Abstract
The stable linearity of eukaryotic chromosomes depends on special characteristics of their ends, the telomeres. Accurate telomere function in turn requires a sustained presence of repeated DNA elements, which are maintained by the enzyme telomerase. The telomerase holoenzyme is composed of both protein and RNA, and its functions rely on proper expression, maturation, trafficking and assembly of these components. Conflicting models for the recruitment of telomerase at telomeres have been proposed; one suggests a local activation of telomerase at short telomeres, while the other proposes that telomerase is recruited only at short telomeres. To discriminate between these models and investigate the cell cycle-dependent regulation of telomerase in living cells, a GFP reporter system to visualize the yeast telomerase RNA has been recently developed. This assay shed new light on the mechanism of recruitment of telomerase to telomeres, and it uncovered a hitherto unrecognized mechanism for restricting telomerase access to telomeres.
Keywords: TLC1 RNA, MS2-GFP, Rif1, Rif2, live cell imaging, telomerase, telomeres, yeast
Introduction
Telomeres are essential nucleoprotein structures localized at the extremities of eukaryotic chromosomes that prevent DNA damage response activation and chromosome end-to-end fusion.1 In the budding yeast Saccharomyces cerevisiae, telomeres are composed of degenerated TG1–3 repeats bound by specific telomere binding proteins.2 Due to inherent problems replicating DNA ends, telomeric DNA shortens during each round of cell division and eventually this failure to maintain telomeric repeats triggers replicative senescence and permanent cell cycle arrest. In order to counteract this telomere attrition, virtually all eukaryotic cells contain an end specialized enzyme called telomerase.3 The telomerase holoenzyme is a ribonucleoprotein complex that possesses a reverse-transcriptase activity and carries its own RNA template.4 In budding yeast, the RNA component of telomerase is called TLC1 and is used as a scaffold for the binding of the catalytic subunit (Est2) and several regulatory proteins, such as Est1, Est3 and the yKu70/80 complex.2,5-8 However, despite the fact that a TLC1 RNA/Est2 complex is sufficient for telomerase activity in vitro, activity in vivo requires other regulatory proteins and cell cycle-dependent post-translational modifications on some of them. These ideas have been well characterized during the last decade as it became clear that telomerase is active only in late S-phase on telomeres.9 However, how these factors recruit and activate telomerase remained unclear.
Telomerase Recruitment and Activation at Telomeres: Conflicting models
Extensive work on this question of how telomerase maintains yeast telomeres nevertheless yielded several general ideas. First, a wealth of genetic data suggests that telomerase is recruited to short, elongation-competent telomeres via a Cdc13-Est1 interaction. This interaction between Est1 and the single-strand telomeric binding protein Cdc13 is indeed essential for telomerase activity at telomeres because a mutation in CDC13, cdc13–2, that causes a loss of this interaction also results in telomere shortening and cell senescence.10 Furthermore, in cells that contain a charge swap mutation in EST1, the est1–60 allele, this senescence phenotype is reversed and telomeres can be maintained. Finally, a fusion between Cdc13 and the catalytic subunit Est2 bypasses the requirement for Est1.11 Est1 and Cdc13 are enriched at telomeres during late S phase of the cell cycle, the time when telomeres are elongated.12 However, other experiments based on chromatin immunoprecipitation (ChIP) and directed against telomerase-associated proteins suggested that the telomerase catalytic subunit Est2 and the TLC1 RNA are both associated with telomeres from G1 to late S phase of the cell cycle.12,13 An enrichment of Est2 at telomeres during these phases raised the possibility that the telomerase enzyme is associated with telomeres during G1 and S phase in an inactive form. Only in late S phase, this telomere-bound telomerase would be locally activated on short telomeres that need to be elongated.
These above concepts are not necessarily mutually exclusive. Nevertheless, they would tend to predict vastly different behaviors for the localization of the telomerase RNP: the first model suggests a free enzyme that only associates with its substrate for productive synthesis rounds in S phase, while the ChIP data supports the idea of a constitutively telomere-tethered enzyme that somehow needs to be activated. In order to discriminate between these two models, one needs to be able directly to visualize telomerase behavior in vivo during the different phases of the cell cycle. Cytological studies have been hampered by the very low abundance of telomerase factors in yeast (i.e., ~30 molecules/cell for the endogenous TLC1 RNA).14 Although direct visualization the TLC1 RNA has been recently achieved using high sensitivity fluorescence in situ hybridization experiments (FISH),15 those studies did not provide a dynamic view of telomerase localization. In this review, we will discuss a recent study that explores the spatio-temporal choreography of the telomere/telomerase interaction in vivo using a newer RNA-tagging approach. The results presented reconcile the telomere/telomerase association models, providing an integrated view of how telomere homeostasis is achieved.16
Live cell imaging of TLC1 RNA dynamics in yeast
As of to date, several methods to visualize RNA dynamics have been developed. Among them, the MS2-GFP technique is the one which has been used mostly to visualize mRNA trafficking in living cells.17 This technique relies on the insertion of multiple MS2 stem loops (up to 24 copies) in the RNA of interest and this tagged RNA now will be specifically bound with high affinity by the MS2 coat protein fused to a fluorescent protein. The increased local concentration of the MS2-fluorescent protein on the target RNA produces a fluorescent focus that can be visualized by fluorescence microscopy. This approach has been successfully applied to visualize the behavior of several mRNAs in different organisms.18-20 We recently adopted it to produce a version of TLC1 bearing ten MS2 stem loops near the 3′ end of the mature RNA.16 Co-expression of this tagged TLC1-10xMS2 RNA with the MS2 coat protein fused to GFP yielded in several fluorescent foci in yeast nuclei (named hereafter TLC1-GFP). FISH experiments directed against TLC1 RNA confirmed that the position of the TLC1-GFP fluorescent foci correspond to the absolute position of the TLC1 RNA. As this construct completely reconstitutes telomerase activity both in vitro and in vivo, we were now in a position to investigate telomerase RNA localization and dynamics during the cell cycle using live cell fluorescence microscopy.
Telomerase in the Act: Divided we fall
As mentioned above, previous ChIP experiments had shown that the catalytic subunit of the telomerase, Est2, is enriched at telomeres in G1 phase of the cell cycle, an enrichment that is dependent on the presence of the TLC1 RNA.13 This result was validated by FISH experiments showing that the TLC1 RNA co-localizes with telomeres clusters in the G1 phase of the cell cycle.15 Unexpectedly, real-time in vivo imaging and tracking of the TLC1-GFP particles in G1 cells documented a much more rapid and diffusive movement of these molecules as compared with telomere dynamics, visualized by lacO/LacI-GFP tagging under the same imaging conditions. Both velocity and diffusion coefficients of the TLC1-GFP foci were higher than those for the telomere (2.5 and 10 times, respectively), indicating that the telomerase RNA is not stably associated with telomeres during G1, and analogous experiments yielded the same conclusion for G2 cells. Dual-color imaging of both TLC1-GFP and Rap1-mCherry, a telomere protein broadly used as a telomere position marker,21 also failed to provide evidence for a stable colocalization of the telomerase RNA and telomere clusters. However, upon further detailed analyses, telomerase/telomere colocalization can be detected in G1, but they occur in an exceedingly transient fashion (< 5 sec). Therefore, the relative dynamics of the TLC1-GFP molecules in G1 does not support the hypothesis of a stable telomere/telomerase association. The previous ChIP and FISH experiments may simply have detected telomerase crosslinked to telomeres during a very transient interaction.
Telomerase in the Act: United we stand
The behavior of the TLC1 RNA during late S phase of the cell cycle, the time when telomeres are elongated, is quite different. Indeed, TLC1-GFP molecules form between 1 and 3 clusters in more than 50% of late S phase cells. Unlike the TLC1-GFP foci in G1, which are around 300 nm in diameter, these clusters are above 600 nm in diameter and contain a 6 to 15-fold enrichment in TLC1-GFP molecules. More importantly, the TLC1-GFP clusters have the same dynamics as telomeres; i.e similar velocities and diffusion coefficients. Thus, TLC1-GFP clusters may represent active telomerase complexes at telomeres. We named these clusters T-Recs for Telomerase REcruitment Clusters. To determine if T-Recs colocalize with telomeres, we performed dual-color imaging of both TLC1-GFP and Rap1-mCherry, and found that T-Recs stably colocalize with telomeres during all the acquisition time (up to 45 sec). Deletion of factors required for telomerase activity at telomeres lead to a strong reduction in T-Rec accumulation in late S phase cells, suggesting that T-Recs represent active telomerase molecules engaged on telomeres. In order to further test this latter idea, artificial shortening of telomeres was induced using a temperature sensitive allele of yku70 grown at 37°C, followed by restoration of Yku-activity by shifting the cells back at 30°C.22 While initially virtually all telomeres are short due to the transient absence of YKu, nearly all telomeres should be elongated following restoration of this protein’s function. We indeed detected an increased number of T-Recs in late S phase cells, but remarkably, most cells still displayed only one T-Rec and a maximum number of five T-Recs per nucleus was observed. It remains unclear why we observed so few T-Recs in this situation where all telomeres needed lengthening. Is there a limited number of telomeres that can be extended per cell cycle? One can also propose that T-Rec formation depends on a minimum number of telomerase molecules engaged per telomere, or that most telomere elongation events are concentrated in one place in the nucleus, irrespective of how many telomeres are involved.
Altogether, these results demonstrate that T-Recs colocalize with telomeres, their number depends on both telomere length and on the presence of regulators of telomerase activity. They thus represent active forms of telomerase elongating telomeres. How T-Recs form, persist and disappear is still unclear. It has been proposed that telomerase acts as a dimer or a multimer in vivo,23 opening the possibility that T-Recs represent a multimerized form of telomerase elongating a telomere. As mentioned above, T-Recs represent sites where telomeres are elongated, like the DNA replication factories observed in mammalian or yeast cells.24 Although it is not clear how many telomeres are present in one T-Rec, the number of T-Rec per cell that was measured (between one and three) is consistent with the previous observation that less than three telomeres should be elongated at each round of cell division.25 Therefore, one T-Rec possibly contains one telomere or two sister telomeres (from a duplicated chromosome).
The observation that a number of telomerase enzymes cluster in T-Recs may also shed new light on the mechanism of action of telomerase on telomeres. Indeed, while yeast telomerase does not show any processivity in vitro, it is known that during one round of telomere elongation, multiple telomerase molecules can extend a single telomere.26 Consistent with this view, the T-Recs may favor the action of multiple telomerase molecules on a single telomere. Such a mechanism may ensure an extensive elongation of a given telomere by increasing the number of engaged telomerase complexes and thus explain how a single telomere can gain up to hundred nucleotides in one cell cycle.25 Disappearance of T-Recs should be controlled by negative regulators of telomerase such as the Pif1 helicase, which removes telomerase from telomeres following elongation.27 However, we do not know if T-Recs are removed with or without a disaggregation step.
Rif1/2 Control the Cell Cycle-Dependent Recruitment of Telomerase at Telomeres
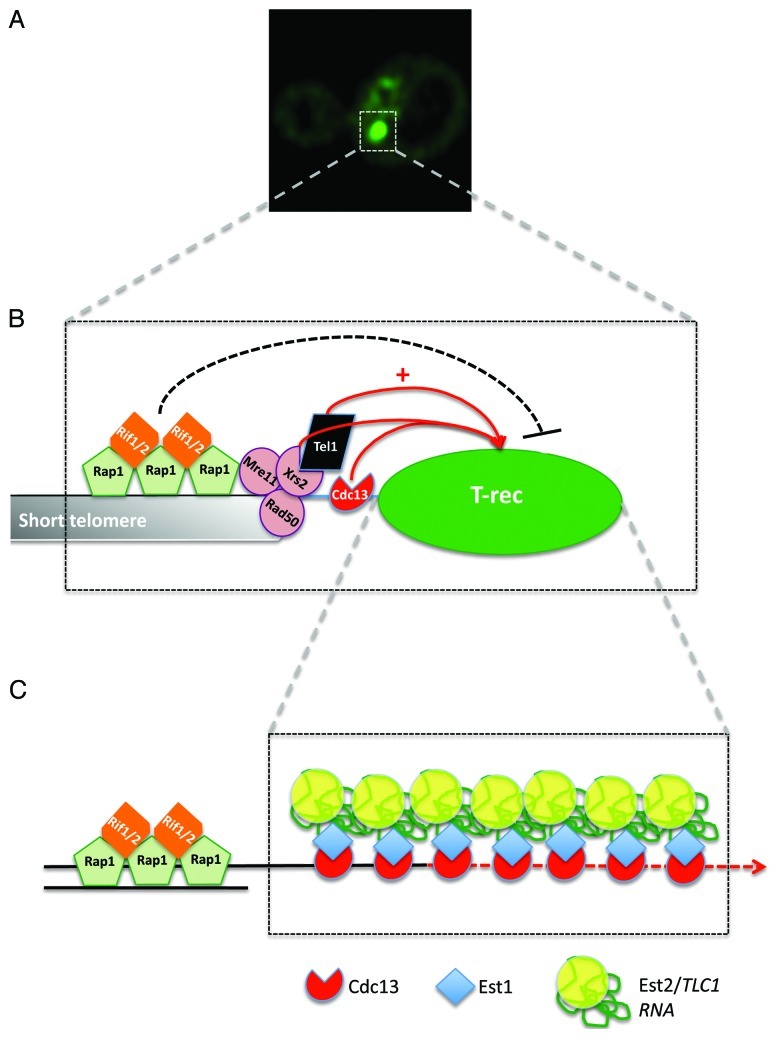
Engaging productive telomere elongation involves first loading of the MRX complex to the ends. The Mre11-Rad50-Xrs2 (MRX) complex is required for proper telomere maintenance and processing by triggering the generation of G-strand extensions and loading of Cdc13.28,29 The Xrs2 subunit of the MRX complex recruits the ATM-related kinase Tel1, which may phosphorylate, among other possible targets, the single-strand telomere DNA binding protein Cdc1330 (but see conflicting results in ref. 31). As mentioned in the introduction, Cdc13 interacts with Est1 to recruit the telomerase holoenzyme to telomeres. The Rif1 and Rif2 proteins are Rap1-interacting proteins that control telomere length by inhibiting Tel1 interaction at long telomeres.32,33 Deletion of either RIF1 or RIF2 induces a telomere hyperelongation phenotype.34 Despite the genetic evidence implicating a Rap1/Rif dependent counting mechanism of telomeric repeats in the regulation of telomere length in vivo,35,36 the details of this mechanism have remained mysterious. Interestingly, while occurrence of T-Recs is restricted to late S phase in wild type cells, they can be detected in all the phases of the cell cycle in RIF deletion mutants.16 Thus, mutations in RIF1 or RIF2 alleviate the cell cycle restriction of T-Rec accumulation. This cell cycle independent formation of T-Recs triggers unregulated telomerase activity, as rif1/2 cells blocked in G1 undergo telomerase-dependent elongation of a short telomere, unlike wild type cells.16 Therefore, the Rif1-Rif2 complex acts as a cell cycle specific inhibitor of telomerase activity by restricting T-Rec formation to the late S phase of the cell cycle. It is possible that in the absence of Rif proteins, telomere-bound MRX loads the Tel1 kinase and stimulates C-strand resection outside of S phase. The resulting G-strand overhangs may trigger T-Rec formation and telomerase activation. In support of this idea, recent data show that Rif1 and Rif2 inhibit the nucleolytic processing of telomeres in G1 and G2 phases of the cell cycle.37 However, it remains to be determined whether the observed T-Recs in rif1/2 cells indeed form outside of S phase or are formed in S phase, never dissolve, and therefore persist after S phase. (Fig. 1)
Figure 1. Model for telomerase recruitment and enrichment at elongating telomere. (A) Example of a Telomerase Recruitment Cluster (T-Rec) in late S phase of the cell cycle (dashed box) (taken from ref. 16). (B) Cell-cycle dependent T-Rec formation on short telomeres depends on the presence of telomerase positive (Tel1, Xrs2, Cdc13) and negative regulators (Rif1/2). (C) Proposed internal architecture of a T-Rec cluster. Successive binding of several telomerase holoenzyme molecules ensures processive elongation of short telomeres in vivo.
Acknowledgments
This work is supported by Canadian Institutes of Health Research grants MOP97874 to R.J.W. and MOP89768 to P.C. F.G. is supported by a post-doctoral fellowship from the Association pour la Recherche sur le Cancer. P.C. is a Senior Scholar from the Fonds de la Recherche en Santé du Québec and R.J.W. holds a Canada Research Chair.
Glossary
Abbreviations:
- ChIP
chromatin immunoprecipitation
- FISH
fluorescent in situ hybridization
- GFP
green fluorescent protein
- MRX complex
Mre11-Rad50-Xrs2 complex
- RNP
ribonucleoprotein complex
- T-Recs
telomerase recruitment clusters
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/21498
References
- 1.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellinger RJ, Zakian VA. Everything You Ever Wanted to Know About Saccharomyces cerevisiae Telomeres: Beginning to End. Genetics. 2012;191:1073–105. doi: 10.1534/genetics.111.137851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autexier C, Lue NF. The structure and function of telomerase reverse transcriptase. Annu Rev Biochem. 2006;75:493–517. doi: 10.1146/annurev.biochem.75.103004.142412. [DOI] [PubMed] [Google Scholar]
- 4.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–98. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 5.Singer MS, Gottschling DE. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266:404–9. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 6.Counter CM, Meyerson M, Eaton EN, Weinberg RA. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci USA. 1997;94:9202–7. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingner J, Cech TR, Hughes TR, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94:11190–5. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stellwagen AE, Haimberger ZW, Veatch JR, Gottschling DE. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 2003;17:2384–95. doi: 10.1101/gad.1125903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009;28:2309–22. doi: 10.1038/emboj.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001;104:387–96. doi: 10.1016/S0092-8674(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 11.Evans SK, Lundblad V. Est1 and Cdc13 as comediators of telomerase access. Science. 1999;286:117–20. doi: 10.1126/science.286.5437.117. [DOI] [PubMed] [Google Scholar]
- 12.Taggart AKP, Teng S-C, Zakian VA. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science. 2002;297:1023–6. doi: 10.1126/science.1074968. [DOI] [PubMed] [Google Scholar]
- 13.Fisher TS, Taggart AKP, Zakian VA. Cell cycle-dependent regulation of yeast telomerase by Ku. Nat Struct Mol Biol. 2004;11:1198–205. doi: 10.1038/nsmb854. [DOI] [PubMed] [Google Scholar]
- 14.Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA. 2006;12:1721–37. doi: 10.1261/rna.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallardo F, Olivier C, Dandjinou AT, Wellinger RJ, Chartrand P. TLC1 RNA nucleo-cytoplasmic trafficking links telomerase biogenesis to its recruitment to telomeres. EMBO J. 2008;27:748–57. doi: 10.1038/emboj.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallardo F, Laterreur N, Cusanelli E, Ouenzar F, Querido E, Wellinger RJ, et al. Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Mol Cell. 2011;44:819–27. doi: 10.1016/j.molcel.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Querido E, Chartrand P. Using Fluorescent Proteins to Study mRNA Trafficking in Living Cells. Methods in Cell Biology: Academic Press, 2008:273-92. [DOI] [PubMed] [Google Scholar]
- 18.Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437–45. doi: 10.1016/S1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- 19.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159–68. doi: 10.1016/S0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 20.Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard J-M, Singer RH, et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol. 2003;13:161–7. doi: 10.1016/S0960-9822(02)01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser SM. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol. 1996;134:1349–63. doi: 10.1083/jcb.134.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravel S, Wellinger RJ. Maintenance of double-stranded telomeric repeats as the critical determinant for cell viability in yeast cells lacking Ku. Mol Cell Biol. 2002;22:2182–93. doi: 10.1128/MCB.22.7.2182-2193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott J, Blackburn EH. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 1997;11:2790–800. doi: 10.1101/gad.11.21.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura E, Blow JJ, Tanaka TU. Live-cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell. 2006;125:1297–308. doi: 10.1016/j.cell.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–35. doi: 10.1016/S0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- 26.Chang M, Arneric M, Lingner J. Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 2007;21:2485–94. doi: 10.1101/gad.1588807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JQ, Monson EK, Teng SC, Schulz VP, Zakian VA. Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science. 2000;289:771–4. doi: 10.1126/science.289.5480.771. [DOI] [PubMed] [Google Scholar]
- 28.Diede SJ, Gottschling DE. Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr Biol. 2001;11:1336–40. doi: 10.1016/S0960-9822(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 29.Larrivée M, LeBel C, Wellinger RJ. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 2004;18:1391–6. doi: 10.1101/gad.1199404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng S-F, Lin J-J, Teng S-C. The telomerase-recruitment domain of the telomere binding protein Cdc13 is regulated by Mec1p/Tel1p-dependent phosphorylation. Nucleic Acids Res. 2006;34:6327–36. doi: 10.1093/nar/gkl786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao H, Toro TB, Paschini M, Braunstein-Ballew B, Cervantes RB, Lundblad V. Telomerase recruitment in Saccharomyces cerevisiae is not dependent on Tel1-mediated phosphorylation of Cdc13. Genetics. 2010;186:1147–59. doi: 10.1534/genetics.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirano Y, Fukunaga K, Sugimoto K. Rif1 and rif2 inhibit localization of tel1 to DNA ends. Mol Cell. 2009;33:312–22. doi: 10.1016/j.molcel.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–60. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 34.Hardy CF, Sussel L, Shore DA. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–14. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 35.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–90. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 36.Levy DL, Blackburn EH. Counting of Rif1p and Rif2p on Saccharomyces cerevisiae telomeres regulates telomere length. Mol Cell Biol. 2004;24:10857–67. doi: 10.1128/MCB.24.24.10857-10867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonetti D, Clerici M, Anbalagan S, Martina M, Lucchini G, Longhese MP. Shelterin-like proteins and Yku inhibit nucleolytic processing of Saccharomyces cerevisiae telomeres. PLoS Genet. 2010;6:e1000966. doi: 10.1371/journal.pgen.1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]