Abstract
Sam68, the Src-associated substrate during mitosis of 68 kDa, belongs to the large class of heteronuclear ribonucleoprotein particle K (hnRNP K) homology (KH) domain family of RNA-binding proteins. Sam68 contains a single KH domain harboring conserved N- and C-terminal sequences required for RNA binding and homodimerization. The KH domain is one of the most prevalent RNA binding domains that directly contacts single-stranded RNA. Sam68 has been implicated in numerous aspects of RNA metabolism including alternative splicing and polysomal recruitment of mRNAs. Studies in mice have revealed physiological roles linking Sam68 to osteoporosis, obesity, cancer, infertility and ataxia. Recent publications have greatly enhanced our understanding of Sam68 mechanism of action in addition to its cellular role. Herein, we will discuss the latest advances in the literature pertaining to obesity and neuronal development.
Keywords: RNA binding proteins, Sam68, adipogenesis, alternative splicing, neurogenesis
Sam68 and Alternative Splicing
Accumulating evidence supports the role of RNA binding proteins (RBPs) to promote a variety of different splicing patterns coupling transcription and splicing regulation.1 Alternative splicing is a key process allowing multiple distinct mRNAs to be generated from a limited number of genes contributing to protein diversity. It enables cell-type or tissue specific functions. The human genome encodes > 500 RBPs, each interacting with RNA with different affinities.2 Considering that RBPs coordinate elaborate networks of RNA-protein and protein-protein interactions that control RNA metabolism, tampering with their RNA-binding function can impact many different genes and pathways, leading to complex and multifaceted phenotypes.3,4 However, despite much progress in identifying alternative splicing events there is still much to uncover about the mechanisms governing specific exon usage in certain tissues and cell-types.
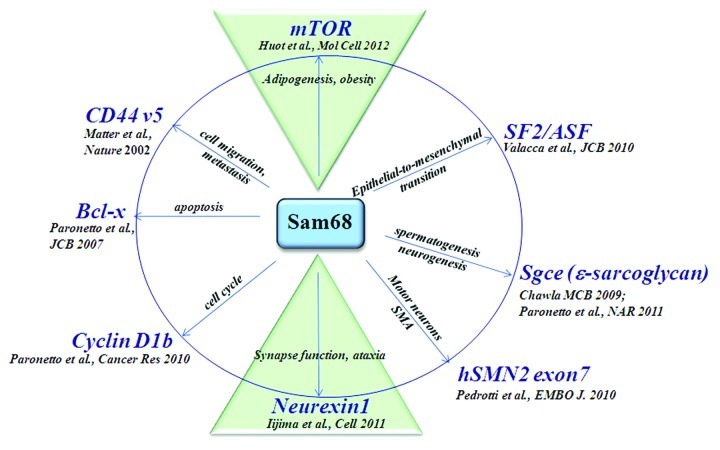
The major function of Sam68 is to regulate alternative splicing by recognizing A/U rich RNA sequences neighboring the included/excluded exon(s).5 Sam68 frequently undergoes several post-translational modifications including serine/threonine and tyrosine phosphorylation, lysine acetylation, arginine methylation and sumoylation. These modifications affect cellular localization, modulate interaction with signaling proteins and RNA binding affinity6 and as such, Sam68 is often referred to as a STAR (Signal Transduction Activator of RNA) protein. An emerging role for Sam68 has been demonstrated in a variety of studies and has been linked to a multitude of pathways (Fig. 1). Clear evidence for the involvement of Sam68 in alternative splicing has been shown in promoting the inclusion of the variable exon 5 (v5) in CD44 correlating with cell migration potential.7,8 Intriguingly, Sam68 was identified as a downstream target of the mitogen activated protein kinase (MAPK) pathway. Stimulation by phorbol esters (PMA) resulted in phosphorylation of Sam68 and enhancement of the splicing activity.
Figure 1. Schematic diagram of the multiple roles for Sam68 in alternative splicing in addition to the recent literature publications.
In addition, Sam68 in conjunction with hnRNPA1 influences the choice of the alternative 5′ splice sites of Bcl-x regulating pro-survival and apoptotic pathways.9 Increased levels of Sam68 promotes the pro-apoptotic isoform, Bcl-x(s) and this is regulated by the p59fyn-dependent phosphorylation of Sam68.9 The role of Sam68 has further been highlighted in spinal muscular atrophy (SMA). SMA is a neurodegenerative disease caused by the loss of α-motor neurons due to mutations within the gene, SMN1.10 A nearly identical gene, SMN2 is unable to compensate for loss of SMN1 due to the presence of a C-to-T transition that creates a docking site for Sam68 binding, promoting the skipping of exon 7 leading to a non-functional protein.11 This mode of action emphasizes the importance of enhancer and silencer sequences in determination of exon usage. The use of dominant negative forms of Sam68 in in vitro cultures was able to restore the splicing of exon 7 and allow accumulation of SMN2 protein and motor neuron survival.11
The use of splicing-sensitive microarrays has allowed the identification of many novel splicing targets in a tissue-specific manner. Sam68 was demonstrated to be involved in the alternative splicing of mRNAs implicated in normal neurogenesis.12 Using a knock-down approach, Chawla and colleagues identified 24 confirmed exons which showed splicing changes by reverse transcription PCR upon depletion of Sam68 in Neuro-2A cells.12 In addition, it was demonstrated that Sam68 was required for maintaining patterns of splicing even after differentiation had occurred. As Sam68 knockout mice exhibit defects in basal motor coordination,4 the alterations in splicing patterns may partly contribute to this defect.
Sam68 was also shown to participate in the epithelial-to-mesenchymal transition by regulating the alternative splicing of SF2/ASF leading to non-sense mediated decay13. Recently, Sette and colleagues identified Sam68 as an important regulator of the proto-oncogene Cyclin D1, a gene frequently dysregulated in prostate cancer cells.14 Sam68 promotes the expression of Cyclin D1b, the isoform with higher oncogenic potential, allowing progression of the cell cycle in PCa prostate cells.
Sam68 integrates signal transduction pathways and RNA metabolism. External cues are able to modulate Sam68 function by affecting protein-protein and protein-RNA interactions. Sam68 is downstream of the epidermal growth factor (EGF) receptor,15,16 hepatocyte growth factor (HGF)/Met receptor,17 leptin18 and tumor necrosis factor (TNF) receptors.19 Sam68 has also been shown to re-localize in the cytoplasm near the plasma membrane, where it functions to transport and regulate the translation of certain mRNAs.20 In particular, in spermatocytes, Sam68 transits into the cytoplasm to regulate the polysomal recruitment of specific mRNAs. Studies have also identified Sam68 as an adaptor protein during cell spreading where it functions to recruit Csk via C-terminal tyrosines to modulate Src kinase activity during cell attachment.21
Transgenic animal models have helped to uncover the critical functional consequences of dysregulated alternative splicing and have facilitated further understanding of the underlying molecular mechanisms that coordinate distinct cellular differentiation programs involved in tissue development. The generation of Sam68-deficient mice has revealed numerous unexpected physiological roles for this RNA binding protein. While most Sam68−/− mice are viable, many die at birth of unknown causes.22 Sam68−/− pups that survive the perinatal period are invariably able to live to old age. Interestingly, Sam68−/− mice have difficulty breeding due to male infertility caused by defects in spermatogenesis,20,22 as Sam68 is implicated as a regulator of co- and post-transcriptional events during male germ cell development.23 Female subfertility has been attributed to improper production of gonadotropin receptor transcripts.24 Sam68 knockout mice are also able to retain their bone mass with aging.22 Although there is ubiquitous expression of Sam68, these studies illustrate the highly specialized role this RNA binding protein plays in various tissues and cell types.
Recently, Scheiffele and colleagues, showed that Sam68 is a key regulator of site-specific and activity-dependent splicing of Neurexin-1 (Nrxn1) within cerebellar neurons.25 Nrxns are a class of synaptic cell surface receptors that contribute to the assembly of functional presynaptic terminals.26-28 Those Nrxn-1 variants containing fragment AS4 exhibit differential interactions with several ligands giving rise to synapse specific functions.29 In vitro analysis revealed Sam68, in addition to homologs SLM1 and SLM2 were able to promote the skipping of exon 20 yielding AS4 (-) isoforms. Within the introns flanking exon 20, numerous Sam68 response elements were identified to cooperate in regulating Nrxn1 splicing. A severe perturbation of Nrxn-1 splice variants was observed in Sam68 knockout brains. Stimulation of cerebellar neurons using the glutamate receptor agonist kainic acid was also dramatically attenuated in these mice indicating Sam68 is required for activity-dependent alternative splicing of Nrxn1 in vivo.25 Interestingly, other alternatively spliced pre-mRNAs that rely on activity-dependent splicing such as Grin1 and Kcnma1 were unaltered suggesting Sam68 is essential for only a specific sub-program of alternative splicing events. This study reveals the contribution of RBPs in the selection of spatial and temporal expression of isoforms.
Sam68-deficient mice exhibit a lean phenotype due to a dramatic reduction in adiposity.30 Small interference RNA (siRNA)-mediated Sam68 depletion in 3T3-L1 cells were unable to differentiate into mature adipocytes as confirmed by Oil-Red-O staining and evaluation of the adipose-specific transcription factor PPARγ. To identify the alternative splicing events regulated by Sam68, genome-wide exon usage profiling in white adipose tissue (WAT) was performed.30 Interestingly, the microarray identified the mammalian target of rapamycin (mTOR) serine/threonine kinase as being differentially alternatively spliced in WAT from Sam68−/− mice. Adipocytes derived from Sam68-deficient cells display intronic polyadenylation activation. Therefore, the absence of Sam68 causes the read-through of the weak 5′ splice site of mTOR intron 5 with transcription termination occurring within intron 5 at a downstream polyadenylation signal (Fig. 2). This ~1kb transcript termed mTOR i5 (intron 5) encodes for a ~25 kDa protein, however, this protein was undetectable using N-terminal mTOR-specific antibodies. Thus in Sam68-deficient cells, less wild type mTOR mRNA is generated because the mTOR gene produces mainly mTORi5. As predicted, Sam68-deficient 3T3-L1 cells have reduced levels of mTOR protein contributing to a reduction in mTORC1 and 2 activity following insulin stimulation, as monitored by ribosomal protein S6 S240/244 phosphorylation and phosphorylation of S473 of Akt, respectively (Fig. 3). To fully validate that the Sam68-induced differentiation defect was caused by a decrease in mTOR expression leading to an impaired activation of the mTORC pathways, the full-length mTOR was reintroduced in Sam68-depleted preadipocytes. Cells transfected with mTOR displayed significant vesicle formation in addition to lipid and triglyceride accumulation. These findings suggest Sam68 behaves as an intronic splicing enhancer for mTOR during adipogenesis. mTOR is a known regulator of cell size and cell proliferation in response to nutrients and various growth factors.31,32 WAT isolated from Sam68 knockout mice revealed a decreased commitment to early adipocyte progenitors and defects in adipogenic differentiation due to aberrant splicing of mTOR. These studies suggest Sam68 is a key regulator of alternative splicing during adipogenesis.
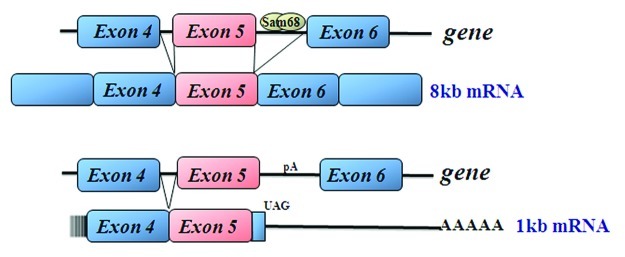
Figure 2. Splicing of mTOR exon4–6 schematic and the intron retention leading to the generation of the mTORi5 transcript.
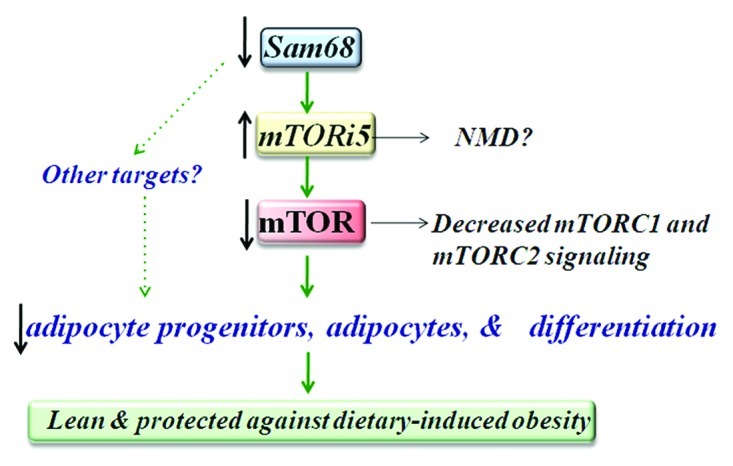
Figure 3. Model of the role of Sam68 in metabolism. Decreased levels of Sam68 results in an increase of the mTORi5 isoform with a corresponding decrease of wild type mTOR. This decrease in mTOR leads to an attenuation of mTORC1 and mTORC2 signaling resulting in decreased adipocyte progenitors, adipocytes and differentiation. In turn, mice ablated for Sam68 remain lean and are protected against dietary-induced obesity.
Sam68 and Cancer
As RBPs are often deregulated in cancer, it is not surprising to also observe an imbalance in spliced isoforms in many cancer types. In fact, certain RBPs involved in alternative splicing are oncogenes.33 There are several studies that have profiled alternative splice events (ASE) in cancer. For example, Venables and colleagues monitored alterations in ovarian and breast cancer compared with normal tissue and demonstrated that nearly half of active events were dysregulated. Notably 525 of these alternative spliced events were common to both tissue types.34 The alternative spliced events were enriched with neighboring FOX2 binding sites. It was shown that a reduced expression level of FOX2 in vitro recapitulates the cancer-associated splicing signature.34 Thus, it was proposed that the RBP, FOX2, acts as a tumor suppressor in breast and ovarian tissue. In an addtional study, 5,183 alternative spliced exons from patient-matched pairs of lung adenocarcinoma tumor tissue and adjacent normal lung tissue were profiled leading to the identification of 4 affected transcripts; VEGFR, MACF1, APP and NUMB.35 Moreover, another 5 (PLD1, DNMT3B, SYNE2, UTRN, FGFR2) previously identified36 were also observed to display changes in the inclusion in 3/5 patient-matched pairs. However, the RBPs contributing to these events remain to be elucidated.
Sam68 was initially shown to have tumor suppressor activity as the downregulation of Sam68 in NIH3T3 murine fibroblasts was associated with metastatic tumor formation in nude mice.37 However, there has been increasing evidence that Sam68 has a pro-oncogenic role and this was recently reviewed.38,39 Briefly, Sam68 haploinsufficiency delays the onset of mammary tumors in mouse models expressing the mammary targeted polyoma middle T antigen oncogene.40 Sam68 is frequently upregulated in human prostate carcinoma, while downregulation effects cell cycle progression and the proliferation rate of LNCaP cells.41 In addition, Sam68 expression has been demonstrated to be markedly overexpressed in human breast cancer cell lines closely correlating with disease progression.42 This apparent dichotomy of activities suggests that the role of Sam68 in regulating tumorigenesis is highly contextual relying on various tissue types or environmental conditions. It remains to be clarified as to how much of the oncogenic properties of Sam68 are due to its RNA binding activity. Further studies are required to ascertain which alternative splicing targets of Sam68 contribute to its tumorigenic properties.
Conclusions
Sam68 has emerged as a key player in contributing to tissue specific differentiation through its RNA binding and alternative splicing functions. The use of animal models has elucidated the role of Sam68 in a variety of unpredictable contexts, in particular its function in adipogenesis and neuronal development. With the addition of high-throughput technologies, insights are offered into the mechanisms by which RBPs contribute to alterative splicing on a genome-wide scale to regulate tissue development. Identification of factors controlling splicing events will define new strategies for understanding diseases and the development of therapies.
Acknowledgments
This work was funded by grant MT-13377 from the Canadian Institutes of Health Research. S.R. is a Chercheur-National of the Fonds de la recherche en Santé du Québec.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/21409
References
- 1.Blencowe BJ. Alternative splicing: new insights from global analyses. Cell. 2006;126:37–47. doi: 10.1016/j.cell.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–86. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–93. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukong KE, Richard S. Motor coordination defects in mice deficient for the Sam68 RNA-binding protein. Behav Brain Res. 2008;189:357–63. doi: 10.1016/j.bbr.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Richard S. Reaching for the stars: Linking RNA binding proteins to diseases. Adv Exp Med Biol. 2010;693:142–57. doi: 10.1007/978-1-4419-7005-3_10. [DOI] [PubMed] [Google Scholar]
- 6.Lukong KE, Richard S. Sam68, the KH domain-containing superSTAR. Biochim Biophys Acta. 2003;1653:73–86. doi: 10.1016/j.bbcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Matter N, Herrlich P, König H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–5. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 8.Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. 2006;26:362–70. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–39. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 11.Pedrotti S, Bielli P, Paronetto MP, Ciccosanti F, Fimia GM, Stamm S, et al. The splicing regulator Sam68 binds to a novel exonic splicing silencer and functions in SMN2 alternative splicing in spinal muscular atrophy. EMBO J. 2010;29:1235–47. doi: 10.1038/emboj.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla G, Lin CH, Han A, Shiue L, Ares M, Jr., Black DL. Sam68 regulates a set of alternatively spliced exons during neurogenesis. Mol Cell Biol. 2009;29:201–13. doi: 10.1128/MCB.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valacca C, Bonomi S, Buratti E, Pedrotti S, Baralle FE, Sette C, et al. Sam68 regulates EMT through alternative splicing-activated nonsense-mediated mRNA decay of the SF2/ASF proto-oncogene. J Cell Biol. 2010;191:87–99. doi: 10.1083/jcb.201001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paronetto MP, Cappellari M, Busà R, Pedrotti S, Vitali R, Comstock C, et al. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Res. 2010;70:229–39. doi: 10.1158/0008-5472.CAN-09-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukong KE, Larocque D, Tyner AL, Richard S. Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem. 2005;280:38639–47. doi: 10.1074/jbc.M505802200. [DOI] [PubMed] [Google Scholar]
- 16.Huot ME, Vogel G, Richard S. Identification of a Sam68 ribonucleoprotein complex regulated by epidermal growth factor. J Biol Chem. 2009;284:31903–13. doi: 10.1074/jbc.M109.018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locatelli A, Lange CA. Met receptors induce Sam68-dependent cell migration by activation of alternate extracellular signal-regulated kinase family members. J Biol Chem. 2011;286:21062–72. doi: 10.1074/jbc.M110.211409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maroni P, Citterio L, Piccoletti R, Bendinelli P. Sam68 and ERKs regulate leptin-induced expression of OB-Rb mRNA in C2C12 myotubes. Mol Cell Endocrinol. 2009;309:26–31. doi: 10.1016/j.mce.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan P, Baltimore D. Sam68 is required for both NF-κB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43:167–79. doi: 10.1016/j.molcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paronetto MP, Messina V, Bianchi E, Barchi M, Vogel G, Moretti C, et al. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J Cell Biol. 2009;185:235–49. doi: 10.1083/jcb.200811138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huot ME, Brown CM, Lamarche-Vane N, Richard S. An adaptor role for cytoplasmic Sam68 in modulating Src activity during cell polarization. Mol Cell Biol. 2009;29:1933–43. doi: 10.1128/MCB.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard S., et al. Ablation of the Sam68 RNA binding protein protects mice from age-related bone loss. 2005;1: e74. doi: 10.1371/journal.pgen.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paronetto MP, Messina V, Barchi M, Geremia R, Richard S, Sette C. Sam68 marks the transcriptionally active stages of spermatogenesis and modulates alternative splicing in male germ cells. Nucleic Acids Res. 2011;39:4961–74. doi: 10.1093/nar/gkr085. [p.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchi E, Barbagallo F, Valeri C, Geremia R, Salustri A, De Felici M, et al. Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development. Hum Mol Genet. 2010;19:4886–94. doi: 10.1093/hmg/ddq422. [DOI] [PubMed] [Google Scholar]
- 25.Iijima T, Wu K, Witte H, Hanno-Iijima Y, Glatter T, Richard S, et al. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147:1601–14. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–16. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–48. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–55. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–43. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 30.Huot ME, Vogel G, Zabarauskas A, Ngo CT, Coulombe-Huntington J, Majewski J, et al. The Sam68 STAR RNA-binding protein regulates mTOR alternative splicing during adipogenesis. Mol Cell. 2012;46:187–99. doi: 10.1016/j.molcel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venables JP, Klinck R, Koh C, Gervais-Bird J, Bramard A, Inkel L, et al. Cancer-associated regulation of alternative splicing. Nat Struct Mol Biol. 2009;16:670–6. doi: 10.1038/nsmb.1608. [DOI] [PubMed] [Google Scholar]
- 35.Misquitta-Ali CM, Cheng E, O’Hanlon D, Liu N, McGlade CJ, Tsao MS, et al. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Mol Cell Biol. 2011;31:138–50. doi: 10.1128/MCB.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venables JP, Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Koh C, et al. Identification of alternative splicing markers for breast cancer. Cancer Res. 2008;68:9525–31. doi: 10.1158/0008-5472.CAN-08-1769. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Li L, Nisson PE, Gruber C, Jessee J, Cohen SN. Neoplastic transformation and tumorigenesis associated with sam68 protein deficiency in cultured murine fibroblasts. J Biol Chem. 2000;275:40195–201. doi: 10.1074/jbc.M006194200. [DOI] [PubMed] [Google Scholar]
- 38.Bielli P, Busà R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18:R91–102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 39.Lukong KE, Richard S. Targeting the RNA-binding protein Sam68 as a treatment for cancer? Future Oncol. 2007;3:539–44. doi: 10.2217/14796694.3.5.539. [DOI] [PubMed] [Google Scholar]
- 40.Richard S, Vogel G, Huot ME, Guo T, Muller WJ, Lukong KE. Sam68 haploinsufficiency delays onset of mammary tumorigenesis and metastasis. Oncogene. 2008;27:548–56. doi: 10.1038/sj.onc.1210652. [DOI] [PubMed] [Google Scholar]
- 41.Busà R, Paronetto MP, Farini D, Pierantozzi E, Botti F, Angelini DF, et al. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene. 2007;26:4372–82. doi: 10.1038/sj.onc.1210224. [DOI] [PubMed] [Google Scholar]
- 42.Song L, Wang L, Li Y, Xiong H, Wu J, Li J, et al. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010;222:227–37. doi: 10.1002/path.2751. [DOI] [PubMed] [Google Scholar]