Abstract
Context
Psychosocial interventions have been shown to enhance pharmacotherapy outcomes in bipolar disorder.
Objective
To examine the benefits of 4 disorder-specific psychotherapies in conjunction with pharmacotherapy on time to recovery and the likelihood of remaining well after an episode of bipolar depression.
Design
Randomized controlled trial.
Setting
Fifteen clinics affiliated with the Systematic Treatment Enhancement Program for Bipolar Disorder.
Patients
A total of 293 referred outpatients with bipolar I or II disorder and depression treated with protocol pharmacotherapy were randomly assigned to intensive psychotherapy (n=163) or collaborative care (n=130), a brief psychoeducational intervention.
Interventions
Intensive psychotherapy was given weekly and biweekly for up to 30 sessions in 9 months according to protocols for family-focused therapy, interpersonal and social rhythm therapy, and cognitive behavior therapy. Collaborative care consisted of 3 sessions in 6 weeks.
Main Outcome Measures
Outcome assessments were performed by psychiatrists at each pharmacotherapy visit. Primary outcomes included time to recovery and the proportion of patients classified as well during each of 12 study months.
Results
All analyses were by intention to treat. Rates of attrition did not differ across the intensive psychotherapy (35.6%) and collaborative care (30.8%) conditions. Patients receiving intensive psychotherapy had significantly higher year-end recovery rates (64.4% vs 51.5%) and shorter times to recovery than patients in collaborative care (hazard ratio, 1.47; 95% confidence interval, 1.08–2.00; P=.01). Patients in intensive psychotherapy were 1.58 times (95% confidence interval, 1.17–2.13) more likely to be clinically well during any study month than those in collaborative care (P=.003). No statistically significant differences were observed in the outcomes of the 3 intensive psychotherapies.
Conclusions
Intensive psychosocial treatment as an adjunct to pharmacotherapy was more beneficial than brief treatment in enhancing stabilization from bipolar depression. Future studies should compare the cost-effectiveness of models of psychotherapy for bipolar disorder.
Trial Registration
clinicaltrials.gov Identifier: NCT00012558
Bipolar disorder is an extremely debilitating illness, in large part because of the difficulty in treating bipolar depressive episodes. Patients experience significantly greater impairment and longer times to recovery from depressive than manic episodes and high levels of residual depressive symptoms between episodes.1–7 The limited efficacy of pharmacotherapy alone8–11 has motivated the study of adjunctive psychosocial interventions. Randomized controlled trials support the efficacy of adjunctive cognitive behavior therapy (CBT),12,13 family-focused treatment (FFT) or similar forms of family psychoeducation,14–18 interpersonal and social rhythm therapy (IPSRT),19 and group psychoeducation20,21 in preventing depressive and manic recurrences, stabilizing symptoms, or enhancing functioning in 1- to 2-year periods. One multicenter effectiveness trial22 found no main effect of CBT on time to recurrence, although post hoc analyses revealed benefits in patients with fewer than 12 episodes.
Despite these important advances, it is unclear whether psychosocial treatments are effective for the acute treatment of depressed bipolar patients in routine practice settings. Family and interpersonal interventions have typically been initiated during or shortly after an acute manic, mixed, or depressive episode to prevent further recurrences,14–17,19 whereas CBT and group psychoeducation have generally commenced after lengthy periods of remission.13,20 Moreover, most studies have been single-site investigations of single treatments compared with routine care conducted in the academic center where the treatment was developed.
We examined the effectiveness of adjunctive intensive psychosocial interventions in the context of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), a National Institute of Mental Health–sponsored study of the effectiveness of treatments for bipolar disorder. Across 15 study sites we randomly assigned patients to receive intensive psychosocial treatment (up to 30 sessions of CBT, IPSRT, or FFT in 9 months) or a minimal psychosocial intervention, collaborative care (CC), consisting of 3 sessions in 6 weeks. All 4 psychosocial treatments included psychoeducation, relapse prevention planning, and illness management interventions. Collaborative care was designed to provide a brief version of the most common psychosocial strategies shown to offer benefit for bipolar disorder.23,24 In contrast, the intensive treatments represented enhanced versions of these core psychoeducational interventions combined with additional treatment targets: disturbances in family relationships and communication in FFT, cognitive distortions and activity and skill deficits in CBT, and disturbances in interpersonal relationships and social rhythms in IPSRT. Consistent with the STEP-BD objective of evaluating interventions in routine practice, therapists were given modest levels of training (a weekend workshop and low-intensity ongoing monitoring) appropriate for a large-scale practical clinical trial.
We hypothesized that compared with adjunctive CC, adjunctive intensive psychosocial intervention would hasten time to recovery from bipolar depression and increase the likelihood of remaining well for 12 months. Secondarily, we explored whether the 3 intensive interventions (FFT, IPSRT, and CBT) differed in their impact on depressive symptoms.
METHODS
PARTICIPANTS
Participants (N = 293) were enrolled in STEP-BD and provided additional separate informed consent to participate in this study. All consents were approved by the respective site’s human research committee and the STEP-BD Data Safety Monitoring Board.25 Initially eligibility was limited to participants who had entered a 26-week double-blind placebo-controlled comparison of a mood stabilizer (defined in the “Pharmacotherapy” section) plus placebo or a mood stabilizer plus a standard antidepressant agent (bupropion or paroxetine) and were also willing to accept randomization to psychosocial treatment (randomized acute depression [RAD] study; n = 236). When it became apparent that these requirements excluded many otherwise appropriate candidates for psychosocial intervention, we initiated the psychosocial acute depression (PAD) study (n=57), which included patients who were ineligible for the pharmacotherapy trial by reason of previous poor response to both of the study antidepressant agents.
INCLUSION AND EXCLUSION CRITERIA
Participants in the RAD and PAD psychosocial studies met the following eligibility criteria: 18 years or older; meets the DSM-IV26 criteria for current bipolar I or II disorder and a current major depressive episode but does not meet the criteria for a DSM-IV mixed episode or depression not otherwise specified; currently being treated with a mood stabilizer or willing to initiate such treatment; not currently undergoing psychotherapy, or, if so, willing to discontinue nonstudy psychotherapy or taper sessions to 1 or fewer per month; speaks English; and willing and able to give informed consent. Patients were excluded only if they required immediate treatment for a current DSM-IV substance or alcohol abuse or dependence disorder (excluding nicotine); were pregnant or planning pregnancy in the next year; had a history of intolerance, nonresponse, or medical contraindication to paroxetine or bupropion; or required initiation of or dose changes in antipsychotic medications.
DIAGNOSTIC EVALUATION
At the patient’s initial evaluation for STEP-BD, certified study psychiatrists administered the Affective Disorders Evaluation, a semistructured interview adapted from the Structured Clinical Interview for DSM-IV, Patient Version.27,28 A second certified clinical interviewer (psychiatrist, psychologist, social worker, or psychiatric nurse) independently interviewed patients using the Mini-International Neuropsychiatric Interview (version 5.0).29 Study diagnoses were based on a consensus between the 2 interviews.
RANDOMIZATION TO TREATMENTS
Eligible participants were randomly assigned to intensive psychosocial treatment (FFT, CBT, or IPSRT) or to the CC control condition. Block randomization included site, bipolar I or II status, and, if also participating in the RAD study, pharmacologic treatment assignment (mood stabilizer with or without a standard antidepressant). All the sites offered CC and 2 of the 3 intensive psychotherapies. Each site chose 1 intensive psychotherapy based on its clinical expertise; the other psychotherapy was assigned randomly.24,25 Of the 15 sites, 10 offered CBT, 9 offered FFT, and 11 offered IPSRT. At the sites offering FFT, randomization was stratified further by whether family members (typically spouses, parents, or siblings) were willing to participate in family treatment. Patients without available family members could be assigned only to IPSRT, CBT, or CC.
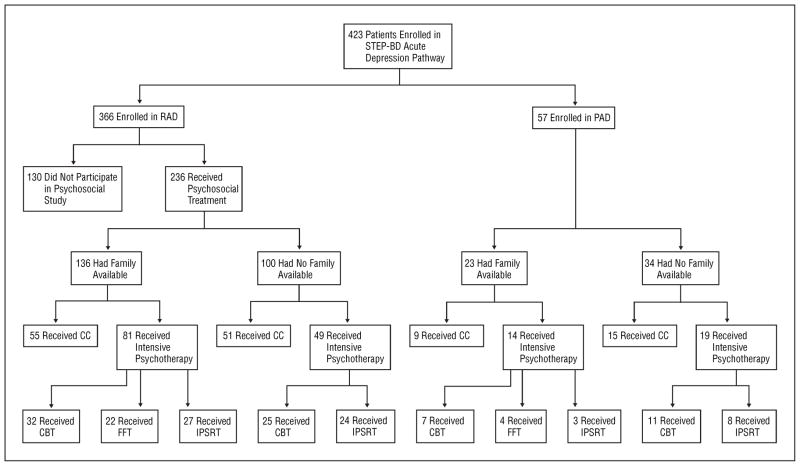
In each stratum, 60% of the eligible patients were randomly assigned to intensive psychotherapy and 40% to CC, resulting in 163 patients being assigned to intensive psychotherapy and 130 to CC (Figure 1). Because only 159 (54.3%) of the 293 patients had family availability, the number randomly assigned to FFT (n=26) was lower than the number assigned to IPSRT (n=62) or CBT (n=75).
Figure 1.
Consort diagram. CBT indicates cognitive behavior therapy; CC, collaborative care; FFT, family-focused therapy; IPSRT, interpersonal and social rhythm therapy; PAD, psychosocial acute depression study; RAD, randomized acute depression study; and STEP-BD, Systematic Treatment Enhancement Program for Bipolar Disorder.
PHARMACOTHERAPY
The 236 patients in the RAD study were randomly assigned to double-blind pharmacotherapy with mood stabilizers (lithium, valproate, and carbamazepine) plus placebo or plus adjunctive antidepressants. The protocol was amended in 2004 to define a mood stabilizer as any Food and Drug Administration–approved antimanic agent. The 57 patients in the PAD trial received treatment in accordance with physician-patient agreement and the STEP-BD guidelines for best-practice evidence-based pharmacotherapy.25
PSYCHOSOCIAL TREATMENTS
Collaborative Care
This control intervention consisted of three 50-minute individual sessions conducted in the 6 weeks after randomization. Participants received a psychoeducational videotape and a workbook that included information about (1) the diagnosis, management, and treatment of bipolar illness; (2) the importance of medication adherence; (3) schedule management (including daily mood charting); (4) typical biases in thinking relevant to mood states; (5) improving relationships through communication skills; and (6) developing a treatment contract geared toward preventing episodes. The CC sessions focused on review of these materials and developing a treatment contract.
Cognitive Behavior Therapy
All intensive treatments consisted of up to thirty 50-minute sessions conducted in 9 months. Individual CBT sessions consisted of (1) psychoeducation regarding the course of bipolar disorder, medication adherence, and stress management; (2) life events scheduling for alleviating inactivity or reducing over-stimulation; (3) cognitive restructuring; (4) problem-solving training; (5) strategies for early detection of and intervention for mood episodes; and (6) selected interventions for comorbidities, if relevant.30 Early sessions focused on monitoring activity and challenging negative thoughts; later sessions focused on challenging dysfunctional beliefs.
Interpersonal and Social Rhythm Therapy
In early sessions of IPSRT, therapists conducted an illness history with a focus on mood episodes associated with disruptions to social routines and sleep/wake cycles (social rhythms).31 A primary problem area was then chosen (ie, grief, role disputes, role transitions, or interpersonal deficits). Therapists acquainted patients with the Social Rhythm Metric,32 a self-report instrument for recording the timing of daily activities (including arising and going to bed), moods, and levels of social stimulation. As treatment progressed, therapists encouraged patients to keep stable social rhythms (eg, when to sleep, exercise, and eat), anticipate events that could disrupt rhythms, and develop plans for continued mood and social rhythm stability. Later in treatment, patients and therapists worked toward interpersonal problem resolution and rehearsed strategies for preventing similar interpersonal problems or social rhythm disruptions in the future.
Family-Focused Therapy
Family-focused therapy began with psychoeducational sessions focused on the symptoms, cause, life course, treatment, and self-management of bipolar disorder.33 Patients and relatives were encouraged to (1) develop a common understanding of precipitants of the index depressive episode, the patient’s vulnerability to future episodes, the need for continuous pharmacotherapy, and the role of stress in provoking episodes and (2) develop a relapse prevention plan involving early intervention for prodromal signs of mania or depression (eg, arranging a pharmacologic reevaluation or de-escalating stressful verbal exchanges). In the intermediate treatment phase, patients and family members participated in communication enhancement exercises designed to reduce levels of negative expressed emotion and rehearse adaptive communication skills.34 In the final phase, families identified, defined, and attempted to solve problems related to the illness (eg, methods to enhance drug adherence) or the home environment.
TRAINING AND MONITORING OF THERAPISTS
Training of STEP-BD therapists was supervised by nationally recognized experts with allegiance to the specific intensive treatments (D.J.M. for FFT; E.F. and Debra Frankel, LCSW, for IPSRT; and M.W.O. and N.A.R.-H. for CBT and CC). Training involved 6-hour workshops supplemented by treatment manuals. After training, therapists could practice any of the modalities assigned to their site. Treatment specialists provided telephone supervision to therapists for the first 2 patients treated in a specific modality. Therapists sent up to 6 audiotaped sessions to the treatment specialists. For CC patients, only 1 session was supervised.
Training was supplemented by monthly case conference calls and ongoing supervision (an average of 2 hours per case). Using modality-specific fidelity scales, treatment specialists judged that 85.6% (143/167) of the CBT sessions, 86.4% (57/66) of the FFT sessions, 82.0% (114/139) of the IPSRT sessions, and 88.8% (79/89) of the CC sessions were acceptable or better in fidelity to the respective treatment models. Low fidelity ratings were evenly spread across the modalities ( ; P =.55). However, 2 study sites accounted for most of the low ratings: of 55 session tapes rated at these sites, 28 (51%) were rated below acceptability thresholds.
ASSESSMENT OF PRIMARY OUTCOMES
The treating psychiatrist assessed clinical status at each out-patient visit using the Clinical Monitoring Form.25 Intraclass interrater reliability coefficients (referenced to gold standard ratings for Clinical Monitoring Form depression and mania items) ranged from 0.83 to 0.99. Clinical status designations were based on the presence or absence of DSM-IV criteria for syndromal depression or mania/hypomania, subsyndromal states (≥3 moderate mood disorder symptoms that did not meet the full DSM-IV criteria for a manic, mixed, or major depressive episode), or recovered status (≤2 moderate symptoms for ≥8 of the previous weeks). These designations allowed for computation of time to recovery and total time in recovery across the year of observation.25,35
At study intake and at quarterly follow-up intervals, independent evaluators conducted interviews with patients covering the previous week to complete the Montgomery-Asberg Depression Rating Scale36 and the Young Mania Rating Scale.37 These ratings were for quality assurance only and were too infrequent to inform the longitudinal analyses planned for the RAD and PAD studies.
STATISTICAL ANALYSIS
All statistical analyses were performed by 2 of us (S.R.W. and M.A.). We used t tests and Kruskal-Wallis tests to compare continuous variables and χ2 tests to compare discrete variables across the RAD vs PAD or intensive vs CC treatments. Time to recovery from major depression was calculated as the number of days from randomization until the patient met the recovery criteria. Analyses were by intention to treat: all randomized patients were included in the at-risk sample, and individuals who did not recover or who terminated prematurely were censored at the point of their final assessment.
Survival curves for time to recovery and time to study dropout (interval from randomization until the last research observation) were compared using the Kaplan-Meier product-limit formula.38,39 Cox proportional hazards models40 were used to assess the independent effect of treatment after controlling for potential confounding effects (site, RAD vs PAD study, family availability, and bipolar I or II status) and included an assessment of the proportionality of the treatment effect.
We hypothesized that patients receiving intensive psychotherapy would be proportionately more likely to be well in any given month of the protocol than patients undergoing CC. We classified each patient, at each monthly interval up to month 12, as well (recovered: ≤2 moderate symptoms on the physician-rated Clinical Monitoring Form for ≥8 weeks; recovering: ≤2 moderate symptoms for <8 weeks) or unwell (subsyndromally or fully manic, depressed, hypomanic, or mixed on the Clinical Monitoring Form). Ordinal logistic mixed-effects regression models41,42 were used to examine this repeated ordinal variable (well, unwell) as a function of treatment group in the intention-to-treat sample, including patients who did not recover during the study year. Secondarily, exploratory analyses examined whether patients in the 4 interventions (CBT, FFT, IPSRT, and CC) differed in time to recovery or the proportion well across time.
RESULTS
STUDY SAMPLE
The 293 participants (mean±SD age, 40.1±11.8 years; range, 17–65 years; 120 males [41.0%] and 173 females [59.0%]) (Table 1) were a subset of the 423 patients who were randomly assigned to experimental treatments for acute depression in the 15 STEP-BD sites (Figure 1). Of 366 patients randomly assigned to pharmacotherapy in the RAD study, 236 (64.5%) agreed to randomization to psychosocial interventions as well. Patients who agreed to psychosocial randomization did not differ significantly from those who refused (n=130) in age, sex, education, self-identified race/ethnicity, bipolar I or II status, number of previous episodes, or age at onset.
Table 1.
Demographic and Illness Characteristics of 293 Bipolar Depressed Patients*
| Variable | Value |
|---|---|
| Age, mean ± SD, y | 40.13 ± 11.77 |
| Female sex | 173 (59) |
| Race | |
| African American | 11 (4) |
| Native American | 1 (0) |
| Asian/Pacific Islander | 2 (1) |
| Other | 3 (1) |
| Hispanic ethnicity | 10 (4) |
| Education >1 y of college | 145 (52) |
| Income <$29 999 | 111 (43) |
| Marital status | |
| Married | 91 (33) |
| Unmarried | 104 (37) |
| Separated | 85 (31) |
| Family available | 159 (54) |
| Diagnosis | |
| Bipolar I | 197 (67) |
| Bipolar II | 90 (31) |
| Bipolar NOS | 5 (2) |
| >10 Previous manic episodes | 192 (66) |
| >10 Previous depressive episodes | 196 (69) |
| Age at illness onset, mean ± SD, y | 16.24 ± 8.44 |
| Baseline MADRS score, mean ± SD† | 21.88 ± 10.13 |
| Baseline YMRS score, mean ± SD† | 5.66 ± 5.70 |
| Depression summary score, mean ± SD‡ | 7.70 ± 2.12 |
| Mania summary score, mean ± SD‡ | 1.17 ± 1.01 |
| Baseline global assessment of functioning, mean ± SD | 53.06 ± 7.87 |
| Duration of index MDE, mean ± SD, d | 206.16 ± 755.46 |
| Rapid cycling in previous year | 79 (29) |
| Medications | |
| Mood stabilizers | 244 (93) |
| Antidepressants | 85 (32) |
| Carbamazepine | 17 (6) |
| Lithium | 120 (46) |
| Lamotrigine | 70 (27) |
| Divalproex sodium | 105 (40) |
| Atypical antipsychotics | 79 (30) |
| Olanzapine-quetiapine | 50 (19) |
| Anxiolytics | 56 (21) |
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; MDE, major depressive episode; NOS, not otherwise specified; YMRS, Young Mania Rating Scale.
Baseline medication regimens were unavailable on 30 patients. Data are presented as number (percentage) unless otherwise indicated. Percentages are not always based on 293 patients owing to missing data.
Refers to scores collected at intake into the study.
Refers to summary scores from the Clinical Monitoring Form25 recorded within 1 week of the date of randomization to treatment.
Baseline medication data were available for 263 (89.8%) of the 293 patients. Of these, 244 (92.8%) began the psychosocial study taking 1 or more mood stabilizers, and 19 (7.2%) were not taking any mood stabilizers; 79 (30.0%) were taking atypical antipsychotics, 85 (32.3%) were taking adjunctive antidepressants, and 56 (21.3%) were taking adjunctive anxiolytics. There were no differences between the RAD psychosocial (n=236), RAD pharmacotherapy-only (n=130), and PAD (n=57) study subsamples on demographic or illness variables except that patients in the PAD trial were less likely to be of minority origin (P = .03) and pharmacotherapy-only patients were more likely to have an income less than $29 999 (P=.005).
BASELINE COMPARISONS OF TREATMENT GROUPS
Table 2 lists the psychosocial treatment assignments as a function of study site. The intensive psychotherapy and CC groups did not differ significantly at the time of psychosocial randomization on demographic, diagnostic, illness history, or current clinical state variables. The 2 groups also did not differ significantly in the proportion of patients being treated at the time of randomization with lithium, divalproex sodium, carbamazepine, lamotrigine, atypical antipsychotics (olanzapine, quetiapine, or any other atypical agent), adjunctive antidepressants, or anxiolytics.
Table 2.
Psychosocial Treatment Assignments as a Function of Study Site
| Site | Psychosocial Treatment Assignment, No. (%)
|
||||
|---|---|---|---|---|---|
| CC | CBT | FFT | IPSRT | Total | |
| Baylor College of Medicine, Houston, Tex | 21 (16) | 0 | 4 (15) | 17 (27) | 42 |
| State University of New York at Buffalo School of Medicine and Biomedical Sciences* | 2 (2) | 0 | 0 | 1 (2) | 3 |
| Case Western Reserve University School of Medicine, Cleveland, Ohio | 16 (12) | 11 (15) | 0 | 10 (16) | 37 |
| University of Colorado Health Sciences Center, Denver | 8 (6) | 9 (12) | 0 | 0 | 17 |
| Cornell University, Weill Medical College, New York, NY* | 1 (<1) | 1 (1) | 0 | 0 | 2 |
| University of Massachusetts Medical School, Worcester | 8 (6) | 0 (0) | 2 (8) | 5 (8) | 15 |
| Massachusetts General Hospital, Boston | 30 (23) | 28 (37) | 12 (46) | 0 | 70 |
| University of Missouri School of Medicine, Kansas City* | 7 (5) | 5 (7) | 2 (8) | 0 | 14 |
| New York University School of Medicine, New York, NY* | 1 (<1) | 0 | 0 | 0 | 1 |
| University of Oklahoma Health Sciences Center, Tulsa | 5 (4) | 5 (7) | 0 | 3 (5) | 13 |
| University of Pennsylvania School of Medicine, Philadelphia | 9 (7) | 7 (9) | 0 | 7 (11) | 23 |
| University of Pittsburgh School of Medicine, Pittsburgh, Pa | 10 (8) | 0 | 4 (15) | 14 (23) | 28 |
| University of Texas Health Science Center, San Antonio | 6 (5) | 3 (4) | 0 | 4 (6) | 13 |
| University of California, San Diego, School of Medicine, La Jolla* | 0 | 0 | 0 | 1 (2) | 1 |
| Stanford University School of Medicine, Stanford, Calif | 6 (5) | 6 (8) | 2 (8) | 0 | 14 |
| Total | 130 | 75 | 26 | 62 | 293 |
Abbreviations: CBT, cognitive behavior therapy; CC, collaborative care; FFT, family-focused therapy; IPSRT, interpersonal and social rhythm therapy.
The site was discontinued from the Systematic Treatment Enhancement Program for Bipolar Disorder.
STUDY ATTRITION AND TREATMENT COMPLETION
Patients in the intensive group began psychosocial sessions a mean ± SD of 17.9 ± 16.1 days after randomization, and those in the CC group began 17.0±10.9 days after randomization (P=.48). Patients in CC attended a mean±SD of 2.2±1.3 of 3 protocol-specified sessions (median, 3.0; range, 0–5; 4 patients received extra sessions for emergencies), whereas patients in the intensive psychotherapy group received a mean±SD of 14.3±11.4 of 30 protocol-specified sessions (median, 13.0; range, 0–30). The mean±SD number of months of intensive psychosocial treatment was 6.8±3.8. Patients in the CBT group attended a mean±SD of 13.3±11.3 sessions (median, 11.0) in a mean±SD of 6.5±4.0 months; in the IPSRT group, 16.7±11.2 sessions (median, 18.5) in 7.2±3.7 months; and in the FFT group, 11.5±11.4 sessions (median, 11.5) in 6.5 ± 2.9 months. Neither the number of sessions (P=.09) nor the months in treatment (P=.53) differed across the intensive groups.
Of the 293 patients, 195 (66.6%) finished the full year of follow-up. Patients in the CC group were as likely to complete the study year (90/130; 69.2%) as patients in the intensive psychotherapy group (105/163; 64.4%) and did not differ in time to study drop-out (log-rank ; P = .35). Likewise, there were no differences among any of the 3 intensive psychotherapies in time to dropout (log-rank ; P = .35). One-year rates of study completion were as follows: FFT, 19/26 (73%); IPSRT, 42/62 (67.7%); CBT, 44/75 (58.7%); and CC, 90/130 (69.2%).
Patients received a mean±SD of 22.6±14.0 sessions of pharmacotherapy from STEP-BD psychiatrists during the study year. The mean ± SD frequency of these sessions did not differ between the intensive psychotherapy (22.7 ± 13.5) and CC (22.5 ± 14.6) groups or across the CBT, FFT, IPSRT, or CC groups (P > .10 for all).
RECOVERY AS A FUNCTION OF TREATMENT GROUP
Of 293 patients, 172 (58.7%) recovered from their depressive episode by the end of the study year, whereas 121 (41.3%) did not recover (n=60) or terminated before a determination of recovery was possible (n=61). The median ± SD time to recovery among the participants who recovered was 122±79 days.
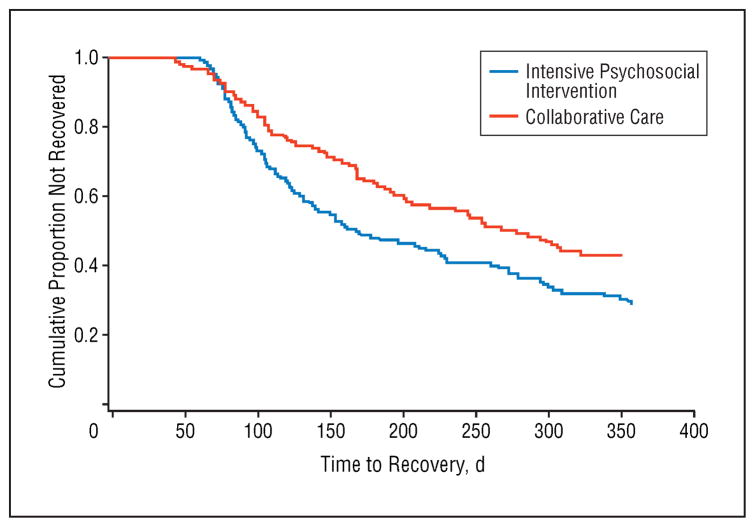
Survival analysis using the Kaplan-Meier method revealed that the cumulative proportion of recovered patients in the intensive psychotherapy conditions was higher than in the CC condition (1-year recovery rate: intensive psychotherapy group, 105/163 [64.4%]; CC group, 67/130 [51.5%]; log-rank ; HR, 1.47; 95% CI, 1.08–2.00; P=.01) (Figure 2). Median±SD time to recovery among patients who recovered was 113±78.2 days in the intensive psychotherapy group and 146 ± 80.0 days in the CC group. The proportionality of risk assumption for the survival curves was upheld ( ; P =.68).
Figure 2.
Time to recovery among 293 bipolar depressed patients assigned to intensive psychosocial intervention or collaborative care. For the intensive group, 50% median time to recovery for the full sample (n = 163), including patients who did not recover, was 169 days (95% confidence interval [CI], 138–230 days); the 25% median was 98 days (95% CI, 88–112 days). For the collaborative care group (n = 130), the 50% median time to recovery was 279 days (95% CI, not calculable); the 25% median was 125 days (95% CI, 105–168 days).
Including study site, assignment to the RAD or PAD study, family availability, and bipolar I or II status as co-variates in a Cox proportional hazards model did not alter the main effect of psychosocial intervention on time to recovery (HR, 1.53; log-rank ; P =.009). Across treatment conditions, RAD trial participants recovered faster than PAD study participants (HR, 1.59; log-rank ; P =.03), and patients with family members (n=159) (Figure 1) recovered more rapidly than those without family members (n=134) (HR, 1.38; log-rank ; P =.047). There were no independent effects of site or bipolar I or II status or interactions of treatment group with these variables on time to recovery (P > .10 for all).
RECOVERY AS A FUNCTION OF TYPE OF INTENSIVE PSYCHOTHERAPY
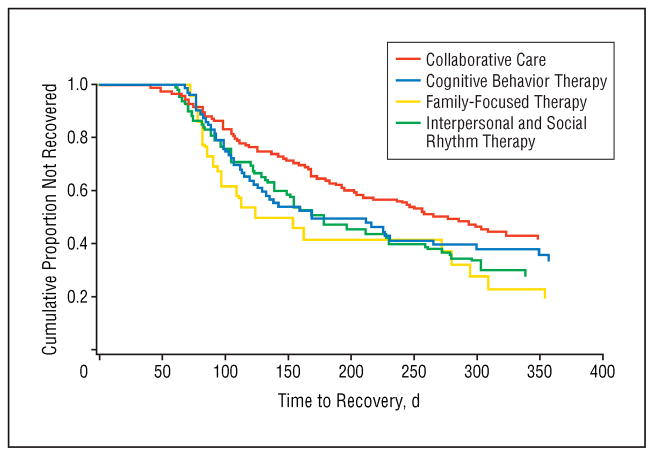
There was a main effect of type of intensive treatment (FFT, CBT, or IPSRT) on time to recovery (log-rank ; P=.046) (Figure 3). Within the 1-year timeframe, 76.9% (20/26) of the patients in the FFT group recovered (HR relative to CC, 1.87), 64.5% (40/62) of the IPSRT patients recovered (HR, 1.48), and 60.0% (45/75) of the CBT patients recovered (HR, 1.34). In comparison, 51.5% (67/130) of the CC patients recovered. The median±SD time to recovery among patients who recovered was 103±94.1 days for FFT, 127.5±76.8 days for IPSRT, 112±72.9 days for CBT, and 146±80.0 days for CC. Results remained significant when site, RAD or PAD study status, and bipolar I or II status were included in a Cox model.
Figure 3.
Time to recovery among 293 bipolar depressed patients assigned to cognitive behavior therapy, interpersonal and social rhythm therapy, family-focused therapy, or collaborative care.
Pairwise comparisons of the 3 intensive modalities revealed no significant differences in time to recovery in the intention-to-treat sample. When considering the sub-sample of patients with family availability, we observed recovery by 1 year in 76.9% (20/26) of the FFT patients (HR relative to CC, 1.40; median±SD days to recovery, 103±94.1), 56.7% (17/30) of the IPSRT patients (HR, 1.16; median±SD days, 105+62.2), 59.0% (23/39) of the CBT patients (HR, 0.98; median±SD days, 106+70.5), and 57.8% (37/64) of the CC patients (median ± SD days, 119+74.6). Finally, among the 3 intensive modalities (n=163), there was no main effect of number of therapy sessions (P=.21) and no interaction between modality and number of sessions on time to recovery (P=.34).
PROPORTION CLASSIFIED AS WELL DURING EACH TREATMENT INTERVAL
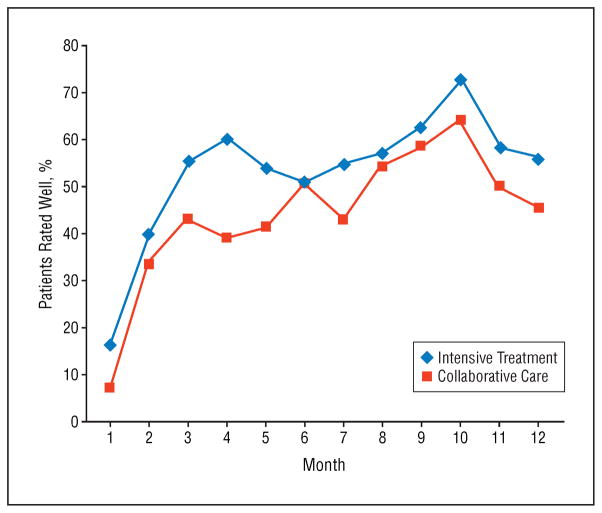
An ordinal logistic mixed-effects regression model revealed that in any given study month, the odds of a patient being well were 1.58 times greater (SE=0.15; 95% CI, 1.17–2.13) in the intensive psychotherapy group than in the CC group (F1,283=9.13; P =.003) (Figure 4). Independent of treatment assignment, patients were more likely to be well in later study months than in earlier months (F11,283=15.65; P < .001). When site, RAD or PAD trial assignment, family availability, and bipolar I or II status were included in the regression model, intensive psychosocial treatment remained associated with a greater likelihood of being well (F1,270=8.80; P =.003; adjusted odds ratio, 1.59; 95% CI, 1.17–2.17), but there were no independent effects of the covariates (P > .10 for all).
Figure 4.
Proportion of patients rated well during each of 12 monthly assessment intervals: intensive psychosocial treatment vs collaborative care.
When the intensive psychotherapy group was stratified according to form of psychotherapy, a main effect of treatment modality was observed on the proportion well (F3,281=3.02; P =.03). Almost identical main effects relative to CC were observed for FFT (odds ratio, 1.60; SE=0.27; 95% CI, 0.94–2.72), IPSRT (odds ratio, 1.61; SE=0.20; 95% CI, 1.09–2.37), and CBT (odds ratio, 1.55; SE=0.19; 95% CI, 1.07–2.25). The main effect of treatment modality was unchanged after adjusting for the effects of site, RAD or PAD study, family availability, and bipolar I or II status (F3,268=3.33; P =.02).
COMMENT
This large multisite randomized trial of bipolar patients treated with mood stabilizers compared 3 types of psychotherapy—CBT, FFT, and IPSRT—with a brief psychosocial treatment in hastening recovery from a depressive episode and maximizing the probability of remaining well during a 1-year period. In contrast to previous trials, patients entered the study early in the development of a major depressive episode (mean Montgomery-Asberg Depression Rating Scale score, 21.9) and, thus, may be more representative of the population of bipolar patients seen for acute care in clinical practice.
Given the increasing acceptance of adjunctive psychosocial interventions for bipolar disorder,43,44 we developed a 3-session comparison condition composed of the many common elements found in existing empirically supported treatments rather than choosing a medication-only control. We found that substituting any 1 of the 3 intensive, specialized, manual-driven interventions for this minimal treatment resulted in clinically significant improvements in time to recovery. Overall, patients were 1.58 times more likely to be well in any study month if they received intensive psychotherapy than if they received CC in addition to their pharmacotherapy.
The present results are consistent with those of previous efficacy trials13–15,19,20,23,24 that found that adjunctive psychotherapy delays recurrences in patients with bipolar disorder. Most of these were single-site randomized controlled trials that required therapists to undergo lengthy periods of training and certification and used time-consuming methods of fidelity monitoring. The benefits observed in the present study were achieved across sites with relatively minimal training and low-intensity supervision. Given the limited benefits of antidepressant medications in patients with bipolar depression who are taking mood stabilizers45 (see also G.S.S., A.A.N., J.R.C., et al, unpublished data, 2007), referral for intensive psychosocial treatment seems to be an especially important addition to clinical care.
In secondary analyses we found no differences among the 3 intensive psychosocial treatments in their capacity to aid and sustain recovery. However, the study was underpowered to detect small effect size differences between each of the intensive modalities. With the observed sample size of 293, a type I error rate of 0.05, a Bonferroni adjustment for 3 comparisons, and 80% power, the intensive modalities would have had to differ from each other by an HR of 3.23 to obtain a statistically reliable treatment effect. Moreover, the sample size needed to identify a statistically significant difference between each of the intensive psychosocial treatments and CC based on the smallest observed effect size of 1.34 (CBT vs CC) would be 445 per group. Focused studies of much larger samples are needed to explore whether the potentially meaningful numerical differences observed between the groups are replicable.
The lack of statistically significant differences between the intensive modalities may also reflect the effect of shared components of the treatments, which are in many ways more striking than their differences.23,46,47 Possibly, future studies will combine the most effective components of the modalities and evaluate hybrid models of psychotherapy.48
Patients in the intensive therapies attended fewer than half (mean, 14.3) of the 30 scheduled sessions. This rate is similar to the frequency that bipolar patients typically obtain in randomized trials (mean, 14 sessions), even when study protocols dictate greater frequencies.22,49 Without an attention control, we cannot determine whether these results are attributable to the specific focus of the intensive psychotherapy sessions or simply the greater number of therapist-patient contacts and, by extension, more opportunities to recognize clinical exacerbations and institute rescue strategies. However, there was no main effect of number of sessions and no interactions between treatment modality and number of sessions on time to recovery. Furthermore, a naturalistic study50 of psychotherapy use in the first 1000 patients to enter the STEP-BD indicated that additional sessions of nonspecialized psychotherapy do not necessarily improve outcome.
Consistent with the evidence-based treatment recommendations of the STEP-BD, approximately 80% of the participants received pharmacologic care concordant with national guidelines (E. Dennehy, PhD, written communication, May 9, 2006); however, the STEP-BD guidelines allowed considerable latitude in drug and dosage selection. The intensive psychotherapy and CC groups were balanced at the time of randomization on the proportion of patients taking each type of mood stabilizer, atypical antipsychotic, or adjunctive agent. Furthermore, the 26-week STEP-BD pharmacotherapy study revealed no differences in time to recovery among patients taking mood stabilizers with or without antidepressants (see G.S.S., A.A.N., J.R.C., et al, unpublished data, 2007). Nonetheless, differences between the intensive and nonintensive psychotherapy conditions in drug choice or dosages might have emerged during the 1-year follow-up. Masking psychiatrists to psychosocial treatment assignments might minimize this source of bias in future studies.
Most of the patients were under the care of a psychiatrist and were receiving mood stabilizers at the time of randomization, and a subset (n=236) were willing and eligible to accept randomized treatment without a standard antidepressant agent. Although few participants were treatment naïve and nearly 70% had a history of more than 10 episodes, it is possible that by pairing the entry criteria for a controlled pharmacotherapy study with a psychosocial intervention study we excluded patients who were highly treatment refractory. Consistent with this possibility, patients who participated in the RAD study had better outcomes than those who did not.
Finally, future trials need to examine the cost-effectiveness of psychosocial interventions. Intensive treatments such as IPSRT, FFT, and CBT, although seeming to be more effective than brief treatments in hastening recovery from episodes, maintaining stability, and delaying recurrences, are also more costly. Treatment-associated costs must be carefully balanced against the potential gains for patients in functioning and quality of life and, possibly, reductions in rates of hospitalization or polypharmacy.
Acknowledgments
Funding/Support: The STEP-BD was funded in part by contract N01MH80001 from the National Institute of Mental Health (Dr Sachs). Support for the development of the psychosocial treatments was provided by grants MH29618 (Dr Frank), MH43931 (Dr Miklowitz), and MH55101 (Dr Miklowitz) from the National Institute of Mental Health and by the National Alliance for Research on Schizophrenia and Depression (Dr Miklowitz).
We thank Debra Frankel, LCSW, for her supervision and coding of the IPSRT sessions.
Footnotes
Author Contributions: Dr Miklowitz verifies that he had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the National Institute of Mental Health. This article was approved by the Publication Committee of the STEP-BD.
Financial Disclosure: Dr Nierenberg has been a consultant to Bristol-Myers Squibb, Genaissance, GlaxoSmithKline, Innapharma, Janssen, Eli Lilly, Novartis, Pfizer, Sepracor, Shire, Somerset, and Sumitomo; has received grant support from Bristol-Myers Squibb, Cedexroth, Cyberonics, Forest, GlaxoSmithKline, Janssen, Lichtwer, Eli Lilly, Pfizer, and Wyeth; and has received honoraria from Bristol-Myers Squibb, Cyberonics, Forest, GlaxoSmithKline, Eli Lilly, and Wyeth.
References
- 1.Gitlin MJ, Swendsen J, Heller TL, Hammen C. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152:1635–1640. doi: 10.1176/ajp.152.11.1635. [DOI] [PubMed] [Google Scholar]
- 2.Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL. The enduring psychosocial consequences of mania and depression. Am J Psychiatry. 1993;150:720–727. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- 3.Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 4.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese JR, Hirschfeld RM, Frye MA, Reed ML. Impact of depressive symptoms compared with manic symptoms in bipolar disorder: results of a U.S. community-based sample. J Clin Psychiatry. 2004;65:1499–1504. doi: 10.4088/jcp.v65n1109. [DOI] [PubMed] [Google Scholar]
- 6.Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ. Bipolar depression: an underestimated treatment challenge. Depress Anxiety. 1997;5:73–83. [PubMed] [Google Scholar]
- 7.Kupfer DJ, Frank E, Grochocinski VJ, Luther JF, Houck PR, Swartz HA, Mallinger AG. Stabilization in the treatment of mania, depression, and mixed states. Acta Neuropsychiatrica. 2000;12:110–114. doi: 10.1017/S0924270800035547. [DOI] [PubMed] [Google Scholar]
- 8.Tohen M, Waternaux CM, Tsuang MT. Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry. 1990;47:1106–1111. doi: 10.1001/archpsyc.1990.01810240026005. [DOI] [PubMed] [Google Scholar]
- 9.Tohen M, Suppes T, Baker RW, Risser RC, Evans AR, Calabrese JR. Olanzapine combined with mood stabilizers in prevention of recurrence in bipolar disorder: an 18-month study. Eur Neuropsychopharmacol. 2002;12(suppl 3):530–537. [Google Scholar]
- 10.Gelenberg AJ, Kane JN, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorders. N Engl J Med. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Sachs GS. Bipolar depression: pharmacotherapy and related therapeutic strategies. Biol Psychiatry. 2000;48:558–572. doi: 10.1016/s0006-3223(00)00980-x. [DOI] [PubMed] [Google Scholar]
- 12.Cochran SD. Preventing medical noncompliance in the outpatient treatment of bipolar affective disorders. J Consult Clin Psychol. 1984;52:873–878. doi: 10.1037//0022-006x.52.5.873. [DOI] [PubMed] [Google Scholar]
- 13.Lam DH, Hayward P, Watkins ER, Wright K, Sham P. Relapse prevention in patients with bipolar disorder: cognitive therapy outcome after 2 years. Am J Psychiatry. 2005;162:324–329. doi: 10.1176/appi.ajp.162.2.324. [DOI] [PubMed] [Google Scholar]
- 14.Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the out-patient management of bipolar disorder. Arch Gen Psychiatry. 2003;60:904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- 15.Rea MM, Tompson M, Miklowitz DJ, Goldstein MJ, Hwang S, Mintz J. Family focused treatment vs. individual treatment for bipolar disorder: results of a randomized clinical trial. J Consult Clin Psychol. 2003;71:482–492. doi: 10.1037/0022-006x.71.3.482. [DOI] [PubMed] [Google Scholar]
- 16.Clarkin JF, Glick ID, Haas GL, Spencer JH, Lewis AB, Peyser J, Demane N, Good-Ellis M, Harris E, Lestelle V. A randomized clinical trial of inpatient family intervention, V: results for affective disorders. J Affect Disord. 1990;18:17–28. doi: 10.1016/0165-0327(90)90113-m. [DOI] [PubMed] [Google Scholar]
- 17.Clarkin JF, Carpenter D, Hull J, Wilner P, Glick I. Effects of psychoeducational intervention for married patients with bipolar disorder and their spouses. Psychiatr Serv. 1998;49:531–533. doi: 10.1176/ps.49.4.531. [DOI] [PubMed] [Google Scholar]
- 18.Fristad MA, Gavazzi SM, Mackinaw-Koons B. Family psychoeducation: an adjunctive intervention for children with bipolar disorder. Biol Psychiatry. 2003;53:1000–1009. doi: 10.1016/s0006-3223(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 19.Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski VJ, Houck P, Scott J, Thompson W, Monk T. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 20.Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, Torrent C, Comes M, Corbella B, Parramon G, Corominas J. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry. 2003;60:402–407. doi: 10.1001/archpsyc.60.4.402. [DOI] [PubMed] [Google Scholar]
- 21.Simon GE, Ludman EJ, Bauer MS, Unutzer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry. 2006;63:500–508. doi: 10.1001/archpsyc.63.5.500. [DOI] [PubMed] [Google Scholar]
- 22.Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H. Cognitive behaviour therapy for severe and recurrent bipolar disorders: a randomised controlled trial. Br J Psychiatry. 2006;188:313–320. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- 23.Miklowitz DJ. A review of evidence-based psychosocial interventions for bipolar disorder. J Clin Psychiatry. 2006;67(suppl 11):28–33. [PubMed] [Google Scholar]
- 24.Miklowitz DJ, Otto MW. New psychosocial interventions for bipolar disorder: a review of literature and introduction of the Systematic Treatment Enhancement Program. J Cogn Psychother. 2006;20:215–230. [PubMed] [Google Scholar]
- 25.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. (Text Revision) (DSM-IV-TR) [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Sachs GS. Use of clonazepam for bipolar affective disorder. J Clin Psychiatry. 1990;51:31–34. [PubMed] [Google Scholar]
- 29.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 30.Otto MW, Reilly-Harrington NA. Cognitive-behavior therapy for the management of bipolar disorder. In: Hofmann SG, Tompson MC, editors. Handbook of Psychosocial Treatments for Severe Mental Disorders. New York, NY: Guilford Press; 2002. pp. 116–130. [Google Scholar]
- 31.Frank E. Treating Bipolar Disorder: A Clinician’s Guide to Interpersonal and Social Rhythm Therapy. New York, NY: Guilford Press; 2005. [Google Scholar]
- 32.Monk TH, Kupfer DJ, Frank E, Ritenour AM. The Social Rhythm Metric (SRM): measuring daily social rhythms over 12 weeks. Psychiatry Res. 1991;36:195–207. doi: 10.1016/0165-1781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- 33.Miklowitz DJ, Goldstein MJ. Bipolar Disorder: A Family-Focused Treatment Approach. New York, NY: Guilford Press; 1997. [Google Scholar]
- 34.Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, Mintz J. Family factors and the course of bipolar affective disorder. Arch Gen Psychiatry. 1988;45:225–231. doi: 10.1001/archpsyc.1988.01800270033004. [DOI] [PubMed] [Google Scholar]
- 35.Otto MW, Simon NM, Wisniewski SR, Miklowitz DJ, Kogan JN, Reilly-Harrington NA, Frank E, Nierenberg AA, Marangell LB, Sagduyu K, Weiss RD, Miyahara S, Thase ME, Sachs GS, Pollack MH STEP-BD Investigators. Prospective 12-month course of bipolar disorder in outpatients with and without comorbid anxiety disorders. Br J Psychiatry. 2006;189:20–25. doi: 10.1192/bjp.bp.104.007773. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 39.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 40.Cox DR. Regression models and life tables. J R Stat Soc [Ser B] 1972;34:187–220. [Google Scholar]
- 41.Hedeker D. A mixed-effects multinomial logistic regression model. Stat Med. 2003;22:1433–1446. doi: 10.1002/sim.1522. [DOI] [PubMed] [Google Scholar]
- 42.Liu LC, Hedeker D. A mixed-effects regression model for longitudinal multivariate ordinal data. Biometrics. 2006;62:261–268. doi: 10.1111/j.1541-0420.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- 43.Keck PE, Perlis RH, Otto MW, Carpenter D, Docherty JP, Ross R. Expert Consensus Guideline Series: treatment of bipolar disorder. Postgrad Med. 2004 Dec;:1–108. [PubMed] [Google Scholar]
- 44.Lembke A, Miklowitz DJ, Otto MW, Zhang H, Wisniewski SR, Sachs GS, Thase ME, Ketter TA. Psychosocial service utilization by patients with bipolar disorders: data from the first 500 participants in the Systematic Treatment Enhancement Program. J Psychiatr Pract. 2004;10:81–87. doi: 10.1097/00131746-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ghaemi SN, Lenox MS, Baldessarini RJ. Effectiveness and safety of long-term antidepressant treatment in bipolar disorder. J Clin Psychiatry. 2001;62:565–569. doi: 10.4088/jcp.v62n07a12. [DOI] [PubMed] [Google Scholar]
- 46.Scott J, Gutierrez MJ. The current status of psychological treatments in bipolar disorders: a systematic review of relapse prevention. Bipolar Disord. 2004;6:498–503. doi: 10.1111/j.1399-5618.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 47.Mansell W, Colom F, Scott J. The nature and treatment of depression in bipolar disorder: a review and implications for future psychological investigation. Clin Psychol Rev. 2005;25:1076–1100. doi: 10.1016/j.cpr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Scott J. Treatment outcome studies. In: Johnson SL, Leahy RL, editors. Psychological Treatment of Bipolar Disorder. New York, NY: Guilford Press; 2004. pp. 226–241. [Google Scholar]
- 49.Lam DH, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, Parr-Davis G, Sham P. A randomized controlled study of cognitive therapy of relapse prevention for bipolar affective disorder: outcome of the first year. Arch Gen Psychiatry. 2003;60:145–152. doi: 10.1001/archpsyc.60.2.145. [DOI] [PubMed] [Google Scholar]
- 50.Miklowitz DJ, Otto MW, Wisniewski SR, Araga M, Frank E, Reilly-Harrington N, Lembke A, Sachs GS. Psychotherapy, symptom outcomes, and role functioning over one year among patients with bipolar disorder. Psychiatr Serv. 2006;57:959–965. doi: 10.1176/ps.2006.57.7.959. [DOI] [PubMed] [Google Scholar]