Abstract
Nanofibrous membranes have drawn considerable interest for filtration applications due to their ability to withstand high fluid flux while removing micro- and nano-sized particulates from solution. The desire to introduce an antibacterial function into water filter applications presents a challenge to widespread application of fibrous membranes because the addition of chemicals or biocides may produce harmful byproducts downstream. Here, we report the development of chitosan-polycaprolactone (PCL) nanofibrous membranes to utilize the natural antibacterial property of chitosan for antibacterial water filtration. Chitosan-PCL fibers with diameters of 200–400 nm and chitosan contents of 25, 50 and 75 wt% were prepared by electrospinning. In a series of bacterial challenge tests, chitosan-PCL fibrous membranes significantly reduced Staphylococcus aureus adhesion compared to PCL fibrous membranes. In water permeability and particulate size removal tests, fibrous membranes with 25% chitosan supported the greatest water flux (~7000 L/hr/m2) with 100% removal of 300-nm particulates, while maintaining the membrane integrity. This study demonstrates the potential of chitosan-PCL nanofibrous membranes as pre-filters for water filtration systems that demonstrate combinatorial filtration and intrinsic antibacterial advantages.
Keywords: microfiltration, nanofiber, chitosan, antibacterial, water permeability
1. Introduction
More than 1 billion people worldwide lack access to affordable, potable water resulting in increased health risks associated with waterborne illnesses (Nations, 2003). Methods currently used to purify water of pathogens and bacteria rely on direct chemical treatments, which can potentially produce harmful byproducts downstream (Gomez, De la Rua, Garralon, Plaza, Hontoria & Gomez, 2006). Consequently, filtration has emerged as a cost effective and chemical-free approach for the decontamination and purification of water supplies (Li & Chase, 2010; Porcelli & Judd, 2010; Sato, Wang, Ma, Hsiao & Chu, 2011). Nanofibrous membranes have increased porosity and an interconnected pore structure that results in increased permeability and thus high-throughput compared to membranes of microfibers. Nanofibrous membranes with pore sizes of 0.45 μm selectively remove bacteria (Gomez et al., 2006), as the bacterium size, i.e. 1.6 × 0.8 μm for Pseudomonas aeruginosa , 2 × 1 μm for Escherichia coli and 0.8 μm for Staphylococcus aureus, are larger than the membrane pore size (Lebleu, Roques, Aimar & Causserand, 2009). In combination with nanofibrous membranes, antibacterial agents are often used to kill or inhibit the growth of bacteria that would otherwise lead to biofouling and decreased filter efficiencies (Botes & Eugene Cloete, 2010). Nanofibrous membranes with an exogenic antibacterial agent, such as polyamide/poly(dimethylimino)(2-hydroxy-1.3-propanedily)chloride (Daels, De Vrieze, Sampers, Decostere, Westbroek, Dumoulin, Dejans, De Clerck & Van Hulle, 2011), cellulose acetate/silver nanoparticles (Lala, Ramaseshan, Bojun, Sundarrajan, Barhate, Ying-jun & Ramakrishna, 2007), poly(vinylidene fluoride)/silver nanoparticles (Yuan, Geng, Xing, Shen, Kang & Byun, 2009), chitosan/polyvinyl alcohol/silver nitrate/TiO2 (Son, Yeom, Song, Lee & Hwang, 2009), and inorganic silica/silver nanoparticles (Kyung, Kwark & Park, 2007) have been developed to release their biocidal agents during filtration. In this study, we report the development of a chitosan-based nanofibrous membrane with inherent antibacterial properties to improve safety and efficacy of filtration.
Chitosan exhibits antimicrobial properties towards bacteria, viruses, and fungi, for which many strains have been assayed (Muzzarelli, Muzzarelli, Tarsi, MIllani, Gabbanelli & Cartolari, 2001; Muzzarelli, Tarsi, Filippini, Giovanetti, Biagini & Varaldo, 1990; Rabea, Badawy, Stevens, Smagghe & Steurbaut, 2003). The antimicrobial mechanism includes the initial deposition of a chitosan coat on the anionic cell wall, with subsequent alteration of biochemical functions, and damage to internal organelles by internalized chitosan oligomers (Kong, Chen, Xing & Park, 2010; Liu, Du, Wang & Sun, 2004; Muzzarelli et al., 1990). Chitosan (in the solid-state as well) exhibits a cationic surface charge at physiological pH values (Hoven, Tangpasuthadol, Angkitpaiboon, Vallapa & Kiatkamjornwong, 2007; Matienzo & Winnacker, 2002; Matsumoto, Yako, Minagawa & Tanioka, 2007), but only a few studies have investigated it as a component in antimicrobial filters. Desai et al, illustrated bacteriostatic reduction on chitosanpolyethylene oxide fibers at pH 7.08, however the fiber membranes lacked structural integrity under a filter pressure of 2 mm Hg (~2 × 10–3 bar, 0.2 kPa), resulting in large tears in the membrane (Desai, Kit, Li, Michael Davidson, Zivanovic & Meyer, 2009). It is therefore of interest to develop an antibacterial, solid-state chitosan-based nanofibrous filter that can sustain filtration pressures, exhibit high permeability, selectively remove particles based on size and provide a safe antibacterial mode of action for water filtration applications.
Polycaprolactone (PCL) is commonly found in tissue engineering applications due to its structural and mechanical stability (Gross & Kalra, 2002; Hollister, 2005). We have previously reported the design and electrospinning fabrication of non-woven nanofibrous membranes comprised of chitosan and PCL with good mechanical and biological properties favorable for tissue regeneration (Cooper, Jana, Bhattarai & Zhang, 2010; Veiseh, Sun, Fang, Bhattarai, Gunn, Kievit, Du, Pullar, Lee, Ellenbogen, Olson & Zhang, 2009). In this study, chitosan-PCL fibrous membranes were prepared with different amounts of chitosan to impart an antibacterial property to the membrane. These fibers were prepared without chemical crosslinking or harmful biocides to demonstrate the intrinsic antibacterial characteristics of chitosan for filtration application. The morphology, mechanical properties, and permeability of the membranes were characterized with scanning electron microscopy (SEM), transmission electron microscopy (TEM), tensile testing, water flow permeability tests and particulate removal tests. A bacterial challenge with Staphylococcus aureus, a Gram-positive bacterium, was performed to evaluate the antibacterial performance of the membranes.
2. Materials and Methods
2.1 Electrospinning Solution Preparation
Chitosan and PCL solutions were prepared separately, and then mixed to create a solution of chitosan-PCL. Chitosan (85% deacetylated, Aldrich, St Louis, MO) was dissolved in 7 wt% trifluoroacetic acid (TFA, Aldrich) and refluxed at 70°C for 3 hours, and PCL was dissolved in 2,2,2, trifluoroethanol (TFE, Aldrich). In preparation of the electrospinning solutions with different polymer concentrations, chitosan (5 and 7 wt%) and PCL (10 and 12 wt%) solutions were prepared. Chitosan-PCL electrospinning solutions were prepared according to Table I. Immediately prior to electrospinning, the chitosan and PCL solutions were mixed to produce a chitosan-PCL solution with the desired final polymer ratio. A 10% PCL solution in TFE was prepared as a PCL electrospinning solution.
Table I.
Solution concentrations used for electrospinning to produce different fiber compositions
| Solution Concentrations Used (wt%) | ||
|---|---|---|
| Fiber Compositions | Chitosan | PCL |
| 100% PCL | 10% | |
| 25% Chitosan, 75% PCL | 7% | 12% |
| 50% Chitosan, 50% PCL | 7% | 12% |
| 75% Chitosan, 25% PCL | 5% | 12% |
2.2 Electrospinning and Characterization of Fibrous Mats
To produce electrospun nanofibers, approximately 2 mL of the solution was placed in a 3 mL syringe. The syringe tip was placed approximately 20 cm from a fiber collector, oriented –25° from the horizontal, and a 22 kV voltage supply was used to charge the solution. The solution was discharged towards a rotating grounded drum (200 rpm) to collect randomly-oriented fibrous mats, with individual fibrous mats being ~100 μm in thickness. The collected mats were allowed to dry overnight under a chemical hood and then were attached to a coverslip using biocompatible poly L-lactide (Boehringer Ingelheim, Germany) dissolved in hexafluoroisopropanol (Aldrich) at 3.5 wt%. Chitosan-PCL non-porous films (as a two-dimensional control) were prepared by spin-coating the dilute chitosan-PCL solution onto a coverslip. All the fibers and films were neutralized with 14% ammonium hydroxide for five minutes to remove residual acid, followed by rinsing three times with DI water for five minutes each. SEM and TEM were used for morphological and phase analysis. For SEM, the samples were coated with gold for 30 seconds with 18 mA current applied to a Pt target. The samples were imaged with a JEOL 7000F SEM (JEOL Ltd., Japan) at an accelerating voltage of 5–10 kV. For TEM analysis, samples were transferred to a PELCO folding copper grid and imaged with a Philips CM100 transmission electron microscope.
2.3 Static Bacterial Challenge
For the bacterial challenge experiments, the samples were disinfected with 70% ethanol prior to bacteria seeding. To evaluate bacterial adhesion and biofilm formation on these fibrous samples, Staphylococcus aureus (ATCC 25693) were incubated with fiber-coated glass coverslips in 24-well tissue culture plates. S. aureus were streaked on agar plates supplemented with 10 g/L trypticase soy broth (TSB, pH 7.2) and incubated at 37°C until growing colonies reached desired sizes. For suspended culture inocula, a single colony sample from the streak plate was collected with a sterile loop, added to 25 mL of 10 g/L TSB, and incubated at 37°C overnight. Bacterial cells from the overnight culture were diluted with 10 g/L fresh TSB to a final concentration of 5 × 104 cells/mL, and then seeded into individual wells of 24-well plates containing fibers and control films (n = 3). The plates were incubated at 37°C under rotation at 125 rpm. At each preset time point (8 and 24 hours of incubation), samples were removed and washed twice with PBS and placed in a new 10 mL tube with 1.5 mL PBS. Each sample was sonicated for 5 seconds three times using a needle ultrasonicator. The solution was then serial diluted and placed on TSB plates and the colony forming units (CFU) counted after 8 and 24 hours (Tomasiewicz, 1980). Data was represented as CFU per area (colony number on plate × dilution factor × 1.5 mL total volume / (0.01 mL sample volume × gross surface area of the glass cover plate 0.785 cm2). After 24 hours of bacterial culture, the membranes were fixed for SEM analysis in 3% glutaraldehyde and dehydrated in an ethanol wash series. The membranes were sputter coated with 7 nm of gold prior to SEM imaging.
2.4 Flow Cell Apparatus and Particulate Testing
Square membranes of ~2 cm in width were cut from the nanofibers mats. These square sample membranes were used for individual water flux testing and particulate-water separation testing with an open-ended filtration setup. The fibrous membrane was sandwiched between two plastic plates with ~1 cm inner diameter holes. The membrane surface was placed horizontally and two tubes (~1 cm inner diameter) were coaxially connected to the holes of the top and bottom plastic plates, respectively, to act as an inlet and outlet for water flow. To create a desired pressure on the membrane surface, the inlet was filled with water to a known head height with respect to the membrane surface. To maintain a constant pressure, water was continuously added to keep the head height constant at the inlet. To test the flow rate through the membranes, an inlet pressure between ~0.75–3 kPa was applied, and the amount of water that passed through the membrane was measured in one minute increments over a 5-min period.
To test the performance of the membranes for particle separation, polystyrene (PS) particles with 100, 300 and 1000 nm diameters (Sigma Aldrich) were used. Individual solutions of the PS beads were prepared at 200 ppm in DI water and were allowed to pass through the membranes. To characterize the removal efficiency of the membranes, the first 2 mL of eluent that passed through the membranes at an initial pressure of ~1.5 kPa were collected. The recovered solution was characterized with a UV-Vis spectrometer operated at 490 nm and the concentration of PS nanoparticles was determined from a calibration curve of known PS/DI water concentrations. The membranes were washed briefly with water to remove excess PS beads and prepared for SEM analysis by drying at 37°C.
2.5 Statistical Analysis
Results for bacterial analysis were presented as means ± standard deviation. Statistical analysis was performed using one-way analysis of variance, followed by post-hoc Student's t-test. Differences were considered significant for values of p < 0.05.
3. Results and Discussion
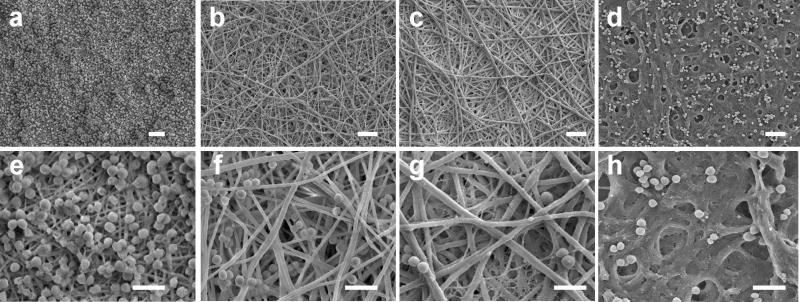
Non-woven, randomly-oriented fibrous chitosan-PCL membranes were prepared by electrospinning chitosan-PCL solutions containing 0, 25, 50 and 75 wt% of chitosan using a previously established rotating drum setup (Veiseh et al., 2009). The volume of the chitosan-PCL solutions used for preparing an electrospun membrane were the same for all of the ratios (~2 mL) and the electrospinning conditions were controlled to produce similar density membranes composed of nanofibers with approximately the same diameter. As shown in the SEM images of Figure 1a-d, uniform nanofibrous membranes were formed from all PCL and chitosan-PCL solutions with fiber diameters of 200–400 nm. Pure PCL fibers were electrospun from a 10 wt% PCL/TFE solution (Figure 1a). Chitosan-PCL fibers with 25 wt% chitosan (Figure 1b), 50 wt% chitosan (Figure 1c) and 75 wt% chitosan (Figure 1d) were electrospun from mixtures prepared from 7 and 12 wt%, 7 and 12 wt%, and 5 and 12 wt% chitosan and PCL solutions, respectively. TEM analysis (Figure 1e-h) indicated the homogeneity of the solid fibers, with no sign of phase separation or structural voids.
Figure 1.
SEM analysis of nanofibrous membranes with various chitosan concentrations. SEM images of electrospun nanofibrous membranes with (a) PCL only, (b) 25% chitosan and 75% PCL, (c) 50% chitosan and 50% PCL, and (d) 75% chitosan and 25% PCL. The scale bars represent 2 μm in (a-d). TEM images of nanofibrous membranes with (e) PCL only, (f) 25% chitosan, (g) 50% chitosan and (h) 75% chitosan. The scale bars represent 400 nm in (e-h).
All the prepared membranes were then assessed as an antibacterial surface with Staphylococcus aureus (SA 25693) as a model bacterium to examine the inhibitory effect of increasing chitosan content in the chitosan-PCL membranes. The membranes were incubated with S. aureus over the course of a 24-hour period. Bacterial cell attachment was quantified by counting the colony-forming units and by SEM imaging.As shown in Table II, compared to the synthetic PCL fiber control, the chitosan-PCL samples induced less S. aureus colonization. At the 24-hour time point, the number of attached S. aureus cells increased compared to the 8-hour time point except for the 75 wt% chitosan membranes, which exhibited no increase from 8 to 24 hrs. PCL control membranes exhibited the largest increase in adherent bacterial numbers. At 8 hrs, all chitosan-containing membranes exhibited approximately half of the adherent cell counts versus the PCL control. The cell adhesion increases for the PCL membrane from 8 to 24 hrs while increases in adherent cell concentration from 8 to 24 hours for chitosan-containing membrane were approximately the same for all four membranes. A previous study of chitosan-PCL films cultured with a gram-positive bacterium, Streptococcus mutans, demonstrated similar results that with increasing chitosan content (i.e. cationic nature) the bacteria growth is reduced but not completely eliminated (Sarasam, Krishnaswamy & Madihally, 2006). Although the study by Sarasam et al. demonstrated that the chitosan-based films are not bactericidal, a reduced bacterial growth in the culture media was reported, suggesting that those chitosan-PCL polyblends leached a growth inhibitory compound into the liquid phase.
Table II.
Colony forming units (CFUs, cell number /cm2) of adhered S. aureus cells on nanofibrous membranes with different chitosan concentrations.
| Cell number/cm2 | ||
|---|---|---|
| Fiber Compositions | 8 hrs | 24 hrs |
| 100% PCL | 4.64 ± 1.20 | 6.45 ± 2.14 |
| 25% Chitosan | 2.02 ± 0.60* | 3.79 ± 0.84 |
| 50% Chitosan | 2.54 ± 0.30* | 3.61 ± 0.28* |
| 75% Chitosan | 2.59 ± 0.9 | 2.92 ± 0.48* |
Indicates significant difference to 100% PCL fiber.
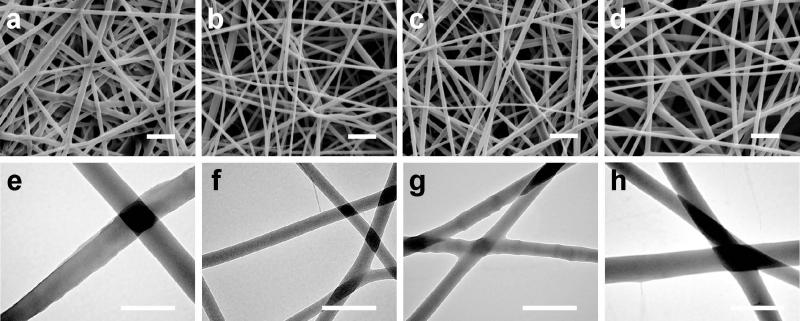
After 24 hours of bacterial culture, the membranes were fixed for SEM analysis to examine S. aureus cell accumulation. As shown in Figure 2a and 2e, a large bacteria population was observed on the PCL fibrous membrane, indicating that the bacteria proliferated into dense colonies. In contrast, S. aureus cells appeared sporadically on chitosan-PCL membranes. Increasing chitosan weight content in chitosan-PCL membranes did not significantly decrease bacterial adhesion (Figure 2b-d and 2f-h). The SEM analysis qualitatively agrees with the colony counts shown in Table II. In this study, the density of bacterial challenge was significantly greater than any situation present in natural environmental conditions, suggesting that the reduction observed within the 24-hour time period may be translated to a dramatically extended filter lifetime for real-world conditions.
Figure 2.
SEM analysis of S. aureus on nanofibrous membranes at low (top row) and high (bottom row) magnifications. (a, e) PCL only, (b, f) 25% chitosan, (c, g) 50% chitosan and (d, h) 75% chitosan. The samples were fixed after 24 hours of culture with an initial bacteria density of 5 × 104 cells/mL in tryptic soy broth medium. Scale bars represent 5 μm (top row) and 2.5 μm (bottom row). Note the excessive fiber swelling in h.
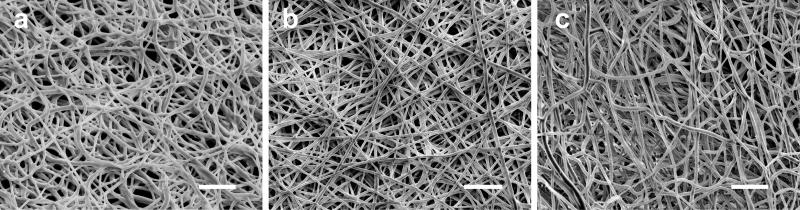
The permeability is particularly important to filtration application as it dictates the amount of fluid that can be processed for a given time and dimension at a defined applied pressure without damaging the mechanical integrity of the filter (Barhate & Ramakrishna, 2007). The advantages of nanofibrous membranes as filters include small pore sizes that reduce particle size exclusion and have a high surface-area to volume ratio, which can result in high flux. In this study, a range of filtration pressures (0.75–3 kPa) was applied to the nanofibrous membranes, which falls within the range of transmembrane pressures for microfiltration membranes (Bjorge, Daels, De Vrieze, Dejans, Van Camp & Audenaert, 2009). Nanofibrous membranes as filters are typically used in conjunction with other supports such as microfibers (Homaeigohar, Buhr & Ebert, 2010); however the focus of this study was only on the pre-filter and its standalone properties. The combination of these chitosan-PCL membranes with a mechanical support could increase the ability to withstand greater transmembrane pressures. In this study, the membranes were exposed to transmembrane pressures of 0.75–3 kPa. Fibrous samples with 50% and 75% chitosan ruptured at 3 kPa. As a result, a pressure of 1.5 kPa was chosen for all subsequent tests. A vertical column of water was applied to the membrane surface at a constant pressure of 1.5 kPa and the amount of water that passed through the membrane was measured in one-minute increments over a five-minute period. Table III lists the evaluated flux rates for PCL, 25 wt% and 50 wt% chitosan fiber membranes. The 75% chitosan membrane was also subjected to the same flux test; however the membrane ruptured at this pressure, which was likely due to chitosan swelling. Thus, we concluded that the 75% chitosan fibrous membrane was not suitable for filtration application under aqueous conditions. The 25% chitosan fiber membrane allowed for the highest fluid flux of ~7000 L/hr/m2. After the flux test, the membrane morphology was examined with SEM (Figure 3). The PCL membrane showed less uniform pore sizes and some pores collapsed corroborating the relatively low flux. The 25% chitosan membrane exhibited uniform pore dispersion. Alternatively, the 50% chitosan showed a compressed structure similar to that of the PCL membrane, which contributed the relatively low flux.
Table III.
Flux of PCL and chitosan-PCL membranes at a water pressure of 1.5 kPa
| Nanofibrous Membrane | Flux (L/hr/m2) |
|---|---|
| PCL | 2756.8 ± 68.9 |
| 25% Chitosan | 6926.8 ± 1143.6 |
| 50% Chitosan | 2629.46 ± 97.3 |
| 75% Chitosan | N/A |
Figure 3.
SEM images of nanofibrous membranes with (a) PCL only, (b) 25% chitosan and (c) 50% chitosan after subjected to water flux for 5 minutes at 1.5 kPa. The scale bars represent 5 μm.
Particulate removal, in addition to flux rate, is another primary measure of filter efficacy. To assess the filtering capabilities of the membranes, a series of particulate suspensions comprising Polysyrene (PS) beads of different sizes were passed through these membrane filters at a set pressure. The 75 wt% chitosan fiber membrane was not included in this study due to significant material swelling and membrane rapture as mentioned above. Solutions containing PS beads of 100, 300 and 1000 nm diameters, respectively, were passed through the membranes at 1.5 kPa, and the first 2 mL of eluent was analyzed by UV-Vis spectroscopy. The removal efficiency was evaluated by comparing the concentration of the eluent with the concentration of the initial solution (200 ppm). As shown in Table IV, all of the membranes completely removed the large 1000-nm diameter PS beads from the solution (100% removal efficiency). With decreasing bead size, the removal efficiency decreased and varied depending on the membrane material. For 300-nm PS beads, the PCL and 50% chitosan membranes had removal efficiencies of ~31.7% and 61.5%, respectively. Alternatively, the 25% chitosan membrane removed 99.6% of the 300 nm PS beads from the solution. The addition of chitosan to PCL altered the electrospinning behaviors of the solution and effectively reduced the fiber diameters and increased the density. As a result, the addition of PCL created a dense, nanofibrous mat surface that effectively restricted passage of the 300-nm PS particles. The removal efficiency was significantly reduced when the PS beads decreased to 100 nm in size (25%, 15% and 10% for the PCL, 25% and 50% membranes, respectively). Notably, the 25% chitosan-PCL fibrous membrane size-exclusion behavior approached the requirements for HEPA filters (99.7% for 300-nm particulates) (Ahn, Park, Kim, Hwang, Lee, Shin & Lee, 2006; Barhate et al., 2007), suggesting the membrane could act as an antibacterial pre-filter for aerosol applications.
Table IV.
Particulate removal efficiency of membranes with different chitosan concentrations.
| Particulate removal efficiency (%) | |||
|---|---|---|---|
| Fiber Compositions | 100 nm | 300 nm | 1000 nm |
| 50% Chitosan | 9.25 | 61.48 | 100 |
| 25% Chitosan | 14.08 | 99.76 | 100 |
| 100% PCL | 30.63 | 31.78 | 100 |
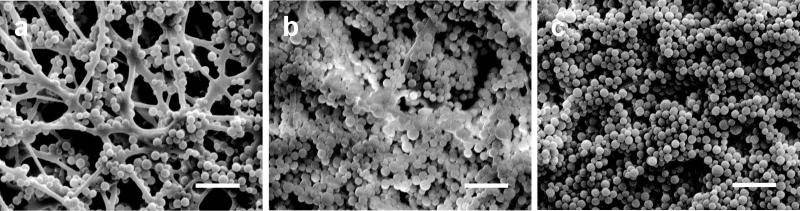
SEM was used to analyze the membrane surfaces after 300-nm particulate removal. As shown in Figure 6a, PS beads adhered strongly to the PCL fibers, wherein the pore size of the membrane was significantly larger than the particle diameter, which could have attributed to its low removal efficiency demonstrated in Figure 4. Alternatively, the PS beads fully covered the 25% (Figure 6b) and 50% (Figure 6c) chitosan membrane surfaces. The resulting beaded surface followed the topography of the fibers, and showed deep penetration into the fiber layers and pores, indicating that the membranes captured the beads, preventing trans-membrane passage. The 25% chitosan-PCL membrane exhibited the best antibacterial behavior, flux performance, and size selectivity down to 300-nm particles. Due to the versatility of the electrospinning method, the porosity, inter-fiber spacing and thickness of a membrane can be controlled to alter the flux and selectivity of a membrane. As a result, the chitosan-PCL membranes can be tailored to specific flux values and particulate removals depending on the application.
Figure 4.
SEM images of nanofibrous membranes containing (a) PCL only, (b) 25% chitosan and (c) 50% chitosan following a particulate flow study with 300-nm diameter PS beads. The scale bars represent 2 μm.
4. Conclusions
Nanofibrous membranes were developed via electrospinning with increasing chitosan content to utilize the antibacterial properties of the natural polymer and size-selectivity of the fibrous membranes. We demonstrated that the incorporation of 25% chitosan into the nanofibrous membrane reduced S. aureus bacterial colonization by 50% compared to membranes made of pure PCL fibers. With increasing chitosan content, the fibers were more susceptible to swelling, preventing their application as a filter. At 25 and 50% chitosan content, the highly porous membranes supported high water permeability that did not damage the membrane morphology. Furthermore, a chitosan-PCL fibrous membrane was able to remove near 100% of 300-nm diameter particles, demonstrating the ability to selectively remove particles and act as a pre-filter. The developed membrane combines the antibacterial behavior of natural chitosan polymer with nanofiber advantages to serve as a candidate for microfiltration applications.
Highlights.
Chitosan-PCL nanofibers exhibit antibacterial activity based on chitosan content.
Chitosan-PCL nanofibrous membranes facilitated high fluid flux.
The membranes successfully removed 300-nm diameter particles from solution.
The chitosan-PCL nanofibers can potentially serve as antibacterial pre-filter for microfiltration.
Acknowledgements
This work is supported in part by UW TGIF and the Kyocera Professor Endowment. Rachael Oldinsky and James Bryers were supported by the NIH (grant number: R01DE018701). Ashleigh Cooper would like to acknowledge the support by the Bank of America Endowed Minority fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn Y, Park S, Kim G, Hwang Y, Lee C, Shin H, Lee J. Development of high efficiency nanofilters made of nanofibers. Current Applied Physics. 2006;6(6):1030–1035. [Google Scholar]
- Barhate R, Ramakrishna S. Nanofibrous filtering media: filtration problems and solutions from tiny materials. J Membrane Sci. 2007;296(1-2):1–8. [Google Scholar]
- Bjorge D, Daels N, De Vrieze S, Dejans P, Van Camp T, Audenaert W. Performance assessment of electrospun nanofibers for filter applications. Desalination. 2009;249(3):942–948. [Google Scholar]
- Botes M, Eugene Cloete T. The potential of nanofibers and nanobiocides in water purification. Critical Rev Microbiol. 2010;36(1):68–81. doi: 10.3109/10408410903397332. [DOI] [PubMed] [Google Scholar]
- Cooper A, Jana S, Bhattarai N, Zhang M. Aligned chitosan-based nanofibers for enhanced myogenesis. J Mater Chem. 2010;20(1):8904–8911. [Google Scholar]
- Daels N, De Vrieze S, Sampers I, Decostere B, Westbroek P, Dumoulin A, Dejans P, De Clerck K, Van Hulle S. Potential of a functionalised nanofibre microfiltration membrane as an antibacterial water filter. Desalination. 2011;275(1-3):285–290. [Google Scholar]
- Desai K, Kit K, Li J, Michael Davidson P, Zivanovic S, Meyer H. Nanofibrous chitosan non-wovens for filtration applications. Polymer. 2009;50(15):3661–3669. [Google Scholar]
- Gomez M, De la Rua A, Garralon G, Plaza F, Hontoria E, Gomez M. Urban wastewater disinfection by filtration technologies. Desalination. 2006;190(1-3):16–28. [Google Scholar]
- Gross RA, Kalra B. Biodegradable polymers for the environment. Science. 2002;297(5582):803–807. doi: 10.1126/science.297.5582.803. [DOI] [PubMed] [Google Scholar]
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- Homaeigohar SS, Buhr K, Ebert K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J Membrane Sci. 2010;365(1-2):68–77. [Google Scholar]
- Hoven VP, Tangpasuthadol V, Angkitpaiboon Y, Vallapa N, Kiatkamjornwong S. Surface-charged chitosan: Preparation and protein adsorption. Carbohydrate Polym. 2007;68(1):44–53. [Google Scholar]
- Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int J Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kyung MD, Kwark Y-J, Park WH. Preparation of Inorganic Silica Nanofibers Containing Silver Nanoparticles. Fibers and Polymers. 2007;8(6):591–600. [Google Scholar]
- Lala NL, Ramaseshan R, Bojun L, Sundarrajan S, Barhate R, Ying-jun L, Ramakrishna S. Fabrication of nanofibers with antimicrobial functionality used as filters: protection against bacterial contaminants. Biotech Bioeng. 2007;97(6):1357–1365. doi: 10.1002/bit.21351. [DOI] [PubMed] [Google Scholar]
- Lebleu N, Roques C, Aimar P, Causserand C. Role of the cell-wall structure in the retention of bacteria by microfiltration membranes. J Membrane Sci. 2009;326(1):178–185. [Google Scholar]
- Li J, Chase HA. Applications of membrane techniques for purification of natural products. Biotechnol Lett. 2010;32(5):601–608. doi: 10.1007/s10529-009-0199-7. [DOI] [PubMed] [Google Scholar]
- Liu H, Du Y, Wang X, Sun L. Chitosan kills bacteria through cell membrane damage. Int J Food Microbiol. 2004;95(2):147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Matienzo LJ, Winnacker SK. Dry Processes for Surface Modification of a Biopolymer: Chitosan. Macromol Mater Eng. 2002;287(12):871–880. [Google Scholar]
- Matsumoto H, Yako H, Minagawa M, Tanioka A. Characterization of chitosan nanofiber fabric by electrospray deposition: Electrokinetic and adsorption behavior. J Colloid Inter Sci. 2007;310(2):678–681. doi: 10.1016/j.jcis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R, Muzzarelli C, Tarsi R, MIllani M, Gabbanelli F, Cartolari M. Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules. 2001;2(1):165–169. doi: 10.1021/bm000091s. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R, Tarsi R, Filippini O, Giovanetti E, Biagini G, Varaldo PE. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother. 1990;34(10):2019–2023. doi: 10.1128/aac.34.10.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nations U. Water for people, water for live: The United Nations World Development Report. UNESCO Publishing/Berghahan Books; 2003. [Google Scholar]
- Porcelli N, Judd S. Chemical cleaning of potable water membranes: A review. Separation and Purification Technology. 2010;71(2):137–143. [Google Scholar]
- Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as Antimicrobial Agent:,Äâ Applications and Mode of Action. Biomacromolecules. 2003;4(6):1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Sarasam AR, Krishnaswamy RK, Madihally SV. Blending chitosan with polycaprolactone: effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7(4):1131–1138. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- Sato A, Wang R, Ma H, Hsiao BS, Chu B. Novel nanofibrous scaffolds for water filtration with bacteria and virus removal capability. Journal of Electron Microscopy. 2011;60(3):201. doi: 10.1093/jmicro/dfr019. [DOI] [PubMed] [Google Scholar]
- Son B, Yeom B-Y, Song SH, Lee C-S, Hwang TS. Antibacterial electrospun chitosan/poly(vinyl alcohol) nanofibers containing silver nitrate and titanium dioxide. J Appl Polym Sci. 2009;111(6):2892–2899. [Google Scholar]
- Tomasiewicz D. The Most Suitable Number of Colonies on Plates for Counting. J Food Prot. 1980;43(4):282–286. doi: 10.4315/0362-028X-43.4.282. [DOI] [PubMed] [Google Scholar]
- Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, Olson J, Zhang M. Specific Targeting of Brain Tumors with an Optical/Magnetic Resonance Imaging Nanoprobe across the Blood-Brain Barrier. Cancer Res. 2009;69(15):6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Geng J, Xing Z, Shen J, Kang I-K, Byun H. Electrospinning of antibacterial poly(vinylidene fluoride) nanofibers containing silver nanoparticles. J Appl Polym Sci. 2009;116(2):668–672. [Google Scholar]