SUMMARY
The LKB1 (also called STK11) tumor suppressor is mutationally inactivated in ~20% of non-small cell lung cancers (NSCLC). LKB1 is the major upstream kinase activating the energy-sensing kinase AMPK, making LKB1-deficient cells unable to appropriately sense metabolic stress. We tested the therapeutic potential of metabolic drugs in NSCLC and identified phenformin, a mitochondrial inhibitor and analog of the diabetes therapeutic metformin, as selectively inducing apoptosis in LKB1-deficient NSCLC cells. Therapeutic trials in Kras-dependent mouse models of NSCLC revealed that tumors with Kras and Lkb1 mutations, but not those with Kras and p53 mutations showed selective response to phenformin as a single agent, resulting in prolonged survival. This study suggests phenformin as a cancer metabolism-based therapeutic to selectively target LKB1-deficient tumors.
INTRODUCTION
The gene encoding the serine/threonine kinase LKB1 was identified originally as the tumor suppressor gene responsible for the inherited cancer disorder Peutz-Jeghers Syndrome (Hemminki et al., 1998). LKB1 is also the second most commonly mutated tumor suppressor in sporadic human lung cancer (after TP53), particularly in multiple subtypes of NSCLC (Sanchez-Cespedes et al., 2002). LKB1 is mutated in at least 15-30% of NSCLCs but the true frequency might be even higher due to difficulties in detecting inactivating lesions (Ding et al., 2008; Gill et al., 2011; Ji et al., 2007). Roughly half of the NSCLC tumors with LKB1 mutation also bear activating KRAS mutations, and current estimates suggest that 7-10% of all NSCLC are co-mutated for KRAS and LKB1 (Ding et al., 2008; The Cancer Genome Atlas). Studies in genetically engineered mouse models have shown that simultaneous activation of KrasG12D and bi-allelic deletion of Lkb1 in the lung dramatically increases tumor burden and metastasis (Carretero et al., 2010; Chen et al., 2012; Ji et al., 2007).
Biochemical and genetic analyses in worms, flies, and mice have shown LKB1 is the major kinase phosphorylating the AMP-activated protein kinase (AMPK) under conditions of energy stress across metazoans (Hardie et al., 2012). AMPK is a highly conserved energy sensor and modulator of cell growth and metabolism that is activated under conditions of low intracellular ATP. Activated AMPK regulates cell growth at least in part through inhibition of mTORC1 signaling achieved through dual phosphorylation of TSC2 (Inoki et al., 2003) and Raptor (Gwinn et al., 2008). AMPK is also hypothesized to maintain energy homeostasis in part by targeting defective mitochondria for autophagy (Egan et al., 2011) and control of fatty acid metabolism (Jeon et al., 2012).
The diabetes therapeutic biguanide compounds metformin and phenformin have been shown to inhibit complex I of the mitochondria (Dykens et al., 2008; El-Mir et al., 2000; Owen et al., 2000), resulting in increases in intracellular AMP and ADP that bind to the gamma regulatory subunit of AMPK and trigger LKB1-dependent phosphorylation of AMPK (Hawley et al., 2010). Consistent with activation of a low energy checkpoint, metformin treatment has been found to reduce tumor growth in xenograft, transgenic, and carcinogen-induced mouse cancer models (Algire et al., 2010; Anisimov et al., 2005; Buzzai et al., 2007; Memmott et al., 2010). Epidemiological studies revealed that diabetic patients taking metformin show a statistically significant reduced tumor incidence (Dowling et al., 2012; Evans et al., 2005). Given the extensive knowledge on the safety and use of metformin, there is increasing interest in using metformin as an anti-cancer agent (Taubes, 2012). Phenformin is a 50-fold more potent inhibitor of mitochondrial complex I than metformin (Dykens et al., 2008; Owen et al., 2000). Moreover, uptake of metformin, but not phenformin, into tissue appears to require the expression of Organic Cation Transporter 1 (OCT1), which is highly expressed in hepatocytes but not elsewhere (Shu et al., 2007). Consistent with greater potency and broader tissue bioavailability, phenformin delayed tumor progression in a Pten+/− spontaneous lymphoma mouse model to a much greater extent than metformin (Huang et al., 2008).
In most settings metabolic stress induces a cytostatic growth arrest, dependent in part on AMPK. However, in cells lacking a functional LKB1 pathway, metabolic stress has been demonstrated to result in rapid apoptosis as the cells are unable to sense the energy stress and activate mechanisms to restore energy homeostasis (Shaw et al., 2004). Similar effects are seen in autophagy-defective cells that are unable to restore metabolism under low nutrient conditions (Jin et al., 2007). Here we directly examine the hypothesis that LKB1-deficient lung tumors may be targeted with metabolic drugs.
RESULTS
Phenformin robustly induces apoptosis in LKB1 deficient NSCLC cell lines
We previously reported that non-transformed murine embryonic fibroblasts and HeLa cervical carcinoma cells deficient of LKB1 exhibit increased apoptosis in response to metabolic stresses including glucose deprivation and the AMP mimetic AICAR (Shaw et al., 2004 ). However, whether tumor cells undergo growth arrest or apoptosis following a particular cellular insult is dependent in part on the full constellation of oncogenic mutations present in the cell. We sought to determine whether NSCLC cell lines bearing KRAS mutations and lacking LKB1 would show enhanced rates of apoptosis following metabolic stress treatments compared to isogenic lines in which wild-type LKB1 had been restored.
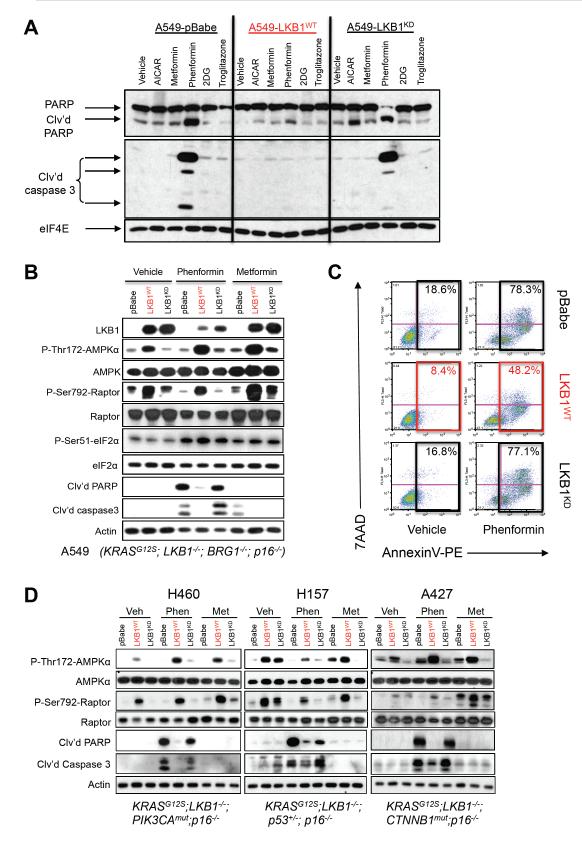
We performed our initial studies using the KRAS mutant and LKB1-deficient NSCLC cell line A549 and derivatives with retrovirally introduced stable expression of wild-type LKB1 cDNA (LKB1WT), a kinase-inactive LKB1 cDNA (LKB1KD), or an empty vector (pBABE). These cells were treated with a variety of drugs with established roles in causing metabolic stress: metformin, phenformin, the cell permeable AMP-mimetic AICAR, the glycolysis inhibitor 2-deoxyglucose, or the thiazolidinedione compound troglitazone that acts as a mitochondrial inhibitor in addition to effects on PPARγ (Hardie et al., 2012). Immunoblotting for cleaved caspase 3 and cleaved PARP as markers of apoptosis induction, we observed that, of the agents tested, only phenformin induced apoptosis in the cells lacking functional LKB1 (Figure 1A). While both biguanides lowered ATP production in A549 cells (Figure S1A), phenformin reduced cellular ATP more, consistent with previous studies (Owen et al., 2000; Dykens et al., 2008). The ATP-lowering effect of both drugs was modestly blunted when wild-type LKB1 was present, consistent with the current model that AMPK acts to restore ATP levels and with previous findings in wild-type and Lkb1-deficient myocytes (Sakamoto et al., 2005). Consistent with a greater impact on ATP levels, phenformin but not metformin induced apoptosis in A549 cells lacking functional LKB1 by multiple measures: cleaved PARP and caspase 3 immunoblot (Figure 1B), activated caspase 3/7 luminescence assay (Figure S1B), and flow cytometry measuring AnnexinV and 7AAD staining (Figure 1C).
Figure 1. Phenformin selectively induces apoptosis in LKB1−/− NSCLC tumor cells.
(A) A549 human NSCLC cells expressing the pBabe vector (A549-pBabe), full length LKB1 (A549-LKB1WT), or kinase dead LKB1 (A549-LKB1KD) were treated with vehicle (DMEM), AICAR (2 mM), metformin (20 mM), phenformin (2 mM), 2DG (10 mM) or troglitazone (25 μM) for 48 hrs. Lysates were immunoblotted with the indicated antibodies.
(B) A549 isogenic cell lines were treated for 24 hrs with vehicle (DMEM), 2 mM phenformin or 20 mM metformin. Lysates were immunoblotted with the indicated antibodies.
(C) Fluorescence-activated cell sorting (FACS) on cells stained with AnnexinV-PE and 7AAD following 48 hr treatment with vehicle or 2 mM phenformin.
(D) H460, H157 or A427 cell lines expressing the pBabe vector (pBabe), full length WT LKB1 (LKB1WT), or kinase dead LKB1 (LKB1KD) were treated for 24 hrs with vehicle (DMEM), 2 mM phenformin or 20 mM metformin. Lysates were immunoblotted with the indicated antibodies. Cancer gene driver mutations found in these cell lines listed under each.
As human daily doses of metformin routinely run between 500 and 1000 mg, and phenformin was given in the range of 50 to 100 mg previously when used clinically, we performed a direct comparison of metformin to phenformin at ratios of 1:1 and 10:1 for in timecourse experiments in isogenic A549 cell lines. At early timepoints (8 or 12 hr) metformin at 2 or 20 mM or phenformin at 2 mM similarly induced AMPK signaling as shown by increased P-AMPK and P-Raptor levels (Figure S1C). However only phenformin induced higher levels of cellular stress, triggering induction of P-Ser51 eIF2α and its downstream target CHOP, and markers of apoptosis at later times (Figure 1B, S1C). eIF2α phosphorylation and upregulation of CHOP is observed in response to a diverse set of cellular stresses, including the unfolded protein response, nutrient deprivation, and mitochondrial stress (Muaddi et al., 2010; Ye et al., 2010; Chae et al., 2012). This suggests that while AMPK activation is a rapid and sensitive sensor of ATP loss, its signal plateaus and does not continue to increase with greater ATP loss, when additional stress sensing pathway like eIF2α kinases may be engaged. Note that LKB1 did not impact P-eIF2α or CHOP levels (Figure 1B, S1C), indicating eIF2α signaling is behaving here as an AMPK-independent pathway.
To examine how widely the sensitivity of cell lines to apoptosis by phenformin treatment is dictated by LKB1, we tested a panel of NSCLC lines that all had KRAS and LKB1 mutations, but each contained additional unique mutations. Isogenic derivatives of each cell line expressing wild-type or kinase-dead LKB1 were established and examined for sensitivity to phenformin or metformin. Despite each cell line containing different additional mutations, their sensitivity to undergo apoptosis following phenformin treatment was dictated by the LKB1 genotype in all cases (Figure 1D, S1B). Protection from phenformin-induced apoptosis was also observed with LKB1-reconstituted NSCLC and cervical cell lines H838 and HeLa, respectively, that are deficient for LKB1 but wild-type for KRAS (Figure S1B). Taken altogether, these data indicate that LKB1 dictates the sensitivity of a variety of cancer cell lines to phenformin-induced metabolic catastrophe regardless of the other tumor mutations present.
Developing genetically engineered mouse models of NSCLC that can be monitored by bioluminescence longitudinally
Though a handful of studies have begun examining metabolic drugs in xenograft models, of particular concern is that subcutaneous placing a mass of tumor cells into an non-vascularized environment and without appropriate basement membrane attachment will itself result in severe metabolic stress and AMPK activation (Jeon et al., 2012; Laderoute et al., 2006), thus altering the very pathways being tested here for their impact on therapeutic outcome. To better model LKB1 deficient tumors, we took advantage of the well-characterized adenoviral cre-induced NSCLC models initiated by a conditionally activated K-ras oncogene (Lox-Stop-Lox-KrasG12D, hereafter called Kras), combined with mice bearing floxed alleles of p53 or Lkb1 (Farago et al., 2012). As previously reported (Chen et al., 2012; Ji et al., 2007), when p53 deficiency or Lkb1-deficiency is combined with Kras activation, there is a dramatic increase in tumor burden leading to metastasis and earlier death,10-12 weeks post-cre administration in both Kras;Lkb1L/L and Kras;p53L/L mice compared to 25+ weeks in Kras mice. To facilitate non-invasive imaging of tumor burden in longitudinal studies on these mice following therapeutic intervention, we crossed Kras, or Kras;Lkb1L/L or Kras;p53L/L mice with a ROSA26 lox-stop-lox luciferase allele (hereafter called Luc) (Safran et al., 2003) to generate Kras;Luc (Kluc), Kras;Lkb1L/L;Luc (KLluc) or Kras ;p53L/L;Luc (KPluc) mice.
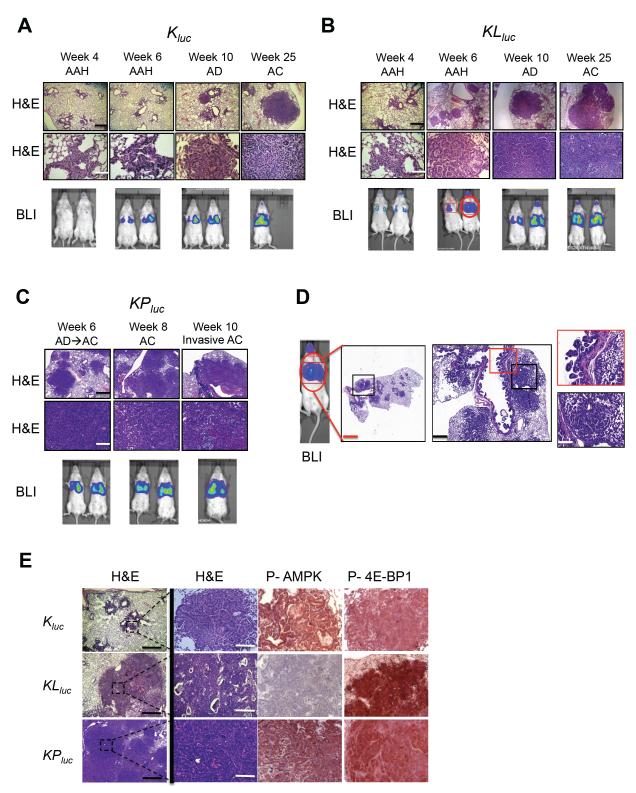
We bred these genotypes into the FVB background whose white coat allowed for noninvasive bioluminescent luciferase imaging without fur removal (Figure S2A-C). On the FVB genetic background, KLluc and KPluc mice exhibited comparable extents of tumor burden and similar latency. We first demonstrated that the bioluminesence output from the ROSA26 encoded luciferase was proportional to tumor burden (see Supplemental Experimental Procedures). Analyzing lung tumor progression in Kluc, KLluc and KPluc mice, we detected bioluminescence as early as 4 weeks in KLluc and KPluc mice and confirmed the presence of adenomas by H&E staining (Figure 2A-D, S2A-C). At 6 weeks, tumors in the KLluc and KPluc mice began to change from adenomatous features to a more malignant tumor phenotype typical of carcinomas (Figure 2D). The most common histologic pattern in both KLluc and KPluc mice were high-grade invasive papillary adenocarcinoma. Squamous cell and adeno-squamous carcinoma were commonly observed tumor subtypes in KLluc mice, as previously noted (Ji et al., 2007). Interestingly, KLluc mice also displayed severe mucinous bronchiolar-epithelial adenocarcinoma (mBEAC) and bronchial mucinous papillary lesions, which were rarely seen in Kluc or KPluc mice (Figure S2D). Of note, LKB1 is frequently mutated in NSCLC patients with mucinous bronchiolar adenocarcinoma (Osoegawa et al., 2011).
Figure 2. Time course of tumor progression in Kluc, KLluc and KPluc lung tumor models.
(A-C) Tumor progression in Kluc (A), KLluc (B), and KPluc (C) murine lung tumor measured by H&E staining or bioluminescence (bottom panel) at the indicated time points. Scale bars (black) = 300 μm. Scale bars (white) = 100 μm. Mice shown are representative of 10-12 mice per genotype.
(D) Detailed analysis of H&E stained lung tumors from a KLluc mouse (red circled mouse from bottom panel B) at 6 weeks post AdCre administration. Scale bars (red) = 1 mm, (black) = 500 μm, (white) = 100 μm.
(E) Immunohistochemical analysis of representative lung tumors from Kluc, KLluc and KPluc mice 10 weeks post AdCre administration. Sections were stained with H&E or the indicated antibodies. Scale bars (black) = 500 μm. Scale bars (white) = 100 μm.
Next we characterized AMPK and mTORC1 signaling in Kluc, KLluc and KPluc lung tumors by immunohistochemical staining for P-AMPK and P-4E-BP1 (Figure 2E). Notably, the KLluc tumors contained the highest degree of P-4E-BP1, consistent with mTORC1 being particularly hyperactive in LKB1-deficient tumors. Lkb1 deletion in the Kras mutant tumors was also accompanied by near complete loss of P-AMPK as seen in many other tissues previously (Figure 2E).
Phenformin activates the AMPK signaling pathway in Kluc and KPluc lung nodules
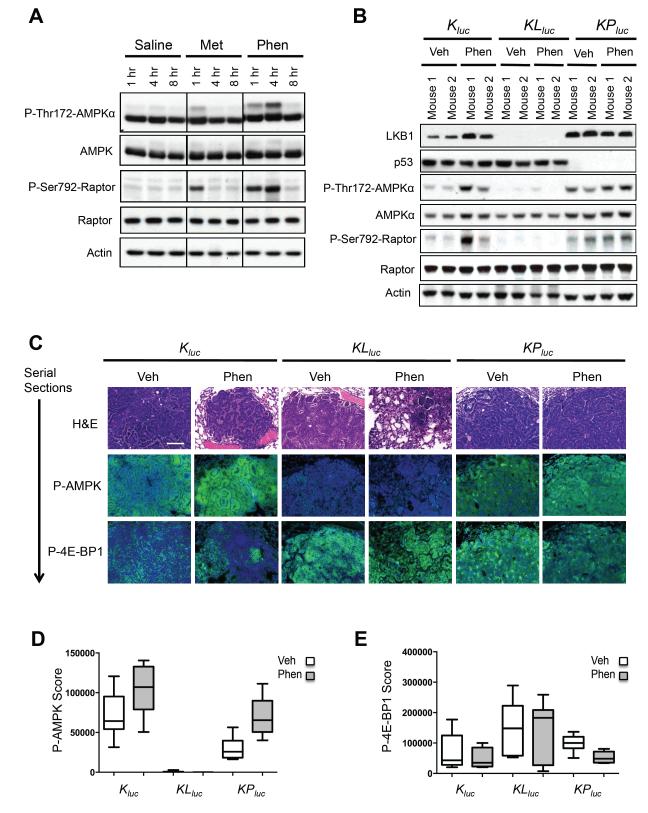
To further examine their prospects as anti-cancer agents, we compared the bioavailability of metformin and phenformin in mouse lung tissue. Phenformin is a more lipophilic molecule compared to metformin (Figure S3A) and, unlike metformin requiring OCT1 for cell entry (Shu et al., 2007), readily permeates cells and tissues (Hawley et al., 2010; Huang et al., 2008). We performed intraperitoneal (i.p.) injections of vehicle, metformin or phenformin on wild type mice and isolated lung tissue at 1, 4, and 8 hours post injection. Immunoblot analysis showed a weaker activation of the AMPK signaling pathway by metformin that only was observed at the 1 hr timepoint, unlike phenformin, which showed a robust activation at 4 hr post-injection (Figure 3A). Mice bearing lung tumors did not tolerate daily i.p. injections over multiple weeks however, therefore phenformin was administered through the drinking water of mice fed ad libitum, as previously described (Appleyard et al., 2012; Huang et al., 2008). Quantitative analysis of P-AMPK staining in KPluc lung tumors from mice treated for two weeks showed greater activation of AMPK with phenformin than metformin (Figure S3B, S3C). As expected, no induction of P-AMPK was ever seen in KLluc tumors. Measurement of metformin and phenformin in blood plasma of mice by mass spectrometry revealed that levels of both were steady over days when administered via drinking water (Figure S3D).
Figure 3. Phenformin treatment robustly activates AMPK pathway in K-Ras driven lung tumors in vivo.
(A) Lung tissue lysates from wild type FVB mice following i.p. injection of saline (0.9%), metformin (300 mg/kg) or phenformin (150 mg/kg) and immunoblotted with the indicated antibodies.
(B) Lysates of lung tumor nodules from Kluc, KLluc and KPluc mice treated with vehicle (water) or phenformin via 5 days ad lib feeding were immunoblotted with the indicated antibodies.
(C) Representative images of hematoxylin and eosin (H&E), P-AMPK or P-4E-BP1 stained Kluc, KLluc and KPluc lung tumor sections. P-AMPK or P-4E-BP1 positively stained cells (in green) and nuclei (in blue). Scale bar = 100 μm.
(D, E) Box plots representing the mean P-AMPK (D) or P-4E-BP1 (E) signal from Kluc, KLluc and KPluc lung tumors treated with vehicle or phenformin. n=6 mice analyzed per treatment group in each genotype (Kluc, KLluc and KPluc) treatment group. All data are represented as the mean ± SEM.
Immunoblot analysis of snap-frozen Kluc, KLluc and KPluc lung tumor nodules revealed that tumors from Kluc, and KPluc mice treated with phenformin had higher levels of AMPK activation compared to vehicle treated mice, while KLluc lung tumors showed a lack of AMPK signaling as expected (Figure 3B). Normal lung lysates from wild type FVB mice given phenformin ad libitum for 2 weeks showed an increase in AMPK signaling and lowered mTORC1 signaling, when compared to the vehicle treated mice (Figure S3E). Phenformin also increased levels of P-eIF2α and its target BiP/Grp78 in normal lung as well as in lung tumors (Figure S3E,F) (the CHOP antibody afore used recognized human but not mouse CHOP). There was no observed difference in IGF-IR/IR or Akt signaling between the vehicle or phenformin treated mice. Analysis of the AMPK activation in lung tumors by quantitative IHC following phenformin treatment paralleled the results observed by immunoblotting (Figure 3C, 3D) and phenformin was observed to modestly reduce the mTORC1 substrate P-4E-BP1 levels in all genotypes (Figure 3C, 3E).
Lkb1−/− lung tumors reveal higher levels of apoptosis and reduced tumor burden following phenformin treatment
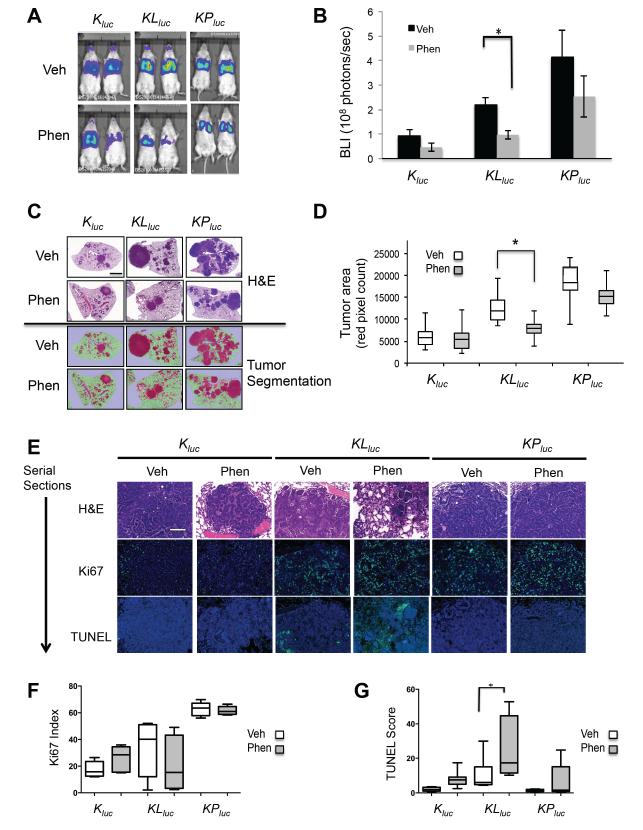
Having established that phenformin had greater bioavailability and was capable of more potently inducing energy stress in tumors than metformin, we next tested phenformin as a single agent therapeutic in the Kluc, KLluc, and KPluc mice. Following tumor induction, we performed bioluminescent imaging (BLI) to monitor tumor growth and sorted mice into equal groups according to their BLI value to normalize the cohorts receiving vehicle or phenformin (Figure S4A-C). We first tested phenformin as a chemotherapeutic by initiating treatment 6 weeks post adenoviral cre delivery for the KPluc and KLluc mice and 9 weeks post adenoviral cre delivery in the Kluc mice (Figure S4D) in order to target aggressively growing tumors transitioning from benign to malignant tumors (Figure 2A-C). After three weeks of treatment, BLI imaging revealed modestly reduced tumor burden in mice of all genotypes receiving phenformin, but the only statistically significant decrease was in the KLluc lung tumors (Figure 4A,B).
Figure 4. Phenformin preferentially inhibits LKB1-deficient lung tumors in vivo.
(A) Representative ventral view images of bioluminescence from Kluc, KLluc and KPluc mice. Bioluminescence imaging (BLI) performed on Kluc (veh n=11, phen n=12 mice), KLluc (veh n=10, phen n=10 mice) and KPluc (veh n=7, phen n=7 mice) following three weeks of treatment with vehicle (water) or phenformin via ad lib feeding.
(B) Average BLI for each treatment group of the Kluc, KLluc and KPluc mice at three weeks treatment.
(C) Representative images of tumor burden in Kluc, KLluc and KPluc mice as shown by H&E stained lung sections (top panel) or the same images tissue segmented and pseudo-colored with Inform software (bottom panel). Scale bar = 1 mm.
(D) Box plots representing the mean tumor burden following 3 weeks of treatment. Tumor burden was calculated by averaging the red pseudocolored tumor area from H&E stained whole lung sections shown in (C) bottom panel.
(E) Representative images of H&E, Ki67 or TUNEL stained Kluc, KLluc and KPluc lung tumor sections. Ki67 or TUNEL positively stained cells (in green) and nuclei (in blue). Scale bar = 100 μm.
(F, G) Box plots representing the mean Ki67 (F) and TUNEL scores (G) from Kluc, KLluc and KPluc mice treated with vehicle or phenformin. (*) Statistical significance (p-value < 0.05) calculated using a non-parametric one-way ANOVA (Tukey test).
All data are represented as the mean ± SEM.
To better quantify tumor burden in mice, we combined BLI with quantitative histological analysis. H&E stained lungs were imaged with morphometric software to quantify the surface area composed of tumor as opposed to normal tissue of representative cross-sections of each lung lobe for each mouse was determined (Figure S4E, F). Importantly, tumor burden determined by histological analysis correlated closely with BLI. KLluc mice showed significant reductions in lung tumor area (Figure 4C,D), while modest reductions in tumor burden were also seen in both Kluc and KPluc mice. Normalization of the tumor burden for each tumor genotype, performed by calculating the ratio of phen:veh tumor mass, showed the largest decrease in tumor burden for the KLluc mice as compared to Kluc and KPluc mice (Figure S4G). In addition, we observed no change in rate of metastasis following phenformin treatment in any of our therapeutic trials. These results demonstrate phenformin as a single agent selectively attenuates Lkb1-deficient lung tumor growth in vivo.
We next analyzed apoptosis and tumor proliferation in the lung tumors by TUNEL and Ki67 staining respectively. Phenformin treatment modestly reduced Ki67 index in KLluc tumors but not Kluc and KPluc tumors (Figure 4E, F) and TUNEL staining revealed that KLluc lung tumors had the highest levels of apoptotic tumor cells following phenformin (Figure 4E,G). Interestingly, KLluc lung tumors also had higher basal levels of apoptosis compared to KPluc and Kluc tumors, perhaps reflecting the pro-survival role of LKB1 in restoring energy homeostasis under conditions of metabolic stress, which may spontaneous arise in the context of a tumor.
Phenformin induced a significant increase in survival and therapeutic response in KLluc mice following long-term treatment
Given the response of late stage KLluc lung tumors to phenformin treatment, we decided to treat earlier stage lung tumors in KLluc and KPluc mice. To better mimic the human clinical use of biguanides, we used oral gavage (o.p.) to deliver daily the maximum tolerated dose of 100 mg/kg phenformin starting at 3 weeks post tumor induction (Figure S5A). As expected, delivery of phenformin by this method resulted in AMPK pathway activation in KPluc but not KLluc tumor nodules (Figure S5B, S5C). Mass spectrometry on phenformin in blood plasma following o.p. delivery demonstrated a rapid uptake of phenformin followed by a steady decrease in blood plasma over twenty-four hours (Figure S5D). Importantly, these FVB mice are on a chow diet and non-diabetic, and the dose of phenformin used following 6 weeks of daily administration did not significantly impact blood plasma insulin levels, glucose levels, or lead to increased blood lactate levels as compared to vehicle treated mice (Figure S5E-H).
The loss of LKB1 function in tumors predicts increased glycolytic metabolism and FDG-PET avidity as shown in gastrointestinal polyps from Lkb1+/− mice (Shackelford et al., 2009) and in NSCLC in mice and humans (Chen et al., 2012). Therefore we explored the use 18F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) as a surrogate endstage biomarker in combination with microCT imaging to longitudinally monitor tumor progression and response in KLluc and KPluc mice following phenformin treatment. KLluc and KPluc mice were given baseline FDG-PET and μCT scans, which were scored by a radiologist blinded to the identities of the mice. Mice in both groups were determined to be negative for tumors by both PET and CT scans and were randomly sorted into treatment groups (Figure 5C).
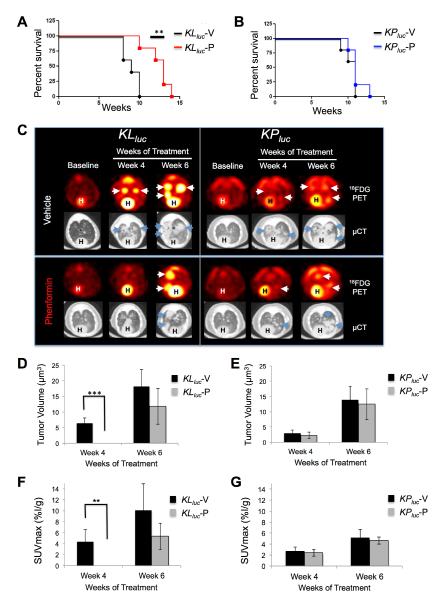
Figure 5. Phenformin significantly increased overall survival and slowed tumor progression in KLluc mice shown by 18FDG-PET and μCT imaging.
(A, B) Kaplan-Meier survival curves for KLluc mice (A) or KPluc mice (B) treated with vehicle (n=5) or phenformin (n=5) for each tumor genotype respectively with a **p-value = 0.008 for KLluc mice determined by Wilcoxon log rank test.
(C) Representative images of micro FDG-PET and CT scans of lungs from KLluc and KPluc mice at baseline (pre-treatment) or following 4 and 6 weeks treatment with vehicle or phenformin. Heart is labeled (H) and tumors are labeled with arrows (white or blue).
(D, E) Mean tumor volume measured by micro CT at 4 and 6 weeks treatment for KLluc (D) and KPluc (E) mice respectively. *** p-value = 0.0009 for KLluc mice as determined by Wilcoxon rank sum test.
(F, G) Mean FDG-PET SUVmax measured at 4 and 6 weeks treatment for KLluc (F) and KPluc mice (F) respectively. **p-value = 0.005 for KLluc mice as determined by Wilcoxon rank sum test. n=5 mice per treatment group of each genotype.
All data are represented as the mean ± SEM.
When phenformin was administered by o.p. for an extended time, it significantly increased survival in KLluc mice compared to KPluc mice (Figure 5A, 5B). Phenformin treatment resulted in delayed tumor progression in KLluc but not KPluc mice as shown by μCT measurements of lung tumor volume over time for individual mice in the study (Figure 5C-E, S5J,K). At 4 and 6 weeks of treatment average lung tumor size was significantly reduced in KLluc mice (Figure 5D). There was a trend towards smaller tumors in the KPluc mice but did not reach statistical significance (Figure 5E). However, at endstage, there was the presence of large, advanced solid tumors of the vehicle and phenformin groups in both KLluc and KPluc tumor genotypes (Figure S5I). The 18FDG uptake into tumors was quantified by the standardized uptake value (SUVmax) at 4 and 6 weeks of treatment. The SUVmax for phenformin treated KLluc lung tumors was significantly reduced compared to vehicle ones (Figure 5F). No difference was seen in the SUVmax for the KPluc mice at these same time points (Figure 5G). These data suggest a therapeutic response in KLluc lung tumors following phenformin treatment, however the SUVmax values in endstage tumors were increased in both KLluc and KPluc mice (Figure S5L,M), suggesting the emergence of phenformin-resistant tumors in KLluc mice (Figure S5J,L).
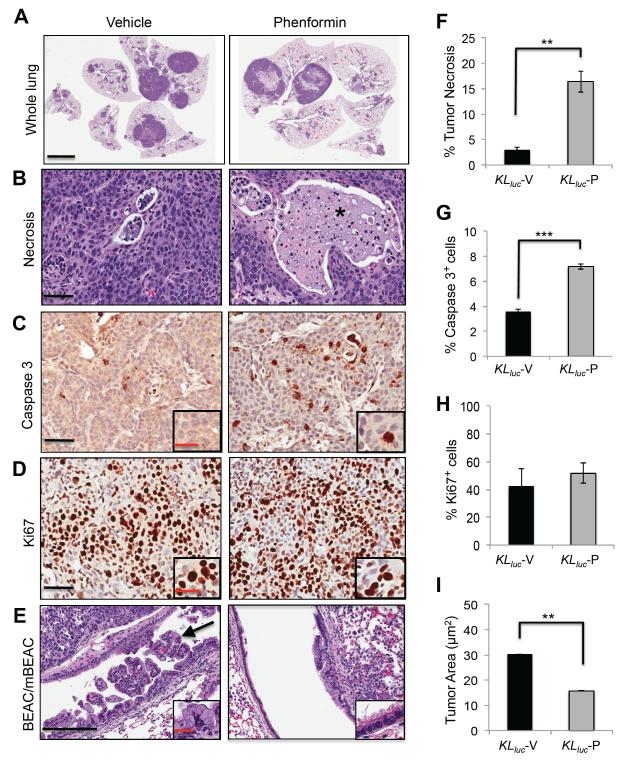
Histopathological analysis of 6 week o.p. phenformin treated lungs revealed that KLluc but not KPluc lung tumors had large regions of necrosis, which was absent in the vehicle-treated controls (Figure 6A and S6A). Further analysis of KLluc lung sections revealed an 8 fold increase in necrotic tumor areas as shown by the presence of necrotic bodies with ghost cells (Figure 6B, 6F). Staining of KLluc and KPluc tumors for cleaved caspase 3 revealed a significant increase in tumor cell apoptosis in both KLluc and KPluc lung tumors following phenformin (Figure 6C, 6G, S6A,S6B), but no difference in Ki67 indices in either genotype following phenformin treatment (Figure 6D, 6H, S6A, S6C). Elevated P-AMPK levels were observed in both tumor and lung parenchyma of phenformin treated KPluc mice (Figure S6D), further reflecting that this dosing regiment was capable of inducing energy stress in these different lung cell populations. Strikingly, the abundant mucinous bronchiolar-epithelial adenocarcinoma (mBEAC), which occluded much of the upper airways in KLluc mice, showed a 2 -fold decrease in surface area in mice receiving phenformin compared to the vehicle group (Figure 6E, 6I). Altogether, these results indicate that phenformin as a single agent induces a tumor response in both KLluc and KPluc lung tumors with Lkb1-deficient tumors showing the most robust overall therapeutic response to phenformin.
Figure 6. Phenformin induces a significant therapeutic response in KLluc lung tumors.
(A) Hematoxylin and eosin (H&E) stained whole lung sections of KLluc mice treated with vehicle (saline) or phenformin, scale bar = 4 mm.
(B-E) Representative images of lung tumors from KLluc mice treated with vehicle (saline) or phenformin. (B) Tumor necrosis, scale bar = 50 μm (C) Activated caspase 3, scale bar= 50 μm. Inset represents magnification of caspase 3 stained cells, scale bar = 25 μm. (D) Ki67 stain, scale bar = 50 μm. Inset represents magnification of Ki67 stained cells, scale bar = 25 μm. (E) Mixture of bronchiolar-epithelial adenocarcinoma (BEAC) and mucinous bronchiolar-epithelial adenocarcinoma (mBEAC) shown with arrow, scale bar = 200 μm. Inset represents magnification of mBEAC cells, scale bar = 50 μm.
(F) Mean percentage of necrotic tumor area in vehicle and phenformin treated KLluc mice. **p-value = 0.002.
(G) Mean percentage of tumor cell apoptosis from vehicle and phenformin treated KLluc mice. ***p-value < 0.0001.
(H) Ki67 index for vehicle and phenformin treated KLluc mice. (I) Total BEAC and mBEAC tumor area (μm2) in vehicle and phenformin treated KLluc mice **p-value < 0.006. n=5 KLluc mice per treatment group (vehicle or phenformin).
Statistical analysis performed using an unpaired Student’s t test.
All data are represented as the mean ± SEM.
Mitochondrial defects may sensitize LKB1-deficient NSCLC tumor cells to phenformin
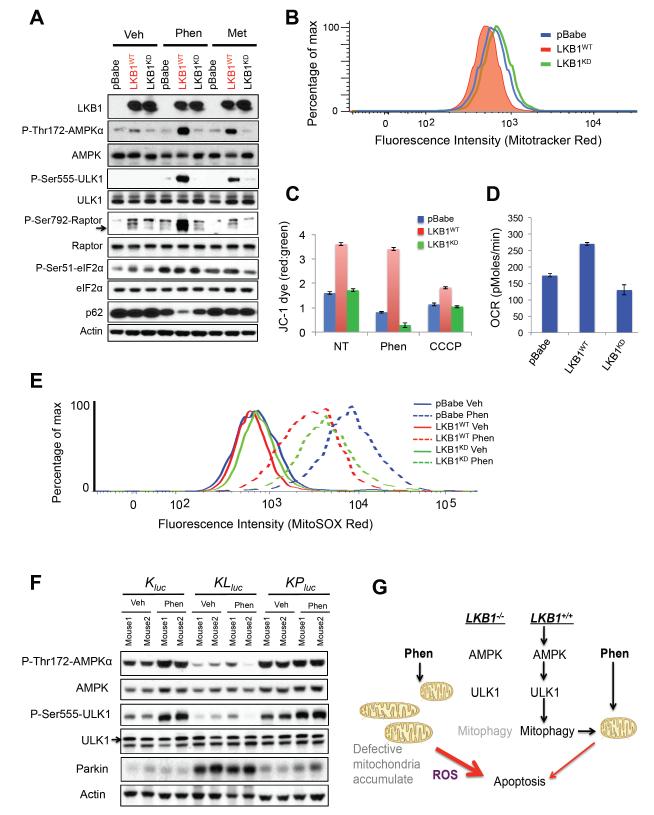
To examine the molecular mechanism behind the selective induction of apoptosis in LKB1-deficient lung cancer cells, we analyzed signals downstream of its main target AMPK. Recently we demonstrated that AMPK phosphorylates and activates the autophagy kinase ULK1, which is required for mitophagy and cell survival under starvation conditions (Egan et al., 2011). We previously observed an accumulation of mitochondria with defective mitochondrial membrane potential (Δψ) in Ampk- and Ulk1-KO fibroblasts and hepatocytes (Egan et al., 2011), similar to that observed in Lkb1-KO hematopoetic stem cells (Narada et al., 2011), so we examined here whether ULK1 and mitochondrial homeostasis might be disrupted in LKB1-mutant NSCLC cells. A549 cells reconstituted with functional LKB1 showed AMPK activation and its phosphorylation of endogenous ULK1 on Ser555 following phenformin treatment, paralleling phosphorylation of Raptor, another AMPK substrate (Figure 7A). Phenformin treatment also resulted in a decrease in levels of the p62/Sqstm1 protein only in the wild-type LKB1-reconstituted cells, indicative of efficient autophagy reliant on LKB1 function (Figure 7A). Importantly, p62 levels were not reduced by metformin here, perhaps reflecting the lower level of AMPK signaling from metformin as compared to phenformin. Expression of functional LKB1 in LKB1-deficient H157 and H838 cells also restored their P-Ser555 ULK1 signal indicating this is a general defect in LKB1-deficient NSCLC cell lines (Figure S7A). As observed previously in AMPK- and ULK1-defective fibroblasts and hepatocytes, here A549, H157, and H838 NSCLC cells defective for LKB1 function exhibited increased mitochondrial content as assayed by flow cytometry for mitotracker red staining (Figure 7B and S7B), suggesting that reconstitution of wild-type LKB1 into these LKB1-deficient NSCLC cell lines may actively induce functional mitophagy. A549-pBabe and A549-LKB1KD cells had impaired Δψ reflecting impaired mitochondrial membrane integrity, as exhibited by the failure of JC-1 dye to accumulate in the mitochondrial membrane when compared to A549-LKB1WT cells (red to green ratio of JC-1 staining in Figure 7C). Following treatment with phenformin, LKB1-deficient tumor cells showed a complete loss of mitochondrial membrane potential, while expression of functional LKB1 fully prevented this loss. In contrast, treatment of with the mitochondrial uncoupler CCCP induced loss of Δψ in all cell lines, though LKB1-reconstituted cells still retained the most mitochondrial membrane potential (Figure 7C). As one measure of functionality of the mitochondria in these cell lines, we examined oxygen consumption as a direct measurement of mitochondrial respiration. Consistent with mitochondrial defects, A549-pBabe and A549-LKB1KD cells had a lower basal rate of oxygen consumption rate (OCR) than the A549WT cells (Figure 7D, S7C). Treatment of these cells with either phenformin or rotenone resulted in inhibition of mitochondrial respiration as shown by an acute drop OCR in levels (Figure S7C, S7D). OCR levels tracked inversely with mitotracker red staining here, consistent with the idea that the accumulated mitochondria in the LKB1-deficient cells are respiration defective.
Figure 7. Mitochondrial defects in LKB1−/− NSCLC confer sensitivity to phenformin.
(A) Lysates from A549-pBabe, A549-LKB1WT, and A549-LKB1KD cells were treated with vehicle (DMEM), phenformin (2 mM) or metformin (20 mM) for 4 hrs and immunoblotted with the indicated antibodies.
(B) FACS analysis of cells from (A) stained with Mitotracker Red.
(C) Cells from (A) stained with JC-1 dye following 4 hrs of treatment with vehicle (DMEM), phenformin (2 mM), metformin (20 mM) or CCCP (100 μM). Mitochondrial membrane potential ( Ψ, red/green ratios) were measured by emission of red and green fluorescence using FACS.
(D) Oxygen consumption rate (OCR) of cells from (A).
(E) Cells from (A) were stained with MitoSOX Red following 16 hrs of treatment with vehicle (DMEM) or phenformin (2 mM) and analyzed by FACS.
(F) Lung tumor lysates from Kluc, KLluc, and KPluc mice treated 5 days with vehicle or phenformin ad lib feeding were immunoblotted with the indicated antibodies.
(G) Model illustrating mitochondrial defects and phenformin induced cell death in LKB1−/− deficient tumor cells.
All data are represented as the mean ± SEM.
Mitochondrial Complex I inhibition has been reported to induce reactive oxygen species (ROS) similar to that seen with Complex III inhibition (Hirst et al., 2008; Kushnareva et al., 2002). Thus, we next examined the release of mitochondrial reactive oxygen species (mtROS) following the treatment with phenformin, metformin, or the complex III inhibitor antimycin A and found that phenformin was a more potent inducer of mtROS than metformin (Figure S7E). Staining of A549-pBabe and A549-LKB1KD cells showed modest elevations in mtROS compared to A549-LKB1WT cells, both basally and following phenformin treatment (Figure 7E). To examine whether the doses of phenformin used in our therapeutic study were effective enough to induce LKB1-dependent effects on mitophagy, we performed western blot analysis of lung tumor nodules from mice with and without phenformin treatment for 5 days and discovered reduced phosphorylation of ULK1 and an accumulation of the mitophagy regulator Parkin in KLluc but not Kluc, or KPluc lung tumors (Figure 7F). These results suggest defects of the AMPK-ULK1 signaling pathway and mitophagy in vivo following loss of LKB1 (Figure 7G), which may help sensitize these tumors to the effects of phenformin on mitochondrial integrity.
DISCUSSION
In this study we have found that the diabetes drug phenformin shows selective and significant anti-tumor activity in genetically engineered mouse models of lung cancer bearing LKB1 mutations. Molecular targeted therapeutics hold great promise for the future of cancer treatment, but for the 15-30% of NSCLC bearing inactivating mutations in LKB1, there has been little in the way of targeted agents to date. As loss of LKB1 results in hyperactivation of mTORC1 in addition to other pro-growth pathways (Shackelford and Shaw, 2009), initial interest has been in rapamycin analogs or other mTOR inhibitors. However, rapamycin treatment of an autochthonous lung tumor model with Kras and Lkb1 mutations similar to the one used here showed a minimal therapeutic effect (Liang et al., 2010), perhaps due to the multitude of pathways deregulated from LKB1 deficiency that can still fuel tumorigenesis even when mTORC1 is suppressed. Hyperactivation of Src family kinases was also discovered as a biochemical marker and potential therapeutic target in Lkb1-deficient lung tumors (Carretero et al., 2010). Combination of the Src inhibitor dasatinib with PI3K and MEK inhibitors in similar mouse lung tumor models showed a synergistic tumor response, though dasatinib as a single agent displayed a minimal effect (Carretero et al, 2010). The fact that phenformin induces therapeutic response as a single agent taken together with the success of dasatinib in combinations suggests that phenformin may also show greater success in defined combinations, which will be a focus for future investigations. Of relevance, a recent study treating the same three NSCLC models used here with a combination of standard-of-care docetaxel with MEK inhibitors revealed that Kras; Lkb1 mutant tumors were selectively resistant to that combination, unlike Kras alone or Kras; p53 mutant tumors (Chen et al., 2012). It is in the context of this body of work that selective sensitivity of Kras; Lkb1 tumors to phenformin as a single agent in aggressive autochthonous models of lung cancer is so notable.
We have been interested in whether one could utilize metabolic stress inducing compounds as anti-cancer agents for LKB1-mutant tumors in their autochthonous setting in the intact organism ever since we observed that LKB1-deficient cells were sensitive to apoptosis induced by metabolic stress in culture (Shaw et al., 2004). We focused our attention on the biguanide family of anti-diabetes therapeutics for their ability to reduce intracellular ATP levels as a result of mitochondrial complex I inhibition. The absence of LKB1 signaling to AMPK means such cells will go for an extended time without activation of this sensor to restore ATP levels. We expect that mitochondrial damage and ATP loss also activate AMPK-independent stress sensing pathways, as we demonstrate here with multiple markers of the eIF2a signaling pathway. These findings are consistent with previous reports of AMPK-independent effects of biguanides (Foretz et al., 2010; Kalendar, 2010). Our results are also consistent with recent studies demonstrating that AMPK mediates cell survival following metabolic stress not only through effects on ATP restoration, but also by restoring NADPH levels, which are needed to neutralize ROS that arise during these stresses (Jeon et al., 2012). Inhibition of mitochondria complex I has been shown to cause ROS induction (Hirst et al., 2008; Kushnareva et al., 2002), so the fact that phenformin is a potent complex I inhibitor may underlie its ROS generation, which LKB1-deficient cells are genetically incapable of neutralizing efficiently. Future studies will be needed to fully assess the contribution of ROS in the LKB1-sensitive apoptosis following phenformin.
The biguanide metformin is the most widely used type 2 diabetes therapeutic worldwide and more recently has become used in treating other insulin resistant conditions including polycystic ovary syndrome (PCOS) (Motta, 2008). Metformin garnered considerable attention as a potential anti-cancer agent once the connection between LKB1 and AMPK was discovered (Birsoy et al., 2012; Taubes, 2012). As AMPK activation can be growth suppressive, diabetics taking these agents daily for decades might have a lowered incidence of cancer due to chronic effects from AMPK-mediated suppression of mTORC1 and other pro-growth pathways, depending on how effective and sustained the clinical doses of metformin are at activating AMPK in different tissues. In addition, a non-cell autonomous mechanism for metformin’s anti-tumor effects may be its ability to lower circulating blood glucose and insulin levels, which also contribute to cancer risk and incidence in some contexts (Pollak, 2010). A number of epidemiological studies have found that diabetics taking metformin exhibit lowered risk of overall cancer compared to those taking other forms of diabetes medication (Dowling et al., 2012; Pollak, 2012). Given these observations, over fifty different clinical trials investigating the use of metformin in oncology are currently underway (www.clinicaltrials.gov). Whether phenformin would yield similar broad anti-cancer results, given that it also effectively lowers blood glucose and also more potently activates AMPK across a wide-variety of tissues, remains to be investigated. As phenformin has a 50-fold greater potency than metformin for mitochondrial complex I inhibition (Owen et al., 2000; Dykens et al., 2008), part of its therapeutic success in cancer models when compared to metformin may be due the fact that at maximal tolerated doses in rodents and clinically utilized doses in humans, one achieves greater ATP loss and ROS induction with phenformin. However, another distinction between these two biguanides is a greater tissue bioavailability for phenformin, which may be due to the apparent reliance of metformin on a tissue-restricted cell-surface transporter, Oct1, to efficiently cross the plasma membrane, unlike the more lipophilic phenformin (Hawley et al., 2010). Phenformin was removed from clinical use for type 2 diabetes due to incidence of fatalities from lactic acidosis (Crofford, 1995; Owen et al., 2000), reported in 64 cases per 100,000 patients per year, with patients bearing compromised kidney function at greatest risk. However, phenformin or another biguanide analog may still find utility as anti-cancer agents as the dosing and shorter duration in cancer therapy would be quite different from its prior clinical use for diabetes. Patients could also be pre-screened for kidney function and other biomarkers to reduce any risk of lactic acidosis. Based on our findings here and previous studies of metformin, it seems most likely that phenformin might have potential use for early stage lung tumors or as an adjuvant therapy following resection of early stage NSCLC tumors. Although phenformin is unlikely to be suitable as a single agent therapy in advanced stage disease, it may synergize with other modalities, which warrants future investigation. A critical need for future clinical studies will be developing biomarkers of metformin and phenformin action in human tissues using clinically achievable doses (Pollak, 2010). Our identification of phospho-Ser51 eIF2α and its targets CHOP and BiP/Grp78 as AMPK-independent markers of phenformin-induced stress may prove valuable for such studies.
Whether LKB1 deficiency confers sensitivity towards other agents that perturb ATP production via effects on mitochondria, glycolysis, or glutaminolysis also remains an interesting area for future targeted therapeutic development. Given the large percentage of sporadic lung, cervical, and endometrial cancers bearing LKB1 mutations, identifying agents selectively targeting LKB1-mutant tumors is a critical goal and one that seems particularly amenable to drugs targeting tumor metabolism.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Antibodies from Cell Signaling Technologies (Beverly, MA) used for immunoblots were diluted 1:1000 and included: LKB1 (D60C5) (#3047), phospho-AMPK Thr172 (#2531), total AMPK α1/2 (#2532), phospho-Raptor Ser792 (#2083), total Raptor (#2280), phospho-ULK1 (ser555) (#5869), eIF4E (#9742), phospho-eIF2α (ser51) (#3398), total eIF2α (#9722), cleaved caspase 3 (#9664), PARP (#9542), cleaved PARP (Asp214) (#5625), cleaved PARP mouse specific (Asp214) (#9544), phospho-S6 (ser255/236) (#4858), S6 (#2217), phospho-4E-BP1 (thr37/46) (#2855), 4E-BP1 (#9644), phospho-IGF-IR/IR (#3024), IGF-IR/IR (#3027), phospho-AKT (ser473) (#4060), AKT (#9272), CHOP (#5554), BiP/GRP78 (#3177). Total ULK1 (A7481 WB 1:1000) and anti actin (A5441 WB1:5000) were purchased from Sigma Aldrich (St Louis, MO). The p53 (CM5) antibody was obtained from Vector labs (VP-P956 WB 1:1000). The p62 antibody was obtained from Progen (#GP62-C). AICAR was obtained from Toronto Research Chemicals (North York, Ontario, Canada). Phenformin, Metformin and 2-deoxyglucose (2DG) and Antimycin A (cat. no. A8674); were purchased from Sigma Aldrich (St. Louis MO). CCCP was obtained from the Molecular Probes JC-1 dye kit (cat no. M34152).
Cell Culture
Cells were incubated at 37C maintained at 5% CO2 and obtained from ATCC. A549, H460, A427, H838 and HeLa cell lines were obtained from ATCC and H157 cells were a kind gift from Dr. John Minna. Cells were grown in DMEM or RPMI 1640 medium (Gibco) plus 10% fetal bovine serum (Hyclone) and penicillin and streptomycin. Retroviral gene expression was performed as described previously (Gwinn et al., 2008). Briefly, for retroviral infection, the pBabe, pBabe-FLAG-LKB1 and pBabe-FLAG-LKB1-KD (Addgene #’s 1764, 8592, 8593) constructs were transfected along with the ampho packaging plasmid into growing HEK293T cells. Viral supernatants were collected 48 hrs after transfection, spun for 5 mins at 3000 rpm, filtered and target cells were infected in the presence of polybrene. Twenty-four hours later cells were selected with puromycin. Cells were plated at a density of 1 ×105 per well in 6 well dishes and grown for 18 hrs before treatment.
Mouse therapeutic trials
Lox-Stop-Lox KrasG12D were obtained from Tyler Jacks at MIT (Jackson et al., 2005) and p53lox/lox mice from the MMHCC (Jonkers et al., 2001). Lkb1lox/lox mice as previously reported (Shaw et al., 2005). Rosa26-Lox-Stop-Lox-Luc mice were obtained from Jackson Laboratories courtesy of Bill Kaelin (Safran et al., 2003). Mice were inbred on an FVB background. Lung tumors were induced by intranasal inhalation of 5×106 p.f.u. adeno-Cre (purchased from University of Iowa adenoviral core) as previously described (Jackson et al., 2005). Mice that displayed with clinical signs of disease such as labored breathing or severe weight loss were euthanized and necropsied. The mean tumor latency were comparable to previous studies with the exception of KPluc mice, which developed large, aggressive lung tumors with the same latency of KLluc mice (Jackson et al., 2005; Ji et al., 2007). In the experiments in Figure 4, mice were administered with vehicle (water), metformin or phenformin (Toronto Research Corporation, Ontario, Canada) ad lib through their drinking water and their daily intake of fluids were monitored. Fresh metformin (1.8 mg/mL) or phenformin (1.8 mg/mL) was administered every other day. Long-term treatments consisted of 3 weeks treatment (schematic in Figure S4D) and acute treatments lasted 5 days. For the intraperitoneal injections in Figure 4A, mice were given one injection of saline (0.9%), metformin (300 mg/kg), or phenformin (150 mg/kg) and euthanized at 1, 4, and 8 hr post injection and lung tissue was isolated. In the therapeutic trials in Figures 5 and 6 (schematized in Figure S5A), mice were administered phenformin (100 mg/kg/day) by o.p. once daily 6 d/week beginning at 3 weeks post delivery of adenovirus expressing Cre. All experimental procedures performed on mice were approved by the Salk Institute and University of California at Los Angeles Institutional Animal Care and Use Committees.
Statistical Analysis Methods
Means were compared and p-values determined by parametric or non parametric analysis of variance (ANOVA) and statistical test details and significance at p <0.05 is indicated in the text and figure legends. Survival curves were computed using the Kaplan-Meier method. All statistical calculations were carried out using GraphPad Prism 5 software (San Diego, CA) or SAS 9.3 (SAS Inc, Cary NC). For analysis of SUVmax and tumor volume from FDG-PET and μCT imaging, comparisons were made between vehicle (V) versus Phenformin (P) treatments at week 7 and week 9. Values that were below the level of detection, referred to as left censored observations were coded with ‘zero’ although the actual value may be above zero but is usually lower than the lowest observed value.
Supplementary Material
Highlights.
Phenformin is a mitochondrial inhibitor that selectively kills LKB1−/− NSCLC cells
LKB1−/− NSCLC cells exhibit defective mitochondria and ROS following phenformin
Phenformin improves tumors and survival in KrasG12DLkb1−/− not KrasG12Dp53−/− mice
eIF2a signaling markers are AMPK-independent biomarkers of biguanide treatment
Significance.
Targeted therapeutics are making significant advances in subsets of NSCLC bearing activated oncogenic targets, such as EGFR and ALK. More prevalent in NSCLC are mutations in the KRAS oncogene coincident with mutations in the LKB1 or p53 tumor suppressor genes. Currently there are limited options for LKB1-mutant tumors. Here we define the hypersensitivity of LKB1-defective cells to metabolic stress and test the therapeutic use of phenformin, a mitochondrial inhibitor and a former type 2 diabetes drug, in mouse models of NSCLC. Phenformin as a single agent reduced tumor burden and prolonged survival in Kras;Lkb1 compound mutant mice but not Kras;p53 mice. These findings suggest phenformin or related agents may find clinical utility to treat NSCLC bearing LKB1 mutations.
ACKNOWLEDGEMENTS
D.B.S. performed all cell experiments and all mouse experiments with assistance from E.A., L.G. and D.S.V. D.B.S. and R.J.S. designed the study, analyzed the data, and wrote the paper. M.L. is board-certified veterinary pathologist, M.C.F and A.S. are board-certified clinical pathologists who performed all pathological analysis. P.S.M. contributed resources and critical feedback on the project. We thank Lawrance Pang and L.W. who performed PET and CT imaging on the mice and J.C. and Dr. Ken Herrmann who reviewed PET and CT scans on the mice. We thank Anne Atkins in Ron Evans lab (Salk) for the HPLC and MS analysis of metformin and phenformin levels in blood. We thank Dr. Jeff Gornbein for statistical analysis and Laurent Vergnes who performed the Seahorse experiments supported by grant S10RR026744. We thank UCLA’s Translational Pathology Core Laboratory and Dr. Clara Magyar for assistance with the Aperio and Definiens software. We thank Dr. Steven M. Dubinett for critical review of the manuscript and feedback. D.B.S. was supported by training grant T32 CA009370 to the Salk Institute Center for Cancer Research and through generous support by the Adler Family Foundation, by the Ahmanson Translational Imaging Division at UCLA’s School of Medicine and by the National Center for Advancing Translational Sciences through UCLA CTSI Grant numbers UL1TR000124 and KL2TR000122. R.J.S. is funded by the NIH R01 DK080425 and P01CA120964, and an American Cancer Society Research Scholar Award (RSG-07-210-01-MGO). This was also supported in part through the Salk CCSG P30 CA014195 and the Samuel Waxman Cancer Research Foundation. We also thank the Leona M. and Harry B. Helmsley Charitable Trust for their generous support.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes seven figures and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2010;30:1174–1182. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- Appleyard MV, Murray KE, Coates PJ, Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR, Thompson AM. Phenformin as prophylaxis and therapy in breast cancer xenografts. British journal of cancer. 2012;106:1117–1122. doi: 10.1038/bjc.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Sabatini DM, Possemato R. Untuning the tumor metabolic machine: Targeting cancer metabolism: a bedside lesson. Nature medicine. 2012;18:1022–1023. doi: 10.1038/nm.2870. [DOI] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, McNamara KL, Brandstetter KA, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford OB. Metformin. N Engl J Med. 1995;333:588–589. doi: 10.1056/NEJM199508313330910. [DOI] [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. Journal of molecular endocrinology. 2012;48:R31–43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol. 2008;233:203–210. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Snyder EL, Jacks T. SnapShot: Lung cancer models. Cell. 2012;149:246–246. e241. doi: 10.1016/j.cell.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochemical Society transactions. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer research. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–383. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-Activated Protein Kinase (AMPK) Is Induced by Low-Oxygen and Glucose Deprivation Conditions Found in Solid-Tumor Microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MC, Ma J, Chen L, Kozlowski P, Qin W, Li D, Goto J, Shimamura T, Hayes DN, Meyerson M, et al. TSC1 loss synergizes with KRAS activation in lung cancer development in the mouse and confers rapamycin sensitivity. Oncogene. 2010;29:1588–1597. doi: 10.1038/onc.2009.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta DA. Metformin in the treatment of polycystic ovary syndrome. Curr Pharm Des. 2008;14:2121–2125. doi: 10.2174/138161208785294609. [DOI] [PubMed] [Google Scholar]
- Muaddi H, Majumder M, Peidis P, Papadakis AI, Holcik M, Scheuner D, Kaufman RJ, Hatzoglou M, Koromilas AE. Phosphorylation of eIF2alpha at serine 51 is an important determinant of cell survival and adaptation to glucose deficiency. Mol Biol Cell. 2010;21:3220–3231. doi: 10.1091/mbc.E10-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoegawa A, Kometani T, Nosaki K, Ondo K, Hamatake M, Hirai F, Seto T, Sugio K, Ichinose Y. LKB1 mutations frequently detected in mucinous bronchioloalveolar carcinoma. Japanese journal of clinical oncology. 2011;41:1132–1137. doi: 10.1093/jjco/hyr102. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. Embo J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Vasquez DS, Corbeil J, Wu S, Leblanc M, Wu CL, Vera DR, Shaw RJ. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004b;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G. Cancer research. Cancer prevention with a diabetes pill? Science. 2012;335:29. doi: 10.1126/science.335.6064.29. [DOI] [PubMed] [Google Scholar]
- Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. The EMBO journal. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.