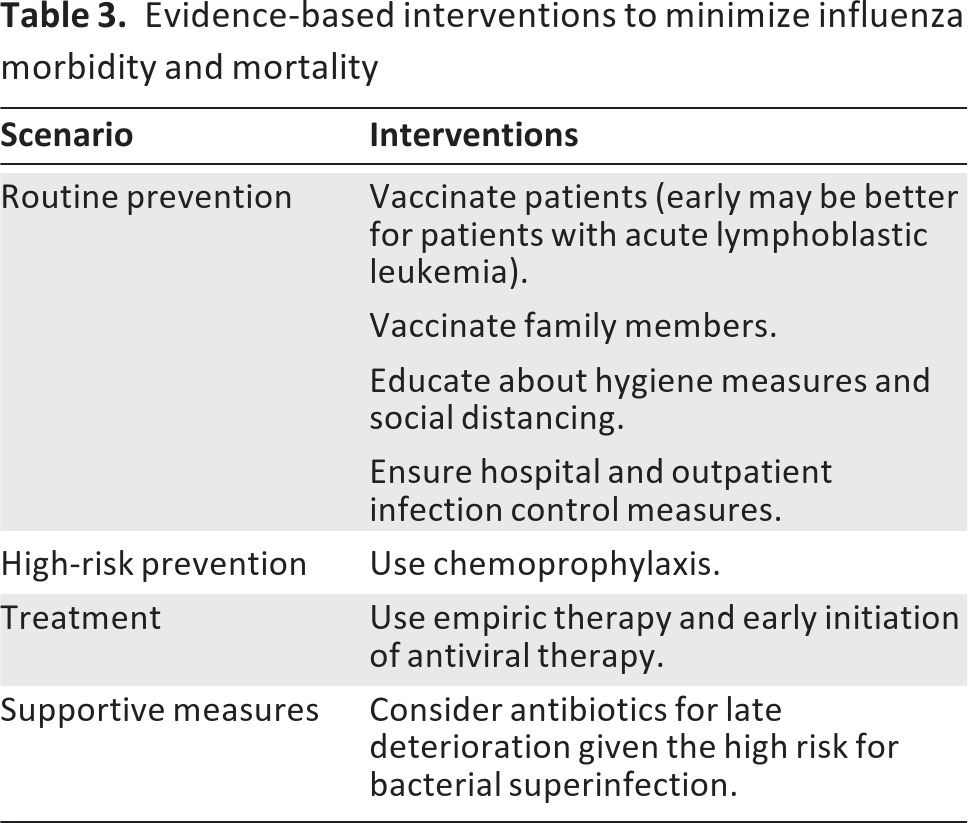
Postexposure prophylaxis has demonstrated efficacy in preventing disease in exposed household or other close contacts. Postexposure prophylaxis is a reasonable practice for high-risk populations if the exposure is known within 48 hours. Use of antiviral medications is critical when there is an institutional outbreak of influenza among patients at high risk for complications secondary to influenza. For active infections, early treatment should be instituted.
It is important to understand similarities and differences with the adult oncology population. Adult oncology patients also benefit from vaccination, although insurance companies do not uniformly cover costs [92–96]. Several studies have implicated rituximab-based protocols as associated with impaired vaccine responses; otherwise, specific protocols have not seemed to affect vaccine responses [97, 98]. Patients with solid tumors, as was seen in children, seem to have slightly better responses than those with hematologic malignancies [99–101]. Early vaccination seems to be optimal for adults as was seen in children [102]. Also concordant with the pediatric experience is the high rate of morbidity and mortality in adult cancer patients with influenza. A case fatality rate of 23% was seen in BMT recipients [104]. In non-BMT patients, case fatality rates have ranged from 10%–30% [2]. Therefore, recommendations for adult patients are largely concordant with those for children.
This review summarizes the current knowledge regarding optimal protection strategies for children with cancer. As survival rates from cancer continue to improve, attention to comorbidities has increased. Optimizing infection control can have significant benefits for patients, so a comprehensive approach to influenza is recommended.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank The Children's Hospital of Philadelphia, the patients, and nurses for their support.
This work was supported by the National Institutes of Health (NO1-AI-50024) and the Wallace Chair of Pediatrics.
Author Contributions
Conception/Design: Kathleen Sullivan, Leslie Kersun, Anne Reilly, Susan Coffin
Collection and/or assembly of data: Kathleen Sullivan, Leslie Kersun, Anne Reilly, Susan Coffin
Data analysis and interpretation: Kathleen Sullivan, Leslie Kersun, Anne Reilly, Susan Coffin
Manuscript writing: Kathleen Sullivan, Leslie Kersun, Anne Reilly, Susan Coffin
Final approval of manuscript: Kathleen Sullivan, Leslie Kersun, Anne Reilly, Susan Coffin
Disclosures
The authors reported no financial relationships.
Section Editors: Rochelle Bagatell: None; John Cunningham: None
Reviewer “A”: Sanofi, Allergan, Peregrine, Pharmacyclics (C/A); Eli Lilly (H); Gilead, Merck, Curis, Onyx, Bristol-Myers Squibb, Amgen, Celgene, Exelixis (O); Epizyme (SAB); PharmaMar (Board of Directors)
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 2.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics. Arch Int Med. 1982;142:85–89. [PubMed] [Google Scholar]
- 4.Tai Y, Lee TC, Chang HL, et al. Epidemiology and outcomes of hospitalization of influenza in the cancer population in Taiwan. J Cancer Res Clin Oncol. 2009;135:1061–1066. doi: 10.1007/s00432-009-0545-0. [DOI] [PubMed] [Google Scholar]
- 5.Seiter K, Shah D, Sandoval C, et al. Prospective evaluation of 2009 H1N1 influenza A in patients admitted with fever to an oncology unit. Infect Control Hosp Epidemiol. 2011;32:815–817. doi: 10.1086/661105. [DOI] [PubMed] [Google Scholar]
- 6.Caselli D, Carraro F, Castagnola E, et al. Morbidity of pandemic H1N1 influenza in children with cancer. Pediatr Blood Cancer. 2010;55:226–228. doi: 10.1002/pbc.22619. [DOI] [PubMed] [Google Scholar]
- 7.Tran D, Science M, Dix D, et al. Pandemic (H1N1) 2009 influenza in Canadian pediatric cancer and hematopoietic stem cell transplant patients. Influenza Other Respi Viruses. 2012;6:e105–e113. doi: 10.1111/j.1750-2659.2012.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasian SK, Park JR, Martin ET, et al. Influenza-associated morbidity in children with cancer. Pediatr Blood Cancer. 2008;50:983–987. doi: 10.1002/pbc.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza Sanchez MC, Ruiz-Contreras J, Vivanco JL, et al. Respiratory virus infections in children with cancer or HIV infection. J Pediatr Hematol Oncol. 2006;28:154–159. doi: 10.1097/01.mph.0000210061.96075.8e. [DOI] [PubMed] [Google Scholar]
- 10.Feldman S, Webster RG, Sugg M. Influenza in children and young adults with cancer: 20 cases. Cancer. 1977;39:350–353. doi: 10.1002/1097-0142(197701)39:1<350::aid-cncr2820390153>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Buchbinder N, Dumesnil C, Pinquier D, et al. Pandemic A/H1N1/2009 influenza in a paediatric haematology and oncology unit: Successful management of a sudden outbreak. J Hosp Infect. 2011;79:155–160. doi: 10.1016/j.jhin.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Chironna M, Tafuri S, Santoro N, et al. A nosocomial outbreak of 2009 pandemic influenza A(H1N1) in a paediatric oncology ward in Italy, October-November 2009. Euro Surveill. 2010;15:19454. doi: 10.2807/ese.15.01.19454-en. [DOI] [PubMed] [Google Scholar]
- 13.Cunha BA, Thekkel V, Krilov L. Nosocomial swine influenza (H1N1) pneumonia: Lessons learned from an illustrative case. J Hosp Infect. 2010;74:278–281. doi: 10.1016/j.jhin.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Treanor JJ. Influenza viruses including avian influenza and swine influenza. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practices of Infectious Diseases. 7th ed. Philadelphia, PA: Elsevier; 2010. pp. 2265–2288. [Google Scholar]
- 15.Klimov AI, Rocha E, Hayden FG, et al. Prolonged shedding of amantadine-resistant influenzae a viruses by immunodeficient patients: Detection by polymerase chain reaction-restriction analysis. J Infect Dis. 1995;172:1352–1355. doi: 10.1093/infdis/172.5.1352. [DOI] [PubMed] [Google Scholar]
- 16.Espinosa-Aguilar L, Green JS, Forrest GN, et al. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: Two centers' experiences. Biol Blood Marrow Transplant. 2011;17:566–573. doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Kersun LS, Coffin SE, Leckerman KH, et al. Community acquired influenza requiring hospitalization: Vaccine status is unrelated to morbidity in children with cancer. Pediatr Blood Cancer. 2010;54:79–82. doi: 10.1002/pbc.22228. [DOI] [PubMed] [Google Scholar]
- 18.Arola M, Ruuskanen O, Ziegler T, et al. Respiratory virus infections during anticancer treatment in children. Pediatr Infect Dis J. 1995;14:690–694. doi: 10.1097/00006454-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Schnell D, Mayaux J, de Bazelaire C, et al. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med. 2010;104:1050–1056. doi: 10.1016/j.rmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Tavil B, Azik F, Culha V, et al. Pandemic H1N1 influenza infection in children with acute leukemia: A single-center experience. J Pediatr Hematol Oncol. 2012;34:48–50. doi: 10.1097/MPH.0b013e3182387d57. [DOI] [PubMed] [Google Scholar]
- 21.Amayiri N, Madanat F. Retrospective analysis of pediatric cancer patients diagnosed with the pandemic H1N1 influenza infection. Pediatr Blood Cancer. 2011;56:86–89. doi: 10.1002/pbc.22805. [DOI] [PubMed] [Google Scholar]
- 22.Elbahlawan L, Gaur AH, Furman W, et al. Severe H1N1-associated acute respiratory failure in immunocompromised children. Pediatr Blood Cancer. 2011;57:625–628. doi: 10.1002/pbc.22973. [DOI] [PubMed] [Google Scholar]
- 23.Kempe A, Hall CB, MacDonald NE, et al. Influenza in children with cancer. J Pediatr. 1989;115:33–39. doi: 10.1016/s0022-3476(89)80325-7. [DOI] [PubMed] [Google Scholar]
- 24.Malik PS, Broor S, Bakhshi S. H1N1 infection in children with hematological malignancies. Indian Pediatr. 2011;48:971–973. [PubMed] [Google Scholar]
- 25.Ozdemir N, Celkan T, Midilli K, et al. Novel influenza a (H1N1) infection in a pediatric hematology oncology clinic during the 2009–2010 pandemia. Pediatr Hematol Oncol. 2011;28:288–293. doi: 10.3109/08880018.2010.550986. [DOI] [PubMed] [Google Scholar]
- 26.Potter MN, Foot AB, Oakhill A. Influenza A and the virus associated haemophagocytic syndrome: Cluster of three cases in children with acute leukaemia. J Clinical Pathol. 1991;44:297–299. doi: 10.1136/jcp.44.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:2094–2101. doi: 10.1200/JCO.2011.38.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabbour E, Saglio G, Radich J, et al. Adherence to BCR-ABL inhibitors: Issues for CML therapy. Clin Lymphoma Myeloma Leuk. 2012;12:223–229. doi: 10.1016/j.clml.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabbour EJ, Kantarjian H, Eliasson L, et al. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87:687–691. doi: 10.1002/ajh.23180. [DOI] [PubMed] [Google Scholar]
- 30.Jeremic B, Shibamoto Y, Milicic B, et al. Impact of treatment interruptions due to toxicity on outcome of patients with early stage (I/II) non-small-cell lung cancer (NSCLC) treated with hyperfractionated radiation therapy alone. Lung Cancer. 2003;40:317–323. doi: 10.1016/s0169-5002(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5:23–31. [PubMed] [Google Scholar]
- 32.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of Ccyr and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–3736. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: Recommendations of the advisory committee on immunization practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 34.Fiore AE, Shay DK, Haber P, et al. Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP), 2007. MMWR Recomm Rep. 2007;56:1–54. [PubMed] [Google Scholar]
- 35.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: Recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 36.Wang K, Shun-Shin M, Gill P, et al. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only) Cochrane Database Syst Rev. 2012;4:CD002744. doi: 10.1002/14651858.CD002744.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols WG, Guthrie KA, Corey L, et al. Influenza infections after hematopoietic stem cell transplantation: Risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 38.Machado CM, Boas LS, Mendes AV, et al. Use of oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34:111–114. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 39.Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124:170–178. doi: 10.1542/peds.2008-0977. [DOI] [PubMed] [Google Scholar]
- 40.Shah DP, El Taoum KK, Shah JN, et al. Characteristics and outcomes of pandemic 2009/H1N1 versus seasonal influenza in children with cancer. Pediatr Infect Dis J. 2012;31:373–378. doi: 10.1097/INF.0b013e3182481ef8. [DOI] [PubMed] [Google Scholar]
- 41.Launes C, Garcia-Garcia JJ, Jordan I, et al. 2009 influenza A H1N1 infections: Delays in starting treatment with oseltamivir were associated with a more severe disease. Pediatr Infect Dis J. 2011;30:622–625. doi: 10.1097/INF.0b013e3182093397. [DOI] [PubMed] [Google Scholar]
- 42.Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza : Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 43.Ison MG, Gubareva LV, Atmar RL, et al. Recovery of drug-resistant influenza virus from immunocompromised patients: A case series. J Infect Dis. 2006;193:760–764. doi: 10.1086/500465. [DOI] [PubMed] [Google Scholar]
- 44.Carr S, Ilyushina NA, Franks J, et al. Oseltamivir-resistant influenza A and B viruses pre- and postantiviral therapy in children and young adults with cancer. Pediatr Infect Dis J. 2011;30:284–288. doi: 10.1097/INF.0b013e3181ff863b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter CC, Poehling KA, Hamilton R, et al. Influenza immunization practices among pediatric oncologists. J Pediatr Hematol Oncol. 2003;25:134–138. doi: 10.1097/00043426-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Smith NM, Bresee JS, Shay DK, et al. Prevention and control of influenza: Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. [PubMed] [Google Scholar]
- 47.Prevention of influenza: Recommendations for influenza immunization of children, 2007–2008. Pediatrics. 2008;121:e1016–1031. doi: 10.1542/peds.2008-0160. [DOI] [PubMed] [Google Scholar]
- 48.Brodtman DH, Rosenthal DW, Redner A, et al. Immunodeficiency in children with acute lymphoblastic leukemia after completion of modern aggressive chemotherapeutic regimens. J Pediatr. 2005;146:654–661. doi: 10.1016/j.jpeds.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 49.Reinhardt D, Houliara K, Pekrun A, et al. Impact of conventional chemotherapy on levels of antibodies against vaccine-preventable diseases in children treated for cancer. Scand J Infect Dis. 2003;35:851–857. doi: 10.1080/00365540310016600. [DOI] [PubMed] [Google Scholar]
- 50.Kosmidis S, Baka M, Bouhoutsou D, et al. Longitudinal assessment of immunological status and rate of immune recovery following treatment in children with all. Pediatr Blood Cancer. 2008;50:528–532. doi: 10.1002/pbc.21327. [DOI] [PubMed] [Google Scholar]
- 51.Chisholm J, Howe K, Taj M, et al. Influenza immunisation in children with solid tumours. Eur J Cancer. 2005;41:2280–2287. doi: 10.1016/j.ejca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Chisholm JC, Devine T, Charlett A, et al. Response to influenza immunisation during treatment for cancer. Arch Dis Child. 2001;84:496–500. doi: 10.1136/adc.84.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross PA, Lee H, Wolff JA, et al. Influenza immunization in immunosuppressed children. J Pediatr. 1978;92:30–35. doi: 10.1016/s0022-3476(78)80065-1. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh YC, Lu MY, Kao CL, et al. Response to influenza vaccine in children with leukemia undergoing chemotherapy. J Formos Med Assoc. 2002;101:700–704. [PubMed] [Google Scholar]
- 55.Lange B, Shapiro SA, Waldman MT, et al. Antibody responses to influenza immunization of children with acute lymphoblastic leukemia. J Infect Dis. 1979;140:402–406. doi: 10.1093/infdis/140.3.402. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzaki A, Suminoe A, Koga Y, et al. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer. 2005;45:831–837. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 57.Porter CC, Edwards KM, Zhu Y, et al. Immune responses to influenza immunization in children receiving maintenance chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:36–40. doi: 10.1002/pbc.10459. [DOI] [PubMed] [Google Scholar]
- 58.Steinherz PG, Brown AE, Gross PA, et al. Influenza immunization of children with neoplastic diseases. Cancer. 1980;45:750–756. doi: 10.1002/1097-0142(19800215)45:4<750::aid-cncr2820450423>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 59.Johnson PR, Feldman S, Thompson JM, et al. Immunity to influenza a virus infection in young children: A comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986;154:121–127. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- 60.Gorse GJ, Belshe RB, Munn NJ. Safety of and serum antibody response to cold-recombinant influenza A and inactivated trivalent influenza virus vaccines in older adults with chronic diseases. J Clin Microbiol. 1986;24:336–342. doi: 10.1128/jcm.24.3.336-342.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Treanor JJ, Mattison HR, Dumyati G, et al. Protective efficacy of combined live intranasal and inactivated influenza a virus vaccines in the elderly. Ann Intern Med. 1992;117:625–633. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- 62.La Montagne JR, Noble GR, Quinnan GV, et al. Summary of clinical trials of inactivated influenza vaccine–1978. Rev Infect Dis. 1983;5:723–736. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- 63.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 64.Hobson D, Curry RL, Beare AS, et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hygiene. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reilly A, Kersun LS, McDonald K, et al. The efficacy of influenza vaccination in a pediatric oncology population. J Pediatr Hematol Oncol. 2010;32:e177–181. doi: 10.1097/MPH.0b013e3181d869f3. [DOI] [PubMed] [Google Scholar]
- 66.Shahgholi E, Ehsani MA, Salamati P, et al. Immunogenicity of trivalent influenza vaccine in children with acute lymphoblastic leukemia during maintenance therapy. Pediatric Blood Cancer. 2010;54:716–720. doi: 10.1002/pbc.22421. [DOI] [PubMed] [Google Scholar]
- 67.Bektas O, Karadeniz C, Oguz A, et al. Assessment of the immune response to trivalent split influenza vaccine in children with solid tumors. Pediatr Blood Cancer. 2007;49:914–917. doi: 10.1002/pbc.21106. [DOI] [PubMed] [Google Scholar]
- 68.Yen TY, Jou ST, Yang YL, et al. Immune response to 2009 pandemic H1N1 influenza virus A monovalent vaccine in children with cancer. Pediatric Blood Cancer. 2011;57:1154–1158. doi: 10.1002/pbc.23113. [DOI] [PubMed] [Google Scholar]
- 69.Bate J, Yung CF, Hoschler K, et al. Immunogenicity of pandemic (H1N1) 2009 vaccine in children with cancer in the united kingdom. Clin Infect Dis. 2010;51:e95–104. doi: 10.1086/657403. [DOI] [PubMed] [Google Scholar]
- 70.Hakim H, Allison KJ, Van De Velde LA, et al. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine. 2012;30:879–885. doi: 10.1016/j.vaccine.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 71.Roos-Van Eijndhoven DG, Cools HJ, Westendorp RG, et al. Randomized controlled trial of seroresponses to double dose and booster influenza vaccination in frail elderly subjects. J Med Virol. 2001;63:293–298. doi: 10.1002/1096-9071(200104)63:4<293::aid-jmv1004>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 72.Falsey AR, Treanor JJ, Tornieporth N, et al. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 73.Flood EM, Rousculp MD, Ryan KJ, et al. Parents' decision-making regarding vaccinating their children against influenza: A web-based survey. Clin Ther. 2010;32:1448–1467. doi: 10.1016/j.clinthera.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Bults M, Beaujean DJ, Richardus JH, et al. Pandemic influenza a (H1N1) vaccination in the Netherlands: Parental reasoning underlying child vaccination choices. Vaccine. 2011;29:6226–6235. doi: 10.1016/j.vaccine.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 75.Lin CJ, Zimmerman RK, Nowalk MP, et al. Parental perspectives on influenza vaccination of children with chronic medical conditions. J Natl Med Assoc. 2006;98:148–153. [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CJ, Nowalk MP, Zimmerman RK, et al. Beliefs and attitudes about influenza immunization among parents of children with chronic medical conditions over a two-year period. J Urban Health. 2006;83:874–883. doi: 10.1007/s11524-006-9084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soyer OU, Hudaverdiyev S, Civelek E, et al. Parental perspectives on influenza vaccination in children with asthma. Pediatr Pulmonol. 2011;46:139–144. doi: 10.1002/ppul.21332. [DOI] [PubMed] [Google Scholar]
- 78.Gnanasekaran SK, Finkelstein JA, Hohman K, et al. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep. 2006;121:181–188. doi: 10.1177/003335490612100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Printza N, Farmaki E, Bosdou J, et al. Pandemic influenza A 2009 (H1N1) vaccination in high-risk children with chronic renal diseases: Acceptance and perceptions. Hum Vaccin. 2010;6:819–822. doi: 10.4161/hv.6.10.12846. [DOI] [PubMed] [Google Scholar]
- 80.Kersun LS, Ingram M, Reilly AF, et al. Parental compliance with the influenza vaccination in children with cancer. Pediatr Blood Cancer. 2009;52:721. [Google Scholar]
- 81.Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: Children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis. 2006;194:S133–S138. doi: 10.1086/507548. [DOI] [PubMed] [Google Scholar]
- 82.Alves Galvao MG, Rocha Crispino Santos MA, et al. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database Syst Rev. 2012;1:CD002745. doi: 10.1002/14651858.CD002745.pub2. [DOI] [PubMed] [Google Scholar]
- 83.Vu D, Peck AJ, Nichols WG, et al. Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: A retrospective case-control study. Clinical Infect Dis. 2007;45:187–193. doi: 10.1086/518985. [DOI] [PubMed] [Google Scholar]
- 84.Chik KW, Li CK, Chan PK, et al. Oseltamivir prophylaxis during the influenza season in a paediatric cancer centre: Prospective observational study. Hong Kong Med J. 2004;10:103–106. [PubMed] [Google Scholar]
- 85.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: A randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 86.Hayden FG, Gubareva LV, Monto AS, et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir family study group. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 87.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 88.Wingard JR. Influenza: Preparedness for an inevitable “emergency” for oncology and BMT units. J Natl Compr Canc Netw. 2008;6:215–222. doi: 10.6004/jnccn.2008.0018. [DOI] [PubMed] [Google Scholar]
- 89.Recommendations for prevention and control of influenza in children, 2011–2012. Pediatrics. 2011;128:813–825. doi: 10.1542/peds.2011-2295. [DOI] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. Preventing infections in cancer patients. [Accessed January 19, 2013]. Available at http://www.cdc.gov/cancer/flu.
- 91.Lessin HR, Edwards KM. Immunizing parents and other close family contacts in the pediatric office setting. Pediatrics. 2012;129:e247–253. doi: 10.1542/peds.2011-2937. [DOI] [PubMed] [Google Scholar]
- 92.Earle CC. Influenza vaccination in elderly patients with advanced colorectal cancer. J Clin Oncol. 2003;21:1161–1166. doi: 10.1200/JCO.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 94.Hottinger AF, George AC, Bel M, et al. A prospective study of the factors shaping antibody responses to the as03-adjuvanted influenza A/H1N1 vaccine in cancer outpatients. The Oncologist. 2012;17:436–445. doi: 10.1634/theoncologist.2011-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mulder SF, Jacobs JF, Olde Nordkamp MA, et al. Cancer patients treated with sunitinib or sorafenib have sufficient antibody and cellular immune responses to warrant influenza vaccination. Clin Cancer Res. 2011;17:4541–4549. doi: 10.1158/1078-0432.CCR-11-0253. [DOI] [PubMed] [Google Scholar]
- 96.Avritscher EB, Cooksley CD, Geraci JM, et al. Cost-effectiveness of influenza vaccination in working-age cancer patients. Cancer. 2007;109:2357–2364. doi: 10.1002/cncr.22670. [DOI] [PubMed] [Google Scholar]
- 97.Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- 98.Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9813-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arrowood JR, Hayney MS. Immunization recommendations for adults with cancer. Ann Pharmacother. 2002;36:1219–1229. doi: 10.1345/aph.1A277. [DOI] [PubMed] [Google Scholar]
- 100.Mackay HJ, McGee J, Villa D, et al. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J Clin Virol. 2011;50:212–216. doi: 10.1016/j.jcv.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Rousseau B, Loulergue P, Mir O, et al. Immunogenicity and safety of the influenza A H1N1V 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: The VACANCE study. Ann Oncol. 2012;23:450–457. doi: 10.1093/annonc/mdr141. [DOI] [PubMed] [Google Scholar]
- 102.Meerveld-Eggink A, de Weerdt O, van der Velden AM, et al. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann Oncol. 2011;22:2031–2035. doi: 10.1093/annonc/mdq728. [DOI] [PubMed] [Google Scholar]
- 103.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: A prospective study from the infectious diseases working party of the European group for blood and marrow transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 104.Kersun LS, Reilly A, Coffin SE, et al. A prospective study of chemotherapy immunologic effects and predictors of humoral influenza vaccine responses in a pediatric oncology cohort. Influenza Other Respi Viruses. 2012 doi: 10.1111/irv.12058. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reilly A, Kersun LS, Luning Prak E, et al. Immunologic consequences of chemotherapy for acute myeloid leukemia. J Pediatr Hematol Oncol. 2013;35:46–53. doi: 10.1097/MPH.0b013e318266c0c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong-Chew RM, Frias MN, Garcia-Leon ML, et al. Humoral and cellular immune responses to influenza vaccination in children with cancer receiving chemotherapy. Oncol Lett. 2012;4:329–333. doi: 10.3892/ol.2012.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shahin K, Lina B, Billaud G, et al. Successful H1N1 influenza vaccination of children receiving chemotherapy for solid tumors. J Pediatr Hematol Oncol. 2012;34:e228–e231. doi: 10.1097/MPH.0b013e318241f7d9. [DOI] [PubMed] [Google Scholar]
- 108.Karras NA, Weeres M, Sessions W, et al. A randomized trial of one versus two doses of influenza vaccine after allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19:109–116. doi: 10.1016/j.bbmt.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carr S, Allison KJ, Van De Velde LA, et al. Safety and immunogenicity of live attenuated and inactivated influenza vaccines in children with cancer. J Infect Dis. 2011;204:1475–1482. doi: 10.1093/infdis/jir561. [DOI] [PubMed] [Google Scholar]
- 110.Cheng FW, Chan PK, Leung WK, et al. Pandemic (H1N1) 2009 vaccine in paediatric oncology patients: One dose or two doses? Br J Haematol. 154:408–409. doi: 10.1111/j.1365-2141.2010.08501.x. 211. [DOI] [PubMed] [Google Scholar]


