Abstract
The objectives of this study were to determine the clinical response to Enterra gastric electric stimulation (GES) in patients with refractory gastroparesis and to determine factors associated with a favorable response.
Methods
This study was conducted in patients undergoing Enterra GES for refractory gastroparesis. Symptoms were scored before and after GES implantation using the Gastroparesis Cardinal Symptom Index (GCSI) with additional questions about abdominal pain and global clinical response.
Results
During an 18-month period, 29 patients underwent GES implantation. Follow-up data were available for 28 patients, with average follow-up of 148 days. At follow-up, 14 of 28 patients felt improved, 8 remained the same, and 6 worsened. The overall GCSI significantly decreased with improvement in the nausea/vomiting sub-score and the post-prandial subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for diabetic patients than idiopathic patients. Patients with main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not.
Conclusions
GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. Three clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these three factors may allow improved patient selection for GES.
Keywords: Gastroparesis, Enterra gastric electric stimulation, Diabetic gastroparesis
Introduction
Many patients with gastroparesis have refractory symptoms of nausea, vomiting, and/or abdominal pain despite conventional treatment with prokinetic agents and antiemetic agents. Gastric electric stimulation (GES) has been investigated as a treatment for patients with medically refractory gastroparesis [1–3].
Enterra GES (Medtronic, Inc.) uses an implantable, programmable neurostimulator with placement of stimulation wires into the stomach to deliver high frequency, low energy stimulation. Two multicenter studies have investigated the clinical effectiveness of this therapy [2, 3]. One of these, a prospective, double-blind, on–off stimulation study, suggested that patients with diabetic gastroparesis might respond more favorably than patients with idiopathic gastroparesis [3]. Symptoms of nausea and vomiting appeared to improve more than other symptoms. The Enterra system received a Humanitarian Device Exemption (HDE) and is used for treatment of refractory gastroparesis in certain centers. Since the HDE approval, some centers have reported favorable results [4, 5], whereas other centers have reported less favorable results [6]. Despite the available literature, clinical factors that predict a favorable response to this treatment have not been adequately addressed.
Temple University Hospital has been using GES in patients with refractory gastroparesis. A validated symptom questionnaire, the Gastroparesis Cardinal Symptom Index (GCSI), has been developed to follow the symptoms of patients with gastroparesis [7, 8]. The aims of this study were to determine the clinical response to GES in patients with refractory gastroparesis using the GCSI questionnaire and to identify factors that may be associated with a favorable response.
Methods
This clinical protocol was conducted prospectively at Temple University Hospital in patients undergoing Enterra GES (Medtronic, Inc.) for refractory gastroparesis under the FDA’s Humanitarian Device Exemption program, which has been approved at our institution by our Institutional Review Board.
Subjects
The study included 29 consecutive patients with refractory gastroparesis who were implanted at our institution under the HDE program over an 18-month period from 3/2004 to 9/2005. All patients had documented delayed gastric emptying and had continued symptoms despite conventional therapy with prokinetic agents and antiemetic agents.
Enterra placement
The Enterra gastric electric stimulator was placed surgically under general sedation, primarily via laparotomy by one of two surgeons (JM and SH). The Enterra GES system consists of a pair of electrodes connected to a pulse generator. The two stimulation leads were inserted into the gastric muscularis propria 1 cm apart along the greater curvature 9.5 and 10.5 cm proximal to the pylorus. An upper endoscopy was performed to ensure that there was no penetration of the wires through the mucosa into the stomach lumen. The impedance (resistance) between the wires was measured to ensure it was in the appropriate range (400–800 ohms). A horizontal incision through the skin in the right lower quadrant was performed, and the distal ends of the stimulating wires were tunneled through the abdominal wall, connected to the neurostimulator with reanalysis of the impedance. The neurostimulator with the distal ends of the stimulating wires was then placed into the RLQ subcutaneous pocket. Both the RLQ incision and the laparotomy incisions were closed, followed by repeat interrogation of the stimulator to determine the impedance of the stimulating system. Patients were hospitalized with a median recovery time of approximately 3 days, for advancing the diet and decreasing analgesic pain medications. The day after surgery, the stimulator was turned on. The pulse generator delivers low energy, 0.1-s trains of pulses at a frequency of 12 cycles per minute. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second.
After hospital discharge, patients were seen 2 weeks later for assessment of the incisions. Then patients were followed at 6 weeks, 3 months, 6 months, 9 months and 12 months after stimulator placement. Patients also continued with their usual medical care including visits with their internist and/or endocrinologist who managed the patient’s other medical problems including diabetes.
Questionnaires
Study subjects completed questionnaires to acquire data prior to Enterra implantation (at baseline) and at subsequent visits (including follow-up periods at 1.5, 3, 6, 9 and 12 months). Baseline was defined as the 2-week period before the surgical implantation of the device. The questionnaires included the Gastroparesis Cardinal Symptom Index (GCSI) scores as well as additional questions concerning abdominal pain. The GCSI evaluates nine symptoms using a Likert scale from 0 (none) to 5 (very severe) [7, 8]. The symptoms include nausea, retching, vomiting, stomach fullness, inability to finish a normal-sized meal, feeling excessively full after meals, loss of appetite, bloating and stomach visibly larger. The symptoms are divided into three subscores (nausea/vomiting, post-prandial fullness and bloating). These subscores are then averaged to compile the total GCSI score, which is between 0 and 5. Questions were also asked about the presence of upper abdominal pain and discomfort with similar scoring to the GCSI symptoms.
For assessment of global clinical response, the following question was utilized: “In thinking about the last 2 weeks, how would you say your stomach/gastroparesis-related problems/symptoms have been compared to the period before you started Enterra gastric electric stimulation?” The possible responses included “improved,” “remained the same,” and “worsened.” Additional questions including nutritional status and use of antiemetic and prokinetic agents, and narcotics were also evaluated. For the purposes of this study, use of narcotic analgesic medications were defined as the daily use of regular narcotic analgesic medications. Adverse events were assessed and recorded throughout the study.
Data analysis
The data were entered into a Microsoft Access database and analyzed using SPSS statistical software. Patients were stratified according to global improvement, and all other variables were assigned to each of three groups (improved, remained the same, worsened). Analyses were performed using paired Student’s t-test, ANOVA, and the Wilcoxon signed rank test where appropriate.
Results
Baseline demographics
Twenty-nine patients with refractory gastroparesis were implanted at our institution over an 18-month period under the HDE guidelines for Enterra GES, that is, refractory symptoms of nausea and vomiting from diabetic or idiopathic gastroparesis. The patients consisted of 25 females and 4 males with a mean age of 40.4 years (range 19–66). Follow-up data were available for 28 of the 29 patients (one patient was lost to follow-up). Table 1 shows the baseline demographic information of the 28 patients with follow-up information. Of the 28 patients, 12 had diabetic gastroparesis, all with insulin-dependence. The remaining 16 were classified as idiopathic. There were no patients with post-surgical gastroparesis in this series. Of the 28 patients, all had documented delayed gastric emptying, with an average of 66.7% gastric retention at 4 h after an egg sandwich meal (range 20–100%; normal < 10%) [9]. The mean body mass index was 22.4, with the range 15.5–32.9.
Table 1.
Demographic information of patients undergoing Enterra gastric electric stimulation (GES); for all patients and stratified by the clinical response to treatment with GES
| All (N = 28) | Improved (N = 14) | Remained the same (N = 8) | Worsened (N = 6) | |
|---|---|---|---|---|
| Age (years ± SD) | 40.3 ± 13.1 | 45.4 ± 14.3 | 32.6 ± 8.3 | 38.3 ± 10.9 |
| Gender | 24 female | 10 female | 8 female | 6 female |
| 4 male | 4 male | 0 male | 0 male | |
| GCSI | 3.27 ± 1.01 | 3.24 ± 0.83 | 2.88 ± 1.13 | 3.87 ± 1.10 |
| Nausea/vomiting subscore | 3.83 ± 1.01 | 3.93 ± 0.75 | 3.58 ± 1.34 | 3.95 ± 1.18 |
| Post-prandial subscore | 3.38 ± 1.20 | 3.16 ± 0.92 | 3.06 ± 1.44 | 4.17 ± 1.16 |
| Bloating subscore | 2.61 ± 1.85 | 2.64 ± 1.59 | 1.88 ± 2.10 | 3.50 ± 1.97 |
| Abdominal pain score | 3.18 ± 1.85 | 2.93 ± 1.73 | 2.62 ± 2.26 | 4.50 ± 0.55 |
| Etiology | 12 diabetic | 7 diabetic | 3 diabetic | 2 diabetic |
| 16 idiopathic | 7 idiopathic | 5 idiopathic | 4 idiopathic | |
| Main symptom | 22 N/V | 12 N/V | 5 N/V | 5 N/V |
| 6 abd pain | 2 abd pain | 3 abd pain | 1 abd pain | |
| Use of narcotics | 13: yes | 4: yes | 6: yes | 3: yes |
| 15: no | 10: no | 2: no | 3: no | |
| Baseline gastric emptying test (% retention 4 h) | 67 ± 17 | 72 ± 16 | 54 ± 19 | 71 ± 11 |
Results reported as mean ± SD
Effect on global response
The mean follow-up for the 28 patients was 4.9 ± 0.3 (SEM) months. The data from a completed questionnaire during the last follow-up were used for the outcome data. In response to the global clinical response question, 14–28 patients (50%) felt their symptoms had improved, 8 (29%) their symptoms remained the same, and 6 (21%) felt that their symptoms had worsened (Fig. 1). The clinical characteristics of the patients in each of the three clinical outcomes to GES (improved, the same, worsened) are shown in Table 1. Of the 12 diabetic patients, 7 (58%) felt their symptoms had improved, 3 (25%) felt their symptoms remained the same, and 2 (17%) felt their symptoms had worsened (Fig. 1). Of the 16 idiopathic patients, 7 (44%) were improved, 5 (31%) remained the same, and 4 (25%) worsened (Fig. 1).
Fig. 1.
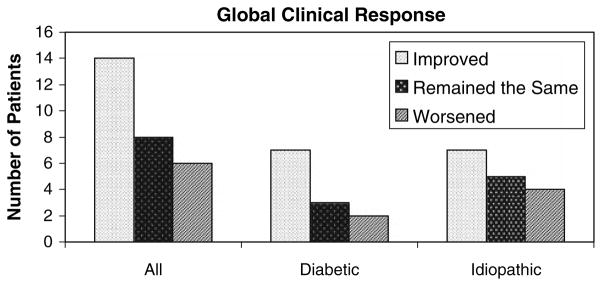
Effect of Enterra gastric electric stimulation on patient’s global clinical response. Overall, 14 of the 28 patients (50%) undergoing GES felt improved, 8 (29%) remained the same, and 6 (21%) felt that their symptoms had worsened. Of the 12 diabetic patients, 7 (58%) felt improved, 3 (25%) remained the same, and 2 (17%) worsened. Of the 16 idiopathic patients, 7 (44%) were improved, 5 (31%) remained the same, and 4 (25%) worsened
Effect of gastric electric stimulation on symptoms using the Gastroparesis Cardinal Symptom Index (GCSI)
Enterra gastric electric stimulation resulted in a significant reduction in GCSI from a baseline score of 3.3 ± 0.2 to 2.7 ± 0.2 (P < 0.05). Among the diabetic subgroup (N = 12), GES resulted in an 18 ± 11% GCSI reduction, from 3.3 ± 0.3 to 2.4 ± 0.2 (P = 0.07). Of the idiopathic patients (N = 16), the GCSI decreased by only 7 ± 9%, from 3.3 ± 0.3 to 3.0 ± 0.3 (P = NS).
In addition to the overall GCSI, GCSI subscores were also analyzed (Fig. 2). GES resulted in a significant decrease (30 ± 7%) in the nausea/vomiting subscore from 3.8 ± 0.2 to 2.6 ± 0.3 (P < 0.01). The post-prandial fullness subscore also significantly decreased from 3.4 ± 0.2 to 3.0 ± 0.3 (P < 0.05). There was no change in the bloating subscore: it remained at 2.6 ± 0.3.
Fig. 2.
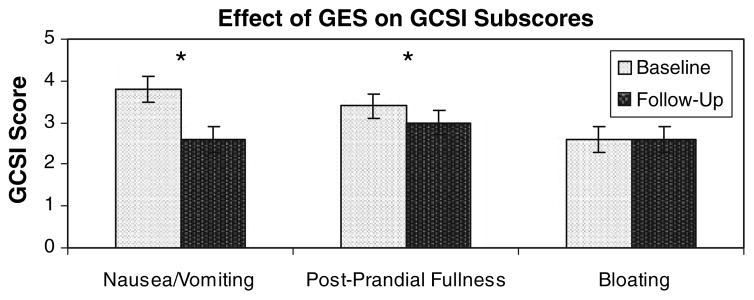
Effect of GES treatment on GCSI subscores. GES resulted in a significant decrease in the nausea/vomiting subscore from 3.8 ± 0.2 to 2.6 ± 0.3 (P < 0.01). The post-prandial fullness subscore significantly decreased from 3.4 ± 0.2 to 3.0 ± 0.3 (P < 0.05). There was no change in the bloating subscore: it remained at 2.6 ± 0.3. Results graphed as mean ± SEM
In response to the added question to the GCSI about abdominal pain, graded on a similar scale of 0 (none) to 5 (very severe), there was no significant difference between the baseline values and the follow-up values. The initial average abdominal pain grade was 3.2 ± 0.35, which slightly increased to 3.3 ± 0.3 at follow-up (P = 0.7).
Results based on main symptom
Of the 28 patients in the study, 22 described nausea and vomiting as the main symptom. For these patients, Enterra stimulation led to a significant decrease in the GCSI from 3.4 ± 0.2 to 2.7 ± 0.2 (P < 0.05) (Fig. 3). Of the six patients that described their primary symptom as abdominal pain, the GCSI was unchanged (3 ± 11% decrease; P = NS). This favorable effect in patients with nausea and vomiting was seen more predominantly in the patients with diabetic gastroparesis rather than in patients with idiopathic gastroparesis (Fig. 3).
Fig. 3.
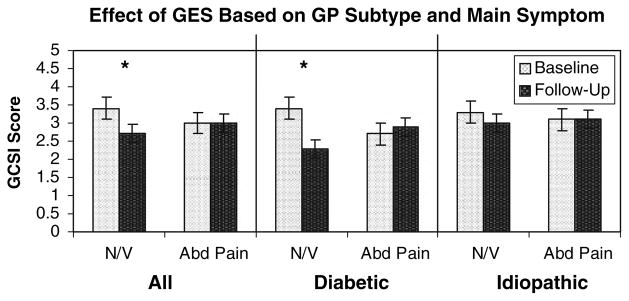
Effect of GES based on gastroparesis subtype and main symptom. Of the 28 patients in the study, 22 described nausea and vomiting as the main symptom. For these patients, Enterra stimulation led to a significant decrease in the GCSI from 3.4 ± 0.2 to 2.7 ± 0.2 (P < 0.05). Of the six patients that described their primary symptom as abdominal pain, the GCSI was unchanged. Results graphed as mean ± SEM. The favorable effect of Enterra GES on clinical symptoms was primarily seen in diabetic patients with main symptom of nausea and vomiting. Results graphed as mean ± SEM
Other factors impacting clinical response
One of the objectives of this study was to determine which patient factors were associated with improved outcomes. Narcotic use was a target of evaluation. Thirteen patients were taking regular daily narcotic analgesic medications at the outset of the study. The 15 patients not using narcotics experienced a significant decrease in the GCSI of 30 ± 8%, from 3.8 ± 0.2 to 2.1 ± 0.4 (P < 0.01), while those on narcotics had no change in the GCSI (9 ± 10% decrease from 3.1 ± 0.3 to 3.1 ± 0.2) (Fig. 4).
Fig. 4.
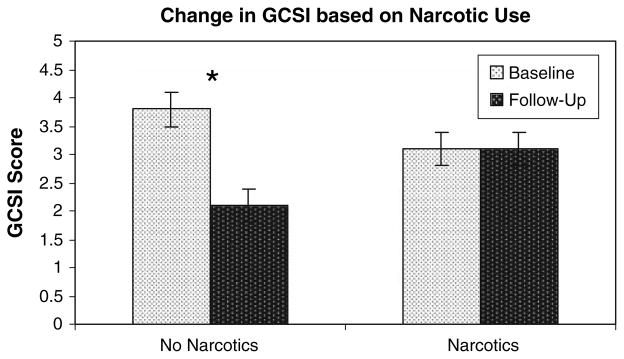
Effect of narcotic analgesic use on clinical response to GES. Use of regular narcotic analgesics reduced the response rate. Thirteen patients were taking some form of narcotic agents at the outset of the study. The 15 patients not using narcotics experienced a significant decrease in the GCSI from 3.8 ± 0.2 to 2.1 ± 0.4 (P < 0.01), while those on narcotics had no change in the GCSI (3.1 ± 0.3 to 3.1 ± 0.2). Results graphed as mean ± SEM
There was no effect of gender, BMI, baseline gastric emptying test or hemoglobin A1c in diabetics on the clinical response. For gastric emptying, there was no correlation with baseline emptying scan and clinical response. All patients had delayed gastric emptying. The mean gastric emptying scans for the improved, same and worsened groups are 72%, 54% and 71%, respectively (Table 1). Of the two patients with gastric retention between 10% and 50% at 4 h, both felt the “same” after implantation. Of the 13 with gastric retention between 50% and 75% retained, 4 were the same, 3 were worse, and 6 were better. Of the six with gastric retention >75% at 4 h, four improved and two worsened.
Adverse events
Of the 28 patients implanted, 9 had subsequent hospitalizations, generally for gastroparesis-related symptoms. All nine of these patients had had multiple admissions to the hospital for gastroparesis-related symptoms prior to implantation. Four patients had a jejunostomy tube placed (three idiopathic gastroparetic patients and one diabetic patient). There was one admission for a bowel obstruction due to J-tube placement. Two patients had their stimulators surgically removed [one as part of a sub-total gastrectomy, the other for a lack of symptom response to the gastric stimulator (both idiopathic gastroparetic patient)]. There was one incidence of deep-vein thrombosis due to central line placement. One PICC line infection occurred (diabetic patient), and another patient had a gram-negative rod bacteremia. Two deaths occurred during the study: one from pancreatic cancer (idiopathic patient) and the other from sepsis (diabetic patient). Two syncopal episodes occurred, as well as one psychotic episode and one seizure.
Discussion
In this single-center study using Enterra GES for treatment of patients with refractory gastroparesis in clinical practice, GES resulted in clinical symptomatic improvement in 50% of patients. Three clinical parameters were found to impact on clinical response: etiology of gastroparesis, main symptom and use of narcotics. Diabetic gastroparetics had a more favorable outcome than idiopathic patients. Patients whose main symptoms were nausea and/or vomiting experienced a more favorable response than those with abdominal pain as their main symptom. Lastly, patients not taking narcotic analgesic medications had better outcome than those patients using narcotics at the study outset.
Enterra gastric electric stimulation is approved for use in patients with refractory symptoms associated with gastroparesis under a FDA compassionate HDE. Two multicenter trials have been conducted to evaluate the efficacy of this high frequency, low energy gastric electrical stimulator in patients with diabetic and idiopathic gastroparesis [2, 3]. In an open label study, 35 of 38 patients (mostly with idiopathic gastroparesis) experienced >80% reductions in nausea and vomiting that persisted for the duration of the observation period (3–15 months) [2]. Although many individuals were able to discontinue enteral or parenteral nutrition, one quarter of patients needed to undergo additional surgeries including sub-total gastrectomy for symptom control and device removal for complications. The second multicenter investigation represents the only sham-stimulation controlled study to date [3]. In this trial, 33 gastroparesis patients (16 idiopathic, 17 diabetic) were randomized to sham versus active stimulation for 1 month each in double-blind, crossover fashion followed by an open label stimulation period to 12 months. During the blinded phase, vomiting frequencies were 14% lower when the device was on compared to times when the device was turned off—a difference reported to be statistically significant. Furthermore, patients preferred the ON period over the OFF period by a three-fold margin. Interestingly, the benefits of treatment were predominantly experienced by the diabetic group—a finding found in this present study reported herein. During the open phase of the study, electrical stimulation produced a 76% reduction in vomiting at 12 months. Approximately 15% of patients required having the device removed or revised due to complications.
There have been other studies reporting on an individual institution’s experience with Enterra GES [4–6, 10–12]. In two single center studies, electrical stimulation has been reported to improve nutritional status, limit the need for prokinetic and antiemetic medications, and reduce the need for hospitalizations and supplemental nutrition [4, 10]. While the published results of Enterra GES are encouraging, the clinical benefits of GES have not been unequivocally demonstrated. Favorable responses have been largely reported by two centers. Some smaller studies have found less favorable responses [6].
The study reported here evaluates the clinical use of Enterra GES for treatment of patients outside of a multi-center clinical trial. Candidates for implantation of the gastric electrical stimulator included patients with chronic diabetic or idiopathic gastroparesis with nausea and vomiting who were not responding to appropriate diet and medication therapy. The clinical response rate to GES in this study (50% patient improvement) is lower than reported in prior multicenter studies, but provides realistic estimates for the response to treatment in clinical practice. The study reported here is a short-term analysis with most patients followed for 4–7 months. In the results reported, patients were not all assessed at the same time due to scheduling difficulties; rather the last available appointment was used for the data collection. Results of other studies suggest favorable responses, if they are to occur, will take place within the initial 3–6 months, although this needs further study. In an abstract with long-term patient follow-up, investigators observed 26% and 44% reductions in nausea and vomiting, respectively, persisting for up to 10 years [11].
In this study, several factors impact on the outcome of GES. Knowledge of these factors may allow better patient education on their expected response to treatment and may allow the physician improved patient selection for treatment with GES. Patients with diabetic gastroparesis responded better (58% of the patients improved) than those with idiopathic gastroparesis (44% of the patients improved). The double-blind study reported by Abell et al. [3] also showed that patients with diabetic gastroparesis did better than patients with idiopathic gastroparesis. In a recent report of the experience at Kansas University Medical Center, similar results to this present study were reported with the clinical improvement greater in diabetic gastroparesis than in idiopathic gastroparesis [13]. In this present study, patients with the main symptom of nausea and vomiting did better than those patients with a main symptom of abdominal pain. Abdominal pain in gastroparesis also does not respond well to prokinetic treatment [14]. This study also showed that the use of narcotic analgesics was associated with a decreased response to Enterra GES. Opioid analgesics are known to delay gastric emptying [15]. Although chronic narcotic analgesic use may reduce the symptomatic benefits of gastric electrical stimulation, the need for opiates should be evaluated on an individual basis and does not necessarily represent an exclusion criterion [16]. In addition, use of narcotics may be related to the presence of abdominal pain, another poor prognostic factor.
This study explored factors that might influence the response to GES. The results suggest a pattern of three predictive factors (etiology of gastroparesis, main symptom, use of narcotic pain medications) for a favorable response to GES. However, the number of factors assessed was high considering the sample size of patients, which although small is actually relatively large for a single center study. The small numbers in the various groups preclude a more elaborate analysis that evaluates interactions between the factors and the relative contribution of each variable. Small numbers can explain why there is a significant difference in GCSI when all patients are analyzed, as compared to subgroups. The patients were followed clinically, and routine follow-up tests such as gastric emptying tests and Hgb-A1c were not routinely obtained. For diabetic patients, glucose control was managed by the patient’s internist or endocrinologists.
Other factors might also be helpful for prognostic information with GES. The presence of interstitial cells of Cajal on full thickness gastric biopsies obtained at the time of stimulator insertion has been suggested to be predictive of clinical response [17]. Generally, this full thickness biopsy is obtained at the time that the stimulator is placed and results are not available to guide treatment. Preliminary reports suggest that the response to temporary gastric stimulation might predict the response to longer-term stimulation [18]. Enterra GES might be helpful in patients with nausea and vomiting from other conditions. Several manuscripts have suggested this might be helpful in patients with postoperative gastroparesis [19, 20].
Although beneficial in half of the patients, some patients experienced adverse events or complications. Several patients not responding to treatment had subsequent jejunostomy feeding tubes placed. Several catheter-related infections were related to the continued need for total parenteral nutrition. The most common reported complication of this form of therapy is infection of the subcutaneous stimulator pocket, which occurred in 5–10% of patients and nearly always requires surgical removal of the device [2, 3]. Other complications include wire breakage, electrode dislodgement or penetration of the stomach, and intestinal obstruction.
In conclusion, this single-center study using Enterra GES for clinical care of patients with refractory gastroparesis reports a symptomatic improvement in half of the patients. Three clinical parameters were found to impact on clinical response: etiology of gastroparesis, main symptom and use of narcotics. Diabetic gastroparetics had a more favorable outcome than idiopathic patients. Patients with their main symptom as nausea and vomiting also experienced a more favorable response than those with the main symptom of abdominal pain. Lastly, patients not taking narcotics also had better outcomes than those patients using narcotics at the study outset. Knowledge of these three factors may allow better education of patients about the efficacy of GES and may also provide improved selection of patients for GES therapy.
Acknowledgments
This study was supported in part by a NIH Midcareer Investigator Award in Patient-Oriented Research to HP Parkman (NIH DK02921).
Contributor Information
Jennifer L. Maranki, Department of Medicine, Gastroenterology Section, Temple University School of Medicine, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA
Vanessa Lytes, Department of Medicine, Gastroenterology Section, Temple University School of Medicine, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA.
John E. Meilahn, Department of Surgery, Temple University School of Medicine, Philadelphia, PA, USA
Sean Harbison, Department of Surgery, Temple University School of Medicine, Philadelphia, PA, USA.
Frank K. Friedenberg, Department of Medicine, Gastroenterology Section, Temple University School of Medicine, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA
Robert S. Fisher, Department of Medicine, Gastroenterology Section, Temple University School of Medicine, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA
Henry P. Parkman, Email: henry.parkman@temple.edu, Department of Medicine, Gastroenterology Section, Temple University School of Medicine, Parkinson Pavilion, 8th Floor, 3401 North Broad Street, Philadelphia, PA 19140, USA
References
- 1.McCallum RW, Chen JDZ, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1988;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 2.Abell T, Custem EV, Abrahamsson H, Huizinga JD, Konturek JW, Galmiche JP, Voeller G, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–221. doi: 10.1159/000068359. [DOI] [PubMed] [Google Scholar]
- 3.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 4.Cutts TF, Luo J, Starkebaum W, Rashid H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term healthcare benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum R. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50(7):1328–1334. doi: 10.1007/s10620-005-2782-7. [DOI] [PubMed] [Google Scholar]
- 6.Jones MP, Maganti K. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol. 2003;98(10):2122–2129. doi: 10.1111/j.1572-0241.2003.07721.x. [DOI] [PubMed] [Google Scholar]
- 7.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13(4):833–844. doi: 10.1023/B:QURE.0000021689.86296.e4. [DOI] [PubMed] [Google Scholar]
- 8.Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Development and validation of a patient-assessed gastroparesis symptoms severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 9.Guo J-P, Maurer AH, Urbain J-L, Fisher RS, Parkman HP. Extending gastric emptying scintigraphy from two to four hours detects more patients with gastroparesis. Dig Dis Sci. 2001;46:24–29. doi: 10.1023/a:1005697422454. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum R. Chronic gastric electrical stimulation for gastroparesis reduced the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328–1334. doi: 10.1007/s10620-005-2782-7. [DOI] [PubMed] [Google Scholar]
- 11.Abell T, Lou J, Tabaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow up. J Parenter Enteral Nutr. 2003;98:277–281. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 12.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27(5):1071–1076. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 13.Lin Z, Sarosiek I, Forster J, McCallum RW. Characteristics of outcomes of gastric electrical stimulation in diabetic, postsurgical and idiopathic gastroparesis. Gastroenterology. 2006;130:A135 (abstract). [Google Scholar]
- 14.Abell T, Al-Juburi A, Rashed H, Mirocha A. 13 years, 214 patients and over 5000 patient months: a long term report on gastric electric stimulation. Gastroenterology. 2005;128(4 Suppl 2):A282 (abstract). [Google Scholar]
- 15.Parkman HP, Gonlachanvit S, Hsu C-W, Kantor S, Knight LC, Boden GH, Maurer AH, Fisher RS. Effect of altering gastric emptying on postprandial glucose following a physiologic meal in type II diabetic patients. Dig Dis Sci. 2003;48:488–497. doi: 10.1023/a:1022528414264. [DOI] [PubMed] [Google Scholar]
- 16.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR, Pasricha PJ, Prather CM, Soffer EE, Twillman R, Vinik AI AMS Gastroparesis Task Force. Treatment of gastroparesis: a multidisciplinary review. Neurogastroenterol Motil. 2006;18(4):263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 17.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Ayinala S, Batista O, Goyal A, Al-Juburi A, Abidi N, Familoni B, Abell T. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 19.Oubre B, Luo J, Al-Juburi A, Voeller G, Familoni B, Abell TL. Pilot study on gastric electric stimulation on surgery-associated gastroparesis: long-term outcome. South Med J. 2005;98:693–697. doi: 10.1097/01.smj.0000168660.77709.4d. [DOI] [PubMed] [Google Scholar]
- 20.McCallum R, Lin Z, Wetzel P, Sarosiek I, Forster J. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3(1):49–54. doi: 10.1016/s1542-3565(04)00605-6. [DOI] [PubMed] [Google Scholar]


