Abstract
Endothelial nitric oxide synthase (eNOS) is a critical modulator of vascular tone and blood flow and plays major roles in liver physiology and pathophysiology. Nitric oxide is widely recognized as one of the key humoral factors important for the initiation of liver regeneration in response to partial hepatectomy. Liver regeneration in response to partial hepatectomy is dependent on the efficiency of growth factor-mediated cell cycle progression. Epidermal growth factor receptor (EGFR) is a critical mediator of multiple hepatic mitogens such as epidermal growth factor, transforming growth factor-α, amphiregulin, and heparin-binding EGF in regenerating livers. However, functional significance of eNOS expressed in hepatocytes and its potential role in EGFR-mediated hepatocyte proliferation remains unexplored. We sought to determine if eNOS is essential for hepatocyte proliferation in response to partial hepatectomy (PH). Our studies with eNOS knockout (eNOS−/−) mice suggest that eNOS activation is essential for efficient induction of early events and elicitation of a robust hepatocyte proliferative response to PH. Moreover, eNOS expression is essential for efficient early induction of matrix metalloprotease-9 (MMP-9), a known mediator of extracellular matrix remodeling and growth factor activation in regenerating livers. Our in vitro studies suggest that eNOS is a critical mediator of EGF-induced hepatocyte proliferation, potentially via its influence on induction of early growth response (Egr-1) and phosphorylation of c-Jun—known mediators of cell cycle progression. EGF-induced eNOS phosphorylation at Ser 1177 is dependent on the phosphorylation and activation of EGFR-PI3K-AKT signaling in hepatocytes. Collectively, these results highlight a hitherto unrecognized role for eNOS activation in hepatocyte proliferation with implications for targeted therapies to enhance liver regenerative response in chronic disorders.
Keywords: liver regeneration, endothelial nitric oxide synthase, epidermal growth factor, hepatocyte proliferation, matrix metalloprotease-9
Despite intensive studies for the past several decades, the molecular identity of key factor(s) that initiate liver regeneration in response to partial hepatectomy remains elusive. Studies with partial hepatectomy of gene-deficient knockout mouse models have uncovered the importance of cytokines and growth factors essential for the stepwise induction of hepatocyte priming and proliferation.[1–2] Nitric oxide is a well known pleiotropic agent influencing multiple aspects of hepatic physiology and pathophysiology and generated via the activation of inducible and endothelial nitric oxide synthase isoforms (iNOS and eNOS), each with distinct modes of activation and co-factor requirements in the liver[3]. Partial hepatectomy and consequent changes in the hemodynamics of remnant livers have led to initial speculations that nitric oxide released in response to elevated shear stress in remnant livers might play a role in the initiation of liver regeneration.[4]
Observations of impaired liver regeneration and hepatocyte survival in iNOS knockout mice provide additional proof for the importance of nitric oxide mediated signaling in liver regeneration.[5] Despite our current understanding of the well-established roles of eNOS in endothelial cell function and vascular physiology, the functional significance of eNOS in liver regeneration has remained largely unexplored. Although first discovered in endothelial cells, many recent studies have confirmed the expression of eNOS in hepatocytes in addition to sinusoidal endothelial cells.[6–10] Primary purpose of this study was, therefore, to determine the functional significance of eNOS expression for efficient hepatocyte proliferation in regenerating livers. Since iNOS mRNA and protein induction in remnant livers does not peak until after several hours after partial hepatectomy[11] and early -events, such as activation of mitogen activated protein kinases (MAPKs) and induction of immediate early gene expression, are detectable in remnant livers within minutes of partial hepatectomy,[1] we reasoned that constitutively expressed eNOS in the endothelial cells and hepatocytes have the capacity to respond instantaneously to an abrupt increase in shear stress in remnant livers—soon after hepatectomy.[12] However, the functional significance of eNOS phosphorylation, activation, and gene expression in liver regeneration is currently unknown. Therefore, a better understanding of eNOS activation and cellular signaling in response to PH will shed new light on possible new therapies targeting liver regeneration.
The findings of this study suggest that eNOS plays a key role in the induction of efficient hepatocyte cell cycle progression and proliferation in response to PH. Early activation of ERK-Egr-1 as well as phospho-c-Jun/AP-1 signaling is critically dependent on eNOS expression in regenerating livers. Moreover, MMP-9 induction in response to PH was attenuated in eNOS−/−, implicating a role for eNOS in extracellular matrix remodeling, essential for latent growth factor activation. Additionally, our in vitro studies provide the first evidence that EGF-mediated proliferation is critically dependent on eNOS expressed in hepatocytes.
Materials and Methods
Animal experiments
C57BL6/J wild-type mice (WT) and eNOS−/− mice in C57BL6/J background were purchased from Jackson lab (Bar Harbor, Maine). Mice were housed in a temperature-controlled animal facility with 12 h light-dark cycles and were maintained on a standard diet and water. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
WT and eNOS−/− adult male mice (10 to 12 weeks) were subjected to 70% PH under light isoflurane anesthesia in the midmorning based on the method described by Higgins and Anderson.[13] Briefly, the left lateral and median lobes were individually ligated and excised, right lateral and caudate lobes (remnant livers) were harvested at various time points (5 min to 8 days). Sham operation consisted of laparotomy and mobilization of liver without ligation or resection of liver lobes.
Western blotting and electrophoretic mobility shift assay (EMSA)
Total protein extracts were obtained by homogenizing liver tissues in total lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 2 mM EDTA, 2 mM EGTA, 1.0% Triton X-100, 0.25% deoxycholate, 1.0 mM phenylmethylsulfonyl fluoride, 1 µg/ml pepstatin, 1.0 µg/ml leupeptin, 1.0 µg/ml aprotinin, 2.0 mM NaF, 2.0 mM activated Na3VO4) and centrifuging at 14,000 rpm for 10 minutes. Nuclear protein extracts were prepared as described previously.[14] Briefly, liver tissues were gently homogenized in lysis buffer (25-mM Tris buffer, pH 7.5 containing 0.5 mM EDTA, 1.5mM MgCl2, 420 mM NaCl and protease inhibitors). Nuclear fractions were isolated by centrifugation at 4000 rpm, and proteins extracted with 25-mM Tris buffer, pH 7.5 containing 1 mM EDTA, 2mM MgCl2, 50 mM KCl, 1 mM DTT and protease inhibitors by incubating at 4° C for 30 minutes and centrifuging at 14,000 rpm for 5 min. A detailed protocol and antibodies used are described in Supplementary Materials and Methods.
Immunohistochemistry
Liver sections were stained for BrdU positive nuclei with the BrdU labeling and detection kit (Roche, Indianapolis, IN) according to manufacturer’s instructions. Ten randomly selected high-power fields (40 X) of liver sections from 4–6 mice per group were analyzed. The number of BrdU positive cells were counted and expressed as a percentage of total number of cells, as visualized by Hematoxylin-Eosin staining.
Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
For evaluation of eNOS gene expression, RNA was isolated from liver tissues harvested at 0.5 to 72h post-PH using Qiagen RNeasy mini kit according to manufacturer’s instructions (Qiagen Sciences, MD). Reverse transcription was performed using 2 µg of total RNA in a first-strand cDNA synthesis reaction with high capacity cDNA reverse transcription kit (Applied Biosystem, Foster city, CA), as recommended by the manufacturer. The cDNA product was amplified by qRT-PCR in ABI prism 7700 sequence-detection system (Applied Biosystem, Foster city, CA) with primers specific for mouse eNOS and cyclophilin described previously.[15]
Hepatocyte isolation and culture
Hepatocytes were isolated from 6–8 week old WT and eNOS−/− male mice by the two-step collagenase perfusion protocol, as described previously with modifications optimized for mice.[16] For hepatocyte proliferation assays, cell preparations with viability over 95% as screened by Trypan Blue exclusion assay, were seeded at a low density of 200,000 cells/35 mm Primaria tissue culture wells (Becton Dickenson Labware, Franklin Lakes, NJ) in Williams E complete media with additives for 3 hrs to ensure hepatocyte adherence to plates. Subsequently, hepatocytes were maintained in Williams E minimal media free from serum and growth factors for 20 hours prior to treatment with growth factors.
Analysis of hepatocyte proliferation in vitro
A detailed protocol of doses and duration of EGF treatment of hepatocytes in vitro and pre-treatment of cell signaling pathway-specific inhibitors, in order to assess the role of eNOS in EGF-mediated mitogenic signaling and proliferation is described in Supplementary Materials and Methods.
Statistical analysis
Data are represented as the mean+/− SD of at least three independent experiments. The statistical significance of difference between groups was analyzed by unpaired Student t test. Values of P<0.05 were considered statistically significant.
Results
Impaired MAPK signaling in eNOS−/− mice after partial hepatectomy
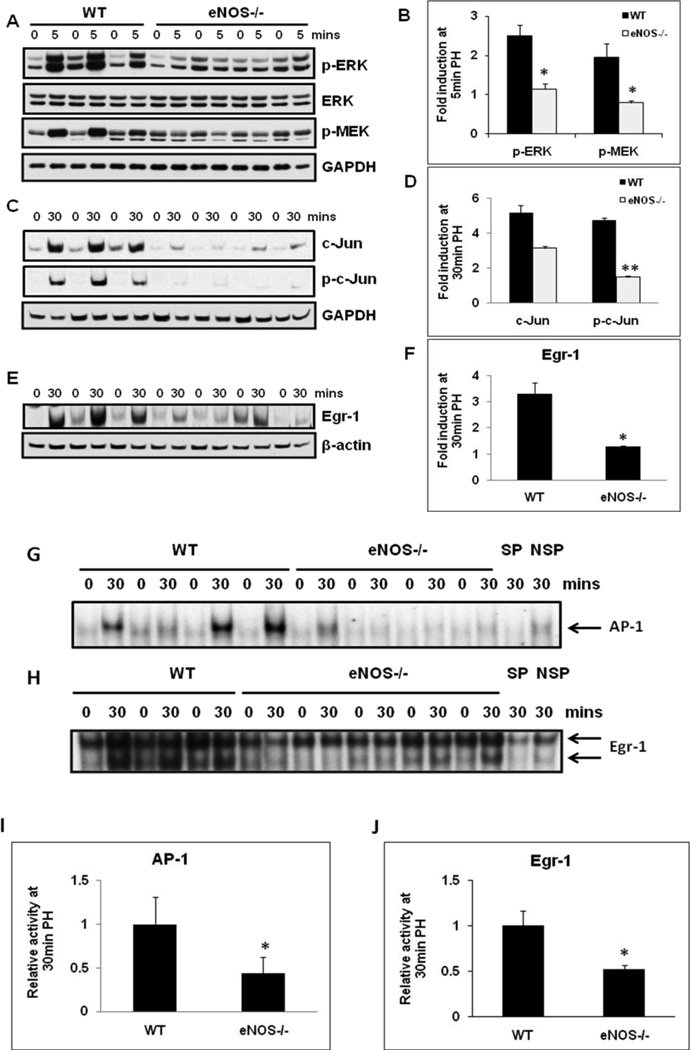
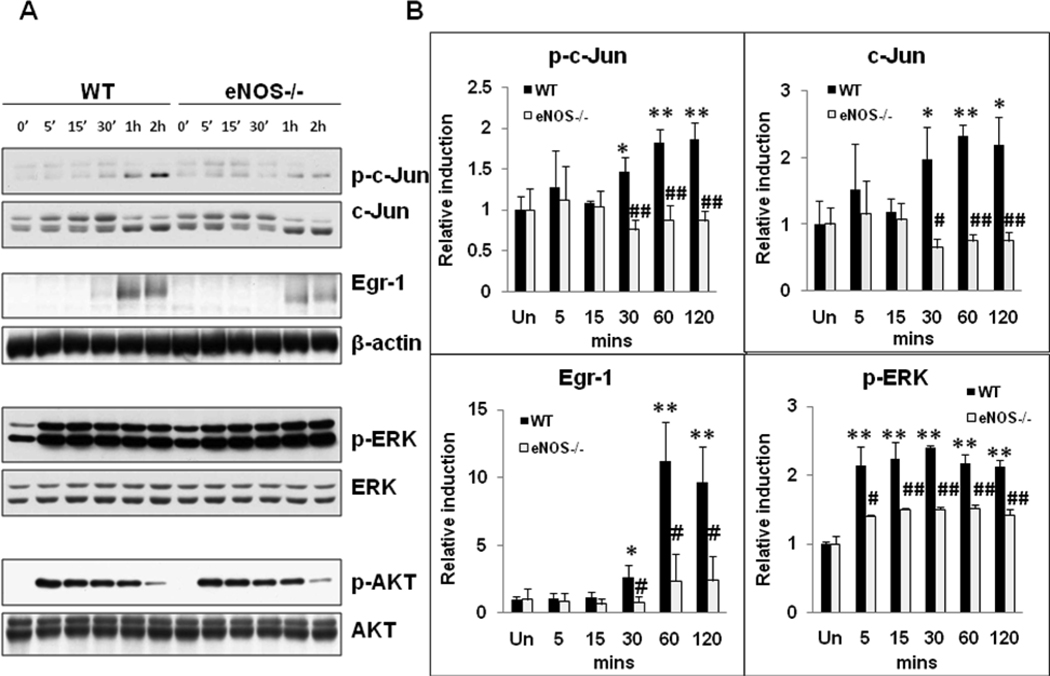
Activation of MAPKs and immediate early genes are hallmarks of early events activated within minutes of PH. In order to evaluate the role of eNOS in the induction of MAPK signaling, total protein extracts isolated from resected lobes (0 min) and remnant livers (5, 30 min post-PH) were analyzed by Western blotting. PH induced robust activation of ERK-MAPK signaling within 5 min of PH (phospho-ERK, 2.5 fold; phospho-MEK, 2.0 fold) in the remnant livers of WT mice. Suggesting a role for eNOS in the early induction of ERK MAPK signaling, activation of ERK MAPK signaling was attenuated in eNOS−/− livers (Fig 1A & B). PH induced immediate early gene c-Jun protein expression and phosphorylation within 30 min in WT livers. However, both c-Jun and phospho-c-Jun induction were attenuated in eNOS−/− livers (Fig.1C & D). Our observations of attenuated early induction of ERK signaling in eNOS−/− mice prompted us to evaluate Egr-1 protein expression (target of ERK signaling) at 30 min post-PH. Mirroring ERK activation, Egr-1 induction at 30 min post-PH was higher in WT mice than that in eNOS−/− mice (3.3 fold in WT vs 1.3 fold in KO mice) (Fig. 1E & F).
Fig. 1. Impaired MAPK signaling in eNOS−/− regenerating livers.
Western blotting for (A) ERK, p-ERK1/2 (Thr202/Tyr204) and p-MEK1/2 (Ser217/221) of total protein extracts of resected lobes (0 min), and remnant livers (5 min post-PH); (C) c-Jun and p-c-Jun (Ser63) of total protein extracts of resected lobes (0 min), and remnant livers (30 min post-PH); (E) Egr-1 of nuclear protein extracts of resected lobes (0 min) and remnant livers (30 min pot-PH); (B, D, F) Fold induction as compared to the respective resected lobes were calculated based on the densitometric analysis of band intensities; (G) EMSA for AP-1 DNA binding activity at 30 min post-PH; (H) EMSA for Egr-1 DNA binding activity at 30 min post-PH; (I, J) Densitometric analysis of AP-1 and Egr-1 DNA binding activity in the remnant livers at 30 min post-PH. The relative DNA-binding activity in remnant livers of eNOS−/− were compared to WT. Data represents mean+/− SD. *P < 0.05; **P < 0.01, vs WT.
AP-1 and Egr-1 DNA binding activity was attenuated in eNOS−/− mice
AP-1 transcriptional activity is induced by growth factors, cytokines, cell-matrix interactions, and a variety of physical and chemical stresses. c-Jun is a component of AP-1 transcription factor, which plays a key role in the regulation of gene expression associated with hepatocyte priming and proliferation. Therefore, AP-1 DNA binding activity of nuclear extracts was determined by EMSA. PH led to induction of AP-1 DNA binding activity in the remnant livers of WT mice. Corresponding to the impairments in the induction of c-Jun and phospho-c-Jun, AP-1 DNA binding activity was attenuated by 57% in eNOS−/− mice (Fig. 1G & I). Egr-1 influences the transcriptional regulation of several genes important for liver regeneration.[17–18] Consistent with impaired ERK and Egr-1 protein induction, Egr-1 DNA-binding activity was also impaired in eNOS−/− mice by 48% at 30min post-PH (Fig 1H & J).
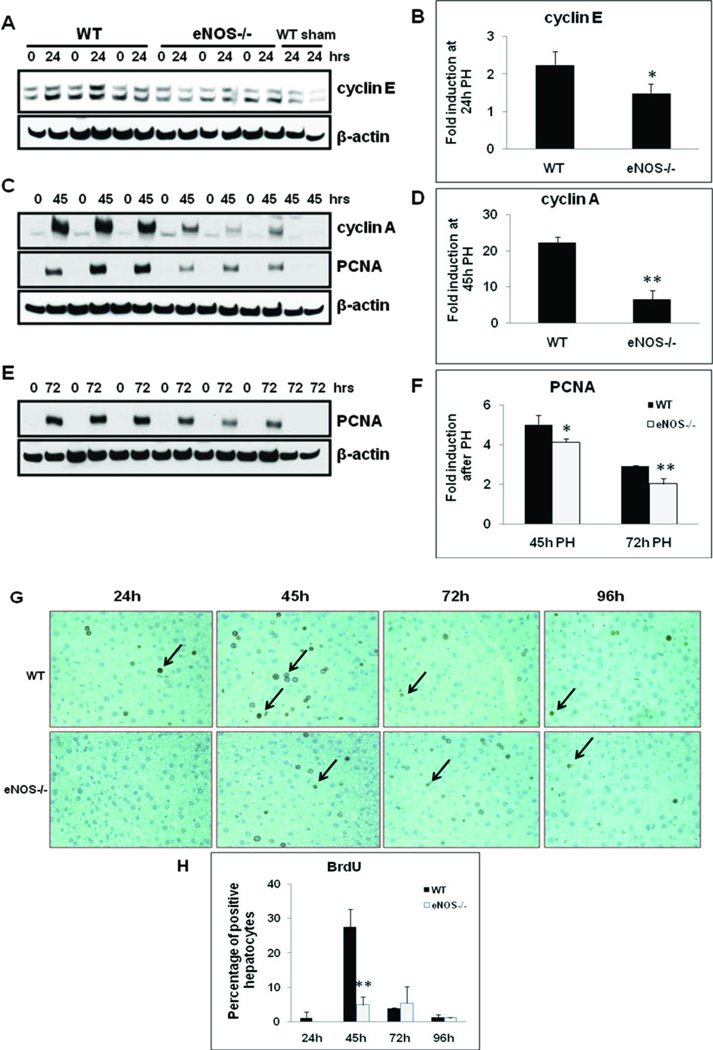
Hepatocyte cell cycle progression in response to PH is impaired in eNOS−/− mice
Early events within minutes of PH help hepatocytes transition from G0 to G1 phase of the cell cycle. In order to determine the role of eNOS in hepatocyte cell cycle progression, induction of key cyclins (cyclins E, G1/S; cyclin A, PCNA, S & G2/M) were analyzed in WT and eNOS−/− livers at 24–72 hours post-PH. As compared to WT, cyclin E induction was impaired in eNOS−/− livers by 34% at 24h post-PH (Fig. 2A & B). PH induced a robust induction of cyclin A protein expression in the remnant livers of WT mice, whereas induction was significantly impaired in eNOS−/− livers with 70% impairment at 45 hrs post-PH (Fig. 2C & D). Correspondingly, the induction of PCNA, a co-factor for DNA replicase and an established marker for DNA replication at S phase, was strongly induced between 45 hours and 72 hours in the WT. In contrast, PCNA induction was significantly attenuated in eNOS−/− with 18% reduction at 45h and 31% reduction at 72h (Fig. 2 C, E & F).
Fig. 2. Impaired hepatocyte cell cycle progression and proliferation in eNOS−/−.
Nuclear protein extracts (20 µg) obtained from livers of WT and eNOS−/− mice subjected to PH were analyzed by Western blotting for (A) cyclin E at 24h; (C) cyclin A and PCNA at 45h; (E) PCNA at 72h. (B, D, F) Fold induction as compared to WT sham at respective timepoints; Data represents mean+/− SD. *P < 0.05; **P < 0.01, vs WT; (G) BrdU immunostaining of liver sections obtained at 24–96h post-PH; (H) BrdU-positive hepatocytes expressed as a percentage of total hepatocytes, by counting 10 high-power fields (40X) of each slide, five animals per group. Data represents mean ± SD, **P < 0.01 versus WT.
Impaired hepatocyte proliferation in eNOS−/− mice
Hepatocyte DNA synthesis was assessed by immunohistochemical analysis for BrdU incorporation from 24–96 hrs post-PH. BrdU-positive hepatocyte nuclei were detectable at 24h and peaked at 45h post-PH in WT livers, with 28 ± 5% BrdU-positive hepatocytes. However, only 5 ± 2% of hepatocytes were BrdU-positive in eNOS−/− mice at 45 h post-PH, 82% fold impairment as compared to WT mice (Fig. 2G & H). At 72 h post-PH, the percentage of BrdU-positive cells was slightly higher in the eNOS−/− mice (4% vs 5%, NS) (Fig. 2 G & H). At 96 h post-PH, BrdU incorporation declined to near basal levels and was comparable between WT and eNOS−/− mice (Fig. 2G & H). Taken together, these results suggest hepatocyte cell cycle progression (Fig 2 A-F) and proliferation (Fig 2G & H) in response to PH is impaired in eNOS−/− mice.
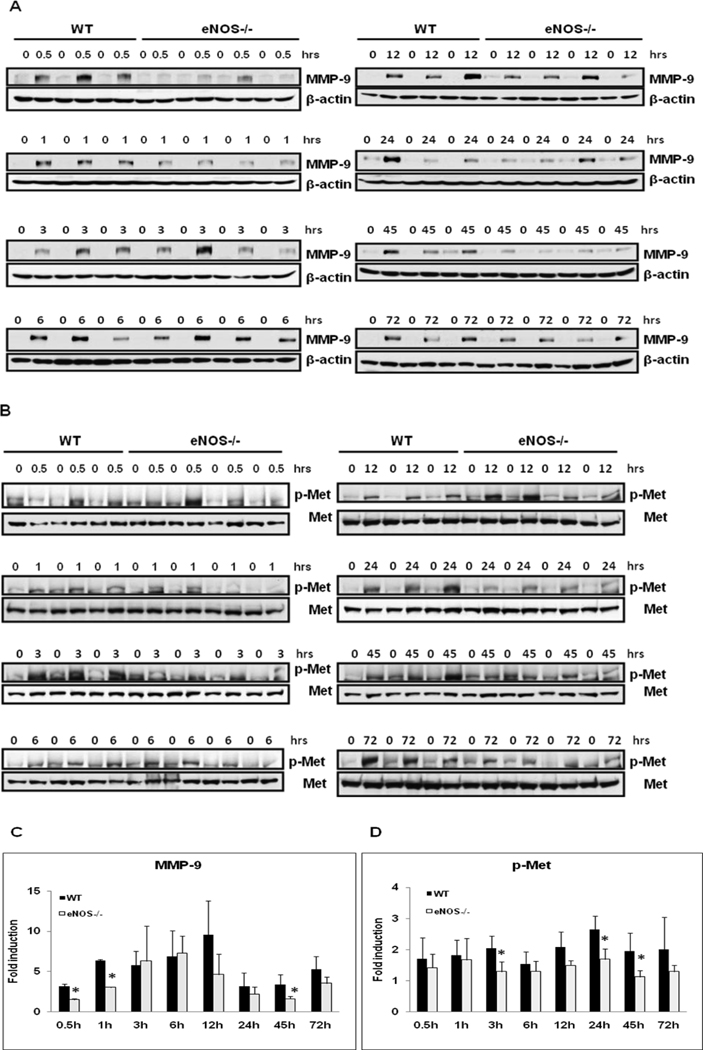
Dysregulation of MMP-9 induction in eNOS−/− mice
Based on the established significance of MMP-9 in extracellular matrix remodeling in regenerating livers, MMP-9 protein expression was analyzed by Western blotting of total liver homogenates of resected lobes (0 min) and remnant livers (0.5–72 hours post-PH).[19] PH induced robust MMP-9 protein expression in WT mice. MMP-9 induction was delayed and significantly attenuated in eNOS−/− mice at 30 min (52.4%), 1 hour (52.1%) and 45 hours (52%) (Fig. 3 A & C). MMP-9 plays a key role in the activation of latent growth factors such as hepatocyte growth factor (HGF). HGF effects on hepatocyte proliferation are mediated via phosphorylation and activation of c-Met, a protooncogene essential for liver regeneration.[20] Corresponding to MMP-9 activation, eNOS−/− regenerating livers exhibit dysregualtion in HGF signaling as evidenced by attenuated induction of c-Met phosphortylation (Tyr1349) at (3 h, 37%; 24 h, 36%; 45 h, 43%) (Fig. 3B & D.)
Fig. 3. Dysregulated MMP-9 Induction and Met phosphorylation in eNOS−/−.
Western blotting of (A) MMP-9 and (B) p-Met (Tyr1349) expression of total protein extracts of resected lobes (0 min) and remnant livers at indicated timepoints post-PH of WT (n=3), eNOS−/− (n=4); (C, D) Densitometric analysis of fold induction as compared to the respective remnant livers. Data represents mean+/− SD. *P < 0.05 vs WT.
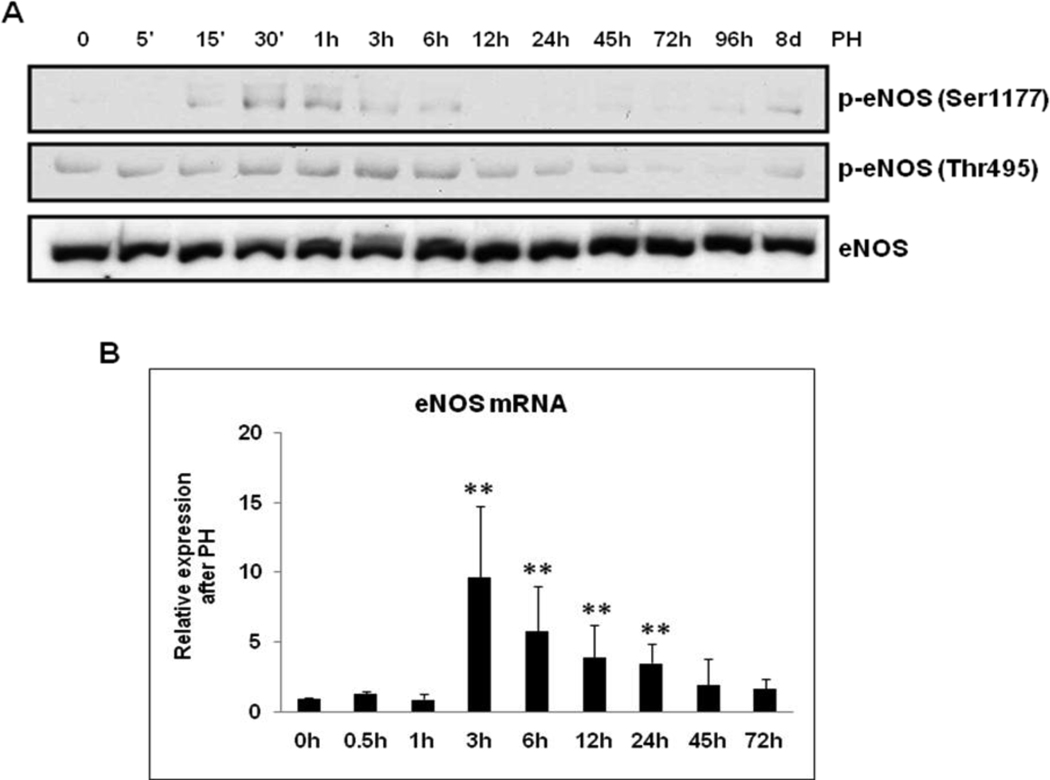
eNOS activation in regenerating livers
Phosphorylation at Ser1177 (activation) and at Thr495 (inhibition) are among the well-characterized post-translational modifications of eNOS.[21] To determine if PH regulates eNOS activity in regenerating livers, eNOS phosphorylation at Ser1177 and Thr495 were analyzed by Western blotting of total lysates. As shown in Fig. 4A, eNOS phosphorylation at Ser1177 was observed early in liver regeneration (15 min to 3 h post-PH; peak at 30 min) and eNOS dephosphorylation at Thr495 was observed later (45 – 96 h post-PH) in WT livers. Total eNOS expression increased slightly after PH.
Fig. 4. eNOS activation in regenerating WT livers.
(A) Total protein extracts of resected lobes (0 min) and remnant livers harvested at indicated time points post-PH were analyzed by Western blotting for the induction of p-eNOS (Ser1177), p-eNOS (Thr495) and eNOS; (B) qRT-PCR analysis of eNOS mRNA expression (n=5). Data represents mean+/− SD. **P < 0.01 vs resected lobes (0 min).
Additionally, eNOS activity can be regulated by transcriptional regulation. To test if PH regulates eNOS mRNA expression, qRT-PCR was performed with RNA isolated from liver tissues (resected and remnant livers at post-PH). eNOS gene expression increased several fold from 3h to 24h, the maximal level was observed at 3h post-PH (7 fold) (Fig. 4B). In order to determine if compensatory iNOS induction plays any role in eNOS−/− regenerating livers, total proteins and RNA isolated from WT and eNOS−/− regenerating livers (15 min–12 h) were analyzed by Western blotting and qRT-PCR for iNOS expression respectively. Our results suggest iNOS protein and mRNA expression were comparable between the WT and eNOS−/− livers (Suppl. Fig. 1. A, B, C). Moreover, EGF (20 ng/ml) treatment (0 – 2h) alone was not sufficient to induce iNOS protein expression in primary hepatocytes isolated from the WT and eNOS−/− mice (Suppl. Fig 1. D). These findings and previous observations that PH led to hepatocyte apoptosis in iNOS−/− mice prompted us to evaluate apoptosis by terminal deoxynucleotidyltransferase-mediated–UTP nick end labeling (TUNEL) assay [5]. Our results suggest that TUNEL-positive apoptotic nuclei were limited to endothelial cells lining large vessels at 24 and 45h post-PH, and comparable between the WT and eNOS−/− (Suppl. Fig. 2. A, B).
Impaired proliferation in response to EGF in eNOS−/− hepatocytes in vitro
To test if eNOS plays any role in EGF-induced hepatocyte proliferation, primary hepatocytes isolated from WT and eNOS−/− mice were treated with EGF (2–20 ng/ml), as shown in Fig. 5A & B. EGF stimulates hepatocytes proliferation, as determined by the induction of cyclin D1 and PCNA at 24h and 48h, which was attenuated in eNOS−/− hepatocytes. Impaired proliferation in eNOS−/− hepatocytes was further validated by BrdU incorporation assay. Accordingly, BrdU incorporation was significantly impaired in eNOS−/− hepatocytes (Fig. 5C & D). In order to evaluate cell viability/apoptosis, hepatocytes were analyzed by TUNEL assay. Analysis of 10 randomly selected fields of view (20X) revealed that TUNELpositive hepatocytes were less than 2% of total aggregate number of hepatocytes (10 filelds of view) in each experimental group and comparable between the WT and eNOS−/− (Suppl. Fig. 3).
Fig. 5. EGF-mediated proliferation is attenuated in eNOS−/− hepatocytes in vitro.
Primary hepatocytes were maintained in serum-free conditions for 16h prior to EGF treatment. Total protein extracts were analyzed by Western blotting for cyclin D1 and PCNA at (A) 24h; (B) 48h. Relative expression is calculated based on densitometic analysis of band intensities, as compared to WT untreated. (C) BrdU immunostaining after 24h of EGF treatment. (D) Percentage of BrdU positive hepatocytes were calculated based on the analysis of 10 fields of view (20X) per coverslip. Results are representative of experiments with three independent hepatocyte preparations. Data represents mean+/− SD. *P < 0.05, **P < 0.01 vs WT untreated; #P<0.05, ##P<0.01 vs WT treated.
EGF-induced mitogenic signaling is attenuated in eNOS−/− hepatocytes in vitro
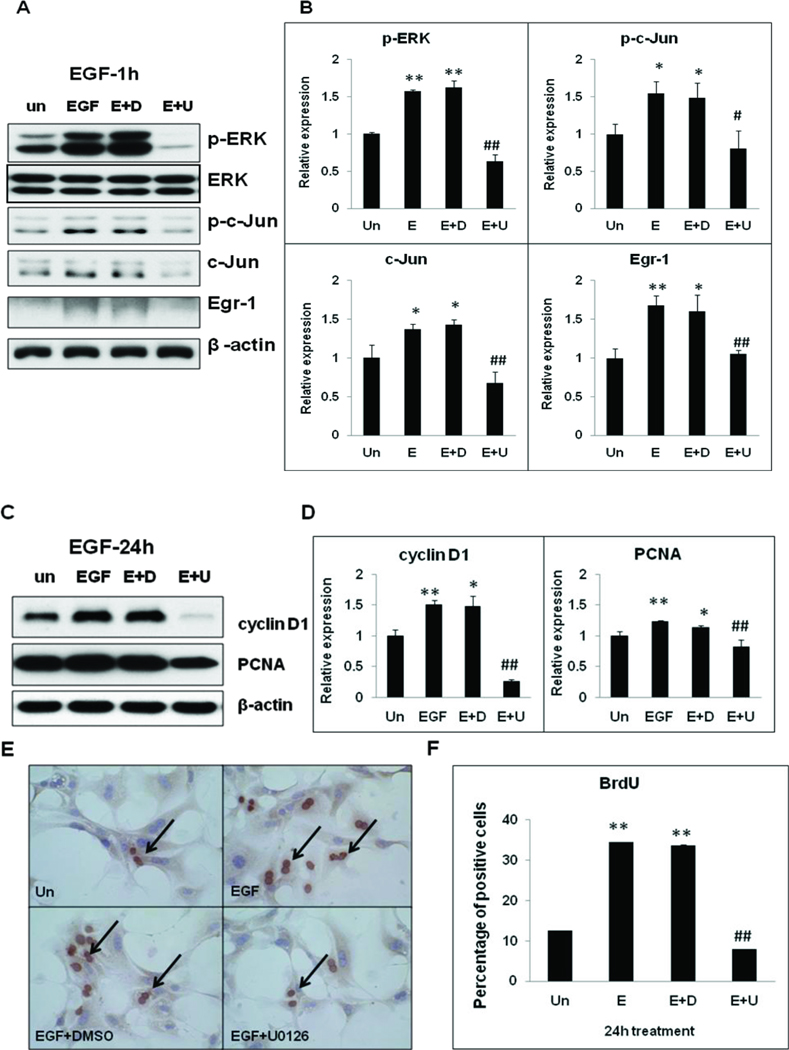
In order to further characterize the role of eNOS in EGF-induced hepatocyte proliferation, primary hepatocytes were treated with EGF (20 ng/ml) for 5 min to 2h, and total protein extracts were analyzed by Western blotting. EGF treatment led to c-Jun phosphorylation (Ser63) and Egr-1 protein expression in hepatocytes with maximal induction observed at 2h (2 fold) and 1h (11 fold, P<0.01) respectively. Interestingly, EGF-induced activation of c-Jun and Egr-1 protein expression was attenuated in eNOS−/− hepatocytes. Moreover, total c-Jun induction was impaired in eNOS−/− hepatocytes. Despite higher basal levels of phospho-ERK in eNOS−/− hepatocytes, EGF-induced ERK activation was attenuated in eNOS−/− hepatocytes at all timepoints tested (5min – 2h) (Fig. 6 A & B).
Figure 6. EGF-mediated mitogenic signaling is attenuated in eNOS−/− hepatocytes in vitro.
Primary hepatocytes were maintained in serum-free conditions for 16h prior to EGF (20 ng/ml) treatment for 5–120 min. (A) Total protein extracts were analyzed by Western blotting for p-c-Jun (Ser63), c-Jun, Egr-1, p-ERK1/2 (Thr202/Tyr204) and p-AKT (Ser473). β-actin, ERK and AKT served as loading controls. Data is representative of experiments performed in triplicate, from three independent hepatocyte isolations. (B) Densitometric analysis of band intensities. Data represents mean+/− SD. *P<0.05, **p<0.01 vs WT untreated; # p<0.05, ## p<0.01 vs WT treated.
In order to evaluate the functional significance of ERK activation in EGF-induced mitogenic signaling, immediate gene expression and cell-cycle progression, primary hepatocytes were treated with MEK/ERK inhibitor (U0126), 30 min prior to EGF treatment. EGF-mediated induction of p-c-Jun, c-Jun and Egr-1 at I hr as well as induction of cell cycle progression (cyclin D1, PCNA, BrdU incorporation at 24 h) were dependent on intact ERK signaling in hepatocytes (Fig. 7 A-F).
Fig. 7. EGF induced hepatocyte proliferation is dependent on intact ERK signaling in vitro.
WT primary hepatocytes maintained in serum-free conditions were treated with U0126 (20 µM) for 30min prior EGF (20 ng/ml) treatment. Total homogenates were analyzed by Western blotting for (A) p-ERK1/2 (Thr202/Tyr204), ERK, p-c-Jun (Ser63), c-Jun and Egr-1 expression at 1h EGF treatment, (C) cyclinD1 and PCNA at 24h EGF treatment. (B, D) Relative expression was calculated based on densitometric analysis of band intensities with reference to untreated. (E) BrdU immunostaining after 24h EGF treatment. (F) Percentage of BrdU positive hepatocytes were calculated based on the analysis of 10 fields of view (20X) per coverslip. Data is representative of experiments performed in triplicate, from three independent hepatocyte isolations. Data represents mean+/− SD. *P<0.05, **p<0.01 vs WT untreated; # p<0.05, ## p<0.01 vs WT EGF treated.
Intact PI3K/AKT signaling is essential for EGF-mediated eNOS phosphorylation and hepatocytes proliferation in vitro
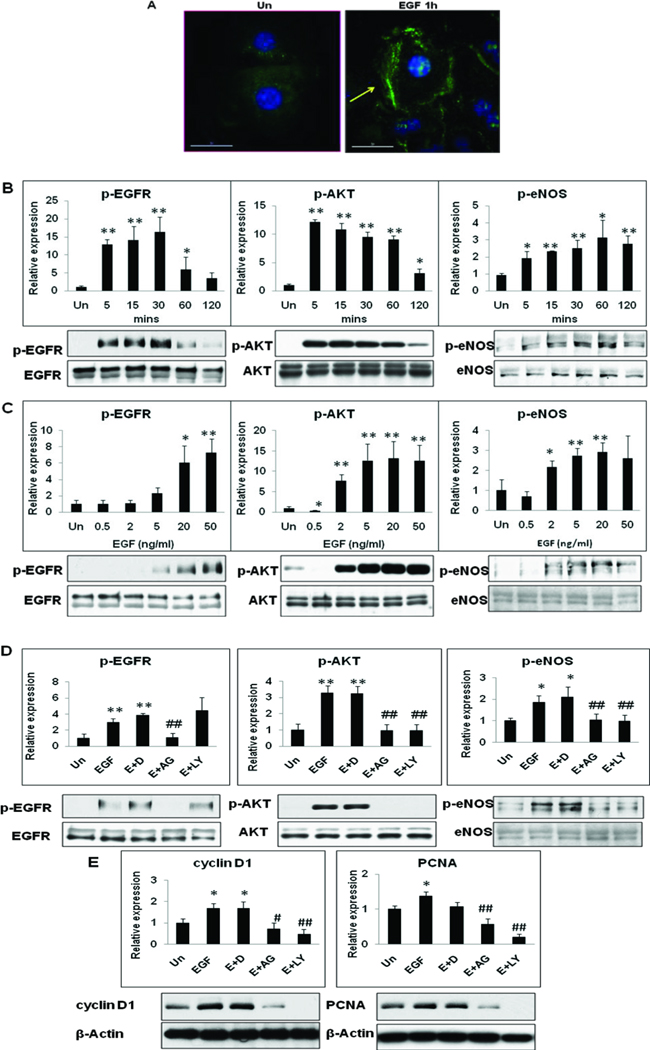
Based on our in vivo observations that PH activates eNOS phosphorylation (Ser1177) in remnant livers, and because it is well established that EGFR activation is critical for efficient hepatocyte proliferation and liver regeneration, we sought to determine if EGF treatment alone was sufficient to induce eNOS phosphorylation in hepatocytes in vitro.[22–23] Immunohistochemical analysis of WT hepatocytes plated on collagen coated coverslips revealed that EGF treatment alone was sufficient to induce eNOS phosphorylation (Fig. 8A). As shown in Fig. 8 B & C, EGF (20 ng/ml) induced a transient and robust p-EGFR Ser1173 expression, with maximal effect at 30 min (16.4 fold). AKT phosphorylation increased dramatically within 5 min of EGF treatment (13 fold). Interestingly, EGF treatment alone was sufficient to induce p-eNOS (Ser1177) expression in hepatocytes, with maximal effect found at 1h (3 fold).
Fig. 8. EGF-mediated eNOS phosphorylation (Ser1177) is dependent on the phosphorylation and activation of EGFR and PI3K/AKT signaling in hepatocytes in vitro.
(A) EGF (1h) treatment induced eNOS phosphorylation (Ser1177) in WT hepatocytes, arrow points to increased immunofluorescence. Western blotting for p-EGFR (Tyr1173), p-AKT (Ser473) and p-eNOS (Ser1177) expression in hepatocytes in response to EGF treatment (B) Time course (20 ng/ml) and (C) Dose-response (1h). Total EGFR, AKT and eNOS served as loading control. (D) Western blotting for p-EGFR (Tyr1173), p-AKT (Ser473) and p-eNOS (Ser1177) expression in WT hepatocytes pre-treated with DMSO (D), AG1478 (AG; 1 µM) or LY294002 (LY; 20 µM) for 30 min prior to EGF (E) treatment for 1h. (E) Western blotting for cyclin D1 and PCNA in hepatocyte pre-treated with inhibitors for 30 min prior to EGF treatment (24 h). Experiments were performed in triplicate with three independent hepatocyte isolations. Data represents mean+/− SD. *P<0.05, **p<0.01 vs WT untreated; # p<0.05, ## p<0.01 vs WT EGF treated.
As expected, pre-treatment with EGFR-kinase inhibitor (AG1578), prior to EGF treatment of hepatocytes resulted in the inhibition of p-EGFR, p-AKT and p-eNOS expression. Interestingly, PI3 kinase inhibitor (LY294002) pre-treatment blocked EGF-induced phosphorylation of AKT (70% decrease) and eNOS (47% decrease) in hepatocytes (Fig. 8 D). Highlighting the importance of EGFR-PI3K-eNOS signaling axis in hepatocyte proliferation, pre-treatment with AG1478 and LY294002 blocked EGF-mediated induction of cyclin D1 and PCNA expression in hepatocytes (Fig. 8E). Furthermore analysis of total liver homogenates of resected and remnant livers by Western blotting for p-AKT and total AKT revealed that AKT activation in repsonse to partial hepatectomy was comparable between WT and eNOS−/− (5 min – 6h) and AKT signaling, a key upstream mediator of eNOS phosphorylation and activation, is intact in eNOS−/− livers (Suppl. Fig. 4).
Discussion
It has been recognized for decades that portal blood flow plays a pivotal role in liver mass restoration following PH. Blood flow to liver mass ratio following two-thirds PH increases dramatically, which results in shear stress-induced NOS activation and NO release. NO can thus serve as a trigger for hepatocyte proliferation.[4] However, the temporal profile of iNOS activation in regenerating livers activation peaks only after 3–6 hours post PH) further validates the current focus on eNOS as a potential mediator of shear stress-induced NO release.[24] Our findings suggest that eNOS activity is subject to both transcriptional and post-translational regulation in regenerating livers. While eNOS expressed in liver sinusoidal endothelial cells and hepatocytes have the capacity to respond to changes in shear stress within minutes of hepatectomy, eNOS activity can also be stimulated via phosphorylation at Ser1177 during the hepatocyte priming phase or via dephosphorylation at Thr495 during peak hepatocyte proliferation and liver regeneration.
Several recent studies suggest that eNOS is expressed in hepatocytes in addition to endothelial cells in the liver. [6–10] However, previous studies with eNOS−/− mice have led to conflicting findings on its potential roles in hepatocyte proliferation in response to PH.[10, 25] Recently, VasquezChantada et al. described a role for LKB1-AMPK in HGF-induced eNOS phosphorylation in hepatocytes and impaired BrdU incorporation in response to PH in eNOS−/−, as compared to WT.[10] Therefore, we undertook a comprehensive approach to determine potential roles of eNOS in hepatocyte proliferation by conducting a systematic analysis of the hepatocyte proliferative response, based on the sequential induction of key cyclins which drive cell cycle progression at G1, G1/S, G2/M phases, as well as BrdU incorporation kinetics over a period of 24–96 hours. Corresponding to early events, hepatocyte cell cycle progression and proliferation in response to PH were significantly attenuated in eNOS−/− livers. Most notably, cyclin E and cyclin A protein induction in response to PH were dependent on eNOS expression. Peak BrdU incorporation reflecting active DNA synthesis, was observed at 45hrs post PH, which was significantly attenuated in eNOS−/− mice.
It should be noted that analysis of BrdU incorporation over extended post-PH time periods confirms lack of compensatory or delayed DNA synthetic responses in eNOS−/− livers and highlights the functional significance of eNOS in hepatocyte proliferation in response to PH. We did not observe any compensatory increase in iNOS protein or mRNA expression in eNOS−/− livers subjected to PH. Our studies confirm previous observations that iNOS isoform does not compensate for eNOS deficiency in regenerating livers.[25] Despite distinct impairments in hepatocyte proliferation, liver growth at 8 days post-PH was comparable between the WT and eNOS−/− livers. During liver regeneration, numerous cytokines, growth factors and transcription factors are activated and function synergistically, which in turn initiate and promote hepatocyte proliferation, angiogenesis, and organ growth.[1,, 2,, 26] Our findings validate the current consensus that multiple redundant mechanisms are in place to ensure liver regeneration in response to hepatic insufficiency and that no single factor is independently sufficient to induce and complete liver regeneration.
Several factors may account for attenuated hepatocyte proliferation in eNOS−/− livers. Firstly, efficient transcriptional activation of cyclins such as cyclin D1, E, and A are dependent on c-Jun/AP-1 activity. Secondly, the ERK signaling cascade is involved in the regulation of G1 phase progression in liver regeneration.[27] The present study reveals a novel role of eNOS-mediated signaling on ERK activation in regenerating livers, as evidenced by impaired MEK/ERK signaling in eNOS−/− mice early (5min) after liver resection. Thirdly, early activation of matrix metalloproteases such as MMP-9 is essential for dissolution of extracellular matrix and proteolytic activation latent growth factors such as HGF.[28–30] Interestingly, our findings suggest that eNOS signaling influences early induction of MMP-9 in regenerating livers, as evidenced by the delay and dysregulation of MMP-9 protein expression in the remnant livers of eNOS−/−.
Efficient hepatocyte cell cycle progression critically depends on the activation of growth factor signaling most notably epidermal growth factor (EGFR).[31] Our in vitro studies suggest that EGF-induced mitogenic signaling and hepatocyte proliferation were significantly attenuated in eNOS−/− hepatocytes. Moreover, EGF treatment alone was sufficient to induce eNOS phosphorylation at Ser1177 in WT hepatocytes, whereas inhibition of EGFR and PI3K-AKT signaling effectively blocked EGF-induced eNOS phosphorylation and hepatocyte proliferation. Our findings suggest that hepatocyte eNOS is a critical mediator of EGF-induced hepatocyte proliferation, potentially via its influence on early induction of Egr-1 and phosphorylation of c-Jun--known mediators of cell cycle progression. EGF-induced eNOS phosphorylation at Ser1177 is dependent on the phosphorylation and activation of EGFR-PI3K-AKT signaling in hepatocytes.
Our in vitro data suggest a modest, but statistically significant attenuation of EGF-mediated induction of ERK signaling in eNOS−/− hepatocytes, in part, as a consequence of elevated basal phospho-ERK level i n e N O S−/− hepatocytes, as compared to WT. Interestingly, HGF-induced activation of ERK signaling in hepatocytes was comparable to EGF (Suppl. Fig. 5). Nevertheless, our in vitro studies with hepatocytes pre-treated with U0126 (MEK1 inhibitor), suggest an important role for MEK-ERK signaling in EGF-induced mitogenic signaling and cell cycle progression in hepatocytes. Moreover, our studies suggest that EGF-treatement alone was not sufficient to induce MMP-9 protein expression in hepatocytes in vitro (data not shown). It is likely that, endothelial cells and other growth factors such as HGF may be responsible for the eNOS-dependent early induction of MMP-9 in regenerating livers. These observations highlight a role for endothelial-eNOS in addition to hepatocyte-eNOS, in the early activation of ERK and MMP-9 expression seen in regenerating livers. The complex interactions between hepatocytes and endothelial cells in eliciting an effective regenerative repsonse to PH are well recognized. Hepatocytes are the first cells to undergo proliferation in response to PH. VEGF-derived from hepatocytes, in turn, activate VEGFR1 in endothelial cells to induce HGF expression (LeCouter et al., 2003). Therefore, it is not surprising to find hepatocyte and endothelial eNOS may have distinct and interdependent redundant roles in order to ensure effective liver regeneration, in response to PH.
In conclusion, the present study highlights the functional significance of eNOS in liver regeneration after partial hepatectomy. Hepatocyte cell cycle progression and proliferation in response to PH is severely impaired in eNOS−/− mice. Impairments in early priming events mediated via the activation MEK-ERK-Egr1 and c-Jun-AP-1 signaling, as well as attenuated induction of MMP-9, may contribute to impaired hepatocyte proliferation in eNOS−/− mice. Induction of phosphorylation as well as transcriptional activation of eNOS in remnant livers further highlights the functional significance of eNOS in liver regeneration. Moreover, our in vitro studies with eNOS−/− hepatocytes suggest that EGF-mediated hepatocyte proliferation is critically dependent on intact EGFR-PI3K/AKT-eNOS signaling. Collectively, these results highlight a hitherto unrecognized role for eNOS activation in hepatocyte proliferation with implications for the development of targeted therapies to enhance liver regenerative response in chronic liver disorders.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by Texas Children’s Hospital Foundation (ST), Public Health Service Grants RO1DK069558 (ST), T32HL007939 (YM), T32DK07644 (YM) and DK56338, which funds the Texas Medical Center Digestive Diseases Center.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- EGFR
epidermal growth factor receptor
- EGF
epidermal growth factor
- HB-EGF
heparin-binding EGF-like growth factor
- VEGF
vascular endothelial growth factor
- PH
partial hepatectomy
- MMP-9
matrix metalloprotease-9
- Egr-1
early growth response-1
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- AP-1
activator protein-1
Footnotes
Disclosures: None
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radomski MW. Nitric oxide: biological mediator, modulator and effector. Ann Med. 1995;27(3):321–329. doi: 10.3109/07853899509002585. [DOI] [PubMed] [Google Scholar]
- 4.Schoen JM, et al. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5(5):453–464. doi: 10.1006/niox.2001.0373. [DOI] [PubMed] [Google Scholar]
- 5.Rai RM, et al. Impaired liver regeneration in inducible nitric oxide synthasedeficient mice. Proc Natl Acad Sci U S A. 1998;95(23):13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gobeil F, Jr., et al. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem. 2006;281(23):16058–16067. doi: 10.1074/jbc.M602219200. [DOI] [PubMed] [Google Scholar]
- 7.Ilan E, Tirosh O, Madar Z. Triacylglycerol-mediated oxidative stress inhibits nitric oxide production in rat isolated hepatocytes. J Nutr. 2005;135(9):2090–2095. doi: 10.1093/jn/135.9.2090. [DOI] [PubMed] [Google Scholar]
- 8.Koti RS, et al. Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. Faseb J. 2005;19(9):1155–1157. doi: 10.1096/fj.04-3220fje. [DOI] [PubMed] [Google Scholar]
- 9.McNaughton L, et al. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A. 2002;99(26):17161–17166. doi: 10.1073/pnas.0134112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez-Chantada M, et al. Evidence for LKB1/AMP-activated protein kinase/ endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology. 2009;49(2):608–617. doi: 10.1002/hep.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Guerra MJ, et al. Nuclear factor kappaB is required for the transcriptional control of type II NO synthase in regenerating liver. Biochem J. 1997;326(Pt 3):791–797. doi: 10.1042/bj3260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales E, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 52(1):54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arab JP, et al. Mild hypothermia does not affect liver regeneration after partial hepatectomy in mice. Liver Int. 2009;29(3):344–348. doi: 10.1111/j.1478-3231.2008.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe-Seyler F, et al. A rapid microscale procedure for the simultaneous preparation of cytoplasmic RNA, nuclear DNA binding proteins and enzymatically active luciferase extracts. Nucleic Acids Res. 1991;19(18):5080. doi: 10.1093/nar/19.18.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai MS, et al. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 51(6):2097–2107. doi: 10.1002/hep.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thevananther S, et al. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39(2):393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 17.Mueller L, et al. The induction of the immediate-early-genes Egr-1, PAI-1 and PRL-1 during liver regeneration in surgical models is related to increased portal flow. J Hepatol. 2002;37(5):606–612. doi: 10.1016/s0168-8278(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, et al. Mitogenic up-regulation of the PRL-1 protein-tyrosine phosphatase gene by Egr-1. Egr-1 activation is an early event in liver regeneration. J Biol Chem. 1999;274(8):4513–4520. doi: 10.1074/jbc.274.8.4513. [DOI] [PubMed] [Google Scholar]
- 19.Olle EW, et al. Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44(3):540–549. doi: 10.1002/hep.21314. [DOI] [PubMed] [Google Scholar]
- 20.Borowiak M, et al. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101(29):10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, et al. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280(4):2562–2568. doi: 10.1074/jbc.M412372200. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104(43):17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoen JM, Lautt WW. iNOS is not involved in shear stress-induced nitric oxide release, which triggers the liver regeneration cascade. Proc West Pharmacol Soc. 2001;44:181–182. [PubMed] [Google Scholar]
- 25.Shergill U, et al. Inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration. Am J Physiol Regul Integr Comp Physiol. 298(5):R1279–R1287. doi: 10.1152/ajpregu.00836.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 27.Dixon M, et al. Inhibition of rat hepatocyte proliferation by transforming growth factor beta and glucagon is associated with inhibition of ERK2 and p70 S6 kinase. Hepatology. 1999;29(5):1418–1424. doi: 10.1002/hep.510290516. [DOI] [PubMed] [Google Scholar]
- 28.Stolz DB, et al. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59(16):3954–3960. [PubMed] [Google Scholar]
- 29.Kim TH, et al. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31(1):75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 30.Mohammed FF, et al. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41(4):857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 31.Scheving LA, et al. Integral role of the EGF receptor in HGF-mediated hepatocyte proliferation. Biochem Biophys Res Commun. 2002;290(1):197–203. doi: 10.1006/bbrc.2001.6157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.