Abstract
The aspartic protease cathepsin-D (cath-D) is a marker of poor prognosis in breast cancer that is overexpressed and hypersecreted by human breast cancer cells. Secreted pro-cath-D binds to the extracellular domain of the β chain of the LDL receptor-related protein-1 (LRP1) in fibroblasts. The LRP1 receptor has an 85-kDa transmembrane β chain and a non-covalently attached 515-kDa extracellular α chain. LRP1 acts by (1) internalizing many ligands via its α chain, (2) activating signaling pathways by phosphorylating the LRP1β chain tyrosine, and (3) modulating gene transcription by regulated intramembrane proteolysis (RIP) of its β chain. LRP1 RIP involves two cleavages: the first liberates the LRP1 ectodomain to give a membrane-associated form LRP1β-CTF and the second generates the LRP1β intracellular domain, LRP1β-ICD, that modulates gene transcription. Here, we investigated the endocytosis of pro-cath-D by LRP1 and the effect of the pro-cath-D/LRP1β interaction on LRP1β tyrosine phosphorylation and/or LRP1β RIP. Our results indicate that pro-cath-D was partially endocytosed by LRP1 in fibroblasts. However, pro-cath-D and ectopic cath-D did not stimulate phosphorylation of the LRP1β chain tyrosine. Interestingly, ectopic cath-D and its catalytically-inactive D231Ncath-D, and pro-D231Ncath-D all significantly inhibited LRP1 RIP by preventing LRP1β-CTF production. Thus cath-D inhibits LRP1 RIP independently of its catalytic activity by blocking the first cleavage. Since cath-D triggers fibroblast outgrowth via LRP1, we propose that cath-D modulates the growth of fibroblasts by inhibiting LRP1 RIP in the breast tumor micro-environment.
Keywords: Animals; Breast Neoplasms; metabolism; pathology; COS Cells; Cathepsin D; metabolism; Cell Line, Tumor; Cell Membrane; metabolism; Cell Proliferation; Cercopithecus aethiops; Endocytosis; Enzyme Precursors; metabolism; Fibroblasts; cytology; enzymology; metabolism; pathology; Humans; Low Density Lipoprotein Receptor-Related Protein-1; chemistry; metabolism; Mammary Glands, Human; cytology; pathology; Neoplasm Invasiveness; Protein Structure, Tertiary; Proteolysis; Tumor Microenvironment
Keywords: cancer, cathepsin D, LRP1, RIP, endocytosis, tyrosine phosphorylation
INTRODUCTION
The lysosomal aspartic protease cathepsin-D (cath-D) is overexpressed and abundantly secreted by human epithelial breast cancer cells (Liaudet-Coopman et al 2006, Rochefort and Liaudet-Coopman 1999, Vetvicka et al 1994). This overproduction in breast cancer is correlated with a poor prognosis (Ferrandina et al 1997, Foekens et al 1999, Rodriguez et al 2005). Human cath-D is synthesized as a 52 kDa precursor that is rapidly converted in the endosomes to form an active, 48 kDa, single-chain intermediate, and then in the lysosomes into the fully active mature protease, composed of a 34-kDa heavy chain and a 14 kDa light chain (Gieselmann et al 1985). The overexpression of cath-D in breast cancer cells leads to the hypersecretion of the 52 kDa pro-cath-D into the extracellular environment (Capony et al 1989, Fusek and Vetvicka 1994, Vignon et al 1986). Cath-D affects both the cancer cells, and the stromal cells of the tumor micro-environment. Human pre-pro-cath-D cDNA transfected in cancer cells promotes cancer cell proliferation, tumor angiogenesis, and tumor growth and metastasis (Berchem et al 2002, Glondu et al 2001). Human pre-pro-cath-D cDNA transfected in cath-D−/− MEF cells induces fibroblast outgrowth (Laurent-Matha et al 2005). Inhibition of cath-D expression in breast cancer cells decreases tumor growth and metastasis (Glondu et al 2002, Ohri et al 2007, Vashishta et al 2007). Human secreted pro-cath-D stimulates breast cancer cell proliferation (Fusek and Vetvicka 1994, Ohri et al 2008, Vetvicka et al 1994, Vignon et al 1986), fibroblast outgrowth (Laurent-Matha et al 2005) and angiogenesis (Hu et al 2008). We have shown that a mutated catalytically-inactive version of cath-D (D231N) is still mitogenic for tumor cells and fibroblasts (Berchem et al 2002, Glondu et al 2001, Laurent-Matha et al 2005). We recently discovered that pro-cath-D is the first ligand that binds to the extracellular domain of the β chain of the LDL receptor-related protein-1, LRP1, in fibroblasts (Beaujouin et al 2010). We also showed that cath-D promotes LRP1-dependent fibroblast outgrowth by a mechanism that is independent of its catalytic activity (Beaujouin et al 2010).
The LRP1 receptor consists of an 85-kDa transmembrane β chain and a non-covalently attached 515-kDa extracellular α chain (Lillis et al 2005, Montel et al 2007, Strickland and Ranganathan 2003). The β chain has an extracellular domain, a trans-membrane region, and a cytoplasmic tail. The extracellular α chain contains binding sites for numerous structurally diverse ligands, including lipoprotein particles, proteases and protease-inhibitor complexes, extracellular matrix proteins, cytokines and growth factors. LRP1 has a well-defined role as a scavenger receptor mediating the endocytosis of more than 40 different extracellular ligands that bind to its α chain. It delivers most, but not all, of these ligands to lysosomes for degradation (Emonard et al 2005, Gonias et al 2004, Herz and Strickland 2001, May et al 2007). It has also been shown that LRP1 is involved in signal transduction by phosphorylation of the tyrosine in the cytoplasmic NPXY motifs of its β chain and modulation of signaling pathways such as the MAP kinase pathway (Barnes et al 2001, Barnes et al 2003, Boucher et al 2002, Boucher and Gotthardt 2004, Hu et al 2006, Loukinova et al 2002, Newton et al 2005, Yang et al 2004). More recent studies have shown that LRP1 influences gene transcription by regulated intramembrane proteolysis (RIP) of its β chain (Kinoshita et al 2003, May et al 2002, von Arnim et al 2005, Zurhove et al 2008). RIP is a process that involves two cleavages, as described for Notch (De Strooper et al 1999), APP (amyloid precursor protein) (De Strooper et al 1998), and LRP6 (Mi and Johnson 2007). The first cleavage, performed by metalloproteinases (Liu et al 2009, Quinn et al 1999, Rozanov et al 2004, Selvais et al 2009, Selvais et al 2011) and by the membrane-associated β-secretase BACE1 (von Arnim et al 2005), occurs in the extracellular region, close to the plasma membrane, and leads to shedding of the LRP1β ectodomain. This produces the membrane-associated carboxyl-terminal fragment LRP1β-CTF, which is then cleaved by γ-secretases within its transmembrane domain (Hass et al 2009). The LRP1β intracellular domain, LRP1β-ICD, is released into the cytosol where it may interact with signaling proteins, translocate to the nucleus, and control gene transcription (Kinoshita et al 2003, May et al 2002, von Arnim et al 2005, Zurhove et al 2008). We have now investigated the mechanisms by which cath-D affects the behavior of LRP1. We studied the endocytosis of pro-cath-D by LRP1, and the effect of the interaction between pro-cath-D and LRP1β on the tyrosine phosphorylation of LRP1β and/or LRP1 RIP. Pro-cath-D was partially endocytosed by LRP1 and cath-D inhibited LRP1 RIP independently of its catalytic activity by competing with the first cleavage. Finally, we obtained evidence that the cath-D-mediated inhibition of LRP1 RIP in fibroblasts could be the mechanism by which cath-D promotes their outgrowth in the breast tumor micro-environment.
RESULTS AND DISCUSSION
Pro-cath-D is partly endocytosed by LRP1 in fibroblasts
While pro-cath-D is mainly internalized by the mannose-6-phosphate (M6P) receptors (Laurent-Matha et al 2002, Laurent-Matha et al 2005), it has been postulated that there are alternative M6P receptor-independent mechanisms (Capony et al 1994, Laurent-Matha et al 2002, Laurent-Matha et al 2005, Rijnboutt et al 1991). Since we recently reported that pro-cath-D interacts with the extracellular domain of the β chain of the LRP1 receptor in fibroblasts (Beaujouin et al 2010), and as LRP1 is an endocytic receptor, we investigated the role of LRP1 in the internalization of secreted pro-cath-D in fibroblasts (Fig. 1). We first analyzed the endocytosis of S35 radiolabelled pro-cath-D secreted by cancer cells in human mammary fibroblasts (HMF) in which endogenous LRP1 synthesis had been blocked by RNA interference (Fig. 1A). Internalized labelled 52 kDa pro-cath-D was first transformed into a 48-kDa endosomal intermediate and then into the 34 kDa lysosomal mature enzyme (Fig. 1A, panel a, lane 1) (Capony et al 1987). Excess M6P prevented pro-cath-D from binding to its M6P receptors and inhibited the internalization of pro-cath-D by 82% (Fig. 1A, panel a, compare lanes 1 and 3). Thus, 18% of pro-cath-D was taken up by HMF fibroblasts in a manner that was independent of the M6P receptors (Fig. 1A, panel a, lane 3), as shown for mouse fibroblasts (Laurent-Matha et al 2005). Inhibiting LRP1 synthesis with siRNA (Fig. 1A, panel b) led to a 50% decrease in reminiscent pro-cath-D internalization (Fig. 1A, panel a, compare lanes 3 and 4). There was also a decrease in pro-cath-D endocytosis in LRP1-silenced fibroblasts (Fig. 1A, panel d) when uptake was measured over 18h (Fig. 1A, panel c, compare lanes 3 and 4, and panel e, for quantification). We then investigated pro-cath-D internalization by LRP1 using mouse embryonic fibroblasts (MEF) that lacked M6P receptors (Fig. 1B). Silencing LRP1 in RM6P−/− MEF cells (Fig. 1B, panel b) partly decreased pro-cath-D endocytosis over 3h (Fig. 1B, panel a, left), and 18h (Fig. 1B, panel a, right, and panel c for quantification). Re-expression of LRP1β chain alone in LRP1-silenced RM6P−/− MEF cells caused accumulation of 52 kDa pro-cath-D (Fig. 1B, panel a, right and left). We obtained further evidence by examining pro-cath-D endocytosis in LRP1−/− MEF cells re-transfected with full-length LRP1 (clone B-41), or with the LRP1β chain in the presence of M6P (Fig. 1C). Re-expressing full-length LRP1 in LRP1−/− MEF cells (Fig. 1C, panel b) stimulated pro-cath-D endocytosis (Fig. 1C, panel a). By contrast, re-expressing the LRP1β chain in LRP1−/− MEF cells (Fig. 1C, panel d) led only to increased 52 kDa pro-cath-D without the concomitant appearance of the 48 kDa endosomal and the 34 kDa lysosomal forms (Fig. 1C, panel c). This suggests that LRP1 requires both its α and its β chains in order to behave as an endocytic receptor. Thus secreted pro-cath-D is partially endocytosed by LRP1 in fibroblasts. Since high (nanomolar) concentrations of pro-cath-D are secreted by cancer cells, LRP1-dependent endocytosis may account for a significant fraction of the pro-cath-D internalized by fibroblasts.
Figure 1. Pro-cath-D is partially endocytosed by LRP1 in fibroblasts.
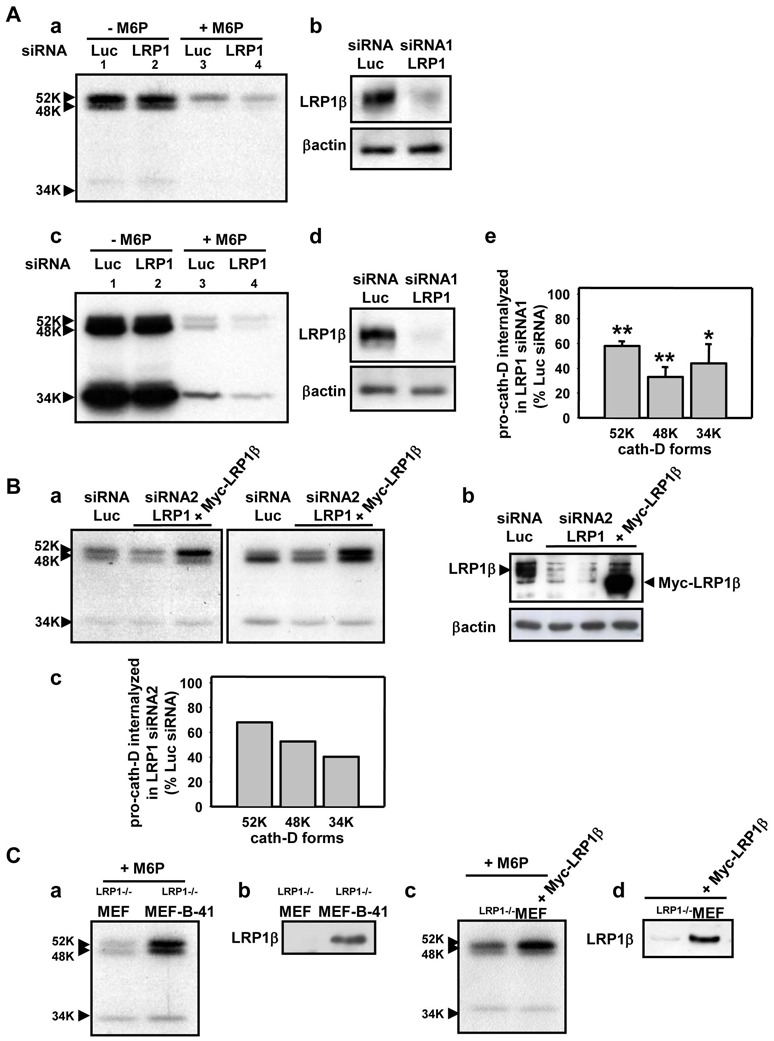
(A) Endocytosis of secreted pro-cath-D by LRP1 in HMF fibroblasts. Conditioned medium containing labelled pro-cath-D was produced by incubating pro-cath-D secreting rat cancer 3Y1-Ad12 cells (Glondu et al 2001) with [S35]methionine and [S35]cysteine (175 μCi/ml [35S]Translabel, MP Biomedicals, Inc.) for 24h. 105 HMF, kindly provided by J. Piette (IGM, Montpellier, France), were transiently transfected with 10 μg human LRP1 or Luc siRNAs using Oligofectamine (Invitrogen). The endocytosis of labelled pro-cath-D was performed 48h post-transfection. For this, siRNA-transfected HMF fibroblasts were incubated for 3h (panel a) or 18h (panel c) with conditioned medium containing labelled pro-cath-D with or without 10 mM M6P. Cells were lysed in 50 mM Hepes [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2 1 mM EGTA, 100 mM NaF, 10 mM sodium pyrophosphate, 500 μM sodium vanadate, and a protease inhibitor cocktail (PLC lysis buffer). The endocytosed labelled pro-cath-D was immunoprecipitated in cell lysates with M1G8 anti-cath-D antibody, that recognizes 52 kDa pro-cath-D, and endosomal 48 kDa and lysosomal 34 kDa cath-D forms processed after endocytosis, and analyzed by SDS-PAGE (panels a and c). The LRP1β expression was monitored 48h post-transfection by immunoblotting with 11H4 hybridoma directed against the C-terminal part of LRP1β chain (ATCC) in HMF fibroblasts transfected with either Luc or LRP1 siRNAs (panels b and d). βactin (Sigma) was used as a loading control. Quantification of the pro-cath-D internalized by LRP1 siRNA1 HMF cells, reflected by the presence of the 52, 48 and 34 kDa bands by immunoblotting, was compared to the pro-cath-D internalized by Luc siRNA HMF cells incubated with M6P after 18h of endocytosis (panel e; mean ± SD of 3 independent experiments). *, p<0.025 versus Luc siRNA HMF cells; **, p<0.0025 versus Luc siRNA HMF cells (Student’s t-test). Duplexes of 21-nucleotide human LRP1 siRNA1 (target sequence AAGCAGTTTGCCTGCAGAGAT, residues 666-684) (Li et al 2003), or firefly luciferase (Luc) siRNA (target sequence AACGTACGCGGAATACTTCGA) were synthesized by MWG Biotech S.A. (France).
(B) Endocytosis of secreted pro-cath-D by LRP1 in RM6P−/− MEF fibroblasts. 2x106 RM6P−/− MEF cells, deficient for both RM6P300 and RM6P46, kindly provided by Prof Kurt Von Figura (Georg-August-University Göttingen, Germany), were transiently transfected with 3 μg Luc siRNA or LRP1 siRNA2 using Nucleofector (AMAXA) with or without 10 μg pcDNA3.1(+)Myc-tagged LRP1β chain (Barnes et al 2003). The endocytosis of labelled pro-cath-D was triggered 24h post-transfection by incubating transfected RM6P−/− MEF cells for 3h (panel a, left) or 18h (panel a, right) with conditioned medium containing labelled pro-cath-D as in (A) without M6P. The LRP1β in RM6P−/− MEF cells was monitored 24h post-transfection by immunoblotting, as in (A) (panel b). Mobility of endogenous LRP1β and transfected Myc-LRP1β was slightly different (panel b). Quantification of the pro-cath-D internalized by LRP1 siRNA2 RM6P−/− MEF cells was compared to the pro-cath-D internalized by Luc siRNA RM6P−/− MEF cells incubated with M6P after 18h of endocytosis (panel c; mean of 2 independent experiments). Duplexes of 21-nucleotide mouse LRP1 siRNA2 (target sequence AAGCAUCUCAGUAGACUAUCA) (Fears et al 2005) was synthesized by MWG Biotech S.A. (France).
(C) Endocytosis of secreted pro-cath-D by LRP1 in LRP1−/− MEF cells transfected with full-length LRP1 or LRP1β chain. LRP1−/− MEF cells stably transfected with full-length LRP1 kindly provided by Prof Dudley Strickland (University of Maryland School of Medicine, Baltimore, USA) (clone B-41) (panel a), or LRP1−/− MEF cells transiently transfected with Myc-LRP1β (panel c), were incubated for 3h with conditioned medium containing labelled pro-cath-D plus 10 mM M6P and endocytosed pro-cath-D was analysed as in (A). The LRP1β in clone B-41 (panel b) and in Myc-LRP1β transiently transfected LRP1−/− MEF cells (panel d) was monitored by immunoblotting as in (A).
Pro-cath-D and ectopic cath-D do not modulate LRP1β chain tyrosine phosphorylation in fibroblasts
The LRP1 at the plasma membrane is located in clathrin-coated pits and lipid rafts (Boucher et al 2002, Wu and Gonias 2005, Zhang et al 2004), and it has been suggested that there are LRP1-induced signal transduction pathways triggered by tyrosine phosphorylation or RIP in lipid rafts (Boucher et al 2002, von Arnim et al 2005, Wu and Gonias 2005). We observed that LRP1β overproduction directs pro-cath-D to the lipid rafts (Beaujouin et al 2010), suggesting that cath-D modulates the tyrosine phosphorylation of LRP1, as shown for the PDGF-BB (Boucher et al 2002, Boucher and Gotthardt 2004, Loukinova et al 2002, Newton et al 2005) and CTGF (Yang et al 2004) growth factors, the tPA serine protease (Hu et al 2006), and in fibroblasts transformed with v-Src (Barnes et al 2001, Barnes et al 2003). We next investigated the effect of pro-cath-D on the tyrosine phosphorylation of LRP1β in fibroblasts. HMF fibroblasts were incubated with conditioned medium containing secreted pro-cath-D (15 nM) (Fig. 2A). Immunoblotting of anti-LRP1β immunoprecipitates with anti-phospho-tyrosine antibodies showed that pro-cath-D did not enhance the tyrosine phosphorylation of LRP1β (Fig. 2A, panel a). As a positive control, LRP1β was strongly tyrosine phosphorylated when cellular protein-tyrosine phosphatases were inhibited with orthovanadate and hydrogen peroxide (Fig. 2A, panel a). Moreover, anti-LRP1β blots showed that the mobility of LRP1β was very different following treatment with orthovanadate and hydrogen peroxide (Fig. 2A, panel b). In contrast, the mobility of LRP1β was unchanged in the presence of pro-cath-D (Fig. 2A, panel b), indicating that secreted pro-cath-D did not influence the tyrosine phosphorylation of LRP1β. Similar results were obtained with HMF cells treated with recombinant pro-cath-D (15 nM) (Fig. 2B). We finally assessed the tyrosine phosphorylation of LRP1β in the presence of ectopic cath-D in cath-D−/− MEF fibroblasts stably transfected with empty (CD55−/− SV40) or cath-D (CD55−/− cath-D) vectors. Anti-LRP1β blots of anti-LRP1β immunoprecipitates showed that the mobility of LRP1β was altered in CD55−/− cath-D cells treated with orthovanadate and hydrogen peroxide (Fig. 2C). However, the mobility of LRP1β in CD55−/− cath-D cells was the same as that of CD55−/− SV40 cells (Fig. 2C), indicating that LRP1β was not tyrosine phosphorylated in cells producing ectopic cath-D. We therefore conclude that secreted pro-cath-D and ectopic cath-D do not modulate the tyrosine phosphorylation of LRP1β chain in fibroblasts.
Figure 2. Pro-cath-D and ectopic cath-D do not modulate tyrosine phosphorylation of LRP1β in fibroblasts.
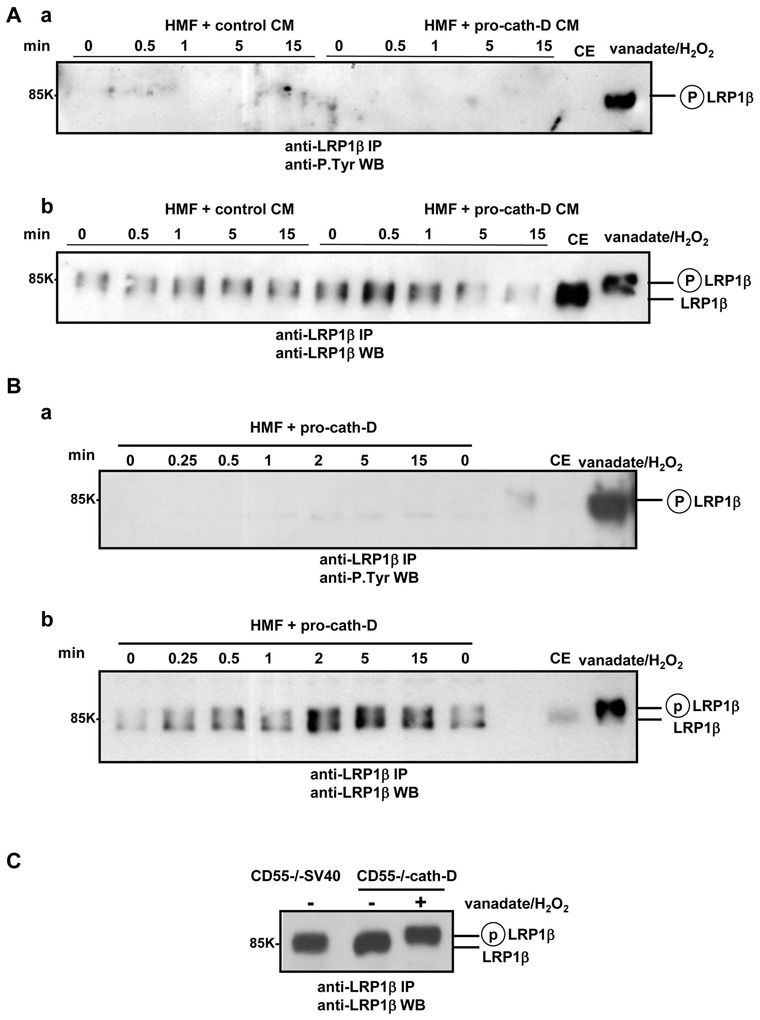
(A) Tyrosine phosphorylation of LRP1β in HMF fibroblasts treated with conditioned medium containing secreted pro-cath-D. COS cells were transiently transfected with 10 μg pcDNA3.1(−)cath-D, or pcDNA3.1(−) plasmids using Lipofectamine (Gibco-BRL). pcDNA3.1(−)cath-D expression plasmid encoding human pre-pro-cath-D has previously been described (Berchem et al 2002, Glondu et al 2001, Glondu et al 2002, Hu et al 2008, Laurent-Matha et al 2005, Vignon et al 1986). Conditioned medium (CM) containing pro-cath-D (15 nM) or not (control) was produced 24h post-transfection by incubating cath-D- or empty vector-transfected COS cells for another 24h in the absence of serum. HMF cells were treated with control or pro-cath-D conditioned medium for the indicated times in the absence of serum. As a positive control of LRP1β tyrosine phosphorylation, cells were treated for 15 min with orthovanadate and hydrogen peroxide (vanadate/H2O2). LRP1β was immunoprecipitated with 11H4 hybridoma in PLC lysis buffer and analysed by immunoblotting using anti-phospho-tyrosine monoclonal antibody 4G10 (Anti-P.Tyr) (Santa Cruz Biotechnology) (panel a) or anti-LRP1β 11H4 hybridoma (panel b). CE, cell extract.
(B) Tyrosine phosphorylation of LRP1β in HMF fibroblasts treated with recombinant pro-cath-D. HMF cells were incubated with 15 nM recombinant pro-cath-D (R&D Systems) for the indicated times in the absence of serum and the tyrosine phosphorylation of LRP1β was analysed as in (A). As a positive control of LRP1β tyrosine phosphorylation, cells were treated for 15 min with vanadate/H2O2.
(C) Phosphorylated LRP1β tyrosine in cath-D-transfected MEF fibroblasts. Tyrosine phosphorylation of LRP1β was analysed in cath-D−/− MEF immortalized mouse fibroblasts stably transfected with empty vector (CD55−/− SV40) or cath-D expression vector encoding human pre-pro-cath-D (CD55−/− cath-D), as previously described (Laurent-Matha et al 2005), as in (A). The positive control of LRP1β tyrosine phosphorylation was CD55−/− cath-D cells treated for 15 min with vanadate/H2O2.
Ectopic cath-D and pro-cath-D inhibits LRP1 RIP in COS cells and in fibroblasts
Since secreted pro-cath-D interacts with the extracellular domain of the LRP1β chain that is cleaved in the first step of RIP, we examined the influence of cath-D on LRP1 RIP. The ectodomain of the LRP1β chain is cleaved by metalloproteinases (Liu et al 2009, Quinn et al 1999, Selvais et al 2009, Selvais et al 2011) and the membrane-associated β-secretase BACE1 (von Arnim et al 2005) during RIP to generate the membrane-associated 25-kDa LRP1-CTF fragment. LRP1β-CTF then becomes a substrate of intramembrane γ-secretases to produce cytoplasmic LRP1β-ICD. This, in turn, diffuses to the nucleus where it modulates transcription (Kinoshita et al 2003, May et al 2002, von Arnim et al 2005, Zurhove et al 2008). As pro-cath-D interacts with the extracellular part of LRP1β at the surface of fibroblasts, we studied the influence of cath-D on the first cleavage of LRP1 RIP (LRP1β-CTF production) (Fig. 3). We used both wild-type and catalytically-inactive cath-D to assess the role of cath-D proteolytic activity. We also measured LRP1β-CTF production in cells treated with the γ-secretase inhibitor DAPT, which blocked the cleavage of LRP1β-CTF into LRP1β-ICD by γ-secretases, in order to analyze the first cleavage of RIP. We first investigated LRP1β-CTF production in COS cells co-transfected with Myc-LRP1β and empty vector (control), cath-D or D231Ncath-D vectors (Fig. 3A, panel a). Co-expression of both Myc-LRP1β and wild-type cath-D or D231Ncath-D resulted in significantly less LRP1β-CTF (Fig. 3A, panel b). Similar experiments using cath-D−/− MEF fibroblasts stably transfected with empty (CD55−/− SV40), wild-type cath-D (CD55−/− cath-D), or D231Ncath-D (CD55−/− D231N) vectors (Fig. 3B, panel a) indicated that both wild-type cath-D and D231Ncath-D significantly inhibited endogenous LRP1β-CTF production (Fig. 3B, panel b). Thus cath-D seems to inhibit RIP by interfering with the first cleavage of LRP1 by a mechanism that is independent of its catalytic activity. We checked these results by investigating the effect of recombinant proteolytically-inactive D231Npro-cath-D on the production of endogenous LRP1β-CTF in HMF fibroblasts (Fig 3C, panel a). Extracellular proteolytically-inactive D231Npro-cath-D significantly inhibited LRP1β-CTF production (Fig 3C, panel b). These findings therefore indicate that pro-cath-D inhibits the first cleavage of LRP1 RIP by a mechanism that is independent of its catalytic activity. As a result we investigated the effect of cath-D and its proteolytically-inactive counterpart on the concentration of LRP1β-ICD. We suspected that this fragment was rapidly degraded in cells, as is LRP1B (Liu et al 2007), because immunoblotting experiments detected no LRP1β-ICD. We therefore used a luciferase reporter assay for LRP1β-ICD production in which the chimeric transcription factor Gal4-VP16 was fused to the carboxy-terminus of LRP1 (May et al 2002, von Arnim et al 2005). Both ectopic cath-D and D231Ncath-D inhibited LRP1β-ICD production in a dose-dependent manner (Fig. 3D, panels a and b). Moreover, anti-cath-D siRNA reversed the cath-D-mediated effect (Fig 3D, panel c). As previously shown (May et al 2002), DAPT also inhibited LRP1β-ICD production (Fig. 3D, panel d). Similar inhibition of LRP1β-ICD production in response to ectopic cath-D was observed in MEF cells (Fig. 3D, panel e). Thus cath-D inhibits LRP1 RIP by a mechanism that is independent of its proteolytic activity, probably by competing with the first RIP cleavage.
Figure 3. Ectopic cath-D and pro-cath-D inhibits LRP1 RIP in COS cells and in fibroblasts.
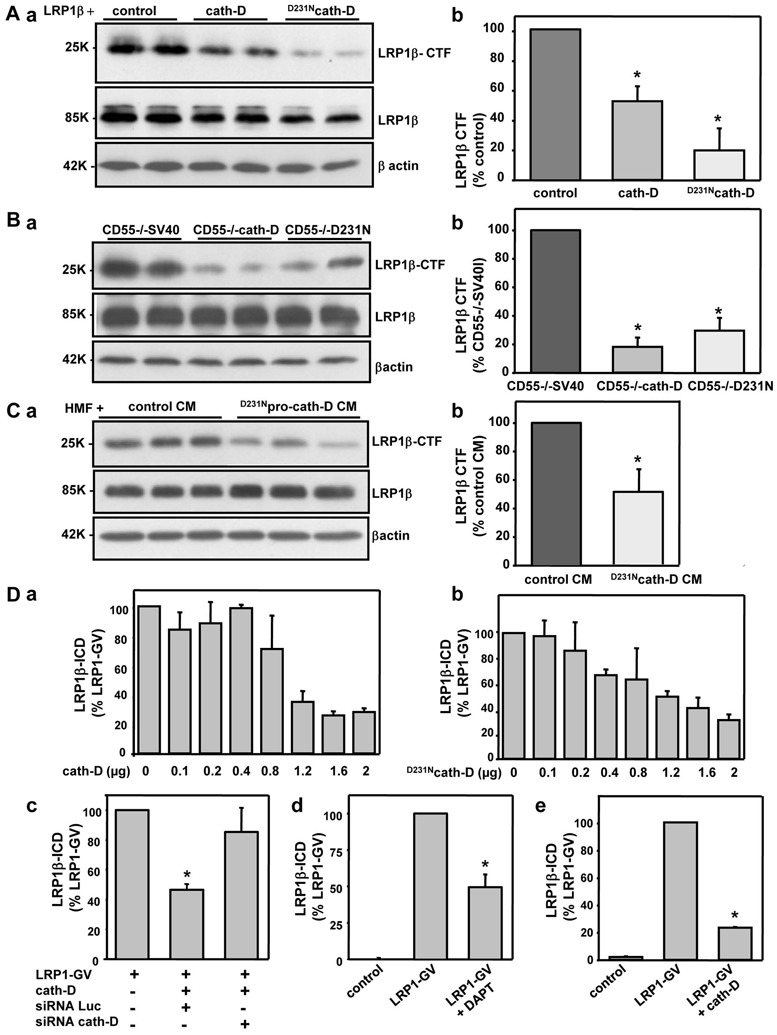
(A) Production of LRP1β-CTF in COS cells co-transfected with cath-D and LRP1β vectors. COS cells were transfected with 10 μg pcDNA3-Myc-LRP1β and 10 μg pcDNA3.1, pcDNA3.1-cath-D, or pcDNA3.1-D231Ncath-D expression vectors. pcDNA3.1(−)cath-D and pcDNA3.1(−)D231Ncath-D expression plasmids encoding human pre-pro-cath-D and pre-pro-D231Ncath-D, respectively, have previously been previously described (Berchem et al 2002, Glondu et al 2001, Glondu et al 2002, Hu et al 2008, Laurent-Matha et al 2005, Vignon et al 1986). 24h post-transfection, cells were incubated with DAPT (20 μM, Sigma) for 24h. Cell extracts in PLC lysis buffer were analysed by immunoblotting with anti-LRP1β 11H4 hybridoma (panel a). Full-length 85-kDa LRP1β and 25-kDa membrane-associated LRP1β-CTF are shown. βactin served as a loading control. Data in panel b are from 3 independent experiments analyzed in duplicate. *, p<0.0005 versus control cells (Student’s t-test).
(B) Production of LRP1β-CTF in MEF fibroblasts stably transfected with cath-D. cath-D−/− MEF cells stably transfected with empty vector (CD55−/− SV40), cath-D (CD55−/− cath-D) or D231Ncath-D (CD55−/−D231N) vectors (Laurent-Matha et al 2005) were treated with DAPT (20 μM) for 24h. Full-length LRP1β and LRP1β-CTF were assayed as in A (panel a). The data in panel b are from 3 independent experiments analyzed in duplicate. *, p<0.0005 versus CD55−/− SV40 cells (Student’s t-test).
(C) Production of LRP1β-CTF in HMF fibroblasts treated with secreted D231Npro-cath-D. COS cells were transiently transfected with 10 μg of pcDNA3.1(−)D231Ncath-D, or pcDNA3.1(−) vectors (Glondu et al 2001) using Lipofectamine (Gibco-BRL). 24h post-transfection, conditioned medium (CM) containing D231Npro-cath-D (15 nM) or unconditioned medium (control) was produced by incubating D231Ncath-D-transfected or empty vector-transfected COS cells for a further 24h in the absence of serum. HMF cells were treated with control or D231Npro-cath-D conditioned medium for 7h in the presence of DAPT (20 μM). Full-length LRP1β and LRP1β-CTF were assayed as in A (panel a). Data from of 2 independent experiments analyzed in triplicate are shown (panel b). *, p<0.025 versus control CM (Student’s t-test).
(D) Production of LRP1β-ICD in COS cells and fibroblasts co-transfected with cath-D and LRP1. COS cells were transiently transfected with pcDNA3.1-LRP1-Gal4-VP16 (LRP1-GV, 0.1 μg), Gal4 DNA binding domain gene reporter (pFR-Luc) (0.4 μg) and CMV-β-galactosidase expression vector (0.4 μg) using Lipofectamine with or without increasing concentrations of pcDNA3-cath-D (panel a) or pcDNA3-D231Ncath-D (panel b) vectors. In rescue experiments, Lipofectamine was used to transiently transfect cells with siRNA cath-D (1 μg) plus cath-D vector (0.4 μg), with LRP1-GV (0.4 μg), pFR-Luc (0.4 μg) and β-galactosidase vector (0.4 μg) (panel c). Human cath-D siRNA (target sequence AAGCUGGUGGACCAGAACAUC, residues 666-684) was described previously (Bidere et al 2003). COS cells were transfected with pFR-Luc and CMV-β-gal with or without LRP1-GV, and plus or minus DAPT (20 μM) for 24h (panel d). MEF cells were transfected using Nucleofector (AMAXA) with LRP1-GV, pFR-Luc and CMV-β-gal with or without cath-D vector (2 μg) (panel e). Luciferase activity was measured 48h later (May et al 2002). The activity of Luciferase relative to β-galactosidase was analysed in triplicate transfections. Mean +/− SD of 3 independent experiments is shown. p<0.005 versus LRP1-GV (Student’s t-test). The vectors used in the LRP1-Gal4-VP16 vector system were kindly provided by D. Strickland (University of Maryland School of Medicine, Baltimore, USA).
Many observations have shown that LRP1 is subject to RIP. A soluble fragment of LRP1, composed of the α chain and the extracellular part of the β chain, has been detected in the blood stream (Quinn et al 1997, Quinn et al 1999). The shedding of the LRP1β-ectodomain is thought to be catalysed by one or more membrane-associated metalloproteases (Liu et al 2009, Quinn et al 1999, Rozanov et al 2004, Selvais et al 2009, Selvais et al 2011) and the membrane-associated β-secretase BACE1 (von Arnim et al 2005). LRP1β-CTF is then cleaved by γ-secretases to give cytoplasmic LRP1β-ICD (May et al 2002). LRP1β-ICD alone is located in the nucleus, and may be associated with the adaptor protein Fe65 and the histone acetyltransferase Tip60 (Kinoshita et al 2003). LRP1β-ICD reduces the transcriptional activity of the APP/Fe65/Tip60 complex in the nucleus, suggesting that it modulates transcription (Kinoshita et al 2003). A few investigations have focused on the biological significance of LRP1β-ICD. Recent studies have suggested that it is involved in ischemic cell death (Polavarapu et al 2008) and the inflammatory response (Zurhove et al 2008). To date, only membrane-associated proteases have been shown to participate in the RIP of LRP1 in an autocrine manner. Interestingly, our findings provide new evidence that LRP1 RIP may be also modulated via a paracrine loop. The pro-cath-D secreted by cancer cells may influence the tumor micro-environment by modulating LRP1 RIP at the fibroblast cell surface.
Cath-D and DAPT inhibit LRP1β-ICD production in human mammary fibroblasts and stimulate HMF fibroblast invasive growth
We have shown that cath-D triggers HMF outgrowth in a LRP1-dependent manner (Beaujouin et al 2010), and the present study indicates that cath-D inhibits LRP1 RIP (Fig. 3). We analysed the interplay between LRP1 RIP and fibroblast outgrowth by evaluating the effect of cath-D and the γ-secretase inhibitor DAPT on LRP1β-ICD production and fibroblast outgrowth in HMF cells. To analyze the role of secreted pro-cath-D on HMF outgrowth, we carried out three-dimensional co-culture assays of HMF co-cultured with cath-D-transfected 3Y1-Ad12 cancer cell lines secreting either no human pro-cath-D (control), or hyper-secreting either human wild-type pro-cath-D (Fig. 4B, panel c). To assess the role of DAPT on HMF outgrowth, 3D culture of HMF was performed in the presence of DAPT (Fig. 4B, panel b). We found that both cath-D and DAPT significantly inhibited LRP1β-ICD production in HMF fibroblasts (Fig. 4A, panels a and b). Cath-D and DAPT also stimulated HMF outgrowth (Fig. 4B, panels a and b, right). Thus inhibiting LRP1β-ICD production may stimulate fibroblast outgrowth. These findings support the hypothesis that LRP1β-ICD restricts fibroblast outgrowth, possibly by regulating transcription. The genes modulated by LRP1β-ICD are presently unknown, although a recent report indicated that LRP1 target genes in the inflammatory response (Zurhove et al 2008). We therefore propose that pro-cath-D hypersecreted by cancer cells triggers fibroblast outgrowth in breast tumor by inhibiting LRP1 RIP (Fig. 4C). It may be particularly pertinent to identify the genes implicated in the control of fibroblast outgrowth that are regulated by LRP1β-ICD and cath-D.
Figure 4. Cath-D and DAPT inhibit LRP1β-ICD production in fibroblasts and stimulate fibroblast outgrowth.
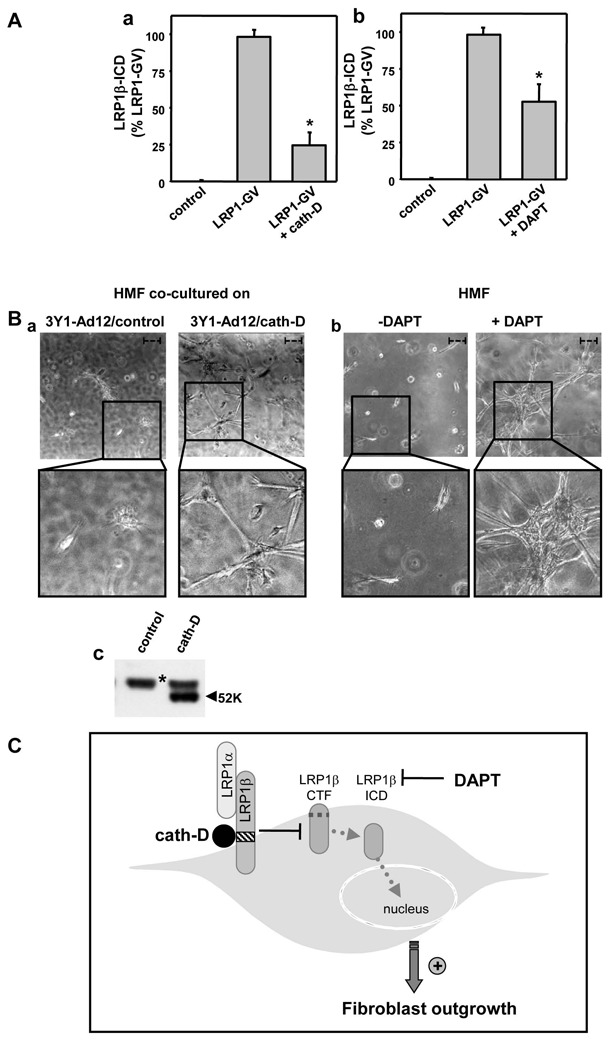
(A) Effect of cath-D and DAPT on LRP1β-ICD production by HMF fibroblasts HMF cells were transiently transfected using Nucleofector (AMAXA) with LRP1-GV, pFR-Luc and CMV-β-gal in the presence or absence of cath-D vector (1.3 μg) as in Fig. 3D (panel a), and were treated with or without DAPT (20 μM) (panel b). Luciferase activity was assayed as described in Fig. 3D. Mean +/− SD of triplicate transfection is shown. p<0.005 versus LRP1-GV (Student’s t-test).
(B) Effect of pro-cath-D and DAPT on HMF fibroblast outgrowth. Phase contrast optical photomicrographs of HMF fibroblasts after 3 days of culture are presented (panels a and b). HMF fibroblasts were embedded in Matrigel with a bottom layer of 3Y1Ad12 cancer cell lines secreting no pro-cath-D (control) (panel a, left), or human wild-type (pro-cath-D) (panel a, right), as previously described (Beaujouin et al 2010). HMF fibroblasts embedded alone in Matrigel were treated with DAPT (20 μM) or not (panel b). High magnifications of the boxed regions are shown below. Bars (---, 50 μm). Pro-cath-D secretion was analyzed after 3 days of co-culture by immunoblotting (panel c). *, un-specific contaminant protein.
(C) Model of action of cath-D and DAPT on LRP1 RIP and fibroblast outgrowth. In RIP, LRP1β chain undergoes ectodomain shedding by membrane-associated proteases (metalloproteases and/or the membrane-associated β-secretase BACE1), generating the membrane-associated LRP1β-CTF fragment. LRP1β-CTF becomes a substrate for intra-membrane cleavage by γ-secretases, producing LRP1β-ICD which is a nuclear transcriptional modulator. We propose that cath-D inhibits RIP-induced LRP1 signaling pathways in a manner independent of its proteolytic activity by competitively inhibiting the first cleavage, leading to decrease LRP1β-CTF production and hence less LRP1β-ICD. The γ-secretase inhibitor DAPT directly blocks LRP1β-ICD production. The reduced concentration of LRP1β-ICD due to cath-D or DAPT inhibition is correlated with stimulation of fibroblast outgrowth, suggesting that the RIP of LRP1 is implicated in the control of fibroblast growth.
Acknowledgments
Grants : This work was supported by the Institut National de la Santé et de la Recherche Médicale, University of Montpellier I, ANR Jeunes Chercheuses, Jeunes Chercheurs and the Ligue Nationale contre le Cancer ; the Association pour la Recherche sur le Cancer provided a fellowship for Mélanie Beaujouin.
We thank Françoise Berthet and Nadia Kerdjadj for secretarial assistance, and Jean-Yves Cance for the photographs. We also thank Vincent Cavaillès for pertinent suggestions for the gene reporter experiments, Hervé Emonard for helpful discussions, and Owen Parkes for English corrections.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Barnes H, Larsen B, Tyers M, van Der Geer P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem. 2001;276:19119–19125. doi: 10.1074/jbc.M011437200. [DOI] [PubMed] [Google Scholar]
- Barnes H, Ackermann EJ, van der Geer P. v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene. 2003;22:3589–3597. doi: 10.1038/sj.onc.1206504. [DOI] [PubMed] [Google Scholar]
- Beaujouin M, Prebois C, Derocq D, Laurent-Matha V, Masson O, Pattingre S, et al. Pro-cathepsin D interacts with the extracellular domain of the beta chain of LRP1 and promotes LRP1-dependent fibroblast outgrowth. J Cell Sci. 2010;123:3336–3346. doi: 10.1242/jcs.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, et al. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5955. doi: 10.1038/sj.onc.1205745. [DOI] [PubMed] [Google Scholar]
- Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, et al. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–31411. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M. LRP and PDGF signaling: a pathway to atherosclerosis. Trends Cardiovasc Med. 2004;14:55–60. doi: 10.1016/j.tcm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Capony F, Morisset M, Barrett AJ, Capony JP, Broquet P, Vignon F, et al. Phosphorylation, glycosylation, and proteolytic activity of the 52-kD estrogen-induced protein secreted by MCF7 cells. J Cell Biol. 1987;104:253–262. doi: 10.1083/jcb.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing, and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904–3909. [PubMed] [Google Scholar]
- Capony F, Braulke T, Rougeot C, Roux S, Montcourrier P, Rochefort H. Specific mannose-6-phosphate receptor-independent sorting of pro-cathepsin D in breast cancer cells. Exp Cell Res. 1994;215:154–163. doi: 10.1006/excr.1994.1327. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Emonard H, Bellon G, de Diesbach P, Mettlen M, Hornebeck W, Courtoy PJ. Regulation of matrix metalloproteinase (MMP) activity by the low-density lipoprotein receptor-related protein (LRP). A new function for an “old friend”. Biochimie. 2005;87:369–376. doi: 10.1016/j.biochi.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fears CY, Grammer JR, Stewart JE, Jr, Annis DS, Mosher DF, Bornstein P, et al. Low-density lipoprotein receptor-related protein contributes to the antiangiogenic activity of thrombospondin-2 in a murine glioma model. Cancer Res. 2005;65:9338–9346. doi: 10.1158/0008-5472.CAN-05-1560. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Scambia G, Bardelli F, Benedetti Panici P, Mancuso S, Messori A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: a meta-analysis. Br J Cancer. 1997;76:661–666. doi: 10.1038/bjc.1997.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens JA, Dall P, Klijn JG, Skroch-Angel P, Claassen CJ, Look MP, et al. Prognostic value of CD44 variant expression in primary breast cancer. Int J Cancer. 1999;84:209–215. doi: 10.1002/(sici)1097-0215(19990621)84:3<209::aid-ijc2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Fusek M, Vetvicka V. Mitogenic function of human procathepsin D: the role of the propeptide. Biochem J. 1994;303 ( Pt 3):775–780. doi: 10.1042/bj3030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V, Hasilik A, von Figura K. Processing of human cathepsin D in lysosomes in vitro. J Biol Chem. 1985;260:3215–3220. [PubMed] [Google Scholar]
- Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H, Liaudet-Coopman E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920–6929. doi: 10.1038/sj.onc.1204843. [DOI] [PubMed] [Google Scholar]
- Glondu M, Liaudet-Coopman E, Derocq D, Platet N, Rochefort H, Garcia M. Down-regulation of cathepsin-D expression by antisense gene transfer inhibits tumor growth and experimental lung metastasis of human breast cancer cells. Oncogene. 2002;21:5127–5134. doi: 10.1038/sj.onc.1205657. [DOI] [PubMed] [Google Scholar]
- Gonias SL, Wu L, Salicioni AM. Low density lipoprotein receptor-related protein: regulation of the plasma membrane proteome. Thromb Haemost. 2004;91:1056–1064. doi: 10.1160/TH04-01-0023. [DOI] [PubMed] [Google Scholar]
- Hass MR, Sato C, Kopan R, Zhao G. Presenilin: RIP and beyond. Semin Cell Dev Biol. 2009;20:201–210. doi: 10.1016/j.semcdb.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- Hu L, Roth JM, Brooks P, Luty J, Karpatkin S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008;68:4666–4673. doi: 10.1158/0008-5472.CAN-07-6276. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003;278:41182–41188. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- Laurent-Matha V, Lucas A, Huttler S, Sandhoff K, Garcia M, Rochefort H. Procathepsin D interacts with prosaposin in cancer cells but its internalization is not mediated by LDL receptor-related protein. Exp Cell Res. 2002;277:210–219. doi: 10.1006/excr.2002.5556. [DOI] [PubMed] [Google Scholar]
- Laurent-Matha V, Maruani-Herrmann S, Prebois C, Beaujouin M, Glondu M, Noel A, et al. Catalytically inactive human cathepsin D triggers fibroblast invasive growth. J Cell Biol. 2005;168:489–499. doi: 10.1083/jcb.200403078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu W, Bu G. Essential role of the low density lipoprotein receptor-related protein in vascular smooth muscle cell migration. FEBS Lett. 2003;555:346–350. doi: 10.1016/s0014-5793(03)01272-9. [DOI] [PubMed] [Google Scholar]
- Liaudet-Coopman E, Beaujouin M, Derocq D, Garcia M, Glondu-Lassis M, Laurent-Matha V, et al. Cathepsin D: newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Lett. 2006;237:167–179. doi: 10.1016/j.canlet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Liu CX, Ranganathan S, Robinson S, Strickland DK. gamma-Secretase-mediated release of the low density lipoprotein receptor-related protein 1B intracellular domain suppresses anchorage-independent growth of neuroglioma cells. J Biol Chem. 2007;282:7504–7511. doi: 10.1074/jbc.M608088200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Tran H, Verbeek MM, Reiss K, Estus S, et al. LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol Neurodegener. 2009;4:17. doi: 10.1186/1750-1326-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, et al. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF. J Biol Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39:219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem. 2007;101:517–529. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- Montel V, Gaultier A, Lester RD, Campana WM, Gonias SL. The low-density lipoprotein receptor-related protein regulates cancer cell survival and metastasis development. Cancer Res. 2007;67:9817–9824. doi: 10.1158/0008-5472.CAN-07-0683. [DOI] [PubMed] [Google Scholar]
- Newton CS, Loukinova E, Mikhailenko I, Ranganathan S, Gao Y, Haudenschild C, et al. Platelet-derived growth factor receptor-beta (PDGFR-beta) activation promotes its association with the low density lipoprotein receptor-related protein (LRP). Evidence for co-receptor function. J Biol Chem. 2005;280:27872–27878. doi: 10.1074/jbc.M505410200. [DOI] [PubMed] [Google Scholar]
- Ohri SS, Vashishta A, Proctor M, Fusek M, Vetvicka V. Depletion of procathepsin D gene expression by RNA interference: a potential therapeutic target for breast cancer. Cancer Biol Ther. 2007;6:1081–1087. doi: 10.4161/cbt.6.7.4325. [DOI] [PubMed] [Google Scholar]
- Ohri SS, Vashishta A, Proctor M, Fusek M, Vetvicka V. The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int J Oncol. 2008;32:491–498. [PubMed] [Google Scholar]
- Polavarapu R, An J, Zhang C, Yepes M. Regulated intramembrane proteolysis of the low-density lipoprotein receptor-related protein mediates ischemic cell death. Am J Pathol. 2008;172:1355–1362. doi: 10.2353/ajpath.2008.070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- Quinn KA, Pye VJ, Dai YP, Chesterman CN, Owensby DA. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP) Exp Cell Res. 1999;251:433–441. doi: 10.1006/excr.1999.4590. [DOI] [PubMed] [Google Scholar]
- Rijnboutt S, Kal AJ, Geuze HJ, Aerts H, Strous GJ. Mannose 6-phosphate-independent targeting of cathepsin D to lysosomes in HepG2 cells. J Biol Chem. 1991;266:23586–23592. [PubMed] [Google Scholar]
- Rochefort H, Liaudet-Coopman E. Cathepsin D in cancer metastasis: a protease and a ligand. Apmis. 1999;107:86–95. doi: 10.1111/j.1699-0463.1999.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Vazquez J, Corte MD, Lamelas M, Bongera M, Corte MG, et al. Clinical significance of cathepsin D concentration in tumor cytosol of primary breast cancer. Int J Biol Markers. 2005;20:103–111. doi: 10.1177/172460080502000204. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Hahn-Dantona E, Strickland DK, Strongin AY. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J Biol Chem. 2004;279:4260–4268. doi: 10.1074/jbc.M311569200. [DOI] [PubMed] [Google Scholar]
- Selvais C, Gaide Chevronnay HP, Lemoine P, Dedieu S, Henriet P, Courtoy PJ, et al. Metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 ectodomain decreases endocytic clearance of endometrial matrix metalloproteinase-2 and -9 at menstruation. Endocrinology. 2009;150:3792–3799. doi: 10.1210/en.2009-0015. [DOI] [PubMed] [Google Scholar]
- Selvais C, D’Auria L, Tyteca D, Perrot G, Lemoine P, Troeberg L, et al. Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J. 2011 doi: 10.1096/fj.10-169508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost. 2003;1:1663–1670. doi: 10.1046/j.1538-7836.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- Vashishta A, Ohri SS, Proctor M, Fusek M, Vetvicka V. Ribozyme-targeting procathepsin D and its effect on invasion and growth of breast cancer cells: an implication in breast cancer therapy. Int J Oncol. 2007;30:1223–1230. [PubMed] [Google Scholar]
- Vetvicka V, Vektvickova J, Fusek M. Effect of human procathepsin D on proliferation of human cell lines. Cancer Lett. 1994;79:131–135. doi: 10.1016/0304-3835(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Vignon F, Capony F, Chambon M, Freiss G, Garcia M, Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986;118:1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, et al. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- Wu L, Gonias SL. The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J Cell Biochem. 2005;96:1021–1033. doi: 10.1002/jcb.20596. [DOI] [PubMed] [Google Scholar]
- Yang M, Huang H, Li J, Li D, Wang H. Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. Faseb J. 2004;18:1920–1921. doi: 10.1096/fj.04-2357fje. [DOI] [PubMed] [Google Scholar]
- Zhang H, Links PH, Ngsee JK, Tran K, Cui Z, Ko KW, et al. Localization of low density lipoprotein receptor-related protein 1 to caveolae in 3T3-L1 adipocytes in response to insulin treatment. J Biol Chem. 2004;279:2221–2230. doi: 10.1074/jbc.M310679200. [DOI] [PubMed] [Google Scholar]
- Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]


