Abstract
Genital Chlamydia trachomatis infections can elicit an inflammatory arthritis in some individuals, and recent surprising studies have demonstrated that only ocular (trachoma) strains, not genital strains, of the organism are present in the synovial tissues of patients with the disease. This observation suggests an explanation for the small proportion of genitally-infected patients who develop Chlamydia-induced arthritis. Other recent studies have begun to identify the specific chlamydial gene products that elicit the synovial inflammatory response during both active and quiescent disease, although much more study will be required to complete the understanding of that complex process of host–pathogen interaction. Several newly developed experimental methods and approaches for study of the process will enable identification of new therapeutic targets, and possibly strategies for prevention of the disease altogether.
Keywords: Chlamydia trachomatis, combination antibiotics, genetics, inflammation, pathogenesis
Chlamydia-induced arthritis
Chlamydia trachomatis is an obligate intracellular bacterial pathogen known to be the etiologic agent for a number of important human diseases. In some underdeveloped regions of the world, specific strains/serovars of the organism cause trachoma, which remains a significant cause of treatable blindness; other strains/serovars of the organism cause genital infections worldwide (for a review see [1]). In the USA, all new genital chlamydial infections must be reported to the CDC by law from all 50 states and the District of Columbia [101]. Recent data from the CDC indicate that more than 3 million new infections are reported each year (see [101]; and also below). Moreover, independent estimates from the WHO indicate that globally, as many as 115 million new genital chlamydial infections occur each year [1,2]. In addition to these primary infections, it has been clear for many years that chlamydial infections can, and often do, cause severe sequelae. These are primarily a function of genital infections with C. trachomatis and include fallopian tubule blockage leading to ectopic pregnancy, pelvic inflammatory disease and other problems with the human female upper reproductive tract, and an inflammatory arthritis, which is the topic of this review (for review see [1,3–6]). The arthritis is classified among the spondyloarthropathies and it has been given several different clinical designations, including Reiter’s disease [7]. Generally it has been referred to as reactive arthritis, and more recently simply as Chlamydia-induced arthritis [8].
Importantly, C. trachomatis is not the only chlamydial species that has been shown to cause joint problems in humans. Chlamydia pneumoniae is, as its name indicates, a respiratory pathogen that was first identified in 1986 and defined as a unique chlamydial species a few years later [9,10]. Epidemiologic data indicate that infection with this organism is extremely common in all populations examined to date, and that reinfection is also common. Some estimates indicate that such pulmonary infections are responsible for as much as half of all community-acquired pneumonia [10,11]. Infections with C. pneumoniae, as with those of C. trachomatis, have also been linked to a number of severe sequelae, including asthmatic bronchitis, chronic obstructive pulmonary disease, atherogenesis and, relevant to the topic of this article, an inflammatory arthritis similar to that elicited by genital infection with C. trachomatis (see [8,12–14]). The clinical aspects of C. pneumoniae-induced arthritis mirror to some extent those characteristic of C. trachomatis-induced arthritis, although several differences are known. While it has not been designated as yet, we assume that the former joint disease will be classified among the spondyloarthritides, as is the latter. Official recognition of C. pneumoniae as a causative agent by the ACR, including diagnostic criteria and treatment preferences, are yet to be formalized.
In this article, the authors summarize the current understanding of the clinical aspects of Chlamydia-induced arthritis, with emphasis on the basic biological factors underlying synovial pathology and the observable aspects of disease phenotype. In that context, we discuss the nature of the chlamydial strains eliciting the disease as well as initial insights regarding the molecular genetic basis for the remitting–relapsing disease phenotype that characterizes many patients with Chlamydia-induced arthritis. Antecedent to that discussion, however, we provide a detailed summary of the current state of understanding regarding chlamydial pathogenesis of the joint as a foundation for what follows. Throughout the text, we suggest lines of research that should provide critical new insights into the synovial pathogenesis process, with the idea that those insights will direct development of new therapeutic approaches to treat, and perhaps to obviate altogether, the disease.
Clinical aspects of Chlamydia-induced arthritis
Reactive arthritis is generally an inflammatory arthritis that occurs within 1–6 weeks after the patient is exposed to one of a panel of triggering bacterial organisms. Historically, two main types of the disease have been recognized: post-venereal and post-dysentery (or post-enteric) [15,16]. Although many triggering organisms have been implicated for both variants, definitive agents for postdysenteric reactive arthritis include various species from the Genera Salmonella, Shigella, Campylobacter and Yersinia [3,15,16]. Chlamydia trachomatis is, of course, the definitive trigger of the postvenereal variant (see [4,5,7]). Moreover as indicated above, C. pneumoniae is now understood to be a triggering organism [8,12,13], along with several additional agents including Ureaplasma urealyticum, Helicobacter pylori and various intestinal parasites. Reports of reactive arthritis secondary to Escherichia coli [17], Clostridium difficile [18] and intravesicular Bacillus Calmette–Guerin [19] have garnered recent attention. Box 1 provides a list of established and probable etiologic agents for the arthritis.
Box 1. Triggering microbes of reactive arthritis.
Definite causes
- ▪ Post-venereal
- – Chlamydia trachomatis
- ▪ Post-enteric
- – Salmonella (S. enteritidis, S. typhimurium, S. bovismorbificans and S. blockley)
- – Shigella (S. flexneri, S. dysenteriae, S. sonnei and S. boydii)
- – Campylobacter (C. jejuni and C. coli)
- – Yersinia (Y. enterocolitica and Y. pseudotuberculosis)
Probable causes
▪ Chlamydophila (Chlamydia) pneumoniae
▪ Ureaplasma urealyticum
▪ Bacille Calmette–Guerin (intravesicular)
Possible causes
▪ Bacillus cereus
▪ Brucella abortis
▪ Clostridium difficile
▪ Escherichia coli
▪ Helicobacter pylori
▪ Hafnia alvei
▪ Lactobacillus
▪ Neisseria meningitidis serogroup B
▪ Pseudomona
▪ Intestinal parasites (Strongyloides stercolis, Taenia saginata, Giardia lamblia, Ascaris lumbricoides, Filariasis and Cryptosporidium)
Other types of inflammatory arthritis in which bacteria may play a causative role
▪ Borrelia burgdorferi (Lyme disease)
▪ Propionbacterium acnes
▪ Streptococcus sp (post-streptococcal reactive arthritis)
▪ Trophyrema whippelii (Whipple’s disease)
Reproduced from [91] with permission from Elsevier.
Regardless of whether the arthritis develops from urogenital, gastrointestinal or other pathogens, clinical symptoms are considered to be congruent. As a type of spondyloarthritis this condition shares features with the other types of spondyloarthritides. Patients develop an inflammatory arthritis that involves peripheral joints, and usually the axial skeleton, particularly also the sacroiliac joints; the arthritis typically affects the large joints of the lower extremities, although any joint can be involved [20,21]. Patients can present with a mono-, oligo- or poly-arthritis, but oligoarthritis is the most typical pattern observed overall. Enthesitis is a common feature of reactive arthritis resulting from any etiologic agent. Other organs including the skin and mucous membranes are often involved [3,22]. Although numbers given in the literature vary, 50–70% of cases of acute reactive arthritis will resolve spontaneously without intervention within the initial 6 months; 30–50% of patients will progress to chronicity [20,21]. Importantly, patients with chronic reactive arthritis deriving from any causative agent often display a remitting–relapsing disease course (e.g., [20–22] and see below). Historically, it has been thought that arthritis from gastrointestinal pathogens affects males and females roughly equally, but that Chlamydia-induced reactive arthritis affects males primarily [22]. This apparent predilection is almost certainly a function, at least in large part, of the official ACR disease definition, which requires a documented prior genital infection with C. trachomatis. However, it is well known that genital chlamydial infections in women are often asymptomatic/subclinical, thus obviating an official diagnosis of Chlamydia-induced reactive arthritis. As indicated, no official diagnostic criteria exist as yet for the inflammatory arthritis elicited by C. pneumoniae.
Epidemiology & incidence of Chlamydia-induced arthritis
As mentioned, the CDC reported that in the USA approximately 3 million new C. trachomatis infections occur each year in the population group 15–44 years of age [101]. The overall incidence of C. pneumoniae infections is not reported, and thus is unknown, but again as mentioned, what data do exist indicate that it is a more common pathogen than C. trachomatis [102]. Interestingly, despite the fact that C. pneumoniae is a more common pathogen than C. trachomatis, our and others’ data indicate that it is a less frequent trigger of inflammatory arthritis. For example, our own studies have indicated that arthritis following C. trachomatis genital infection accounts for as much as 50% of all reactive arthritis, but that only approximately 12% of the clinically similar arthritis is attributable to C. pneumoniae infection (e.g., see [5,12,23,24] for review). In a few cases, patients with inflammatory arthritis due to chlamydial infection are coinfected with both C. trachomatis and C. pneumoniae (e.g., [25]). Moreover, published data indicate that only approximately 4% of patients who experience an acute genital chlamydial infection will develop inflammatory arthritis [3,4,22]. Calculations using this rate and the annual incidence of three million new C. trachomatis infections each year in the USA, indicate that more than 120,000 cases of acute C. trachomatis-induced reactive arthritis should occur in the USA per year. This does not include arthritis resulting from C. pneumoniae infections or arthritis in patients with an age outside the range specified above. If accurate, the estimated annual incidence of Chlamydia-induced arthritis in the USA is approximately equal to, and perhaps somewhat higher than, that of rheumatoid arthritis [26]. Consistent with this contention, a 2002 study in Sweden found the annual incidence of chlamydial arthritis to be higher than that of rheumatoid arthritis [27]. Because much epidemiologic data demonstrate that the number of patients diagnosed with rheumatoid arthritis greatly exceeds that for patients diagnosed with Chlamydia-induced arthritis, the impact of the latter certainly is significantly underappreciated.
One reason for the underdiagnosis of Chlamydia-induced arthritis is related to the fact that the acute disease often resolves spontaneously, prior to consultation with a physician for joint discomfort (see above). Furthermore, an over-reliance on the classic triad of symptoms, on HLA-B27 positivity, on the more subtle clinical presentation in women, or on all these in combination in the context of the lack of clear and specific diagnostic criteria [20,28] also contributes to the problem. Furthermore, patients can be reluctant to reveal or discuss a genital C. trachomatis infection. In addition, as discussed above, a compelling explanation for underdiagnosis of Chlamydia-induced arthritis stems from the frequently asymptomatic nature of many such genital infections, particularly in women (e.g., [7,29]). The lack of a clinically apparent preceding genital chlamydial infection obfuscates the diagnosis, as well as compromising the very definition of the condition.
Strains/serovars involved in arthritogenesis
An issue that has never been adequately resolved in relation to Chlamydia-induced arthritis concerns the low incidence of the acute disease following genital chlamydial infection, and the observation that only approximately half of those who do develop the acute disease progress to chronicity (see references [15,20,21], and also above). As mentioned, C. trachomatis is a human pathogen, and its strains are generally divided into ocular and genital groups, referred to as serovar groups since initially they were defined serologically. Serovar was defined as a function of the structure of the ompA gene product, which is the major outer membrane protein, and serovar-specific monoclonal antibodies to this protein were used to differentiate strains in infected tissue samples. More recently, serovars have been elucidated in clinical samples by DNA sequence determination of the ompA gene cloned from the sample, followed by in silico translation to determine the predicted amino acid sequence of the protein, particularly in regions targeted by the differentiating monoclonal antibodies [30,31].
The ocular chlamydial serovar group includes serovars A, B, Ba and C, while the genital group includes serovars D–K, plus the lymphogranulosum venereum group (LGV) (e.g., [32]). The tacit assumption has always been that, since the inflammatory arthritis follows genital infection, the inciting organisms must belong to the genital serovar group. We recently performed an extensive DNA sequence determination of multiple cloned ompA genes from each of 36 patients with well-defined chronic Chlamydia-induced arthritis. The experimental intention was simply to assess DNA sequence diversity at that well-studied locus within individual patient samples, and as we predicted that diversity was indeed low. However, when we asked which specific serovars were involved via comparison of our sequences to the known ompA sequences in the databases, we were surprised to find that all sequences from each patient derived virtually exclusively from ocular group organisms [33]. Interestingly, in that study we did identify a few cloned sequences in which some DNA exchange had apparently taken place so as to give minor characteristics of genital serovar genome structure in the predominantly ocular serovar genome. The overall genome structure differs somewhat between ocular and genital group organisms at ompA and other chromosomal regions, and those differences are almost certainly responsible in some as yet unknown fashion for the ability of ocular group organisms to disseminate from the genital system to the joint, and once at that site to elicit severe inflammation. More detailed and extensive study of the genetic component of C. trachomatis infecting synovial tissue in additional patient samples will be required to fully elucidate the mechanism(s) underlying chlamydial dissemination from the urogenital system to the joint. Unknown attributes of the host genetic background may also influence dissemination to the joint in some individuals. These differences either individually or in concert also probably influence the characteristic remitting–relapsing phenotype of patients with the chronic arthritis, again as developed below. Table 1 presents a summary of strains identified in our study.
Table 1.
Chlamydia trachomatis strains/serovars identified in synovial tissues of arthritis patients.
| Strain/serovar† | Number of patients | Range of disease duration |
|---|---|---|
| A | 2 | 15–18 months |
| B | 1 | Not available |
| C | 33 | 0.5–96 months |
Serovar determined by DNA sequence at ompA, chromosome structure at trpA and the cytotoxin locus; see [35] for details.
In the absence of additional data, we argue at this point that the relatively low incidence of acute inflammatory arthritis among patients with a documented genital chlamydial infection is primarily a function of the presence or absence of ocular serovar organisms in the genital inoculum leading to infection. That is, infection of the human genital tract almost certainly does not involve a clonal population of chlamydiae. Rather, the inoculum often, if not always, includes some serovar diversity, with a majority of such inocula including only one or more genital serovars and others, a minority, having a component (probably a small component) of ocular group organisms. Genital infections by ocular serovars are known but rare, supporting the contention that the majority of genital inocula do not include any ocular group organisms [34,35]. We contend that the acute inflammatory arthritis develops only in that minority of patients whose genital inocula include ocular serovar organisms (for further discussion see [33]). However, this hypothesis does not explain the observation that only approximately half of patients with the acute disease progress to chronicity. We predict that the explanation will be found to be complex and will include small genome sequence differences among the synovial population of infecting ocular organisms, as yet unknown aspects of the host genetic background, and the host–pathogen interaction that these genetic components engender. Elucidating these interactions and their genetic underpinnings will comprise experimental questions of significant interest for future studies, in our view. We note in conclusion, however, that determination of whether cervical or urethral infections include a component of ocular serovar chlamydiae is a promising avenue of approach to identifying patients at risk for development of the inflammatory arthritis.
Fundamental molecular genetic aspects of chlamydial pathogenesis
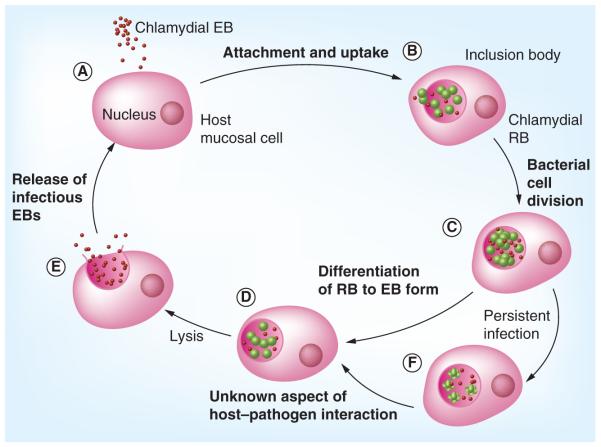
The basic outline of the process underlying chlamydial pathogenesis during primary infection of the genital tract has emerged over the many years of study it has undergone. Not surprisingly, that pathogenesis is a function of the biology of active infection by C. trachomatis – that is, it is a function of details attendant on the developmental cycle. The cycle is initiated by attachment of the extracellular form of the organism, the elementary body, to target host cells, upon which the organisms are taken into the host cell by an active process. The primary host cell type is the epithelial or epithelia-like cell, but many other cell types can be infected as well [6,36,37]. The receptor to which chlamydiae attach on the host cell surface has been an important target of research for decades, and a number of host surface molecules have been implicated (e.g., [38–40]). One recently published report indicated that the receptor for C. pneumoniae attachment on endothelial cells is the lectin-like oxidized LDL receptor [41]. That observation is consistent with as yet unpublished results from this laboratory for attachment of either C. pneumoniae or C. trachomatis on epithelial cells (Gerard HC et al. Chlamydia pneumoniae uses apolipoprotein E and the LDL receptor family for host cell attachment [2012], Manuscript in preparation; also see [42–45]). If these receptor data are confirmed by other groups it would provide some explanation for several as yet unexplained aspects of chlamydial infection, including the ability of these organisms to elicit phagocytosis in normally nonphagocytic cells. Regardless, once in the host cell cytoplasm, the organisms reside within a membrane-bound vesicle for the duration of their intracellular tenure. Within the inclusion, each elementary body undergoes a transcriptionally-determined ‘differentiation’ process yielding the vegetative growth form of the organism, the reticulate body. Each of these undergoes seven to eight cell divisions. Near the end of the cell division process approximately 80% of reticulate bodies de-differentiate back to the elementary body form, and at approximately 48 h postinfection those new extracellular forms are released to the external milieu by host cell lysis or exocytosis (for review see [6,46]). For C. pneumoniae, the cycle requires approximately 72 h for completion. Figure 1 presents a graphic representation of the chlamydial developmental cycle.
Figure 1. The chlamydial developmental cycle.
(A) In the first phase, EBs locate and attach to a host cell. (B) Following attachment, the organism is brought into a membrane-bound vesicle in the host cell cytoplasm. Within this inclusion, each EB reorganizes into a RB. (C) During the intracellular phase, each RB undergoes several rounds of cell division, at the termination of which, (D) most reorganize back to the EB form. (E) Newly formed EB are released from the host cell by exocytosis or host cell lysis to propagate further infection. (F) The host adaptive response has a significant effect on whether the infection enters a persistent state.
EB: Elementary body; RB: Reticulate body.
Reproduced with permission from [92].
Recent elegant studies from many groups have illuminated the means by which invading chlamydiae influence the host cell and its biochemical processes during the active infection process. Genome sequence data have clearly demonstrated that C. trachomatis possesses a type III secretion system, by which the organism injects various effector proteins into the host cell at the attachment stage [47]. The total panel of injected proteins and their detailed functions in uptake into the host cells remain to be determined. However, for just one example, evidence for injection of a toxin encoded by the C. trachomatis gene designated toxB was published several years ago [48]. A number of chlamydial proteins, including Tarp and others, have an important function in the uptake/invasion process leading to sequestration of the organisms in their cytoplasmic inclusions (e.g., [49–52]). A protease designated CPAF is produced, which acts on both chlamydial and host proteins [53,54], and the organism also produces a protein designated CADD, which binds to host cell death receptors to influence the apoptotic process [55s]. Recent reviews from a number of sources highlight these and other aspects of interaction with their immediate host cells by chlamydiae (e.g., [56]).
In vivo chlamydial infections elicit a strong inflammatory response, although that response is often more clinically apparent in men than in women, at least for urogenital infections (see above). A major surprise from the various full-genome sequencing programs that have been published over the last decade and a half is that the chlamydial chromosome encodes not one, but three versions of the highly proinflammatory Hsp60 proteins [47,57]. The authentic gene, which is nearly identical to that from E. coli and other bacteria, is groEL, and it is found in an operon with groES, as in E. coli [47]; these genes are designated CT110 and CT111, respectively, in the C. trachomatis genome sequence. The other two Hsp60-encoding genes are distantly linked to CT110/CT111 and are designated CT604 and Ct755. While they are clearly the result of gene duplication events, their sequences are not identical to that of CT110 or to each other. CT604 and CT755, interestingly, do not exist in individual operons, and no CT111/groES-like genes are present in their immediate vicinities. The three Hsp60-encoding genes are expressed early in the developmental cycle, are transcribed fully independently of one another throughout that cycle, and show high levels of expression throughout the cycle [58]. Without question these gene products are largely, but almost certainly not exclusively, responsible for eliciting the host inflammatory response, which includes high levels of production of IFN-γ, TNF-α and other proinflammatory mediators. Host signaling pathways triggered during active chlamydial infection by other proteins from the bacterium have also been extensively studied (e.g., [59]).
Chlamydial pathogenesis of the joint
As a number of investigators have pointed out, in many instances the initial elicitation of disease by a pathogen during primary infection is simply a preliminary for establishment of a longer-term habitation of the host [60,61]. The authors of this article and others have argued this to be the case for genital infection by C. trachomatis, and it is also almost certainly is true for ocular infections by this pathogen and pulmonary infection by C. pneumoniae. Specifically, production of the Hsp60 and other gene products by chlamydiae during urethral or cervical chlamydial infection elicits a number of responses from the host, including a Th1-type immune response [5,6,11,32,62,63]. Most importantly for this discussion is that monocytic cells are attracted to the site of infection, where they take up elementary bodies with the intention of disposing of them, as is their function with invading organisms (e.g., [6]). Infection of monocytes, however, does not proceed as usual. For reasons that we do not understand, following internalization of chlamydial elementary bodies into the monocyte inclusion, the normal course of phagosome–lysosome fusion does not take place [13,64,65]. Instead, within the cytoplasmic inclusion, the elementary bodies undergo the initial stages of the differentiation process to the reticulate body form; over the first 24 h or so during uptake and within the inclusion the course of chlamydial development appears to be relatively normal. Specifically, transcriptome analyses over time during the first day post-infection of normal human monocytes in culture demonstrate that a large number of genes encoding products necessary for the differentiation to reticulate bodies are expressed as they are during the initial stage of normal active infection [Belland RJ et al. The Chlamydia trachomatis transcriptional control program governing entry into the persistent infection state (2012), Manuscript in preparation]. These include genes specifying components of the protein synthetic system, various transporters, proteins to be inserted into the inclusion membrane, the three Hsp60 proteins and many others. Importantly, more than 200 genes encoding proteins of unknown function are also expressed, and it is currently believed that many, if not most, of these contribute to virulence and pathogenesis in some fashion. Importantly, Chlamydia-infected monocytes are frequently extravasated from the genital tract, by which means they disseminate to other sites using the monocytes as a vehicle [13,58,63].
During the 40 h or so after the differentiation process, chlamydiae within monocytic cells enter an unusual infection state designated ‘persistence’ [13,64,66]. Our data from patient samples and from studies of an in vitro model system of this state strongly suggest that chlamydiae within the circulating monocytes reach the joint in the persistent state [13,67]. That is, no aspects of synovial pathogenesis are a function of normal active infection as occurs in the genital tract. Rather, joint pathogenesis results from the biology of chlamydial persistence and the interaction of the organisms with the host cell in that infection state. Our transcriptome analyses demonstrated that the transition from normal active infection to the persistent state often involves severe downregulation expression of many of the genes upregulated during the first 24 h post-infection, with adjustment of transcript levels for a panel of genes encoding lipid-modification enzymes, ABC transporters, some components of the transcription and translation systems, and others (e.g., [68–70]). Surprisingly, however, we could identify no panel of genes that appeared to be specifically and solely involved in/responsible for the transition to persistence for C. trachomatis; this is in contrast to the situation for other bacterial pathogens known to utilize a persistent infection phase, such as Mycobacterium tuberculosis and others [71]. Box 2 provides a partial panel of chlamydial genes that show significantly altered regulation of expression during persistent infection.
Box 2. Chlamydial genes that show altered expression during persistent infection.
- ▪ Selected genes showing increased transcript levels
- – CT604† Hsp60 paralog 2
- – CT393 proS‡ proline tRNA synthase§
- – CT437 fusA elongation factor G
- – CT727 zntA cation-transporting ATPase
- – CT414 pmpC outer membrane protein C
- ▪ Selected genes showing decreased transcript levels
- – CT755 Hsp60 paralog 3
- – CT681 ompA major outer membrane protein
- – CT739 ftsK cell division-related protein
- – CT760 ftsW cell division-related protein
- – CT313 tal transaldolase
- – CT014 cydB cytochrome oxidase d subunit II
†Genome sequence designation.
‡Gene name.
§Genetic function encoded.
In vitro and in vivo studies of persistence did identify one modulation of expression for a set of genes of particular interest in the biology of chlamydial persistence in the joint. Transcript analyses targeting the three Hsp60-encoding genes demonstrated high levels of expression for each of them during normal active infection, with expression levels of the CT604 and CT755 genes exceeding that of the authentic original groEL (CT110) gene [58]. By contrast, studies of the monocyte model of chlamydial persistence demonstrated that transcript levels from CT604 were actually increased in that state relative to their levels during active infection, but mRNA levels from CT755 were severely attenuated [58]. Indeed, even using extremely sensitive PCR assays it was difficult to identify any transcripts from CT755 during established persistent infection. We confirmed that these data from the in vitro model system accurately reflected the situation in synovial tissue samples from patients with well-documented chronic Chlamydia-induced arthritis. Given these observations, we hypothesized that the CT604 gene product functions in some as yet unknown manner to facilitate the transition to persistence, and that attenuation of the level of the CT755 gene product during that transition indicated its possible function in maintaining the active infection state for chlamydiae [58]. Using a new dendrimer-based system for the manipulation of gene expression in C. trachomatis, we are now testing these contentions [72].
At this point our understanding of the fine details relating to the molecular genetic basis underlying chlamydial pathogenesis of the joint is relatively limited. Given that a significant host synovial inflammatory response is characteristic in patients with active chronic Chlamydia-induced arthritis, it seems clear that the CT110- and CT604-encoded Hsp60 proteins are involved in eliciting that inflammatory response, whereas the CT755 gene product is not. Details relating to which genes among the more than 200 in the C. trachomatis genome that encode proteins of unknown function also contribute to elicitation of the inflammatory response remain to be determined. We point out in this context that, given the insertion of chlamydial proteins into the inclusion membrane and into host cell itself via the type III secretion system and other means during the infection process (see above), chronic synovial pathogenesis and its consequent inflammation must result from an extensive process of host–pathogen interaction. We view this interaction as a sort of molecular genetic conversation between pathogen and host cell that ends in a balance, which we understand as persistent long-term chlamydial infection of synovial tissue. We currently have little or no understanding of the details of the conversation, but transcriptome analyses that are currently underway, and use of the new system for modulation of chlamydial gene expression will be critical in sorting out these details.
Remitting–relapsing Chlamydia-induced arthritis
As mentioned above, many patients with chronic Chlamydia-induced arthritis display a remitting–relapsing disease phenotype, with quiescent periods of disease lasting for weeks to years in some cases [4,5,20,21,67]. Clinical samples that we and others have analyzed over the last many years have, of course, been obtained from individuals in the active disease phase, and thus all information currently available regarding the behavior of persistently infecting chlamydiae is confined to that disease state. It would be of significant interest to understand the molecular genetic and other details governing the cycle of active to quiescent to active disease, since knowledge of those details might allow therapeutic interventions to elicit quiescence and/or to obviate progression back to active disease in those in the quiescent phase. A recent study from this group examined a limited number of issues regarding chlamydial and host gene expression during quiescence in several patients with well-documented chronic Chlamydia-associated arthritis [73]. A question of primary interest was whether the organism is actually present in synovial tissue during quiescent disease, and quantitative PCR assays targeting the chlamydial chromosome in those patient samples indicated that the organism is indeed present, although the bacterial load is several-fold lower during remission than during active disease. Interestingly, however, assessment of transcript levels from the three Hsp60-encoding genes in those samples showed that messengers from CT110 (groEL) and CT604, and presumably the translation products encoded, were at, or above, the levels seen in chlamydiae during active disease; transcripts from the CT755 gene were extremely low in the samples from patients in quiescent disease, as they are in samples from those with active disease [74]. Messengers encoding host IL-10, IFN-γ and TNF-α were somewhat below levels seen in synovial tissue samples from those with active disease, but mRNA encoding MCP-1 and RANTES were either at approximately the same level as in active disease or in the case of the latter significantly higher in some samples ([73,74]; also see [75]). Unfortunately, data regarding the histopathology of the tissue samples from patients in quiescent disease was not available in this study.
While these data provide the first information regarding chlamydial and host genetic behavior during quiescent disease, they provide virtually no insight into why remission was the case. That is, although the infecting organisms were present in the samples at relatively low levels in all patient samples, they were still producing high levels of mRNA encoding the two relevant Hsp60 proteins, and the host response was not significantly attenuated from that reported in tissues from patients suffering active disease, at least in terms of messengers encoding proinflammatory cytokines and chemokines. Thus at this point it is clear that the simplest explanation of quiescence cannot be the case; that is, that the infecting chlamydiae are in some sort of dormant, metabolically inactive state during remission, and that inflammatory molecules therefore are not present in the synovium. The true explanation for remission, and any strategy to exploit aspects bacterial or host behavior for therapeutic purposes, must await further investigation.
Treatment of Chlamydia-induced arthritis
Only a few prospective randomized trials have assessed various therapeutic strategies to treat Chlamydia-induced arthritis. Generally, clinical data suggest that NSAIDs and corticosteroids are useful, especially in early or mild disease (e.g., [76]). Both disease-modifying antirheumatic drugs and antibiotics have been assessed as potential therapeutic options for patients with chronic disease and/or those with more severe disease symptoms. To our knowledge, the only traditional disease-modifying antirheumatic drug to be studied in any formal manner for treatment of the disease is sulfasalazine. Earlier studies [77,78] found that this drug was marginally efficacious in patients with chronic reactive arthritis [77], but not useful for treatment of acute disease [78]. However, in some studies no distinction was made between those with chlamydial or post-enteric disease. In one study in which the etiologic groups were separated, individuals with disease derived from both fared equally.
Anti-TNFα therapy for the treatment of Chlamydia-induced arthritis has been a topic of interest over the last decade or so, although no large-scale randomized trials have been carried out to assess the efficacy of this approach. One case report and one small open-label study using etanercept did, however, suggest some clinical benefit to patients with the disease [79,80]. The latter trial did include synovial biopsies to assess chlamydial DNA by PCR before and after treatment. Of the three patients who were PCR positive for synovial chlamydiae before treatment, two became PCR negative on therapy and one remained positive. Interestingly, two patients who were negative at baseline became PCR positive for chlamydiae while on etanercept. In a preliminary study, one of the authors of this study assessed relative bacterial load in paired synovial tissue samples procured before and after several months of treatment with etanercept from a patient with Chlamydia-induced arthritis. Quantitative RT-PCR analyses demonstrated that the second biopsy sample included a bacterial load several-fold higher than that of the pretreatment sample [Hudson AP, Unpublished Data]. Thus, while more study concerning the use of this biological modifier is required, some data regarding post-chlamydial arthritis raise concern.
As reviewed extensively elsewhere, many studies, including some that were large and well structured, have indicated that treatment with antibiotics of patients with Chlamydia-induced arthritis is relatively ineffective (e.g., [81,82]). This lack of efficacy certainly results in large part from the observation that persistently infecting chlamydiae are refractory (not resistant) to standard courses of antibiotics, including azithromycin and others; indeed studies of treatment of Chlamydia-infected cell cultures with certain antibiotics has demonstrated that such treatment elicits persistent infection rather than clearing the organism (e.g., [83]). Recently, combination antibiotic therapy has been examined as a treatment for Chlamydia-induced arthritis. In an open-label pilot study, one of the authors of this study demonstrated that a combination of doxycycline and rifampin was superior to doxycycline alone in patients with suspected Chlamydia-induced arthritis [84]. A more recent 6-month, double-blind, placebo-controlled study of patients with the disease analyzed two combinations of antibiotics, doxycycline/rifampin and azithromycin/rifampin [85]. The results showed both combinations to be superior to placebo. Patients were randomized only if they were PCR positive for chlamydiae on peripheral blood mononuclear cells or synovial tissue. Nearly two-thirds of the patients studied responded to combination antibiotics, whereas only approximately one-fifth of those randomized to placebo responded. Importantly, PCR analysis showed that more patients on combination antibiotics cleared their synovial chlamydial infection compared with those on placebo. While larger studies are required, these initial observations do suggest that combination antibiotic therapy may be a fruitful strategy to pursue for the treatment of patients with Chlamydia-induced arthritis.
Conclusion
Significant progress has been made over the last 5 years or so in understanding the fundamental mechanisms of chlamydial pathogenesis during both primary active infection and persistent infection following dissemination to distant sites such as the synovium. Much of this increased knowledge has resulted from studies of the basic biology of the organisms, including elucidation of genome structure and differences in such structure among chlamydial strains/isolates and among chlamydial species, the realization that chlamydiae can and do sometimes exchange genetic information to account for some of those genome structure differences, detailed large-scale gene expression studies, extensive cell biological analyses illuminating details of influences of the pathogen on its host cell and vice versa, and others. From these studies it is clear that the host–pathogen interplay during both normal active infection and persistent infection is complex, and that further understanding of its complexities will be required before new avenues of therapeutic approach can be envisioned and productively pursued. Regarding Chlamydia-induced arthritis, the bacterial products that elicit the characteristic inflammatory response in the joint are being defined, and further insights into the nature and specific effects of those gene products on the host will inform current and future treatment options. Of potentially significant interest is the very initial insight into the genetic behavior of pathogen and host during the remitting phase of the chronic arthritis, since if molecular details underlying the transitions between active and quiescent disease can be exploited it should provide a means by which disease development or relapse can be manipulated to advantage. Other studies make clear that progress has been made in treatment in terms of combination antibiotic therapy, as a function of identification of the nature of the chlamydial strains/serovars that appear to be the specific causative agents for disease development, and in terms of bacterial genetic and related strategies for entry into the persistent infection state (e.g., [86]).
A significant question at this point concerns the sources from which new insights will come vis-à-vis chlamydial pathogenesis and host–pathogen interaction. We contend that one source that has not yet been exploited extensively centers on the evolution of chlamydiae, both in and of themselves and in terms of host relationships. An interesting recent study of chlamydial evolution may provide a starting point for such investigation [87]. Another potentially fruitful source will result from the development of systems for genetic manipulation of growing C. trachomatis and its related pathogens. Over the last 5 years three systems for transformation of chlamydiae have been published, and these hold promise for elucidation of the functions of the many proteins encoded by genes of currently unknown function [72,88,89]. Other means of genetic manipulation of these organisms are also becoming available, which expand importantly our means of analysis of host–pathogen interaction (e.g., see [90]). Of course, a panel of well-developed genetic and biochemical methods already exists for the assessment of host cell responses to both active and persistent chlamydial infection. Certainly, study focused on these aspects of host biology must be an integral part of any research program to develop new strategies for anti-Chlamydia therapies. Thus, given new experimental tools and the fresh points of view concerning pathogenesis that they provide, the control of both active and persistent chlamydial infection as they operate to induce inflammatory arthritis should be amenable to clinical control.
Future perspective
Recent studies indicate that only ocular strains of C. trachomatis are responsible for elicitation of synovial inflammation, and development of PCR-based or other assays will allow the determination of whether any given patient’s genital inoculum includes a component of ocular strain chlamydiae. Thus it will be possible to routinely and rapidly identify those patients at high risk for development of the arthritis. Given that the genomes of ocular and genital strains of C. trachomatis possess significant differences, determination of which gene product(s) are responsible for eliciting synovial inflammation will allow specific targeting of that/those gene products to obviate disease induction. New genetic and other experimental methods will identify the host–pathogen genetic program responsible for the remitting–relapsing disease phenotype often seen in patients with Chlamydia-induced arthritis, permitting manipulation of the program to force remitting and/or obviate relapsing disease. Finally, combination antibiotic therapy will be the standard of treatment for genital, and also ocular, chlamydial infections, and probably pulmonary infections caused by the respiratory pathogen C. pneumoniae.
Executive summary.
Chlamydia-induced arthritis
▪ Genital infections with Chlamydia trachomatis are extremely common in the USA and worldwide; engender often severe sequelae, including an inflammatory arthritis.
▪ In addition to infections with C. trachomatis, pulmonary infection with C pneumoniae has been shown to cause a similar inflammatory arthritis.
Clinical aspects of Chlamydia-induced arthritis
▪ Grouped with the spondyloarthropathies; inflammatory arthritis, can be caused by infection with bacteria in addition to chlamydiae, but clinical presentation is similar in all cases.
▪ Develops 1–6 weeks after infection, axial skeleton usually affected as are peripheral joints of the lower body, usually an oligoarthritis; enthesitis is common.
▪ Minority of individuals who acquire a genital or pulmonary chlamydial infection will develop the arthritis, and only perhaps half of them will advance to chronic disease.
▪ ACR disease definition indicates that Chlamydia-induced arthritis is primarily a disease of males, but this is inaccurate. Diagnosis requires a documented genital chlamydial infection, but such infections are commonly subclinical in women.
Epidemiology & incidence of Chlamydia-induced arthritis
▪ Incidence of new genital chlamydial infections is high, >3 million/year in the USA; incidence of pulmonary infection with C. pneumoniae is not known.
▪ Although C. pneumoniae infection is probably more common than infection by C. trachomatis, the latter organism accounts for the large majority of arthritis cases.
▪ Chlamydia-induced arthritis is underdiagnosed for a number of reasons, including resolution of disease prior to referral to rheumatologist.
▪ The incidence of Chlamydia-induced arthritis is probably similar to that of rheumatoid arthritis in the USA.
Strains/serovars involved in arthritogenesis
▪ Different strains of C. trachomatis elicit ocular disease (trachoma) versus genital disease.
▪ Recently published observations indicate that contrary to expectation genital strains are not found in synovial tissue of patients with Chlamydia-induced arthritis.
▪ Only ocular strains are present, possibly providing an explanation for the unusually low incidence of post-urogenital arthritis.
Basic molecular genetic aspects of chlamydial pathogenesis
▪ Pathogenesis is a function of details of the chlamydial developmental cycle; organisms attach to host cells perhaps by LDL receptors, induce phagocytosis, reside within membrane-bound inclusions in the host cytoplasm and produce highly immunogenic products within the inclusion.
▪ The key to understanding chlamydial pathogenesis overall lies in understanding host–pathogen interaction at the molecular genetic/biochemical level.
▪ During normal active infection, any products produced by intracellular chlamydiae that influence host cell processes must be defined.
▪ Inflammatory response is elicited at least in large part by production of the three chlamydial hsp-60 gene products.
▪ At this point it is unclear what other products elicit inflammation and or host cell damage.
Chlamydial pathogenesis of the joint
▪ Inflammation in the genital tract elicited by primary chlamydial infection causes attraction of monocytic cells; chlamydiae infect monocytes, extravasation of infected cells allows dissemination to the joint.
▪ Within monocytes, chlamydiae somehow stop phagosome–lysosome fusion, allowing organisms to survive; enter a persistent phase of infection.
▪ Persistence characterized by unusual panel of genes expressed, extensive but poorly understood aspects of host–pathogen interaction.
▪ Especially important is unusual expression of the three hsp-60 genes, which may be responsible for the transition from active infection to persistence; and for continuing inflammation once resident in synovial tissue.
Remitting–relapsing Chlamydia-induced arthritis
▪ Most information relating to chlamydial pathogenesis of the synovium is derived from studies of tissues from patients with active disease.
▪ Recent studies with samples from patients with quiescent (remitting) disease indicate a highly complex explanation that still needs to be elucidated.
▪ Quiescence is not due to a simple lack of highly immunogenic gene products produced by chlamydiae, or to lack of proinflammatory host mediators.
Treatment of Chlamydia-induced arthritis
▪ Single antibiotic therapy has proved to be ineffectual; biological modifiers have shown some success but may be contraindicated for a number of reasons.
▪ NSAIDs and standard disease-modifying antirheumatic drugs have been used for treatment but require more formal study.
▪ Recent studies indicate that combination antibiotic therapy is promising for treatment.
Conclusion
▪ Much has been learned over the last 5 years regarding chlamydial pathogenesis.
▪ New experimental approaches will allow even more detailed understanding of host–pathogen interaction and thus pathogenesis.
Footnotes
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Whittum-Hudson JA, Hudson AP. Human chlamydial infections: persistence, prevalence, and prospects for the future. Nat. Sci. Soc. 2005;13:371–382. [Google Scholar]
- 2.WHO Department of Reproductive Health and Research . Prevalence and Incidence of Selected Sexually Transmitted Infections. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 3.Sieper J. Pathogenesis of reactive arthritis. Curr. Rheumatol. Rep. 2001;3:412–418. doi: 10.1007/s11926-996-0012-8. [DOI] [PubMed] [Google Scholar]
- 4.Zeidler H, Kuipers JG, Köhler L. Chlamydia-induced arthritis. Curr. Opin. Rheumatol. 2004;16:380–392. doi: 10.1097/01.bor.0000126150.04251.f9. [DOI] [PubMed] [Google Scholar]
- 5.Carter JD, Inman RD, Hudson AP. Chlamydia and chronic arthritis. Ann. Med. 2011 doi: 10.3109/07853890.2011.606830. doi:10.3109/07853890.2011.606830. (Epub ahead of print) ▪▪ A recent thorough review of clinical and other aspects of Chlamydia-induced arthritis.
- 6.Ward ME. Mechanisms of Chlamydia-induced disease. In: Stephens RS, editor. Chlamydia – Intracellular Biology, Pathogenesis, and Immunity. ASM Press; Washington DC, USA: 1999. pp. 171–210. [Google Scholar]
- 7.Carter JD, Gerard HC, Espinoza L, et al. Chlamydiae as etiologic agents for chronic undifferentiated spondyloarthropathy. Arthritis Rheum. 2009;60:1311–1316. doi: 10.1002/art.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter JD, Hudson AP. The evolving story of Chlamydia-induced reactive arthritis. Curr. Opin. Rheumatol. 2011;22:424–430. doi: 10.1097/BOR.0b013e32833a43a2. [DOI] [PubMed] [Google Scholar]
- 9.Grayston JT, Campbell LA, Kuo CC, et al. A new respiratory tract pathogen Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 1990;161:618–625. doi: 10.1093/infdis/161.4.618. [DOI] [PubMed] [Google Scholar]
- 10.Kuo CC, Jackson LA, Campbell LA, Grayston JT. Chlamydia pneumoniae (TWAR) Clin. Microbiol. Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen M. Pathogenic mechanisms and epidemiology of Chlamydia pneumoniae. Eur. Heart J. 1993;14:57–61. [PubMed] [Google Scholar]
- 12.Schumacher HR, Gérard HC, Arayssi TK, et al. Lower prevalence of Chlamydia pneumoniae DNA compared with Chlamydia trachomatis DNA in synovial tissue of arthritis patients. Arthritis Rheum. 1999;42:1889–1893. doi: 10.1002/1529-0131(199909)42:9<1889::AID-ANR13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Gérard HC, Whittum-Hudson JA, Carter JD, Hudson AP. Molecular biology of infectious agents in chronic arthritis. Rheum. Dis. Clin. North Am. 2009;35:1–19. doi: 10.1016/j.rdc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Hahn DL, Azenabor AA, Beatty WL, Byrne GI. Chlamydia pneumoniae as a respiratory pathogen. Front. Biosci. 2002;7:e66–e76. doi: 10.2741/hahn. [DOI] [PubMed] [Google Scholar]
- 15.Kvien TK, Glennås A, Melby K, et al. Reactive arthritis: incidence, triggering agents, and clinical presentation. J. Rheumatol. 1994;21:115–122. [PubMed] [Google Scholar]
- 16.Hill Gaston JS, Lillicrap MS. Arthritis associated with enteric infection. Best Prac. Res. Clin. Rheumatol. 2003;17:219–239. doi: 10.1016/s1521-6942(02)00104-3. ▪ Thorough description of reactive arthritis elicited by enteric pathogens.
- 17.Townes JM, Deodhar AA, Laine ES, et al. Reactive arthritis following culture-confirmed infections with bacterial enteric pathogens in Minnesota and Oregon: a population-based study. Ann. Rheum. Dis. 2008;67:1689–1696. doi: 10.1136/ard.2007.083451. [DOI] [PubMed] [Google Scholar]
- 18.Birnbaum J, Bartlett JG, Gelber AC. Clostridium difficile: an under-recognized cause of reactive arthritis? Clin. Rheumatol. 2008;27:253–255. doi: 10.1007/s10067-007-0710-2. [DOI] [PubMed] [Google Scholar]
- 19.Tinazzi E, Ficarra V, Simeoni S, Artibani W, Lunardi C. Reactive arthritis following BCG immunotherapy for urinary bladder carcinoma: a systematic review. Rheumatol. Int. 2006;26:481–488. doi: 10.1007/s00296-005-0059-2. [DOI] [PubMed] [Google Scholar]
- 20.Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum. Dis. Clin. North Am. 2009;35:21–34. doi: 10.1016/j.rdc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher HR. Chlamydia-associated arthritis. Isr. Med. Assoc. J. 2000;2:532–535. [PubMed] [Google Scholar]
- 22.Inman RD. Reactive and enteropathic arthritis. In: Klippel JH, editor. Primer on the Rheumatic Diseases. Springer Science/Arthritis Foundation; New York, NY, USA: 2008. pp. 217–223. [Google Scholar]
- 23.Hannu T, Puolakkainen M, Leirisalo-Repo M. Chlamydia pneumoniae as a triggering infection in reactive arthritis. Rheumatology (Oxford) 1999;38:411–414. doi: 10.1093/rheumatology/38.5.411. [DOI] [PubMed] [Google Scholar]
- 24.Contini C, Grilli A, Badia L, Guardigni V, Govoni M, Seraceni S. Detection of Chlamydophila pneumoniae in patients with arthritis: significance and diagnostic value. Rheumatol. Int. 2011;31:1307–1313. doi: 10.1007/s00296-010-1460-z. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo A, Domenico MD, Carratelli CR, Paolillo R. Role of Chlamydia and Chlamydophila infections in reactive arthritis. Int. Med. 2012;51:113–117. doi: 10.2169/internalmedicine.51.6228. [DOI] [PubMed] [Google Scholar]
- 26.Doran MF, Pond GR, Crowson CS, O’Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 27.Soderlin MK, Borjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M. Annual incidence of inflammatory joint disease in a population based study in southern Sweden. Ann. Rheum. Dis. 2002;61:911–915. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations of reactive arthritis: results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany. J. Rheumatol. 2000;27:2185–2192. [PubMed] [Google Scholar]
- 29.Rich E, Hook EW, Alarcon GS, Moreland LW. Reactive arthritis in patients attending an urban sexually transmitted disease clinic. Arthritis Rheum. 1996;39:1172–1177. doi: 10.1002/art.1780390715. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y, Zhang YX, Watkins NG, Caldwell HD. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 C. trachomatis serovars. Infect. Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole E, Lamont I. C. trachomatis serovar differentiation by direct sequence analysis of variable segment 4 region of the major outer membrane protein gene. Infect. Immun. 1992;60:1089–1094. doi: 10.1128/iai.60.3.1089-1094.1992. ▪ A complete description of the modern means of serovar determination for Chlamydia trachomatis.
- 32.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia – Intracellular Biology, Pathogenesis, and Immunity. ASM Press; Washington DC, USA: 1999. pp. 139–169. [Google Scholar]
- 33.Gerard HC, Stanich JA, Whittum-Hudson JA, Schumacher HR, Carter JD, Hudson AP. Patients with Chlamydia-induced arthritis have ocular (trachoma), not genital, serovars of C. trachomatis in synovial tissue. Microb. Pathogen. 2011;48:62–68. doi: 10.1016/j.micpath.2009.11.004. ▪▪ The surprising first description of ocular serovars in synovial tissues from reactive arthritis patients.
- 34.Mittal A. Serovar distribution of Chlamydia trachomatis isolates collected from the cervix: use of the polymerase chain reaction and restriction endonuclease digestion. Br. J. Biomed. Sci. 1998;55:179–183. [PubMed] [Google Scholar]
- 35.Workowski KA, Stevens CE, Suchland RJ, et al. Clinical manifestations of genital infection due to Chlamydia trachomatis in women: differences related to serovar. Clin. Infect. Dis. 1994;19:756–760. doi: 10.1093/clinids/19.4.756. [DOI] [PubMed] [Google Scholar]
- 36.Gaydos CA, Summersgill JT, Sahney HH, Ramirez JA, Quinn TC. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect. Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreses-Werringloer U, Gérard HC, Whittum-Hudson JA, Hudson AP. C. pneumoniae infection of human astrocytes and microglial cells in culture displays an active, rather than a persistent, growth phenotype. Am. J. Med. Sci. 2006;332:168–174. doi: 10.1097/00000441-200610000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Kuo CC, Lee A, Jiang SJ, Yaraei K, Campbell LA. Inoculation of Chlamydia pneumoniae or Chlamydia trachomatis with ligands that inhibit attachment to host cells reduces infectivity in the mouse model of lung infection: implication for anti-adhesive therapy. Microbes Infect. 2007;9:1139–1141. doi: 10.1016/j.micinf.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Su H, Watkins NG, Zhang YX, Caldwell HD. Chlamydia trachomatis-host cell interaction: role of the chlamydial major outer membrane protein as an adhesion. Infect. Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ting LM, Hsia RC, Haidaris CF, Bavoil PM. Interaction of outer envelope proteins of Chlamydia psittaci GPIC with the HeLa cell surface. Infect. Immun. 1996;63:3600–3608. doi: 10.1128/iai.63.9.3600-3608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell LA, Puolakkainen M, Lee A, Rosenfeld ME, Garrigues HJ, Kuo CC. Chlamydia pneumoniae binds to the lectin-like oxidized LDL receptor for infection of endothelial cells. Microbes Infect. 2012;14:43–49. doi: 10.1016/j.micinf.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerard HC, Whittum-Hudson JA, Hudson AP. C. pneumoniae utilizes apoE and the LDL receptor family for host cell attachment. In: Chernesky M, et al., editors. Human Chlamydial Infections. Proceedings of the 11th Symposium on Human Chlamydial Infections; International Chlamydia Symposium; San Francisco, CA, USA. 2006. pp. 153–156. [Google Scholar]
- 43.Dautry-Varsat A, Subtil A, Hackstadt T. Recent insights into the mechanisms of chlamydial entry. Cell Microbiol. 2005;7:1714–1722. doi: 10.1111/j.1462-5822.2005.00627.x. ▪▪ Excellent summary of known aspects of attachment and entry of chlamydial elementary bodies to host cells.
- 44.Hybiske K, Stephens RS. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect. Immun. 2007;75:3925–3934. doi: 10.1128/IAI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl Acad. Sci. USA. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol. Rev. 2005;29:949–959. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Stephens RS, Kalman S, Lammel C, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 48.Belland RJ, Scidmore MA, Crane DD, et al. Chlamydia trachomatis cytotoxicity associated with complete and partial cytoxin genes. Proc. Natl Acad. Sci. USA. 2001;98:13984–13489. doi: 10.1073/pnas.241377698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jewett TJ, Miller NJ, Dooley CA, Hackstadt T. The conserved Tarp acting binding domain is important for chlamydial invasion. PLoS Pathog. 2010;6:e1000997. doi: 10.1371/journal.ppat.1000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Betts HJ, Wolf K, Fields KA. Effector protein modulation of host cells: examples in the Chlamydia spp arsenal. Curr. Opin. Microbiol. 2009;12:81–87. doi: 10.1016/j.mib.2008.11.009. ▪ Excellent discussion of how chlamydiae influence host cell processes.
- 51.Moore ER, Mead DJ, Dooley CA, Sager J, Hackstadt T. The trans-Golgi syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology. 2011;157:830–838. doi: 10.1099/mic.0.045856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valdivia RH. Chlamydial effector proteins and new insights into chlamydial cellular microbiology. Curr. Opin. Microbiol. 2008;11:53–59. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Jorgensen I, Bednar MM, Amin V, et al. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe. 2011;10:21–32. doi: 10.1016/j.chom.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong G. Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways. Front Biosci. 2011;2:14. doi: 10.3389/fmicb.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenner-Liewen F, Liewen H, Zapata JM, Pawlowski K, Godzik A, Reed JC. CADD, a Chlamydia protein that interacts with death receptors. J. Biol. Chem. 2002;277:9633–9636. doi: 10.1074/jbc.C100693200. [DOI] [PubMed] [Google Scholar]
- 56.Saka HA, Valdivia RH. Acquisition of nutrients by chlamydiae: unique challenges of living in an intracellular environment. Curr. Opin. Microbiol. 2010;13:4–10. doi: 10.1016/j.mib.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Read TD, Brunham RC, Shen C, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR-39. Nucl. Acids Res. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gérard HC, Whittum-Hudson JA, Schumacher HR, Hudson AP. Differential expression of the three Chlamydia trachomatis hsp60-encoding genes in active vs persistent infection. Microb. Pathogen. 2004;36:35–39. doi: 10.1016/j.micpath.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 59.O’Connell CM, AbdelRahman YM, Green E, et al. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infect. Immun. 2011;79:1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhen M, Eriksson S, Clements M, Bergström S, Normark SJ. The basis of persistent bacterial infection. Trends Microbiol. 2003;11:80–86. doi: 10.1016/s0966-842x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 61.Gefen O, Balaban NQ. The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol. Rev. 2009;33:704–717. doi: 10.1111/j.1574-6976.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 62.Taylor BD, Darville T, Tan C, Bavoil PM, Ness RB, Haggerty CL. The role of Chlamydia trachomatis polymorphic membrane proteins in inflammation and sequelae among women with pelvic inflammatory disease. Infect. Dis. Obstet. Gynecol. 2011;2011:989762. doi: 10.1155/2011/989762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuipers JG, Zeidler H, Köhler L. How does Chlamydia cause arthritis? Rheum. Dis. Clin. N. Am. 2003;29:613–629. doi: 10.1016/s0889-857x(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 64.Schoborg RV. Chlamydia persistence: a tool to dissect Chlamydia-host interactions. Microbes Infect. 2011;13:649–662. doi: 10.1016/j.micinf.2011.03.004. ▪▪ Recent and thorough discussion of chlamydial persistence and how it might be exploited to understand other aspects of chlamydial infection.
- 65.Hackstadt T. Cell biology. In: Stephens RS, editor. Chlamydia – Intracellular biology, Pathogenesis, and Immunity. ASM Press; Washington DC, USA: 1999. pp. 101–138. [Google Scholar]
- 66.Whittum-Hudson JA, Swanborg RH, Hudson AP. Persistent microbial infection and the genesis of chronic disease. Biol. Int. 2003;45:9–17. [Google Scholar]
- 67.Whittum-Hudson JA, Gérard HC, Schumacher HR, Hudson AP. Pathogenesis of Chlamydia-associated arthritis. In: Bavoil P, Wyrick P, editors. Chlamydia – Genomics and Pathogenesis. Horizon Bioscience, Inc.; CO, USA: 2007. pp. 475–504. [Google Scholar]
- 68.Gérard HC, Krauß-Opatz B, Rudy D, et al. Expression of Chlamydia trachomatis genes required for DNA synthesis and cell division in active vs. persistent infection. Mol. Microbiol. 2001;41:731–741. doi: 10.1046/j.1365-2958.2001.02550.x. [DOI] [PubMed] [Google Scholar]
- 69.Byrne GI, Ouellette SP, Wang Z, et al. Chlamydia pneumoniae expresses genes required for DNA replication but not cytokinesis during persistent infection of HEp-2 cells. Infect. Immun. 2001;69:5423–5429. doi: 10.1128/IAI.69.9.5423-5429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gérard HC, Freise J, Rudy D, et al. Chlamydia trachomatis genes whose products are related to energy metabolism are expressed differentially in active vs. persistent infection. Microb. Infect. 2002;4:13–22. doi: 10.1016/s1286-4579(01)01504-0. [DOI] [PubMed] [Google Scholar]
- 71.Gérard HC, Whittum-Hudson JA, Schumacher HR, Hudson AP. Synovial Chlamydia trachomatis up-regulates expression of a panel of genes similar to that transcribed by Mycobacterium tuberculosis during persistent infection. Ann. Rheum. Dis. 2006;65:321–327. doi: 10.1136/ard.2005.042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mishra MK, Gérard HC, Whittum-Hudson JA, Hudson AP, Kannan RM. Dendrimer-enabled modulation of gene expression in Chlamydia trachomatis. Mol. Pharm. 2012;9:413–421. doi: 10.1021/mp200512f. [DOI] [PubMed] [Google Scholar]
- 73.Gérard HC, Carter JD, Hudson AP. C. trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic Chlamydia-induced arthritis. Am. J. Med. Sci. 2012 doi: 10.1097/MAJ.0b013e3182648740. doi:10.1097/MAJ.0b013e3182648740. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gérard HC, Wang Z, Whittum-Hudson JA, Bardin T, Schumacher HR, Hudson AP. Cytokine and chemokine mRNA produced in synovial tissue chronically infected with C trachomatis and Chlamydia pneumoniae. J. Rheumatol. 2002;29:1827–1835. [PubMed] [Google Scholar]
- 75.Kotake S, Schumacher HR, Arayssi TK, et al. IFN-γ, IL-10, and IL-12 p40 gene expression in synovial tissues from patients with recent-onset Chlamydia-associated arthritis. Infect. Immun. 1999;67:2682–2686. doi: 10.1128/iai.67.5.2682-2686.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flores D, Marquez J, Garza M, Espinoza LR. Reactive arthritis: newer developments. Rheum. Dis. Clin. N. Am. 2003;29:37–59. doi: 10.1016/s0889-857x(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 77.Clegg DO, Reda DJ, Weisman MH, Cush JJ, Vasey FB, Schumacher HR., Jr. Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter’s syndrome). A department of veterans affairs cooperative study. Arthritis Rheum. 1996;39:2021–2027. doi: 10.1002/art.1780391211. [DOI] [PubMed] [Google Scholar]
- 78.Egsmose C, Hansen TM, Andersen LS, Beier JM, Christensen L, Ejstrup L. Limited effect of sulfasalazine treatment in reactive arthritis: a randomized double blind placebo controlled trial. Ann. Rheum. Dis. 1997;56:32–36. doi: 10.1136/ard.56.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schrafranski MD. Infliximab for reactive arthritis secondary to Chlamydia trachomatis infection. Rheumatol. Int. 2010;30:679–680. doi: 10.1007/s00296-009-0965-9. [DOI] [PubMed] [Google Scholar]
- 80.Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher HR., Jr. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum. 2005;53:613–617. doi: 10.1002/art.21323. [DOI] [PubMed] [Google Scholar]
- 81.Carter JD. Reactive arthritis: defined etiologies, emerging pathophysiology, and unresolved treatment. Infect. Dis. Clin. N. Am. 2006;20:827–847. doi: 10.1016/j.idc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Sieper J, Fendler C, Laitko S, Sörensen H, Gripenberg-Lerche C, Hiepe F. No benefit of long-term ciprofloxacin treatment in subjects with reactive arthritis and undifferentiated oligoarthritis: a three month, multicenter, double-blind, randomized, placebo-controlled study. Arthritis Rheum. 1999;42:1386–1396. doi: 10.1002/1529-0131(199907)42:7<1386::AID-ANR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 83.Dreses-Werringloer U, Padubrin I, Jürgens-Saathoff B, Hudson AP, Zeidler H, Köhler L. Persistence of Chlamydia trachomatis is induced by ciprofloxacin and orofloxacin treatment in vitro. Antimicrob. Agents Chemother. 2000;44:3288–3297. doi: 10.1128/aac.44.12.3288-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carter JD, Valeriano J, Vasey FB. A prospective, randomized 9-month comparison of doxycycline vs. doxycycline and rifampin in undifferentiated spondyloarthritis: with special reference to Chlamydia-induced arthritis. J. Rheumatol. 2004;31:1973–1980. [PubMed] [Google Scholar]
- 85.Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis. Arthritis Rheum. 2010;62:1298–1307. doi: 10.1002/art.27394. ▪▪ Formal trial demonstrating on a small scale that combination antibiotic therapy is promising for treatment of Chlamydia-induced arthritis.
- 86.Lo CC, Xie G, Bonner CA, Jensen RA. The alternative translational profile that underlies the immune evasive state of persistence in Chlamydiaceae exploits differential tryptophan contents of the protein repertoire. Microbiol. Mol. Biol. Rev. 2012;76:405–443. doi: 10.1128/MMBR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clarke IN. Evolution of Chlamydia trachomatis. Ann. NY Acad. Sci. 2011;1230:e11–e18. doi: 10.1111/j.1749-6632.2011.06194.x. [DOI] [PubMed] [Google Scholar]
- 88.Binet R, Maurelli AT. Transformation and isolation of allelic exchange mutants of C. psittaci using recombinant DNA introduced by electroporation. Proc. Natl Acad. Sci. USA. 2009;106:292–297. doi: 10.1073/pnas.0806768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathogens. 2011;7:e1002258. doi: 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen BD, Valdivia RH. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc. Natl Acad. Sci. USA. 2012;109:1263–1268. doi: 10.1073/pnas.1117884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carter JD. Reactive arthritis: defined etiologies, emerging pathophysiology, and unresolved treatment. Infect. Dis. Clin. North Am. 2006;20(4):827–847. doi: 10.1016/j.idc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Swanborg RH, Boros DL, Whittum-Hudson JA, Hudson AP. Molecular mimicry and horror autotoxicus: does chlamydial infection elicit autoimmunity? Exp. Rev. Mol. Med. 2006;8:1–23. doi: 10.1017/S1462399406000160. [DOI] [PubMed] [Google Scholar]
▪ Websites
- 101.Chlamydia/I> – CDC fact sheet. www.cdc.gov/std/Chlamydia/STDFact-Chlamydia.htm.
- 102.Chlamydia pneumoniae. www.cdc.gov/ncidod/dbmd/diseaseinfo/chlamydiapneumonia_t.htm.