Dear Sirs
Elevation of inflammatory markers such as C-reactive protein (CRP) and serum amyloid A (SAA) are associated with increased cardiovascular risk (1–4). The broad overlap of risk factors between cardiovascular risk and venous thromboembolic disease (VTE) (5–8) and the theoretical association of inflammation with VTE has stimulated research of inflammatory markers and VTE (9–11). The association of CRP with VTE has been variable and inconsistent (10, 11).
SAA is known best for its role during the acute phase response to an inflammatory stimulus such as infection, tissue injury, and trauma. During active inflammation, the concentration of SAA in plasma can increase up to 1,000-fold within 24 hours (12) and decline after 4–5 days, and then return to the baseline after 10–14 days (13, 14). SAA elevations have been associated with estrogen therapy, metabolic syndrome including obesity and type 2 diabetes (15–19). Recent studies suggest that SAA could act as a pro-inflammatory factor by stimulating its receptors on cell surfaces to release cytokines (20–22). Furthermore, SAA induces tissue factor (TF) on monocytes and endothelial cells (23, 24), suggesting SAA could initiate the coagulation system. These potential biological properties of SAA may link inflammation to thrombotic diseases. In one subgroup of patients, SAA was associated with primary anti-phospholipid syndrome and the number of recurrent VTE events (25). We found that plasma SAA levels from individual normal donors were correlated with several parameters of endogenous thrombin potential assays (Table 1S), suggesting that endogenous plasma SAA levels might be associated with plasma coagulability. Since hypercoagulability is causally linked to VTE, we hypothesized that plasma levels of SAA may be associated with the risk of VTE.
In assessing this hypothesis, we measured both plasma SAA and CRP levels using 113 VTE patients and 113 age and sex matched controls from the Scripps VTE registry (26–29). The protocol was approved by the Institutional Review Board, and subjects provided written informed consent. Blood was collected > 3 months after diagnosis of acute thrombosis to avoid the influence of acute phase reactions caused by the thrombotic event itself. The VTE study population included 50.4% idiopathic cases, 31.9% recurrent cases and 60.2% had pulmonary embolism. Comprehensive clinical characteristics and risk factors are described in Supplement Materials and Methods and Table 2S. VTE patients had significantly higher levels of SAA compared to matched controls (p<0.0001, Figure 1A). The higher levels of SAA plasma concentration (>90th, >75th and >67th percentile of control) were associated with VTE with an odds ratio (OR) of 8.4 (95 % CI, 3.3–21), 8.4 (95 % CI, 3.3–21), 5.4 (95% CI, 2.7–11) and 4.0 (95% CI, 2.1–7.7), respectively (Table 3S, Model I). The significance of the OR at 90th, 75th and 67th percentile cut-off remained after adjustment for parameters which may be associated with both SAA level and VTE risk including estrogen treatment, obesity, type II diabetes and anti-phospholipid syndrome (5, 6, 8, 15–19) (Table 3S, Model III) (6.8 (95 % CI 2.7–17), 4.5 (95% CI, 2.2–9.4) and 3.4 (95% CI, 1.7–6.6), respectively). When other known VTE risks including factor V Leiden, prothrombin nt G20210A polymorphism, history of VTE, hypertension, and current smoking (5, 6, 8), were added in the model, the ORs still remained significant (Table 3S, Model IV) as the OR at 90th, 75th and 67th percentile cut-off were 9.3 (95 % CI, 2.9–30), 11 (95% CI, 3.5–35) and 6.3 (95% CI, 2.3–18), respectively.
Figure 1. Plasma SAA and CRP levels in VTE patients and controls.
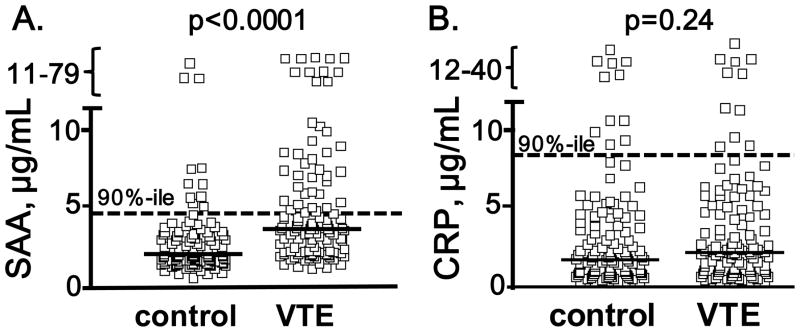
Plasma SAA (A) and CRP (B) levels were measured for 113 VTE patients and 113 age and sex matched controls using standard ELISA methods. Solid thick lines indicate median values and the dotted lines indicate values for the 90th percentile of the control values. VTE patients had significantly higher levels of SAA compared to the matched controls (2.12 μg/ml, interquartile range (IQR) 1.66 to 3.22 μg/ml) versus 3.70 μg/ml (IQR 2.27 to 6.54 μg/ml) (p<0.0001, Mann-Whitney test). In contrast, there was no statistical difference in CRP levels between VTE (2.20 mg/L, IQR 0.95 to 5.46 μg/ml) and control (1.68 μg/L, IQR 0.85 to 4.11 μg/ml) (p=0.23, Mann-Whitney test).
In the control group, SAA levels were correlated significantly with another acute-phase inflammatory protein, CRP (r = 0.56, p<0.0001). Therefore, we tested if the association of SAA with VTE was primarily or only secondarily associated with CRP. Notably, after adjustment for CRP, the OR at 90th, 75th and 67th percentile cut-off for the association of elevated SAA with VTE was not significantly altered 8.4 (95% CI, 3.3–21), 5.5 (95% CI, 2.7–11) and 4.1 (95% CI, 2.1–8.2), respectively (Table 3S Model II). Thus, we found that a high level of plasma SAA was associated with VTE for a substantial percentage of VTE patients. Moreover, we did not find a significant association of plasma CRP levels with VTE (p=0.23, Mann-Whitney test) (Figure 1B). High levels of plasma CRP (>90th percentile of control) were not associated with increased risk of VTE (OR = 0.92, 95%CI, 0.40–2.1). These data suggest that elevated SAA is associated with VTE and that in this study population, SAA appears to be independent of the widely studied inflammatory biomarker, CRP as well as known VTE risk factors or condition or comorbidities that influence the development of VTE.
This study has several potential limitations. Our control cohort consisted of predominantly healthy medical or research institute employees who might have less chronic inflammation and may not be representative of the general population and it could be postulated that the control SAA values are artificially low. However, it is noteworthy that other inflammatory markers, CRP and fibrinogen, did not show any differences between groups, suggesting that there was no identifiable difference in inflammation based on these inflammation biomarkers. The timing of a blood drawn in relation to a clinical event can raise an issue of validity of data conclusions but the biological half-life of SAA suggests that the carry-over effect of acute phase reactions caused by the thrombotic event itself can be discounted with the plasma collected > 3 months after diagnosis of acute thrombosis. Finally, retrospective studies of SAA plasma levels from this case-control study cannot distinguish whether the observed differences are causal, consequential, or incidental although the ability of SAA to induce tissue factor (23, 24) and our findings that thrombin generation parameters in ETP assays of plasma was correlated with SAA levels (Table 1S) provide some degree of biologic plausibility for potential procoagulant actions of SAA.
In summary, SAA is an acute phase protein whose increased plasma levels are suggested to be associated with blood coagulability and VTE. Future studies are needed to verify that elevated SAA is a biomarker for VTE.
Supplementary Material
Acknowledgments
Source of support: This work was supported by the National Institutes of Health grants HL021544 and HL101034, and by the Ministerio de Ciencia e Innovación, PS09/00610 and Red RECAVA RD06/0014/0004, Spain.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Johnson BD, Kip KE, Marroquin OC, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement of healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Eng J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 5.Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56:1–7. doi: 10.1016/j.jacc.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Dentali F, Squizzato A, Ageno W. The metabolic syndrome as a risk factor for venous and arterial thrombosis. Semin Thromb Hemost. 2009;35:451–7. doi: 10.1055/s-0029-1234140. [DOI] [PubMed] [Google Scholar]
- 8.Di Minno MN, Tufano A, Ageno W, et al. Identifying high-risk individuals for cardiovascular disease: similarities between venous and arterial thrombosis in perspective. A 2011 update. Intern Emerg Med. 2012;7:9–13. doi: 10.1007/s11739-011-0582-y. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez AL, Wojcik BM, Wrobleski SK, et al. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. 2012;33:371–82. doi: 10.1007/s11239-012-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–5. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- 11.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30:1672–8. doi: 10.1161/ATVBAHA.109.198473. [DOI] [PubMed] [Google Scholar]
- 12.Hua S, Song C, Geczy CC, et al. A role for acute-phase serum amyloid A and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Rep. 2009;14:187–96. doi: 10.1179/135100009X12525712409490. [DOI] [PubMed] [Google Scholar]
- 13.Gabay C, Kushner I. Mechanisms of disease: acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 14.Takata S, Wada H, Tamura M, et al. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community-acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers. 2011;16:530–535. doi: 10.3109/1354750X.2011.607189. [DOI] [PubMed] [Google Scholar]
- 15.van Rooijen M, Hansson LO, Frostegård J, et al. Treatment with combined oral contraceptives induces a rise in serum C-reactive protein in the absence of a general inflammatory response. J Thromb Haemost. 2006;4:77–82. doi: 10.1111/j.1538-7836.2005.01690.x. [DOI] [PubMed] [Google Scholar]
- 16.Abbas A, Fadel PJ, Wang ZA, et al. Contrasting effects of oral versus transdermal estrogen on serum amyloid A (SAA) and high-density lipoprotein-SAA in postmenopausal women. Arterioscler Thromb Vasc Biol. 2004;24:e164–7. doi: 10.1161/01.ATV.0000140198.16664.8e. [DOI] [PubMed] [Google Scholar]
- 17.Jylhävä J, Haarala A, Eklund C, et al. Serum amyloid A is independently associated with metabolic risk factors but not with early atherosclerosis: the Cardiovascular Risk in Young Finns Study. J Intern Med. 2009;266:286–95. doi: 10.1111/j.1365-2796.2009.02120.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumon Y, Suehiro T, Itahara T, et al. Serum amyloid A protein in patients with non-insulin-dependent diabetes mellitus. Clin Biochem. 1994;27:469–73. doi: 10.1016/0009-9120(94)00044-v. [DOI] [PubMed] [Google Scholar]
- 19.Leinonen ES, Hiukka A, Hurt-Camejo E, Wiklund O, Sarna SS, Mattson Hultén L, Westerbacka J, Salonen RM, Salonen JT, Taskinen MR. Low-grade inflammation, endothelial activation and carotid intima-media thickness in type 2 diabetes. J Intern Med. 2004;256:119–27. doi: 10.1111/j.1365-2796.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 20.He R, Shepard LW, Chen J, et al. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–9. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 21.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–81. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 22.Lee MS, Yoo SA, Cho CS, et al. Serum amyloid A binding to formyl peptide receptor-like 1 induces synovial hyperplasia and angiogenesis. J Immunol. 2006;177:5585–94. doi: 10.4049/jimmunol.177.8.5585. [DOI] [PubMed] [Google Scholar]
- 23.Cai H, Song C, Endoh I, et al. Serum amyloid A induces monocyte tissue factor. J Immunol. 2007;178:1852–60. doi: 10.4049/jimmunol.178.3.1852. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Zhou S, Heng CK. Impact of serum amyloid A on tissue factor and tissue factor pathway inhibitor expression and activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:1645–50. doi: 10.1161/ATVBAHA.106.137455. [DOI] [PubMed] [Google Scholar]
- 25.Ames PRJ, Antinolfi I, Ciampa A, et al. Primary antiphospholipid syndrome: a low-grade auto-inflammatory disease? Rheumatology. 2008;47:1832–7. doi: 10.1093/rheumatology/ken382. [DOI] [PubMed] [Google Scholar]
- 26.Deguchi H, Pecheniuk NM, Elias DJ, et al. High density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112:893–9. doi: 10.1161/CIRCULATIONAHA.104.521344. [DOI] [PubMed] [Google Scholar]
- 27.Pecheniuk NM, Deguchi H, Elias DJ, et al. Cholesteryl ester transfer protein genotypes associated with venous thrombosis and dyslipoproteinemia in males. J Thromb Haemost. 2006;4:2080–2. doi: 10.1111/j.1538-7836.2006.02099.x. [DOI] [PubMed] [Google Scholar]
- 28.Pecheniuk NM, Elias DJ, Deguchi H, et al. Elevated plasma fibronectin levels associated with venous thromboembolism. Thromb Haemost. 2008;100:224–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung MC, Albers JJ, Kennedy H, et al. Association of Apo(a) isoform size with dyslipoproteinemia in male Venous Thrombosis patients. Clin Chim Acta. 2010;411:1279–83. doi: 10.1016/j.cca.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


