Abstract
Aldehyde dehydrogenase 3A1 (ALDH3A1) is a recently characterized corneal crystallin with its exact functions still being unclear. Expressing recombinant human ALDH3A1 has been difficult in Escherichia coli (E. coli) because of low solubility, yield and insufficient purity issues. In this report, we compared different E. coli expression strategies (namely the maltose binding protein; MBP- and the 6-his-tagged expression systems) under conditions of auto-induction and co-expression with E. coli’s molecular chaperones where appropriate. Thus, we aimed to screen the efficiency of these expression strategies in order to improve solubility of recombinant ALDH3A1 when expressed in E. coli. We showed that the MBP- tagged expression in combination with lower-temperature culture conditions resulted in active soluble recombinant ALDH3A1. Expression of the fused 6-his tagged-ALDH3A1 protein resulted in poor solubility and neither lowering temperature culture conditions nor the auto-induction strategy improved its solubility. Furthermore, higher yield of soluble, active native form of 6-his tagged-ALDH3A1 was facilitated through co-expression of the two groups of E. coli’s molecular chaperones, GroES/GroEL and DnaK/DnaJ/GrpE. Convenient one step immobilized affinity chromatography methods were utilized to purify the fused ALDH3A1 hybrids. Both fusion proteins retained their biological activity and could be used directly without removing the fusion tags. Taken together, our results provide a rational option for producing sufficient amounts of soluble and active recombinant ALDH3A1 using the E. coli expression system for conducting functional studies towards elucidating the biological role(s) of this interesting corneal crystallin.
Introduction
Human crystallin ALDH3A1 is an NADP(+)-dependent enzyme existing as a dimer of 54 kDa subunits. The protein is contained in high amounts in the cornea of mammals ranging between 5–50% of the total water-soluble protein content (depending on species) but is almost absent in the cornea of other species [1]–[3]. Similar to other catalytically active enzymes recruited as taxon-specific corneal crystallins [4], ALDH3A1 is a metabolic enzyme catalyzing the oxidation of aldehydes to their corresponding acids demonstrating high substrate specificity for medium-chain saturated and un-saturated aldehydes [5]. Its metabolic activity appears to be related with the protein’s protective role in corneal epithelium against oxidative damage caused by aldehyde by-products of lipid peroxidation under conditions of cellular stress, e.g. UV-induced oxidative stress [6], [7]. However, constitutive expression of ALDH3A1 in the mammalian cornea exceeds, by far, the levels required for a pure metabolic function. Thus, other potential roles are being explored including: (i) generation of the antioxidant NADPH [8], (ii) direct absorption of UV-energy [9], (iii) scavenging of reactive oxygen species (ROS) [10] and (iv) possession of chaperone-like activity [3], suggesting that ALDH3A1 may contribute to the optical properties of the cornea as well [1], [3]. Furthermore, its absence has been linked with cataract phenotype and ocular oxidative damage in ALDH3A1-null mice [11], whereas recent studies implicate its involvement in cell homeostatic pathways, such as apoptosis, cell cycle regulation, proteasome degradation and DNA damage response [6], [7], [11], [12].
The purpose of the present study was to employ E. coli recombinant methods in order to produce substantial amounts of human ALDH3A1 with the aim to direct future studies towards elucidating the biological functions of ALDH3A1. To this end, E. coli is the preferred organism for heterologous protein expression due to its many advantages including: (i) the ability to grow quickly into high cell densities, (ii) the requirement of non expensive carbon sources and (iii) its extensively studied physiology [13], [14]. In addition, the wider range of commercial products available for all steps of expression and purification using E. coli makes this system even more operable. However, miss-folding and aggregation of recombinant proteins within inclusion bodies in bacteria hinders the successful production of many eukaryotic proteins [13], [15]–[17]. Furthermore, on the occurrence of insolubility issues, both the type of fusion tag and the purification method to be used, constitute critical parameters. In addition, although known tags like maltose binding protein (MBP), can contribute to greater protein solubility [18], [19], culture temperature conditions (during induction) also appear to be a detrimental factor in the production of native protein [20]. Furthermore, methods like auto-induction can be used for easier handling of cultures in combination with high protein yield [21] whereas co-expression of certain bacterial molecular chaperones can assist in the conformational process of the native protein [22].
In the present study, we report the expression of soluble MBP-fused and his-tagged recombinant human crystallin ALDH3A1 in substantial amounts, in E. coli, and their purification to homogeneity.
Materials and Methods
Materials
Vectors pMAL-c2X and pET-26b(+) were purchased by New England Biolabs (Beverly, MA, USA) and Novagen (EMD Millipore Corporation, Billerica, MA, USA) respectively. All primers were obtained by Invitrogen (Carlsband, CA, USA) while the restriction enzymes and the chaperone plasmid set were from Takara (Shiga, Japan). Pfu polymerase and the DNA ligase kit were purchased from Fermentas (Burlington, ON, Canada). Amylose resin was purchased by New England Biolabs (Beverly, MA, USA), while Ni-NTA resin by Qiagen (Venlo, Netherlands). Medium for bacterial cultures along with antibiotics and inducers were purchased either from Applichem (Darmstadt, Germany) or from Sigma-Aldrich Co. (Taufkirchen, Germany). Protease inhibitors as well as the chemicals for the ALDH3A1 enzymatic activity were obtained by Sigma-Aldrich Co. For western blotting, PVDF membranes were purchased from Millipore (Bedford, MA, USA), whereas the chemiluminescence reagents were from Thermo Scientific (Rockford, IL, USA) and the autoradiography films from Genesee Scientific (San Diego, CA, USA). Rabbit polyclonal antibody against human ALDH3A1 was obtained from Abgent (San Diego, CA, USA) and the goat anti-rabbit IgG horseradish peroxidise conjugated antibody was purchased by Millipore (Bedford, MA, USA).
pMAL/ALDH3A1 Vector Construction
To construct the pMAL/ALDH3A1 expression vector, the ΔpCEP4Δ/ALDH3A1 plasmid (containing a full-length of human ALDH3A1 cDNA) was used as the template for the polymerase chain reaction [6], [23]. The following two primers were synthesized in order to: (i) amplify the entire coding sequence and (ii) introduce an EcoRI restriction site at the 5′ end and a HindIII site on the 3′ end: 1.forward primer: 5′-CTGAATTCAGCAAGATCAGCGAG-3′ and, 2.reverse primer: 5′-CTAAGCTTTCAGTGCTGGGTCAT-3′. The PCR conditions for the amplification were: 94°C for 60 sec and further 94°C for 30 sec, 60°C for 60 sec, 72°C for 90 sec (for 30 cycles) and a final step at 72°C for 10 min. The EcoRI HindIII fragment of the PCR product was inserted into the EcoRI and HindIII sites of the pMAL expression vector. The resulting vector, pMAL/ALDH3A1 (Figure 1A) was verified by restriction digestion and sequencing from both ends of the inserted fragment.
Figure 1. Cloning of the coding sequence of ALDH3A1 into the expression vectors.
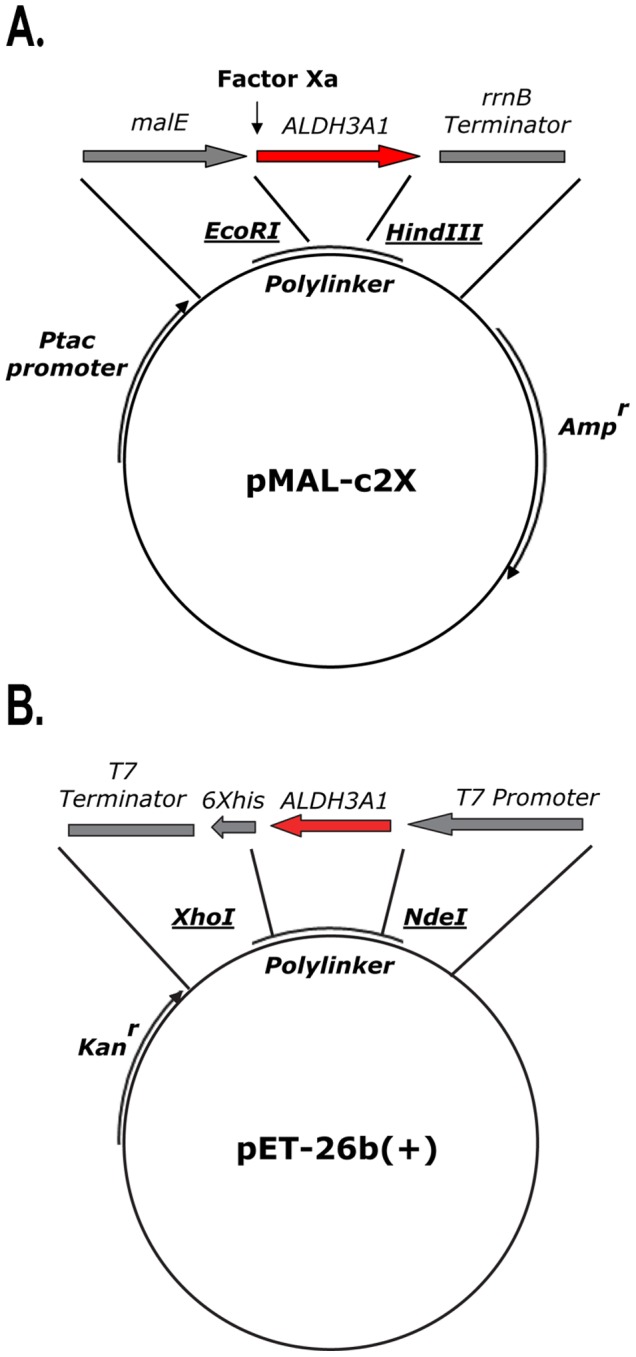
(A) Construction of the pMAL/hALDH3A1 vector. The HindIII/EcoRI fragment of the PCR product containing the coding region of human ALDH3A1 was inserted into the HindIII and EcoRI sites of the pMAL vector. (B) Construction of the pET-26b (+)/hALDH3A1 vector. The NdeI/XhoI fragment of the PCR product containing the coding region of human ALDH3A1 was inserted into the NdeI and XhoI sites of the pET-26b(+) vector.
ALDH3A1-MBP Tagged Expression
For the expression of ALDH3A1 protein tagged with MBP, BL21(-) E.coli cells were transformed with the pMAL/ALDH3A1 construct and incubated in LB broth (with 0.2% glucose and 100 µg/ml ampicillin for selection). The medium was inoculated with an overnight culture (1∶100 dilution) and the culture was incubated under shaking at 37°C until an OD600 = ∼ 0.5 was reached. Subsequently, 0.5 mM IPTG was added and the culture was further incubated at 37°C or 25°C for 6 hours.
pET-26b(+)/hALDH3A1 Vector Construction
The coding sequence of human ALDH3A1 was amplified once more from the ΔpCEP4Δ/hALDH3A1 plasmid by PCR. The following two primers were designed so as to introduce an NdeI restriction site at the 5′ end and an XhoI restriction site at the 3′ end of the ALDH3A1 coding sequence: 1.forward primer: 5′-GGGAATTCCATATGAGCAAGATCAGCGAG-3′ and, 2.reverse primer: 5′-CCGCTCGAGGTGCTGGGTCAT-3′. The PCR conditions used for amplification were: initial denaturation at 95°C (90 sec), denaturation at 95°C (30 sec), annealing at 56°C (90 sec), extension at 72°C (120 sec) for 30 cycles and an extra step of 10 min incubation at 72°C. The NdeI/XhoI fragment of the PCR product was inserted into the NdeI and XhoI sites of the of pET-26b(+) expression vector. The resulting vector of pET-26b(+)/ALDH3A1 (Figure 1B) was verified by restriction digestion and sequencing from both ends of the inserted fragment.
ALDH3A1 his-tagged Expression
BL21(DE3) E.coli transformed with pET-26b(+)/ALDH3A1 were cultured at 37°C, in the presence of 30 µg/ml kanamycin for plasmid selection, and when OD600 reached ∼ 0.5, 0.5 mM IPTG was added and incubation continued at 18–37°C for 6 hours.
Auto-induction
For auto-induction, ZYM-5052 medium was used containing 1% tryptone, 0.5% yeast extract, 25 mM Na2HPO4, 25 mM KH2PO4, 50 mM NH4Cl, 5 mM Na2SO4, 2mM MgSO4, 0.5% glycerol, 0.05% glucose and 0.2% α-lactose as previously described [21]. An overnight culture of BL21(DE3) transformed with pET-26b(+)/ALDH3A1 was used for inoculation at a dilution of 1∶1000. Cultures were incubated (in ZYM-5052 medium with 100 µg/ml kanamycin) at 18°C, 25°C and 37°C, for 9 hours.
Molecular Chaperone Co-expression
The pET-26b(+)/ALDH3A1 transformed BL21(DE3) E.coli were re-transformed with one of the set’s plasmids: pG-KJE8, pGro7, pKJE7, pG-Tf2 and pTf16 and cultured in LB broth with 20 µg/ml chloramphenicol, along with 30 µg/ml kanamycin for the selection of the transformed clones. For protein expression, cells were incubated in shaking cultures at 37°C and in the presence of the appropriate chaperone inducer (0.5 mg/ml L-arabinose and/or 5 ng/ml tetracycline) for allowing the chaperones to be already expressed at the time of ALDH3A1 induction (Table 1). When culture reached OD600 ∼ 0.6, 0.5mM IPTG was added and the incubation proceeded for 6 hours at 25°C.
Table 1. Description of the chaperone plasmids used in the study.
| Plasmid | Resistance Marker | Inducer | Chaperones | Molecular Weights |
| pG-KJE8 | Chloramphenicol (20 µg/ml) | L-arabinose (0.5 mg/ml) tetracycline (5 ng/ml) | dnaK-dnaJ-grpE/groES-groEL | dnaK-70 kDa dnaJ-40 kDa grpE-22 kDa groES-10 kDa groEL-60 kDa |
| pGro7 | Chloramphenicol (20 µg/ml) | L-arabinose (0.5 mg/ml) | groES-groEL | groES-10 kDa groEL-60 kDa |
| pKJE7 | Chloramphenicol (20 µg/ml) | L-arabinose (0.5 mg/ml) | dnaK-dnaJ-grpE | dnaK-70 kDa dnaJ-40 kDa grpE-22 kDa |
| pG-Tf2 | Chloramphenicol (20 µg/ml) | tetracycline (5 ng/ml) | groES-groEL/tig | groES-10kDa groEL-60 kDa tig-56 kDa |
| pTf16 | Chloramphenicol (20 µg/ml) | L-arabinose (0.5 mg/ml) | tig | tig-56 kDa |
ALDH3A1 Enzymatic Activity
ALDH3A1 activity determinations were carried out using a spectrophotometer (Libra S22, Biochrom Ltd, Cambridge, UK) by monitoring NADPH production at 340 nm as described previously [6]. Briefly, a total of 1 ml reaction containing sodium pyrophosphate (100 mM, pH 8.0), 1 mM pyrazole and 2.5 mM NADP+ (co-enzyme) and recombinant ALDH3A1 at various concentrations were prepared and incubated at 25°C. The reaction was initiated using benzaldehyde as a substrate (to a final concentration of 0.5 mM) and was monitored as an increase in NADPH at 340 nm for 5 min. Enzyme activity was calculated using a molar extinction coefficient of 6.22 mM−1/cm−1 for NADPH. Enzyme specific activities are expressed as nmoles of NADPH/min/mg protein.
Purification of the ALDH3A1/MBP Recombinant Protein
Cells were harvested through centrifugation at 4.000xg, at 4°C for 20 min and lysed in 20 mM Tris-HCl pH 7.4, 200 mM NaCl and 1 mM EDTA (with the addition of the protease inhibitors: 100 µg/ml PMSF, 0.5 µg/ml leupeptin, 0.5 µg/ml aprotinin and 1 µg/ml pepsatin) by sonication for 8s using the UP50H sonifier by Hielscher Ultrasonics GmbH (Teltow, Germany) at an intermediate setting (cycle 1, 70% amplitude). The lysates were cooled on ice for 30s and the procedure was repeated for a total of 6 cycles. Crude extract was isolated by centrifugation of samples at 9.000xg (4°C) for 30 min and further applied to an amylose resin column, already equilibrated with the lysis buffer. Recombinant protein was eluted after the addition of lysis buffer with 10 mM maltose. The presence of ALDH3A1 throughout E. coli expression and purification steps was determined by SDS-PAGE and western blot analysis.
Purification of the ALDH3A1/6xHis Recombinant Protein
Cells were collected and placed in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 1% Tween-20, 20 mM imidazole, pH 8.0) in the presence of protease inhibitors as mentioned above. Purification was conducted via affinity chromatography, by Ni-NTA resin. For the two washes, the concentration of imidazole in the buffer was increased to 40 and 70mM respectively. Bounded protein was eluted through the addition of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 300 mM imidazole, pH 8.0).
Western Blotting
Elution parts of ALDH3A1 purification were subjected to SDS-PAGE electrophoresis. The separated proteins were then transferred to a PVDF membrane (Polyvinylidene), which was subsequently blocked with 5% of non-fat dry milk in TBST solution (100 mM Tris, 150 mM NaCl, 0.1% Tween-20). Membrane was subsequently incubated overnight (at 4°C) with the primary, polyclonal anti-ALDH3A1 antibody at a dilution of 1∶500 in 5% non-fat dry milk in TBST, while the secondary horseradish peroxidase conjugated goat anti-rabbit IgG was used in a dilution of 1∶5000 (1 hour incubation). Finally, the protein bands were visualized by the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) for alkaline phosphatase-conjugated secondary antibody as described by the manufacturer.
Results
Expression of MBP Fused ALDH3A1 Leads to High Yield and Sufficient Solubility
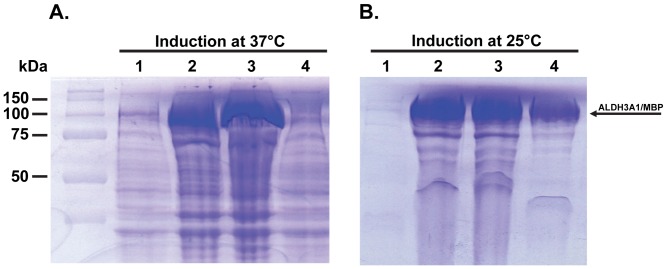
Initially, we tested the expression of the MBP tagged ALDH3A1, through the pMAL-c2X system, given that MBP is known to contribute to increased solubility of the heterologous produced proteins. As it is shown (Figure 2A), ALDH3A1 was expressed at high yields, but with extremely low solubility, when the induction occurred at 37°C (Table 2). After lowering cultivation temperature to 25°C, during induction time (a common strategy to overcome insolubility), significant improvement in ALDH3A1 solubility was observed by increasing from 2.5% at 37°C to 35.5% at 25°C (Figure 2B; Table 2). The recombinant MBP-fused ALDH3A1 was found to be functionally active when tested for the presence of ALDH3A1 enzymatic activity even though it was tagged with the 42kDa MBP (Table 3, specific activity of the crude extract).
Figure 2. ALDH3A1 heterologous expression through the pMAL-c2X expression system.
SDS-PAGE pattern showing induction of ALDH3A1 expression at (A) 37°C and (B) 25°C. Samples were subjected to SDS-PAGE and stained with Coomassie blue. Lane 1, whole cell lysate prior IPTG induction; Lane 2, whole cell lysate 6 hours after IPTG induction; Lane 3, insoluble fraction; Lane 4: soluble fraction, respectively, 6 hours after IPTG induction. The arrow indicates the position of ALDH3A1/MBP protein.
Table 2. Comparison of different E.coli strategies for production of soluble recombinant ALDH3A1.
| Method of expression | Induction temperature (°C ) | Solubility (% of the induced protein)a |
| ALDH3A1/MBP tagged | 37 | 2.5±0.59 |
| 25 | 35.47±3.29 | |
| ALDH3A1/6xHis tagged | 37 | 2.02±0.57 |
| 25 | 3.45±1.26 | |
| 18 | 2.73±0.48 | |
| ALDH3A1/6xHis tagged (autoinduction) | 37 | 3.11±0.34 |
| 25 | 4.02±0.11 | |
| 18 | 2.96±0.59 | |
| ALDH3A1/6xHis tagged pG-KJE8 | 25 | 19.54±1.01 |
| ALDH3A1/6xHis tagged pGro7 | 25 | 11.63±1.03 |
| ALDH3A1/6xHis tagged pKJE7 | 25 | 4.36±0.36 |
| ALDH3A1/6xHis tagged pG-TF2 | 25 | 3.75±0.48 |
| ALDH3A1/6xHis tagged pTF16 | 25 | 6.29±1.01 |
The % solubility values reported are mean of three different experiments.
Table 3. Purification of MBP-tagged recombinant human ALDH3A1 from E. coli.
| Purification Steps | Total Protein (mg) | Yield (%) | Specific Activityb (mU/mg protein) | Purification (fold) |
| Crude supernatanta | 62.5 | 100 | 90 | 1 |
| Amylose resin column | 3.2 | 5.12 | 250 | 2.77 |
The starting material was 250 ml of crude E. coli supernatant.
One milliunit (mU) of activity was defined as the amount of activity that oxidized of 1 nmol of NADPH/min at 25°C. Representative results of three different isolation procedures.
Expression of his-tagged ALDH3A1 Leads to High Protein Yield but Insufficient Solubility
Although the MBP tag facilitated the expression of the target heterologous protein, there are further limitations considering the usage of the MBP-fused recombinant proteins including the fact that a number of assays do not allow for the use of a tag with the size of MBP. To this end, the usage of the specialized protease, factor Xa, could solve the problem but the required extra step of the purification process could be a limitation. Consequently, we sought to examine the expression of recombinant human ALDH3A1 fused with a rather smaller but very common 6-histidines tag. Although the resulted recombinant his-tagged ALDH3A1 was expressed at remarkably higher rates, almost the whole amount of the recombinant protein was insoluble and trapped into the inclusion bodies (Figure 3A, Table 2). Unlike the case of MBP-fused expression, lowering the induction temperature at 25°C (Figure 3B) and at 18°C (Figure 3C) did not improve significantly the solubility of the recombinant protein (Table 2).
Figure 3. ALDH3A1 heterologous expression through the pET-26b(+) expression system.
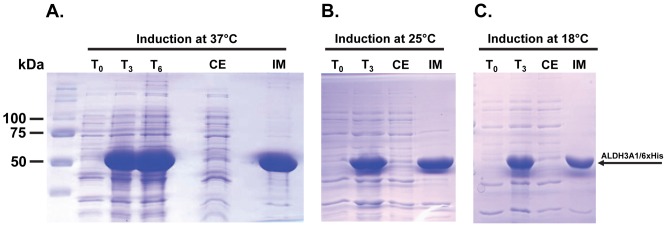
Induction at (A) 37°C, (B) 25°C and (C) 18°C. Samples were subjected to SDS-PAGE and stained with Coomassie blue. T0: Total cell extract form bacterial culture prior of protein induction, T3: Total cell extract 3 hours after induction, T6: Total cell extract 6 hours after induction, CE: Crude extract of lysed cells 6 hours after induction, IM: Insoluble matter of lysed cells 6 hours after induction. The arrow indicates the position of ALDH3A1/6xHis protein.
Auto-induction Leads to Enhanced Expression Levels of his-tagged ALDH3A1 but Poor Solubility
In order to improve the solubility of the his-tagged recombinant ALDH3A1, we employed the auto-induction protocol described recently by Studier et al [21]. An important factor during heterologous protein expression is the need for a strict control of protein induction and the retention of cultures’ viability for as long as possible. The auto-induction protocol requires specialized culture media which in combination with high rates of aeration allows for a firm hold of the induction, an equilibrated pH and a subsequent elongated viability even at extremely high cell densities. Isopropyl β-D-1-thiogalactopyranoside (IPTG) induction is not applied in this case, as glucose, glycerol and lactose are all included in the media. Glycerol is used as an efficient carbon and energy source, which contributes to the growth of cells. On the other hand, as long as glucose is available in the media and usually until the late log phase, bacteria do not metabolize lactose. Near saturation, though, and when glucose is depleted, cells metabolize lactose to the inducer allo-lactose and the induction of the protein begins then manually with no extra addition. Due to the high viability of cultures, the yield of recombinant protein is extremely high.
In the case of ALDH3A1, while the expression of the protein was sufficiently rich under conditions of auto-induction, almost all of the produced ALDH3A1 was found in the inclusion bodies, as inactive aggregates (Figure 4). The soluble fraction of the expressed ALDH3A1 estimated to represent approximately 3% of the induced protein at 37°C (Table 2). Lowering the temperature from 37°C to 25°C and 18°C did not improve any further the solubility of the recombinant protein (Figure 4; Table 2).
Figure 4. ALDH3A1 heterologous expression through autoinduction with the pET-26b(+) expression system.
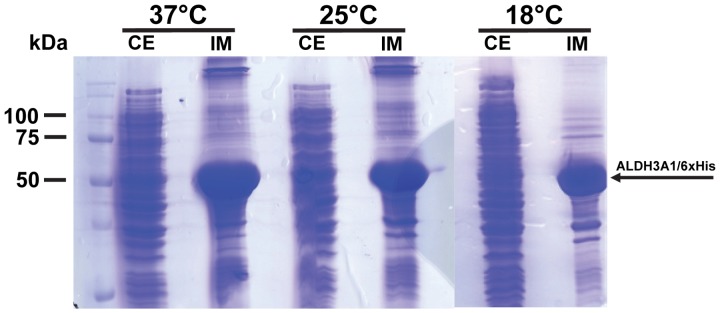
Samples were subjected to SDS-PAGE and stained with Coomassie blue. 37°C, 25°C and 18°C: different induction temperatures, CE: Crude extract of lysed cells 9 hours after inoculation, IM: Insoluble matter of lysed cells 9 hours after inoculation. The arrow indicates the position of ALDH3A1/6xHis protein.
Molecular Chaperones’ Co-expression Facilitates the Production of Soluble Recombinant his-tagged ALDH3A1
Molecular chaperones play an important role in the conformation process of newly synthesized proteins. In the case of heterologous expressed proteins, which commonly exhibit solubility problems and misfolding, the presence of chaperones could become even more of a necessity. E.coli affords a variety of proteins that could be characterized as chaperones, and amongst them, the GroEL/GroES and the DnaK/DnaJ/GrpE are considered to be key groups. The above, along with the Trigger factor could be co-expressed with a heterologous protein and assist to its production in native and active forms. As these chaperone molecules are normally expressed at low levels in prokaryotic cells, heterologous over-expression of eukaryotic proteins with chaperones has been shown to improve the solubility of the overexpressed proteins in E.coli [24].
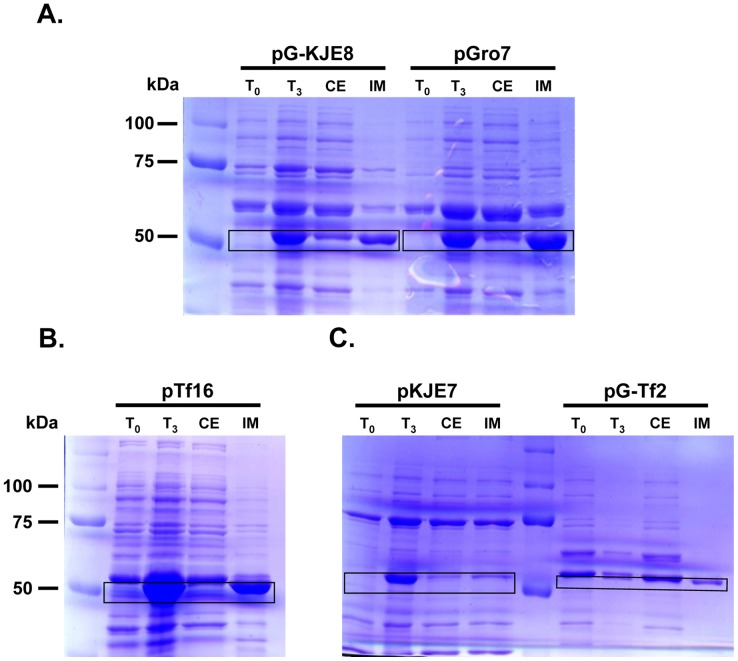
In this study, we used different combinations of chaperones to enhance the solubility of the recombinant his-tagged ALDH3A1 in BL21(DE3) E. coli (Table 1). The solubility was improved in the cases of co-expression with the pG-KJE8 (approximately 20%), and pGro7 (approximately 12%) plasmids (Figure 5; Table 2). However, the expression levels of soluble his-tagged ALDH3A1 were significantly lower (3.7%) when the recombinant protein was co-expressed in the presence of pG-Tf2 plasmid (Figure 5C; Table 2). On the other hand, negligible enhancement in solubility was observed when his-tagged ALDH3A1 was co-expressed with pTf16 (approximately 6%) and pKJE7 (approximately 4%) (Figure 5B/C; Table 2). Our results demonstrate that the presence of chaperone complexes especially those contain GroES and GroEL (e.g. plasmids pGKJE8 and pGro7) increased the solubility of the recombinant protein. In the presence of Tig chaperone, the solubility of his-tagged was significantly impeded. Combination of GroES/GroEL and dnaK/DnaJ/GrpE chaperone groups exhibited the most profound effect on his-tagged ALDH3A1 solubility. Lower induction temperatures or addition of ethanol did not facilitate any further the protein’s solubility (data not shown).
Figure 5. Co-expression of ALDH3A1 with molecular chaperones.
(A) Co-expression of pG-KJE8/pGro7 plasmid’s chaperones, (B) Co-expression of pTf16 plasmid’s chaperones, (C) Co-expression of pKJE7/pG-Tf2 plasmid’s chaperones. Samples were subjected to SDS-PAGE and stained with Coomassie blue. T0: Total cell extract form bacterial culture prior of protein induction, T3: Total cell extract 3 hours after induction, CE: Crude extract of lysed cells 3 hours after induction, IM: Insoluble matter of lysed cells 3 hours after induction. The recombinant proteins expression is presented in the boxes.
Purification of the Recombinant Human Fused ALDH3A1 Hybrids
Recombinant human fused ALDH3A1 hybrids were produced and purified from E.coli using the MBP-fused expression strategy (under low temperature conditions during protein induction) and the his-tagged ALDH3A1 expression strategy (under conditions of co-expressing the pG-KJE8) both of which produced soluble recombinant ALDH3A1 at sufficient levels.
Purification of MBP-fused recombinant human ALDH3A1 was conducted with the use of affinity chromatography. Protein supernatant obtained from E. coli lysate was applied to amylose resin column in column buffer (see Methods). MBP-fused ALDH3A1 was then eluted from the column in the same buffer containing 10 mM maltose and appeared in the elution fractions (Figure 6). This convenient step of immobilized affinity chromatography resulted in purified recombinant MBP-fused ALDH3A1 of sufficient homogeneity. The final yield was approximately 5% from the initial 62.5 mg of crude protein and purification of the recombinant protein was 2.8-fold (Table 3).
Figure 6. Protein expression and purification of recombinant MBP fused ALDH3A1.
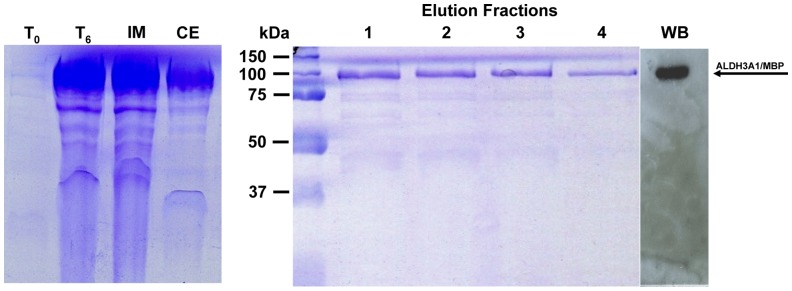
SDS-PAGE analysis at various stages of purification of recombinant MBP-fused ALDH3A1 using amylose resin chromatography (Coomassie blue staining). T0: Total cell extract form bacterial culture prior of protein induction, T6: Total cell extract 6 hours after IPTG induction, IM: Insoluble matter of lysed cells 6 hours after IPTG induction, CE: Crude extract of lysed cells 6 hours after IPTG induction, Elution fractions: purified recombinant ALDH3A1 eluted from amylose resin column. WB: western immunoblotting of purified recombinant ALDH3A1/MBP. The arrow indicates the position of the MBP-fused ALDH3A1 recombinant protein at approximately 92 kDa.
Isolation of his-tagged recombinant human ALDH3A1 was achieved by convenient one step affinity chromatography using nickel nitrilotriacetic (Ni-NTA) resin and elution in buffer containing 300 mM imidazole resulting in purification of his-tagged recombinant human ALDH3A1 to homogeneity (Figure 7). The final yield was approximately 7% from the initial 36 mg of crude protein and purification of the recombinan protein was 5.8-fold (Table 4).
Figure 7. Protein expression and purification of recombinant his-tagged ALDH3A1.
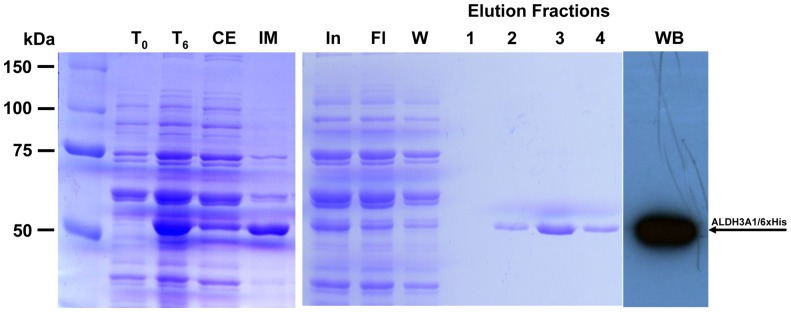
SDS-PAGE analysis at various stages of purification of recombinant his-fused ALDH3A1 using Ni-affinity chromatography (Coomassie blue staining). T0: Total cell extract form bacterial culture co-expressing pG-KJE8 along with ALDH3A1 prior to IPTG induction, T6: Total cell extract 6 hours after IPTG induction, CE: Crude extract of lysed cells 6 hours after IPTG induction, IM: Insoluble matter of lysed cells 6 hours after IPTG induction, In: Input of the column, Fl: flowthrough part, W: wash part, Elution fractions: purified recombinant protein eluted from Ni-NTA column. WB: western immunoblotting of purified recombinant ALDH3A1/6xHis. The arrow indicates the position of the recombinant his-tagged ALDH3A1 at approximately 51 kDa.
Table 4. Purification of his-tagged recombinant human ALDH3A1 from E. coli.
| Purification Steps | Total Protein (mg) | Yield (%) | Specific Activityb (mU/mg protein) | Purification (fold) |
| Crude supernatanta | 36 | 100 | 200 | 1 |
| Ni-NTA column | 2.43 | 6.75 | 1150 | 5.75 |
The starting material was 250 ml of crude E. coli supernatant.
One milliunit (mU) of activity was defined as the amount of activity that oxidized of 1 nmol of NADPH/min at 25°C. Representative results of three different isolation procedures.
Discussion
Previous attempts to express active recombinant human ALDH3A1 in E. coli have failed primarily because of low solubility, yield and insufficient purity issues. In this study, we compared different E. coli fusion expression strategies (the MBP- and the 6-his-tagged expression) under various conditions intending to increase their efficiency for producing soluble recombinant ALDH3A1. We have shown that the MBP-tagged expression in combination with lower- temperature culture conditions resulted in active soluble recombinant ALDH3A1. Expression of the fused his-tagged ALDH3A1 protein resulted in poor solubility and neither lowering temperature culture conditions nor auto-induction conditions improved solubility. Furthermore, higher yield of soluble, fully active native form of his-tagged ALDH3A1 was facilitated through the co-expression of the two groups of E.coli’s molecular chaperones GroES/GroEL and DnaK/DnaJ/GrpE. Convenient one-step immobilized affinity chromatography methods were utilized to purify the fused ALDH3A1 hybrids to sufficient homogeneity. To our knowledge, this is only the second time that recombinant techniques have been used to produce human crystallin ALDH3A1. We have previously utilized the baculovirus expression system to produce recombinant human ALDH3A1 in order to overcome the insolubility problems occurred at our initial attempts to express the protein in E. coli. The baculovirus infection system in Sf9 cells in combination with 5′ AMP sepharose chromatography resulted to extremely high yield of recombinant ALDH3A1 with sufficient solubility [5]. However, while it is true that the baculovirus expression systems allow for high yields of structurally and functionally foreign proteins in insect cells, their high cost, complexity and requirement of specific equipment and trained personnel pose limitations thus providing reasoning for the development of alternative recombinant expression strategies.
Insolubility is the number one issue of recombinant proteins expressed in E. coli. The reason for this is believed to be their non-native, aggregation-prone conformation and their subsequent expression as inactive forms in the inclusion bodies. Important parameters include the interactions between the hydrophobic patches of newly synthesized unfolded polypeptides, which are influenced by the rate of protein synthesis along with the cellular microenvironment of expression. Therefore, factors considered important during protein synthesis are usually related with the expression vector used, the induction parameters and the cultivation conditions [17].
The selection of the appropriate tag for the fusion of the heterologous protein usually depends on the desired method of chromatography and the experimental needs of the protein’s utilization. On the other hand, protein solubility depends on the type of the fusion tag to be used. Tags known for their contribution to solubility are the MBP, the NusA and the GST (glutathione S-transferase) [19]. MBP, in particular, is considered to be one the most effective and has been widely used. Although, MBP appears to facilitate the correct formation of disulfide bonds in the newly synthesized proteins, the exact mechanism through which it improves the solubility of the target heterologous proteins remains unknown [25]. Indeed, in our case, the expression of MBP-fused ALDH3A1 in combination with lower induction temperature in E. coli resulted in much better solubility compared to his-tagged ALDH3A1 expression under all different strategies tested.
While the establishment of a highly productive system with strict control elements is a well known and crucial issue, another prominent, but often underestimated factor is the need for high cell density cultures with enhanced viability [16]. Even though E. coli cultures are easy to be cultivated, the limited sources of oxygen and nutrition elements as well as the increased energy needs under conditions of protein induction, lead to stress and inadequately operated metabolism. This is the reason why the induced protein expression occurs during the exponential rather than the stationary phase, but even in this case, problems could also occur and the yield of production could be significantly restrained [13]. Auto-induction, a technique introduced by Studier et al [21], was designed so as to solve the above restrictions. Contrary to the classic method, protein expression is induced near saturation phase, when cultures have a high cell density, by lactose supplementation in the media instead of IPTG. Induction prior to saturation is prevented by glucose. The specially formulated media required along with proper aerization conditions are responsible for balancing the pH of the cell cultures and the growth of cells to extremely high densities without loss of viability. Bibliography is full of variable examples of correctly expressed proteins using the auto-induction method. The tumor necrosis factor family member APRIL [26], long repetitive resilin-based proteins [27], the psychrophilic TAB5 alkaline phosphatase [28], an Asc-1 homologue [29], the human epoxide hydrolase [30], the holotoxin Stx2 [31], and globin chains from Arenicola marina [32] are just a few of the recombinant produced proteins to name. In the case of recombinant ALDH3A1 (his-tagged ALDH3A1) however, the employment of auto-induction method resulted in higher protein expression levels but unfortunately it did not improve the protein’s solubility.
Considering cultivation conditions, several different strategies have been demonstrated, in everyday laboratory practice, for the enhancement of protein solubility. The most commonly and easily tested one is with no doubt the low-temperature culture which was proved to be miraculous in a variety of cases [20], [33] including ours as well by enhancing the solubility of the MBP-fused ALDH3A1. On the other hand, temperature is known to correlate with the production of active protein through a variety of mechanisms. Hydrophobic interactions, the basic driving force of inclusion bodies formation depend on temperature. Furthermore, (i) the temperature-dependent expression of molecular chaperones, (ii) the reduction of protein synthesis rate, (iii) the different folding kinetics and (iv) the lower activity of specific proteases [17], [18] can also contribute to the enhanced yield of active recombinant proteins.
Co-expressing the recombinant protein with chaperones has been exploited as the most effective way and a quality control system to increase the solubility of recombinant proteins in E. coli [34]–[37]. Molecular chaperones facilitate the correct conformation of newly synthesized proteins and contribute in the retention of their native folding. Among them, DnaK/DnaJ/GrpE along with GroES/GroEL are the most commonly used systems for the expression of soluble proteins [22], [38]. Basically, they are ATP-dependent folding chaperones which induce the partial unfolding and re-folding of non-native proteins [17]. Trigger factor, on the other hand, associates with the synthesized proteins as soon as they leave ribosome and through its interaction with their exposed hydrophobic patches averts their subsequent aggregation [14], [38]. Co-expression of molecular chaperones resulted in enhanced solubility and production of recombinant rice plant catalase A [39] active ribonuclease inhibitor [40], human scramblase 1 [41] and zeta-crystallin [42]. Solubility of his-tagged ALDH3A1 was significantly improved under conditions of co-expressing the pG-KJE8) suggesting that dnaK/dnaJ/grpE and groES/groEL are the essential chaperones for the correct folding of recombinant human ALDH3A1 (his-tagged ALDH3A1) when over-expressed in E. coli.
In summary, soluble MBP-fused and his-tagged recombinant human ALDH3A1 proteins have been successfully expressed in E. coli and purified to homogeneity. Both fusion proteins retained their biological activity and so can be used directly without removing the fusion tags. The methods described in this study permit the production of substantial amounts of the recombinant human ALDH3A1 for conducting functional studies on the biological role of this interesting crystallin, which exists in high concentrations in the cornea of certain mammalian species.
Acknowledgments
This study represents part of the doctoral thesis of Ms Georgia-Persephoni Voulgaridou. The authors would like to thank Dr. Vasiliki Fadouloglou for her valuable advice and suggestions.
Funding Statement
This research has been co-financed by the European Union (European Social Fund–ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Estey T, Piatigorsky J, Lassen N, Vasiliou V (2007) ALDH3A1: a corneal crystallin with diverse functions. Exp Eye Res 84: 3–12. [DOI] [PubMed] [Google Scholar]
- 2. Pappa A, Sophos NA, Vasiliou V (2001) Corneal and stomach expression of aldehyde dehydrogenases: from fish to mammals. Chem Biol Interact 130–132: 181–191. [DOI] [PubMed] [Google Scholar]
- 3. Piatigorsky J (2001) Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the “refracton” hypothesis. Cornea 20: 853–858. [DOI] [PubMed] [Google Scholar]
- 4. Piatigorsky J (2000) Review: A case for corneal crystallins. J Ocul Pharmacol Ther 16: 173–180. [DOI] [PubMed] [Google Scholar]
- 5. Pappa A, Estey T, Manzer R, Brown D, Vasiliou V (2003) Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J 376: 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pappa A, Chen C, Koutalos Y, Townsend AJ, Vasiliou V (2003) Aldh3a1 protects human corneal epithelial cells from ultraviolet- and 4-hydroxy-2-nonenal-induced oxidative damage. Free Radic Biol Med 34: 1178–1189. [DOI] [PubMed] [Google Scholar]
- 7. Black W, Chen Y, Matsumoto A, Thompson DC, Lassen N, et al. (2012) Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal. Free Radic Biol Med 52: 1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atherton SJ, Lambert C, Schultz J, Williams N, Zigman S (1999) Fluorescence studies of lens epithelial cells and their constituents. Photochem Photobiol 70: 823–828. [PubMed] [Google Scholar]
- 9. Estey T, Cantore M, Weston PA, Carpenter JF, Petrash JM, et al. (2007) Mechanisms involved in the protection of UV-induced protein inactivation by the corneal crystallin ALDH3A1. J Biol Chem 282: 4382–4392. [DOI] [PubMed] [Google Scholar]
- 10. Uma L, Hariharan J, Sharma Y, Balasubramanian D (1996) Corneal aldehyde dehydrogenase displays antioxidant properties. Exp Eye Res 63: 117–120. [DOI] [PubMed] [Google Scholar]
- 11. Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, et al. (2007) Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. J Biol Chem 282: 25668–25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pappa A, Brown D, Koutalos Y, DeGregori J, White C, et al. (2005) Human aldehyde dehydrogenase 3A1 inhibits proliferation and promotes survival of human corneal epithelial cells. J Biol Chem 280: 27998–28006. [DOI] [PubMed] [Google Scholar]
- 13. Chou CP (2007) Engineering cell physiology to enhance recombinant protein production in Escherichia coli. Appl Microbiol Biotechnol 76: 521–532. [DOI] [PubMed] [Google Scholar]
- 14. Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115: 113–128. [DOI] [PubMed] [Google Scholar]
- 15. de Marco A, Deuerling E, Mogk A, Tomoyasu T, Bukau B (2007) Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam RS, Tisi D, Levy MS, Lye GJ (2007) Framework for the rapid optimization of soluble protein expression in Escherichia coli combining microscale experiments and statistical experimental design. Biotechnol Prog 23: 785–793. [DOI] [PubMed] [Google Scholar]
- 17. Sahdev S, Khattar SK, Saini KS (2008) Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Mol Cell Biochem 307: 249–264. [DOI] [PubMed] [Google Scholar]
- 18. Georgiou G, Valax P (1996) Expression of correctly folded proteins in Escherichia coli. Curr Opin Biotechnol 7: 190–197. [DOI] [PubMed] [Google Scholar]
- 19. Guo W, Cao L, Jia Z, Wu G, Li T, et al. (2011) High level soluble production of functional ribonuclease inhibitor in Escherichia coli by fusing it to soluble partners. Protein Expr Purif 77: 185–192. [DOI] [PubMed] [Google Scholar]
- 20. Semba H, Ichige E, Imanaka T, Atomi H, Aoyagi H (2010) Efficient production of active form recombinant cassava hydroxynitrile lyase using Escherichia coli in low-temperature culture. Methods Mol Biol 643: 133–144. [DOI] [PubMed] [Google Scholar]
- 21. Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41: 207–234. [DOI] [PubMed] [Google Scholar]
- 22. Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T (1998) Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli. Appl Environ Microbiol 64: 1694–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bunting KD, Townsend AJ (1996) Protection by transfected rat or human class 3 aldehyde dehydrogenase against the cytotoxic effects of oxazaphosphorine alkylating agents in hamster V79 cell lines. Demonstration of aldophosphamide metabolism by the human cytosolic class 3 isozyme. J Biol Chem 271: 11891–11896. [DOI] [PubMed] [Google Scholar]
- 24. Ma N, Wei L, Fan Y, Hua Q (2012) Heterologous expression and characterization of soluble recombinant 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Actinosynnema pretiosum ssp. auranticum ATCC31565 through co-expression with Chaperones in Escherichia coli. Protein Expr Purif 82: 263–269. [DOI] [PubMed] [Google Scholar]
- 25. Tait AR, Straus SK (2011) Overexpression and purification of U24 from human herpesvirus type-6 in E. coli: unconventional use of oxidizing environments with a maltose binding protein-hexahistine dual tag to enhance membrane protein yield. Microb Cell Fact 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu S, Wang Y, Liu Y, Mo W, Song H, et al. (2009) Expression and purification of APRIL by auto-induction. Protein Expr Purif 68: 49–53. [DOI] [PubMed] [Google Scholar]
- 27.Renner JN, Kim Y, Cherry KM, Liu JC (2011) Modular cloning and protein expression of long, repetitive resilin-based proteins. Protein Expr Purif. [DOI] [PubMed] [Google Scholar]
- 28. Lu Z, Chen W, Liu R, Hu X, Ding Y (2010) A novel method for high-level production of psychrophilic TAB5 alkaline phosphatase. Protein Expr Purif 74: 217–222. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Hald H, Ernst HA, Egebjerg J, Christensen KV, et al. (2010) Over-expression, purification and characterization of an Asc-1 homologue from Gloeobacter violaceus. Protein Expr Purif 71: 179–183. [DOI] [PubMed] [Google Scholar]
- 30. Nishi K, Kim IH, Ma SJ (2010) Expression of the human soluble epoxide hydrolase in Escherichia coli by auto-induction for the study of high-throughput inhibition assays. Protein Expr Purif 69: 34–38. [DOI] [PubMed] [Google Scholar]
- 31. Tu W, Cai K, Gao X, Xiao L, Chen R, et al. (2009) Improved production of holotoxin Stx2 with biological activities by using a single-promoter vector and an auto-induction expression system. Protein Expr Purif 67: 169–174. [DOI] [PubMed] [Google Scholar]
- 32. Harnois T, Rousselot M, Rogniaux H, Zal F (2009) High-level production of recombinant Arenicola marina globin chains in Escherichia coli: a new generation of blood substitute. Artif Cells Blood Substit Immobil Biotechnol 37: 106–116. [DOI] [PubMed] [Google Scholar]
- 33. Choi MS, Saxena A, Chilukuri N (2010) A strategy for the production of soluble human senescence marker protein-30 in Escherichia coli. Biochem Biophys Res Commun 393: 509–513. [DOI] [PubMed] [Google Scholar]
- 34. Hartl FU, Martin J (1995) Molecular chaperones in cellular protein folding. Curr Opin Struct Biol 5: 92–102. [DOI] [PubMed] [Google Scholar]
- 35. Welch WJ, Brown CR (1996) Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5: 781–791. [DOI] [PubMed] [Google Scholar]
- 37. Oganesyan N, Ankoudinova I, Kim SH, Kim R (2007) Effect of osmotic stress and heat shock in recombinant protein overexpression and crystallization. Protein Expr Purif 52: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22: 1399–1408. [DOI] [PubMed] [Google Scholar]
- 39. Ray M, Mishra P, Das P, Sabat SC (2012) Expression and purification of soluble bio-active rice plant catalase-A from recombinant Escherichia coli. J Biotechnol 157: 12–19. [DOI] [PubMed] [Google Scholar]
- 40. Siurkus J, Neubauer P (2011) Heterologous production of active ribonuclease inhibitor in Escherichia coli by redox state control and chaperonin coexpression. Microb Cell Fact 10: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sahu SK, Rajasekharan A, Gummadi SN (2009) GroES and GroEL are essential chaperones for refolding of recombinant human phospholipid scramblase 1 in E. coli. Biotechnol Lett 31: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 42. Goenka S, Rao CM (2001) Expression of recombinant zeta-crystallin in Escherichia coli with the help of GroEL/ES and its purification. Protein Expr Purif 21: 260–267. [DOI] [PubMed] [Google Scholar]