Abstract
Background/Objectives
The primary goal was to determine whether repetitive functional electrical stimulation (FES) for unilateral foot drop increases tibialis anterior (TA) muscle size compared with an untreated baseline and the contralateral side in cerebral palsy (CP). Secondary goals were to determine whether positive changes in muscle size and gait, if found, accumulated during the 3 intervals during which participants used the device. FES devices differ from traditional orthoses that often restrict muscle activation and may exacerbate weakness, promote continued dependence on orthoses, or precipitate functional decline.
Methods
Participants were 14 independent ambulators with inadequate dorsiflexion in swing, with a mean age of 13.1 years, evaluated before and after the 3-month baseline, 1-month device accommodation, 3-month primary intervention, and 3-month follow-up phases. The FES device (WalkAide) stimulated the common fibular nerve to dorsiflex the ankle and evert the foot while monitoring use. TA muscle ultrasound, gait velocity, and ankle kinematic data for barefoot and device conditions are reported.
Results
Ultrasound measures of TA anatomic cross-sectional area and muscle thickness increased in the intervention compared with baseline and with the contralateral side and were maintained at follow-up. Maximum ankle dorsiflexion decreased at baseline but improved or was maintained during the intervention phase with and without the device, respectively. Muscle size gains were preserved at follow-up, but barefoot ankle motion returned to baseline values.
Conclusions
This FES device produced evidence of use-dependent muscle plasticity in CP. Permanent improvements in voluntary ankle control after repetitive stimulation were not demonstrated.
Keywords: cerebral palsy, electrical stimulation, gait, ankle, ultrasound, muscle thickness, cross-sectional area
Introduction
Cerebral palsy (CP) encompasses a group of disorders characterized by motor impairments that result from an abnormality or injury to the brain during early development. Individuals with CP exhibit a wide range of motor disabilities; however, approximately 70% of those with CP achieve the ability to ambulate during childhood, albeit with difficulty.1 Muscle weakness is pervasive in CP, with the ankle joint affected in virtually all patients.2,3 Most ambulatory children with CP are prescribed ankle-foot orthoses (AFOs) to improve ankle position and stability for standing and walking. AFOs typically provide support for or passively assist a weak muscle—for example, position the ankle in dorsiflexion in swing to facilitate toe clearance—but may also restrict wanted motions—for example, active dorsiflexion in swing or plantarflexion during push-off. As a result, AFOs may exacerbate weakness in both muscle groups over time, leading to continued dependence on orthoses, or precipitate functional or structural decline with growth or aging. One of the most consistent findings across investigations of traditional AFOs is reduced ankle power.4 Olney and coauthors5 cautioned against prescribing treatments in CP that may further weaken children who were already weak. Using a device that instead repetitively stimulates the weak muscle without restricting voluntary muscle activation at that joint may reverse these trends.
Muscle atrophy and connective tissue alterations such as increased collagen6 and adipose infiltration7 have been reported in CP. Despite knowledge that muscles are one of the most plastic tissues in the body, the extent to which these adverse changes may be reversible remains unknown. Recent evidence suggests that muscle size is directly related to mobility levels and degree of physical activity in CP.8 It has also been shown that voluntary resistance training can increase muscle size in CP.9 However, positive effects from training are only possible for those who have adequate voluntary muscle strength and control to participate in the types of exercises or activities that could be beneficial. Electrical stimulation is an alternative therapeutic approach that has been used successfully to increase muscle strength and size in children and adults with spinal cord injury,10,11 even in the absence of voluntary control, but this has not yet been shown in CP.
Functional electrical stimulation (FES) refers to delivery of electrical impulses to a nerve or muscle sufficient to produce desired joint motion during performance of a motor task. FES can also be used to augment repetitive task-specific practice, thereby improving underlying motor control.12,13 Several studies have delivered electrical stimulation to ankle muscles, aiming to improve gait in CP with intensity ranging from below the sensory threshold14 to that producing antigravity movement.15 The latter intensity level is used in commercial devices for foot drop in persons with central nervous system disorders such as stroke.16 Prior to this study, no clinical trials had yet been published on the effectiveness of any available commercial devices for foot drop, or excessive swing phase equinus, in CP.
Data on user acceptability and orthotic effects of an FES device, that is, whether it improves foot drop in CP, have been reported previously.17,18 The primary goal here was to examine the therapeutic effects, that is, alterations at the muscle or joint level that may improve the effectiveness of the device over time or that may persist even when the device is not worn. Specifically, we examined the effect of device use on tibialis anterior (TA) muscle size and pennation angle (PA) as assessed by ultrasound. We hypothesized that repetitive daily TA stimulation on the more affected side over the 3-month intervention phase would increase muscle size compared with the 3-month baseline phase and the contralateral side.
We also hypothesized that positive effects of FES on muscle size, ankle dorsiflexion, and gait velocity when walking barefoot or with the device would accumulate or be maintained over all time intervals during which participants used the device, with no improvement during baseline. We further hypothesized that resultant changes in muscle size would be related to the amount of time the device was used and to the amount of positive changes in ankle dorsiflexion and gait speed in the same time intervals.
Methods
Participants
Participants included 14 out of a total sample of 21 enrolled in a clinical trial evaluating the use of the WalkAide device (Innovative Neurotronics Inc, Austin, Texas) for improving foot drop in children with unilateral or asymmetrical CP. The presence of foot drop was determined by clinical observation of the treating physician and confirmed by gait analysis after enrollment. Of 21 patients, 7 were excluded here because they either did not complete the entire 10-month study (n = 5) or had poor or missing ultrasound data for 1 or more time points (n = 2). The remaining 14 children had complete muscle ultrasound data from all 5 assessments over the 10-month study; 13 had a diagnosis of hemiplegia, and 1 had diplegia. Of these, 8 were male, and the mean age was 13.1 ± 3.56 years (range, 8–19 years). All were able to walk independently with 8 classified in Gross Motor Function Classification System (GMFCS) level I and 6 in level II. Two participants used unilateral AFOs, and 3 used supramalleolar orthosis (SMOs)—2 unilateral and 1 bilateral—prior to enrollment. All but 1 unilateral SMO user discontinued the use of orthoses after receiving the device. Exclusion criteria were botulinum toxin injection to calf muscles within 4 months prior to enrollment or orthopedic surgery in the previous year. The study was approved by the institutional review board at the National Institutes of Health in Bethesda, Maryland. Written informed consent was obtained from participants older than 18 years of age and parents of minors. Written assent was obtained from all minors.
Procedures
Each assessment was performed on a single day lasting 3 to 5 hours, including breaks for rest and snacks as needed (Figure 1). For the baseline phase, assessments were conducted at months 0 and 3 to evaluate change over time in the absence of this device. Each was then given the device for their more affected side, instructed in its use, and allowed to accommodate to it for 1 month, with the goal of gradually increasing use to 6 hours per day by month 4. Next, each was asked to use the device for 3 months, which was the primary intervention phase (months 4 to 7). No additional therapy was provided during the study. At month 7, all were given the choice to continue using the device or not using the device until the follow-up evaluation at month 10. Outcome measures included the following: (1) muscle ultrasound measures of muscle thickness (MT), cross-sectional area (CSA), and PA on the FES side at each time point and on the contralateral side for months 3, 4, and 7; and (2) gait velocity and maximum ankle dorsiflexion in swing when walking at self-selected and fast speeds barefoot (all 5 time points) and with the FES device (months 4, 7, and 10).
Figure 1.
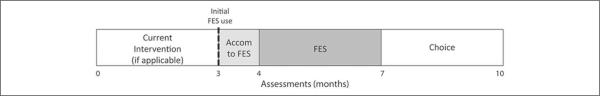
Illustrates the overall 10-month-long study design, with the light gray shading indicating the device accommodation period and the darker gray shading indicating the intervention period when all were required to use the device. The 5 assessment time points are also shown. Abbreviation: FES, functional electrical stimulation.
FES Device Set-up
The WalkAide delivers asymmetrical biphasic surface electrical stimulation to the common fibular (formerly peroneal) nerve, triggered by an individually programmed tilt sensor to improve foot clearance during swing. TA is the major ankle dorsiflexor and also inverts the foot. The fibular muscles primarily evert the foot, with some contribution to plantarflexion. The stimulating electrode was initially placed posterior and distal to the fibular head to stimulate the nerve as it wraps around the fibular head just prior to decussating into superficial and deep branches. The second “passive” electrode completing the electrical circuit was placed over the proximal TA muscle belly to target the deep branch for maximal dorsiflexion. Minor adjustments in electrode placement (location and spacing) can more selectively activate either the TA or the fibular muscles to achieve desired motion and improve comfort. Larger electrode sizes were also used to maximize comfort and were changed weekly. Electrode locators were placed inside the cuff of the device by the clinician once the best location was determined, which eliminated the need for the family to determine this each time. Participants controlled stimulation amplitude using the dial on the device and were instructed to use the highest level that maximized dorsiflexion, yet was still comfortable. Amplitude was gradually increased to tolerance throughout the accommodation phase.
Table 1 lists stimulation parameters adjustable by the clinician for each participant with their range throughout the study. A short pulse width was programmed (25 or 50 μs) for greater comfort. A short ramp-up of stimulation improved comfort and decreased elicitation of a plantarflexor stretch response. Small increases in amplitude and pulse width were made in some to gain more dorsiflexion as tolerance increased. Adjustments to electrode location were made at each assessment as needed.
Table 1.
Stimulation Parameters Programmed by the Clinician for Each Participant From the End of the Device Accommodation Period (First Value) to the End of the Study (Second Value in Range)
| Participant | Pulse Frequency, Hz | Pulse Width, μs | Minimum Time, s | Maximum Time,s | Wait Time, s | On Ramp, s | Off Ramp, s |
|---|---|---|---|---|---|---|---|
| 1 | 25 | 50 | 0.3–0.6 | 1.4–1.5 | 0.2–0.3 | 0.2–0.3 | 0–0.2 |
| 2 | 25 | 50 | 0.2 | 1.0 | 0.2 | 0 | 0 |
| 3 | 25 | 50 | 0.2 | 1.5 | 0.2 | 0.3 | 0.3 |
| 4 | 25 | 25 | 0.3 | 1.3 | 0.2 | 0.2 | 0 |
| 5 | 25 | 25–50 | 0.2 | 1.4 | 0.2 | 0.1 | 0 |
| 6 | 25 | 25 | 0.2 | 1.4 | 0.2 | 0 | 0–0.2 |
| 7 | 25 | 25–50 | 0.2 | 1.4 | 0.2 | 0–0.2 | 0 |
| 8 | 25 | 25–50 | 0.2 | 1.4 | 0.2 | 0.2 | 0 |
| 9 | 25 | 50 | 0.3 | 1.5 | 0.3 | 0.3–0.5 | 0 |
| 10 | 25 | 50 | 0.2 | 1.4 | 0.2 | 0–0.5 | 0 |
| 11 | 25 | 50 | 0.2 | 1.4 | 0.2–0.3 | 0 | 0 |
| 12 | 25 | 50 | 0.2 | 1.4 | 0.2 | 0–0.3 | 0.2–0.3 |
| 13 | 25 | 25–50 | 0.2 | 1.4 | 0.2 | 0 | 0.3 |
| 14 | 25 | 50 | 0.2 | 1.4 | 0.2 | 0 | 0 |
Data Procurement
Real-time musculoskeletal ultrasound imaging was performed to examine muscle architecture of the TA. The more affected (FES) leg was scanned at all time points, and the contralateral leg was scanned only at the 3-, 4-, and 7-month assessments. For ultrasound imaging (SonixTOUCH, Ultrasonix Medical Corporation, Richmond, BC, Canada), participants were in a supported sitting position with 60° knee flexion and 0° ankle dorsiflexion and were instructed to relax. Muscle contractions were easily detected in real time via ultrasound. Ultrasound gel was applied liberally to provide acoustic coupling and reduce muscle compression. Most TA images were taken by 1 of 2 ultrasound operators shown to have excellent intrarater as well as interrater reliability,19 with a third trained by them to serve as backup. Images were centered on the thickest region of the muscle belly as determined by visual inspection. A 2-dimensional B-mode ultrasound device with a 5- to 14-MHz linear array transducer was used to record images from which measurements of TA MT, PA, and anatomic CSA were obtained. Longitudinal images were taken with the probe oriented in the sagittal plane and perpendicular to the skin for MT and PA. Cross-sectional images were taken with the probe oriented in the transverse plane and perpendicular to the skin, with 2 images for each view.
Three-dimensional kinematic data were collected with a 10-camera motion capture system (Vicon, Lake Forest, California), which tracked the movement of 34 reflective markers placed on pelvic and lower-extremity landmarks at 120 Hz. Participants walked down the middle of an 8-m walkway. All were instructed to walk at a “normal, comfortable pace” and “as fast as possible,” respectively, for 5 trials each. Two different conditions were analyzed here: barefoot and with the FES device plus shoes; however, the FES device was not provided until on or after the 3-month assessment, so full FES data sets are only available from months 4 to 10.
Data Processing
Analysis of ultrasound images was performed with Medical Image Processing, Analysis and Visualization (MIPAV) software (National Institutes of Health, Bethesda, Maryland) by 1 of 2 trained individuals. The image from each pair that showed the clearest picture for analysis of all measures was chosen. MT was calculated at the midpoint of the ultrasound image as the perpendicular distance from the deep to the superficial aponeuroses. PA was measured as the positive angle between the central intramuscular septum and the line of the clearest fascicle. The perimeter of the TA was manually traced in the software for each cross-sectional image, from which CSA was calculated.
Marker data and anthropometric measurements were used to create a participant-specific anatomic model in Visual 3D (C-Motion, Inc, Gaithersburg, Maryland), from which gait variables were computed, which included velocity and maximum dorsiflexion angle in swing for barefoot and device conditions at both freely selected and as-fast-as-possible speeds. The average of 5 gait cycles was used for analyses.
Statistical Analyses
To address the first hypothesis that muscle size increased as a result of the FES intervention, repeated measures analysis of variance (ANOVA) procedures were performed comparing each muscle ultrasound value at times 0 and 3 (no treatment baseline) with those at times 4 and 7 (primary intervention period) with time (pre or post) and study phase (baseline or intervention) as within-subject factors. As an additional treatment to the no-treatment comparison, ultrasound values across legs were compared using repeated-measures ANOVA with time (4 or 7 months) and side (treated/more-affected side or untreated/less-affected side) as within-subject factors.
Repeated-measures ANOVA was also used to examine the effect of time (5) for ultrasound and gait variables. Change scores were computed for each time interval for the muscle size variables and correlated with the mean amount of daily stimulation and with change scores in the kinematic and temporal-spatial gait variables, both barefoot and with the device in the same time interval. A P value <.05 was used for all analyses.
Results
Tables 2 and 3 list the mean and standard deviation for outcome measures across time points. All 14 children wore the device during the primary intervention period with a mean of 5.85 ± 2.0 h/d, close to the study goal of 6 h/d. At month 7, all 14 chose to continue using the device, and no amount of daily use was specified. Use data during follow-up were not available for 3 participants (log not reset in 2 and failed in 1), with the remaining 11 reporting a mean duration of use of 4.34 ± 3.0 h/d (mean including all 14 = 3.40 ± 3.2 h/d).
Table 2.
Group Means and Standard Deviations for Each Variable at Each Time Point, Organized by Outcome Category: Muscle Ultrasound Measures for the Side on Which the Device Was Worn
| Month | MT, cm | PA, deg | CSA, cm2 |
|---|---|---|---|
| 0 | 1.71 (0.20) | 10.09 (2.78) | 3.05 (0.65) |
| 3 | 1.69 (0.21) | 10.95 (2.53) | 3.10 (0.65) |
| 4 | 1.74 (0.23) | 11.79 (1.88) | 3.17 (0.73) |
| 7 | 1.87 (0.27) | 12.69 (2.46) | 3.65 (0.98) |
| 10 | 1.90 (0.23) | 13.42 (1.92) | 3.79 (0.95) |
Abbreviations: MT, muscle thickness; PA, pennation angle; CSA, cross-sectional area.
Table 3.
Group Means and Standard Deviations for Each Variable at Each Time Point, Organized by Outcome Category: Gait Variables for BF and FES (WalkAide Device) Conditions
| Velocity, m/s |
Max DF, deg |
||||
|---|---|---|---|---|---|
| Month | SS | Fast | SS | Fast | |
| BF | 0 | 1.05 (0.14) | 1.55 (0.29) | −0.14 (3.88) | 0.57 (5.50) |
| 3 | 1.10 (0.13) | 1.55 (0.22) | −1.79 (4.06) | −1.74 (4.62) | |
| 4 | 1.14 (0.18) | 1.62 (0.21) | −0.91 (3.18) | −1.05 (4.33) | |
| 7 | 1.11 (0.10) | 1.60 (0.20) | −1.32 (3.49) | −1.18 (4.10) | |
| 10 | 1.14 (0.11) | 1.68 (0.20) | −2.57 (3.37) | −2.50 (4.06) | |
| FES | 0 | N/A | N/A | N/A | N/A |
| 3 | N/A | N/A | N/A | N/A | |
| 4 | 1.26 (0.16) | 1.68 (0.20) | 2.44 (3.20) | 2.38 (4.25) | |
| 7 | 1.26 (0.14) | 1.66 (0.22) | 5.96 (5.78) | 4.59 (6.05) | |
| 10 | 1.25 (0.13) | 1.70 (0.22) | 4.13 (4.57) | 3.53 (4.54) | |
Abbreviations: BF, barefoot; FES, functional electrical stimulation; MT, muscle thickness; PA, pennation angle; CSA, cross-sectional area; Max DF, maximum dorsiflexion in swing; SS, self-selected walking speed; Fast, fastest walking speed.
Ultrasound Data
The FES intervention led to increases in muscle size but not PA as compared with baseline or the contralateral leg. In comparing the baseline and primary intervention phase, significant main effects for time (P < .001 and =.04) and phase (P = .025 and .003) and an interaction (P = .03 and .005) were found for MT and CSA, respectively. Both were virtually unchanged at baseline but increased during the FES phase (P = .001 and .002, respectively). PA demonstrated significant main effects for time (P = .004) and phase (P = .029), with increases over time for both phases and higher angle values in the treatment interval. The amount of change over time in PA between phases did not differ (P = .85).
Similar results were found in the comparison across legs. PA showed a significant main effect for time only (P = .003), with no difference between sides (P = .14). CSA showed a main effect for time and side with an increase during the treatment interval only on the more affected side (P = .002). MT on the more affected side also increased significantly during that interval (P = .001), whereas MT on the unaffected side was slightly but not significantly less (P = .68), so no main effect for time was found. MT did show a main effect for side and interaction between time and side (P < .001 for both).
To address the secondary hypotheses proposing that changes would be maintained or accumulate over the treatment intervals, outcomes on the more affected side were compared over time. TA MT and CSA values were normalized by body weight to account for changes resulting from growth and then compared across time. A main effect for time was found, with significant increases in size between times 3 and 10 for both parameters (P < .001) and additionally between times 3 and 7 for MT only (P < .001); see Figure 2 and Table 2. Although mean normalized CSA continued to increase slightly from months 7 to 10, this was not significant (P = .07).
Figure 2.
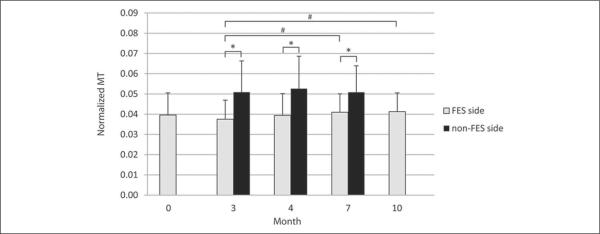
Muscle thickness (MT) at each measured time point for the more affected side on which the functional electrical stimulation (FES) device was worn as well as that on the non-FES side. Significant differences between sides (*) or on the more affected side across time (#) are indicated here.
Muscle size measures (MT and CSA) on the more affected side were significantly smaller at all measured time points using paired t tests (P < .05 for all comparisons). MTs of the more-affected side were 74.8%, 75.7%, and 81.3% of the less-affected side at times 3, 4, and 7, respectively. The CSAs of the more affected side were 61.6%, 63.8%, and 72.5% of the less affected side, showing relative differences across sides diminishing as treatment continued.
Gait Data
No difference in mean normalized velocity was found across time for either gait speed condition. After accommodation, 12 of 14 participants showed greater maximum dorsiflexion in swing in the device plus shoes condition compared with shoes alone. The 2 participants who did not show an improvement had a difference of less than 1° between conditions.
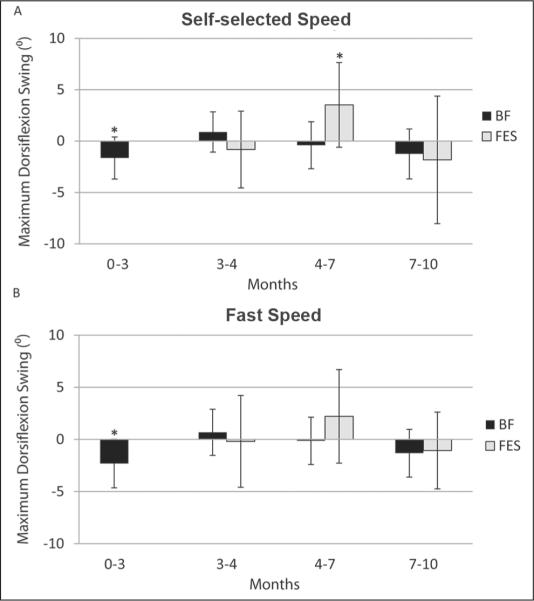
For barefoot walking at self-selected speed, maximum ankle dorsiflexion decreased during baseline (P = .01), with no significant decline during the primary intervention period (P = .712). By 10 months, a significant decline (P = .007) was seen as compared with time 4. Whereas the 10-month value for ankle dorsiflexion maximum was significantly less than at study entry (P = .004), there was no difference in this measure compared with the second baseline (P = .18).
Fast barefoot walking also showed declines in maximum dorsiflexion only during baseline (P = .003). The 10-month value was also less than at study entry (P = .004), but did not differ from that at the second baseline (P = .86), with no decline during the primary intervention period (P = .85). Figure 3 depicts changes in ankle dorsiflexion maximum for barefoot and FES conditions across each interval.
Figure 3.
Change across the 4 study intervals listed by month: (0–3 = baseline, 3–4 = device accommodation, 4–7 = primary intervention, 7–10 = follow-up) in maximum ankle dorsiflexion in swing in both the barefoot (BF) and functional electrical stimulation (FES) device conditions during (A) self-selected and (B) fast speed trials. Significant changes within each interval for either condition (*) are indicated here.
For the FES device over time, only data from months 4, 7, and 10 were available. Improvement from month 4 to 7 in maximum dorsiflexion during self-selected walking with the device was significant (+3.0°, P = .02) but did not change from time 7 to 10 months. For fast walking with the device, no significant differences in maximum ankle dorsiflexion were found across time.
Relationship of Muscle Changes to Gait Measures
No significant correlations were found between changes in muscle size and ankle motion or gait velocity with or without the FES device across any of the time intervals, nor were there correlations with the amount of device use and magnitude of changes.
Discussion
Muscle size, when either normalized or compared with the opposite side to account for increases caused by growth, increased as hypothesized as a result of the intervention. Changes during the primary intervention period reached significance when compared with baseline or the same time interval on the contralateral side. Muscle size was maintained during follow-up with all 14 participants continuing to use the device, although mean daily use was considerably less. Percentage increases in CSA and MT were approximately 19.9% and 7.0%, respectively, over the intervention period when compared with the larger baseline value, with additional nonsignificant increases of 4.8% and 2.0% during follow-up. Decreased muscle size on the more affected leg compared with the contralateral leg is evidence that the TA either failed to develop normally or atrophied on that side as a result of weakness, spasticity, or poor selective control or perhaps because of residual effects of partial immobilization from long-term orthotic use.
This study is the first evidence showing that regular use of FES could “grow” muscle in CP, demonstrating use-dependent plasticity. McNee and colleagues9 were the first to show increases in muscle size as a result of voluntary muscle training in CP, with increases in volume after progressive resistance exercise to the gastrocsoleus muscles of 16% after 5 weeks and an additional 7% in the second 5 weeks. Whereas no direct numerical comparisons are possible across studies because of differences in muscles and training regimens, this study supported their finding that direct muscle training in CP can increase muscle size. The slower rate of increase in the second half of each program may reflect a waning response to the same stimulus in the muscle over time because the muscle had grown and thus should require proportionate increases in the stimulus for similar changes or because of diminished amounts of exercise or stimulation, as seen in our study. However, mean muscle size was at least maintained over the second 3-month period, even with diminished use in several participants. Although we suspect that muscle size is likely to revert to or approach baseline levels in the absence of training, this will require further investigation.
PAs increased over time regardless of side and intervention phase, suggesting that this was the result of physical growth and that higher values in the intervention period were likely because these data were collected later in time. The lack of response to intervention seems surprising because muscle size increased, similar to what happens in resistance training, which typically increases size and PA. However, electrical stimulation preferentially targets fast twitch fibers, so effects may also be similar to those from high-velocity training, which produces the opposite effect (decrease) on PA20 and may reverse the effect from size increases.
Orthotic effects of the device were reported previously in this sample18: that is, it improved dorsiflexion compared with shoes only, with no differences in gait velocity. Gait velocities for both free- and fast-speed trials were unchanged throughout the entire study period and were unrelated to whether the device was being used. This analysis extended the comparison to barefoot walking and to baseline and follow-up periods. One explanation for the lack of an improvement in gait speed is that this sample of independent ambulators was already walking at close to normal velocity on enrollment, unlike a similar study in stroke that reported increased speed in slower patients,16 so the potential for further improvement was limited from the outset. We recommend that future studies include monitoring for increases in amount of walking activity or for decreased trips and falls because these may be the most likely direct functional benefits of using these devices for more functional users.
Ankle kinematic data for barefoot walking showed a fairly consistent pattern of initial decline during baseline, maintenance during intervention, and a return toward the lowest (second) baseline value at follow-up. This was in contrast to the FES condition where a significant improvement was seen during intervention that was at least maintained during follow-up. From these data, it appears that intense and repetitive use of FES may lead to improvement in ankle motion over time when the device is worn regularly for 5 to 6 h/d, although some improvement may have been a result of increased stimulation amplitude or pulse width in a few participants. Wearing the FES device for several hours per day may also help prevent decline in ankle function when walking without the device, but this effect may also lessen over time or if intensity of the intervention wanes. The lack of significant or sustained improvement in barefoot ankle motion over the study period seems to indicate that selective motor control at the ankle was not permanently improved by the repetitive stimulation. This is not surprising because repetition was generated externally by the device rather than “internally” through the voluntary effort of the participant and thus could not be considered task-specific practice. In fact, it was noted that some participants actually decreased their amount of active dorsiflexion while using the device and may have “learned” instead to let the device take over ankle control. The particular device used here does have an exercise mode that could be used to augment voluntary repetitive practice of ankle dorsiflexion, which may be more effective in improving selective control, thus potentially decreasing the need for an orthosis. A study by Daly and colleagues12 in patients poststroke demonstrated that FES was effective in enhancing lower-extremity coordination when used in combination with intense task-specific treadmill walking practice, with effects persisting at least 6 months posttraining.
The changes in muscle size were more pronounced and consistent—and in some cases in the opposite direction—than those in ankle kinematics, so these were not found to be related in this sample. If the primary underlying problem is poor selective motor control, an increase in muscle size could lead to increases in electrically stimulated strength and motion with the device but not when barefoot.
Conclusions/Implications
Broader implications include support for expanding the use of electrical stimulation in CP to prevent muscle atrophy or to maintain antagonist muscle size, both by stimulating the weaker muscle at the joint and by not restricting activation in the stronger muscle. The latter strategy of weakening the stronger muscle to enable the weaker antagonist is used frequently in CP—for example, surgical muscle releases and botulinum toxin injections to stronger muscles at a joint—as well as in current orthotic approaches. The strategy proposed here seems far more likely to promote function over the long term and counteract the often devastating functional effects of growth or aging on many with CP. Tolerability issues, although not problematic here, are still a major concern when using FES; however, using stimulation to augment existing muscle control may be feasible even in larger muscles (eg, to promote knee extension in crouch). Greater incorporation of electrical stimulation devices to augment motor skill training should also be explored.
Acknowledgments
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Intramural Research Program at the NIH Clinical Center. Innovative Neurotronics provided the devices to all participants.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Beckung E, Hagberg G, Uldall P, Cans C. Probability of walking in children with cerebral palsy in Europe. Pediatrics. 2008;121:187–192. doi: 10.1542/peds.2007-0068. [DOI] [PubMed] [Google Scholar]
- 2.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- 3.Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev Med Child Neurol. 2010;52:264–269. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 4.Morris C, Condie D, Fisk J. ISPO Cerebral Palsy Consensus Conference Report. Prosthet Orthot Int. 2009;33:401–402. doi: 10.3109/03093640903311400. available free at www.ispoweb.org. [DOI] [PubMed] [Google Scholar]
- 5.Olney SJ, MacPhail HE, Hedden DM, Boyce WF. Work and power in hemiplegic cerebral palsy gait. Phys Ther. 1990;70:431–438. doi: 10.1093/ptj/70.7.431. [DOI] [PubMed] [Google Scholar]
- 6.Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001;43:314–320. doi: 10.1017/s0012162201000597. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. J Pediatr. 2009;154:715–720. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau NG, Simpson KN, Teefey SA, Damiano DL. Muscle architecture predicts maximum strength and is related to activity levels in cerebral palsy. Phys Ther. 2010;90:1619–1630. doi: 10.2522/ptj.20090377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNee AE, Gough M, Morrissey MC, Shortland AP. Increases in muscle volume after plantarflexor strength training in children with spastic cerebral palsy. Dev Med Child Neurol. 2009;51:429–435. doi: 10.1111/j.1469-8749.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 10.Gargiulo P, Reynisson PJ, Helgason B, et al. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res. 2011;33:750–758. doi: 10.1179/1743132811Y.0000000007. [DOI] [PubMed] [Google Scholar]
- 11.Johnston TE, Modlesky CM, Betz RR, Lauer RT. Muscle changes following cycling and/or electrical stimulation in pediatric spinal cord injury. Arch Phys Med Rehabil. 2011;92:1937–1943. doi: 10.1016/j.apmr.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Daly JJ, Zimbelman J, Roenigk KL, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. 2011;25:588–596. doi: 10.1177/1545968311400092. [DOI] [PubMed] [Google Scholar]
- 13.Hu XL, Tong KY, Li R, et al. Effectiveness of functional electrical stimulation (FES)-robot assisted wrist training on persons after stroke. Conf Proc IEEE Eng Med Biol Soc. 2010;58:19–22. doi: 10.1109/IEMBS.2010.5627471. [DOI] [PubMed] [Google Scholar]
- 14.Carmick J. Managing equinus in children with cerebral palsy: electrical stimulation to strengthen the triceps surae muscle. Dev Med Child Neurol. 1995;37:965–975. doi: 10.1111/j.1469-8749.1995.tb11951.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Linden ML, Hazlewood ME, Hillman SJ, Robb JE. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr Phys Ther. 2008;20:23–29. doi: 10.1097/PEP.0b013e31815f39c9. [DOI] [PubMed] [Google Scholar]
- 16.Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and non-progressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–167. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 17.Durham S, Eve L, Stevens C, Wins D. Effect of functional electrical stimulation on asymmetries in gait of children with hemiplegic cerebral palsy. Physiotherapy. 2004;90:82–90. [Google Scholar]
- 18.Prosser LA, Curatalo LA, Alter KE, Damiano DL. Acceptability and effectiveness of a foot drop stimulator in children and adolescents with cerebral palsy. Dev Med Child Neurol. doi: 10.1111/j.1469-8749.2012.04401.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland DC, Prosser LA, Bellini LA, Alter KE, Damiano DL. Tibialis anterior architecture, strength, and gait in individuals with cerebral palsy. Muscle Nerve. 2011;44:509–517. doi: 10.1002/mus.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe T, Kumagai K, Brechue WF. Fascicle length of leg muscles is greater in sprinters than distance runners. Med Sci Sports Exerc. 2000;32:1125–1129. doi: 10.1097/00005768-200006000-00014. [DOI] [PubMed] [Google Scholar]