Abstract
The colony stimulating factor-1 (CSF-1) receptor (CSF-1R) directly regulates the development of Paneth cells (PC) and influences proliferation and cell fate in the small intestine (SI). In the present study, we have examined the role of CSF-1 and the CSF-1R in the large intestine, which lacks PC, in the steady state and in response to acute inflammation induced by dextran sulfate sodium (DSS). As previously shown in mouse, immunohistochemical (IHC) analysis of CSF-1R expression showed that the receptor is baso-laterally expressed on epithelial cells of human colonic crypts, indicating that this expression pattern is shared between species. Colons from Csf1r null and Csf1op/op mice were isolated and sectioned for IHC identification of enterocytes, enteroendocrine cells, goblet cells and proliferating cells. Both Csf1r−/− and Csf1op/op mice were found to have colon defects in enterocytes and enteroendocrine cell fate, with excessive goblet cell staining and reduced cell proliferation. In addition, the gene expression profiles of the cell cycle genes, cyclinD1, c-myc, c-fos, and c-myb were suppressed in Csf1r−/− colonic crypt, compared with those of WT mice and the expression of the stem cell marker gene Lgr5 was markedly reduced. However, analysis of the proliferative responses of immortalized mouse colon epithelial cells (lines; Immorto-5 and YAMC) indicated that CSF-1R is not a major regulator of colonocyte proliferation and that its effects on proliferation are indirect. In an examination of the acute inflammatory response, Csf1r +/− male mice were protected from the adverse affects of DSS-induced colitis compared with WT mice, while Csf1r +/− female mice were significantly less protected. These data indicate that CSF-1R signaling plays an important role in colon homeostasis and stem cell gene expression but that the receptor exacerbates the response to inflammatory challenge in male mice.
Introduction
Colonic and small intestinal (SI) crypts share a range of architectural and molecular features and for convenience are often discussed interchangeably. Each generates the three cell lineages; the enterocytes, which secrete hydrolases and absorb nutrients, [1] mucin-producing goblet cells [2], [3] and the less common enteroendocrine cells that secrete a spectrum of mediators including serotonin, secretin and substance P [4], [5]. Similarly, both crypt compartments are regulated by Wnt signaling and dysregulation of this pathway initiates adenoma formation [6]. However, there are as several important differences. The SI possesses PC, which reside as a cluster of up to 16 cells at the SI crypt base [7] that out live the cells of the other three SI lineages and are absent in the colonic crypt. In addition, the SI contain villi, absorptive structures (predominantly enterocytes) supported by approximately 6 crypts, that increase the epithelial surface area of the SI [8].
We have recently shown that the cytokine colony stimulating factor receptor (CSF-1R) is required for PC development and that CSF-1R-deficient mice possess deficits in SI enterocytes and enteroendocrine cells and excess goblet cell production, as well as substantively reduced crypt proliferative capacity and stem cell niche maintenance [9]. Here we demonstrate a role for CSF-1R in the colon, thereby uncoupling the CSF-1R-dependence of PC from the effects of CSF-1R on enterocyte proliferation and lineage specification.
In vivo CSF-1 regulation has been investigated in the CSF-1-deficient osteopetrotic (Csf1op/op) mutant mouse, [10] which possesses an inactivating mutation in the Csf1 gene [11], [12], [13], [14] and in CSF-1R-deficient Csf1r−/− mice with a targeted deletion of the only cellular receptor for CSF-1 [15], [16]. These studies unequivocally established that CSF-1 is the primary regulator of macrophage, [17] osteoclast [16] and Langerhans [18] cell production via CSF-1R signaling [15]. Using approaches pioneered by investigators of the hematopoietic system, we previously showed that CSF-1 supported the colony formation of fetal and new born colonocytes in vitro and that CSF-1R is expressed on the basal lateral surface of mouse colonic crypts [19]. In parallel with the defects we have reported in the SI of mice with defective CSF-1R signaling [9], we now show that these mice also have proliferative deficits in the colonic crypts. Furthermore, the expression of the intestinal stem cell marker gene, Lgr5 and proliferation-associated genes indicative of progenitor cell activity are also reduced. In addition, using an established model for inflammatory bowel disease, we show that heterozygous loss of the Csf1r gene is protective in male mice. Collectively, these studies support a central role for CSF-1R signaling in gastrointestinal homeostasis and disease.
Results
The CSF-1R is Expressed in Human Colonic Crypts
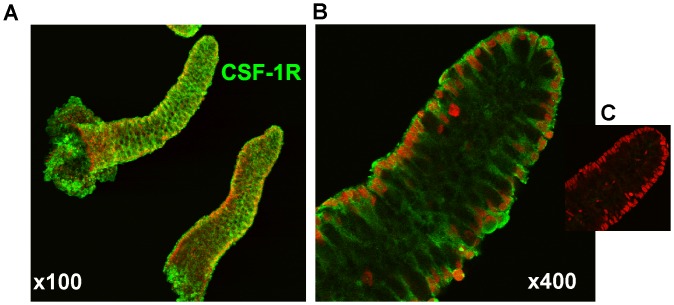
We have previously reported the baso-lateral presence of CSF-1R on isolated mouse colonic crypts [19]. When human crypts were similarly examined using a human CSF-1R-specific antibody and counter-stained with propidium iodide essentially identical images were generated ( Figure 1 ). These data and our previous report of SI defects in mice with ablated CSF-1R signaling prompted us to investigate the role of the CSF-1R in the colon.
Figure 1. Isolated human colonic crypts display robust expression of cell surface CSF-1R.
(A) Low power and (B) high power confocal images stained with FITC-conjugated anti-CSF-1R antibody (green) or (C) secondary antibody alone and counter-stained with propidium iodine (red).
Altered Colon Metrics and Cell Fate in Colons of CSF-1- and CSF-R- Deficient Mice
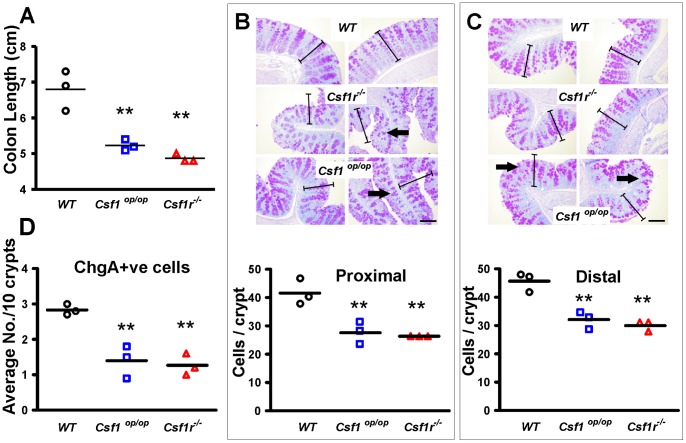
Colons of two-week old Csf1r−/− and Csf1op/op mutant mice and matching wild type (WT) littermate controls were excised from cecum to anus. Three mice of each genotype were evaluated and both mutants were found to have significantly shorter colons (p>0.01), commensurate with their reduced overall size ( Figure 2A ). Tissue was processed for cytochemistry and IHC. Proximal and distal colon regions were evaluated separately. In both regions it was evident that the mucosal thickness in mutant mice was less than that of WT mice ( Figure 2B–C ). Enumeration of cell nuclei in definable crypts revealed that the number of cells per crypt was significantly less in mutants compared to WT ( Figure 2B–C ). PAS (Periodic Acid Schiff) staining suggested that the degree of mucin staining was greater in sections from the mutant mice ( Figure 2B–C ). To explore this observation further, section were also stained with Alcian Blue and the level of mucin staining in the mutant colons was consistently observed to be greater than in WT colons (Figure S1). Staining of sections for Chromogranin A, to evaluate the number of enteroendocrine cells for the whole colon, revealed that both mutant mice had fewer enteroendocrine cells than WT (p<0.01) ( Figure 2D ). These data indicate that the mutant mice have shorter colons, with fewer enterocytes (the predominant cell lineage in the crypt), fewer enteroendocrine cells and greater mucin production.
Figure 2. Two-week old Csf1r−/− and Csf1op/op mutant mice have shorter colons, fewer cells per crypt and fewer enteroendocrine cells.
(A) Colon lengths for each genotype are represented (n = 3). (B,C) Colon sections from proximal (B) and distal (C) regions were stained with PAS to highlight neutral mucin production. The crypt length bars defined in WT colons (B) were duplicated and superimposed on the images in (C) to illustrate the shortened crypts of the mutant mice. Bars = 50 µm. Lower panels show the number of cells per crypt cross section. (D) Average numbers of enteroendocrine cells as visualized by Chromogranin A immunohistochemistry are plotted. Means are depicted by horizontal bars, n = 3 per group, (**P<0.01 using unpaired two-tailed t-tests).
Defective Cell Proliferation in Csf1r −/− and Csf1op/op Colonic Epithelia
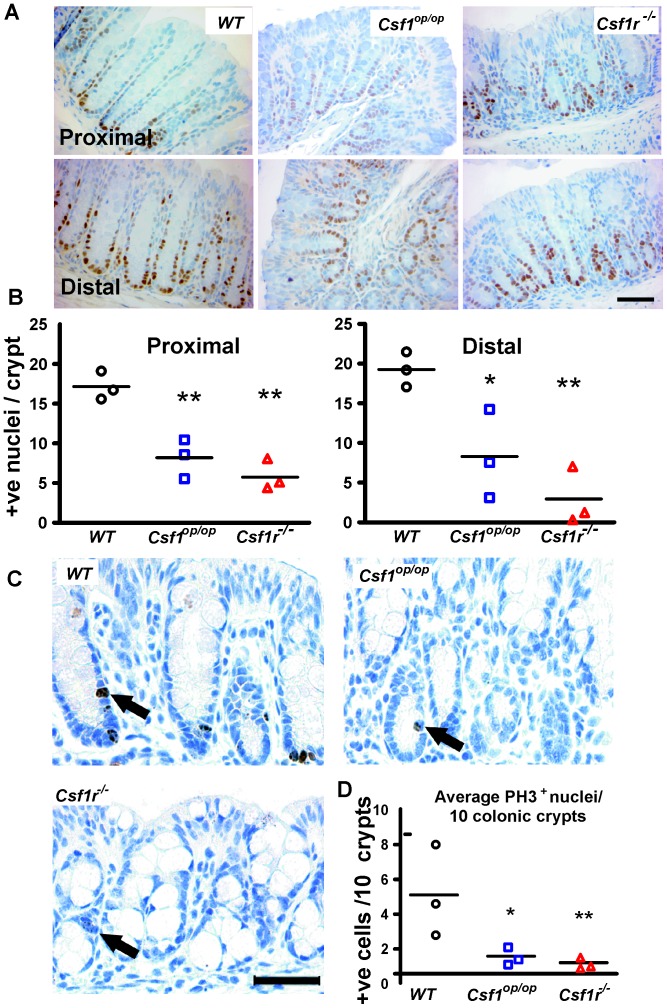
In view of the shorter crypts in the mutant mice, we postulated that proliferation within their crypts was defective. To measure this, sections were stained for proliferating cell nuclear antigen (PCNA). Images of these sections ( Figure 3A ) suggesting that there are fewer positive cells in the mutant crypts was confirmed by counting positive nuclei per crypt (proximal; P<0.01), (distal; Csf1op/op; P<0.05, Csf1r−/−; P<0.01) ( Figure 3B ). PCNA identifies cells that are in cell cycle but does not provide information about whether cells are progressing through the cell cycle. Accordingly, sections were additionally stained for the G2/M marker, phospho-histone 3 (PH3) which showed that the mutant crypts had fewer cells that had progressed to these later phases of the cell cycle (distal plus proximal: Csf1op/op; P<0.05, Csf1r−/−; P<0.01) ( Figure 3C–D ).
Figure 3. Decreased proliferation in Csf1r−/− and Csf1op/op mouse colonic epithelium.
(A) Representative PCNA immunostaining in proximal and distal colon sections are shown. (B) Enumeration of the total number of positively stained nuclei reveals that average number of proliferating cells/crypt is significantly reduced in proximal (left panel) and distal (right panel) colons of Csf1op/op and Csf1r−/−, compared to WT, mice. Bar = 50 µm (n = 3). (C) Staining with the G2/M phase marker, phosphorylated histone H3 (PH-3, black arrows) also shows decreased numbers of cells in cycle per crypt in both mutants. Bar = 50 µm. Means are depicted by horizontal bars, n = 3 per group, **P<0.01, *P<0.05, using unpaired two-tailed t-test. (D) Quantitation the PH-3+ staining where means are depicted by horizontal bars, (n = 3 per group). Having predicted a reduced level of PH3 in Csf1r−/− crypts data was analyzed using one-tailed t-tests, (**P<0.01, *P<0.05).
Reduced Expression of Cell Cycle Genes in Csf1r −/− Crypt Epithelial Cells
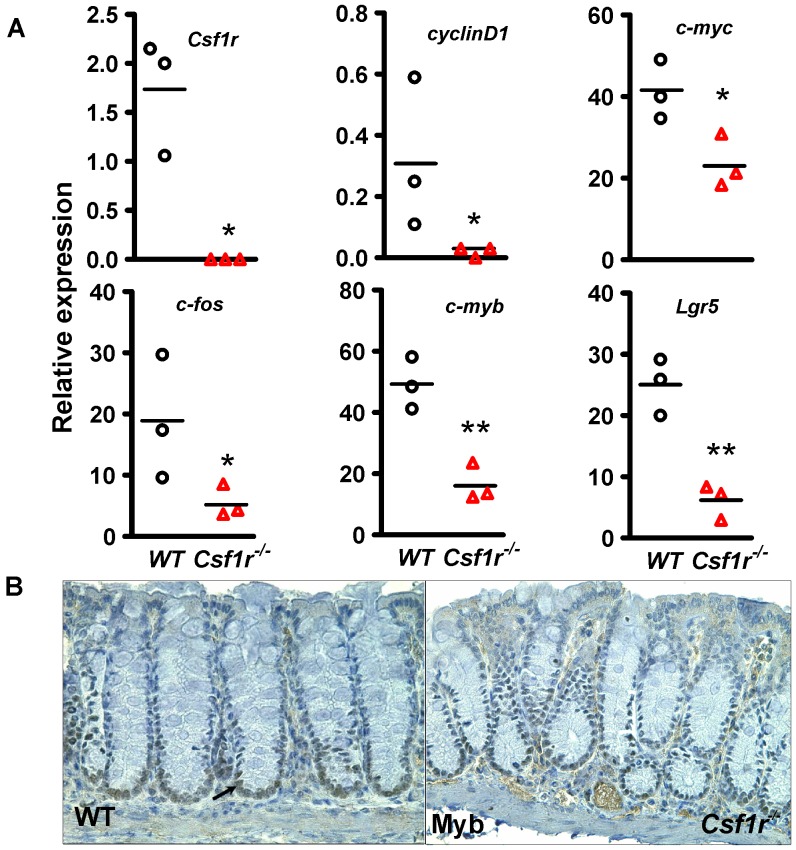
As the defect in proliferation was slightly worse in Csf1r −/− mice, we focused attention on colons from these mice to investigate the expression of genes involved in cell cycle progression. Colonic crypts from WT and Csf1r−/− mice were isolated for qRT-PCR analysis. As expected Csf1r expression was undetectable in the Csf1r−/− mice. The expression of growth factor receptor target genes, c-myc and cyclinD1 [20], [21] and the immediate early response target gene c-fos [22] were expressed at lower levels (P<0.05) in the colonic epithelium of Csf1r−/− compared to WT mice ( Figure 4 ). As we had previously reported a role for c-myb in colon homeostasis [23] this gene was also evaluated and found to be expressed at a lower level (P<0.003). Finally, the intestinal stem/progenitor marker Lgr5 [24] which we have found to be in part regulated by Myb [25] was similarly found to be significantly under-expressed (P<0.002) ( Figure 4A ). Immunochemical staining of Myb ( Figure 4B ) confirmed that Myb protein expression was lower in the Csf1r−/− mice. This evaluation of genes associated with proliferation confirms that the reduced proliferation found in the Csf1r−/− colonic crypts and their correspondingly shorter length is correlated with reduced cell cycle gene expression. These data also highlight the importance of the CSF-1R in maintaining intestinal stem cell gene expression.
Figure 4. Csf1r−/− colonic crypts show reduced expression of cell cycle gene mRNAs compared to WT colonic crypts.
(A) RNA from WT and Csf1r−/− crypts was subjected to qRT-PCR analysis for expression of Csf1r, the cell cycle genes cyclinD1 and c-myc, c-myb and the immediate early gene, c-fos, as well as the intestinal stem cell marker, Lgr5. Expression of all six genes was reduced in the Csf1r−/− compared with WT crypts, (n = 3, *P<0.05; **0.01). (B) Immunohistochemical staining for Myb. In addition to a shorter crypt length, the expression of Myb, which has a direct effect on this metric [23], is less extensive in Csf1r−/− crypts. Black arrow indicates Myb positive nucleus. Means are depicted by horizontal bars, n = 3 per group and having predicted a reduced level of gene expression in Csf1rKO crypts data were analyzed using one-tailed t-tests**P<0.01, *P<0.05.
Modest Proliferation Colonic Epithelial Cells in Response to CSF-1R Ligands in vitro is Consistent with Indirect Regulation of Epithelial Cell Proliferation by these Ligands in vivo
To further explore the role of the CSF-1R signaling in colonic epithelial cells, we examined the response of cells of the immortalized, colonic epithelial cell lines YAMC [26] and Immorto-5 [19] to growth factor stimulation following serum starvation. Under these conditions, pregnant mouse uterus extract (PMUE), a good source of CSF-1 [19], [27], [28] stimulated robust YAMC growth (Figure S2A), phospho-ERK1/2 induction (Figure S2B) and c-myb, c-myc, cyclinD1, Ets-2 and c-jun gene expression (Figure S3). Cytokine antibody arrays confirmed the presence of CSF-1 in PMUE, but also demonstrated the presence of several other factors (Figure S2C). However, attempts to replicate the level of growth stimulation with either purified recombinant CSF-1, or purified IL-34, which also activates the CSF-1R [29], were only partially successful (Figure S2D–E). Although these experiments were carried out with cell lines, the lack of a strong proliferative response to CSF-1R ligands raised the possibility that the in vivo requirement of the CSF-1R for epithelial cell proliferation is indirect. As recent studies have shown that PC, the CSF-1R-responsive cells of the SI [9], produce two key epithelial cytokines Wnt3 and R-Spondin [30], we tested the ability of these factors to stimulate proliferation of our colonic epithelial cells. Consistent with an indirect effect of CSF-1 on epithelial cell proliferation, robust cell proliferation was seen with R-spondin, with or without CSF-1 plus Wnt3a, but not with Wnt3a alone (Figure S2F).
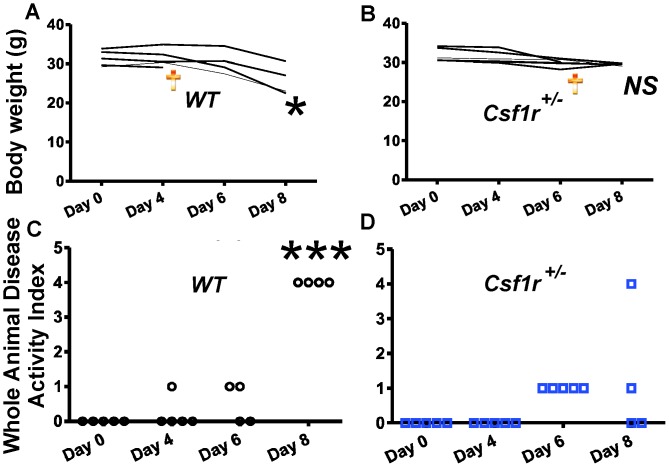
Loss of a Single Csf1r Allele in Male Mice Alleviates DSS-induced Colitis
As pre-treatment of mice with neutralizing anti-CSF-1 antibodies protects them from DSS-induced colitis [31], we investigated whether loss of CSF-1R expression also afforded protection. Since Csf1r−/− FVB/NJ mice do not normally survive beyond 1 month of age, we queried whether loss of one Csf1r allele in Csf1+/− mice was sufficient. WT (Csf1+/+) and Csf1+/− mice were provided with water containing 2% w/v DSS ad libitum for 8 days and evaluated for inflammatory responses. While the weights of male WT male mice at day 8 had decreased significantly by ∼20% (mean +/− SEM; Day 0∶31.42 g +/−0.90, Day 8∶25.78 g +/−1.91; P<0.02; t-test) ( Figure 5A ), with one mouse dying at day 5, the weights of the Csf1r (mean +/− SEM; Day 0∶32.00 g +/−0.71, Day 8∶29.50 g +/−0/13; P = NS) ( Figure 5B ), with one mouse dying at day 7. In contrast, neither WT nor Csf1r+/− females exhibited significant DSS-induced weight loss and remained healthy after the 8-day treatment (data not shown). To further assess the effects of DSS treatment, we monitored the Whole Animal Disease Activity Index [31] as described in the Materials and Methods. Female mice of both genotypes showed no observable inflammation (data not shown). The most profound differences between the WT and Csf1r+/− male mice were noted at day 8 ( Figure 5C–D ), when male Csf1r+/− mice exhibited fewer symptoms compared to WT mice (p<0.001; ANOVA). Others have also reported that male mice are more susceptible to DSS-induced colitis, but not specifically in FVB/n mice [32], although this strain is differentially adversely affected in mdr1a mutant males [33].
Figure 5. Loss of one Csf1r allele in male mice is protective for weight loss and symptoms associated with DSS-induced colitis.
Eight to 10-week old FVB/NJ male mice were given water with 2% w/v DSS ad libitum for 8 days and their body weight changes monitored during this period. (A–B) Significant changes in body weight of WT but not in Csf1r+/− mice was evident at day 8 of treatment. (C–D) Whole Animal Disease Activity Index for (WADI) WT (C) and Csf1r+/− (D) mice based on the modified criteria reported by Marshall et al (2007) [31] and described in the Methods. One WT mouse died at day 5 and one Csf1r+/− mouse died at day 7 whereas WT mice overall showed significantly elevated WADI compared to Csf1r+/− mice (*P<0.001, ANOVA with Bonferroni’s Multiple Comparison Test). Female mice of both genotypes show no observable clinical symptoms of inflammation (data not shown).
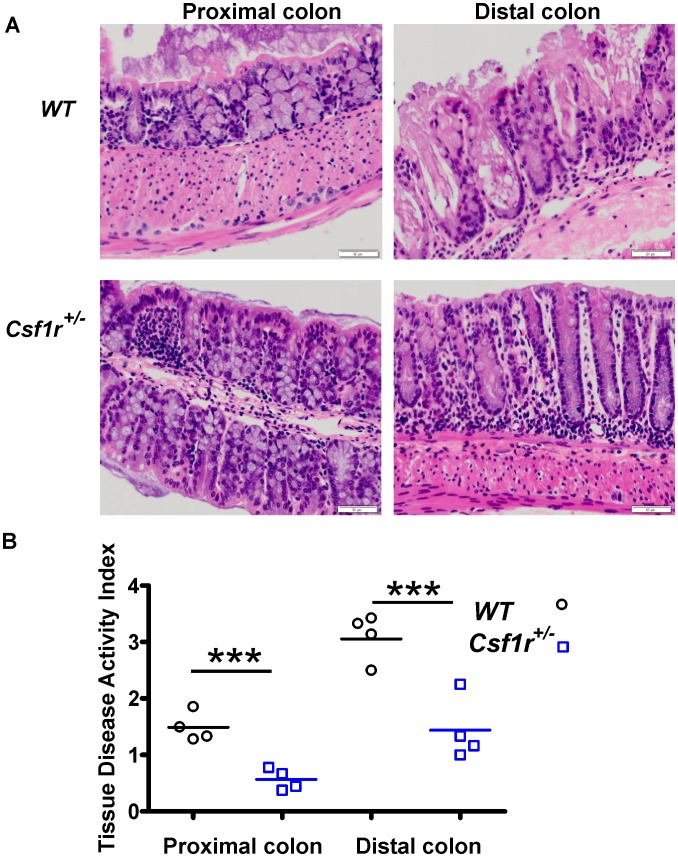
Further morphological evaluation of the SI and colons of WT and Csf1r+/− male mice failed to reveal significant differences in SI crypt or villi morphology, cell number or intestinal length (Figure S4). However, DSS treatment had pronounced effects on the colon as assessed using the modified criteria of Dieleman et al (1998) [34] ( Figure 6A ). Crypt damage was more severe in the distal colon compared to proximal regions and damage was observed in both WT and Csf1r+/− male mice. However, Csf1r+/− mice have significantly less crypt damage throughout the colon compared to WT mice (P<0.001 and 0.003; proximal and distal colon respectively; t-test) ( Figure 6B ). These data indicate that reduction of the Csf1r gene dosage has a dramatic impact on the inflammatory response mediating damage associated with exposure to DSS.
Figure 6. Male Csf1r+/− mice show less colonic epithelial damage compared to WT male mice.
(A) Colons were separated into proximal and distal regions and stained with H & E, (Bar = 100 µm). (B) Crypt damage is more severe in the distal colon compared to proximal regions and is more severe in WT mice. Colonic crypt damage in each section (Tissue Disease Activity Index) was graded according to the modified criteria of Dieleman et al (1998) [34], as described in the Methods (n = 4, **P<0.01; ***P<0.001;data analyzed using two-tailed t-tests).
Discussion
The maintenance of intestinal homeostasis requires the orchestration of many signaling pathways which may also be activated when the gastrointestinal tract is subjected to damage or stress [35], [36]. The recent discovery of the importance of the CSF-1R in SI homeostasis and differentiation [9] indicates that pathways downstream of this receptor could also be involved. The most obvious role of the CSF-1R in gut epithelium appears to be regulation of the development and maintenance of PC. However, CSF-1R deficiency is also associated with decreased Lgr5+ mRNA expression and decreased proliferation and altered cell fates of epithelial cells [9]. The discovery that Lgr5+ cells have the capacity to regenerate SI crypts and villi in vitro [37], that these cells are embedded among the PC in the crypts [37], that PC produce epithelial growth factors [30] and support Lgr5+ve intestinal stem cell propagation in vitro [37] and that there is local regulation of PC via CSF-1-expressing cells within the crypt [9], has drawn attention to the relatively long-lived PC population [38]. Indeed, PC appear to play an important role in responding to tissue damage and in activating the quiescent stem cell pool [39]. While PCs are not normally found in colon, because we observed decreased proliferation and altered cell fates of SI epithelial cells in CSF-1 - and CSF-1R -deficient mice, [9] we decided to examine the effects of CSF-1 and CSF-1R deficiency in normal colon and in mice with DSS-induced colitis. As discussed below, despite the absence of PC in colon, we observe defects in epithelial proliferation and differentiation that parallel those found in the SIs of CSF-1/CSF-1R-deficient mice.
In contrast to the localization of CSF-1R+ cells (PC) to the crypt base of the SI, in both the mouse [19] and human ( Figure 1 ) colon the receptor is expressed throughout the crypt, despite the localization of Lgr5+ve cells in small numbers at the base of the colonic crypt [37]. It has been proposed that a mucous secreting non-goblet cell in the colonic crypt base is the PC equivalent cell [40] and recently it has been suggested that the cell surface antigen CD24 may mark the PC equivalent cells in the colon [30]. However, we found no significant difference in the expression of CD24a mRNA or of the mRNA encoding the PC product, R-spondin1, in Csf1r−/− and WT colonic crypts (Figure S5). (We could not detect CD24 by IHC or confocal microscopy in WT mouse colon despite its strong expression in the SI, Akçora, et al, In Press).
Previously we showed that expression of the cell surface CSF-1 isoform could completely rescue the CSF-1-deficient phenotype within the SI and that the CSF-1-reporter expressing cells were localized in the crypts adjacent to PC, suggesting that regulation of PC by CSF-1 was local, if not juxtacrine [9]. Recently, we specifically deleted the Csf1r gene in the mouse intestinal epithelium and found a reduction in PC, Lgr5 expression, cell proliferation and altered cell fate that phenocopied those observed in mice with the global Csf1r deletion (Akçora, et al, In Press). As PCs appear to be the only CSF-1R-expressing cells of the SI epithelium, these results are consistent with an aberrant regulation by PC of the proliferation and fate of epithelial cells resulting from disruption of the direct, local regulation of PCs by CSF-1. Thus it appears that it is through its action in PCs that the CSF-1R plays such a critical role in the SI. In colon, despite the lack of PCs, we have shown that, as in the SI, CSF-1R deficiency results in decreased cell proliferation, reduction in the expression of cell cycle genes and altered cell fate. Most importantly, as in SI, we have found that the expression of the mRNA for Lgr5, the most compelling stem cell molecular marker in the GI tract to date, is markedly reduced in the colonic crypts of Csf1r−/− compared with WT mice. Our failure to demonstrate robust CSF-1 stimulation of the proliferation of cells of two colonic epithelial cell lines, compared with their robust response to R spondin, suggests that, as in SI, epithelial cell proliferation is not directly regulated through the CSF-1R, despite the broader expression of the CSF-1R in colonic crypts. Thus these experiments demonstrate a critical role for the CSF-1R in the development of the colonic epithelium that does not appear to be a direct effect of regulation of the proliferation and differentiation of epithelial cells. In colon, it is possible that the CSF-1R is required for the development of cells with a function analogous to that of intestinal stem cell supporting PCs.
CSF-1 has been implicated in a range of inflammatory contexts [41]. A role for CSF-1 in GI inflammatory responses has been shown by the demonstration that administration of an anti-CSF-1 neutralizing antibody in mice is partially protective in DSS-induced colitis [31]. We used a genetic approach to examine the role of CSF-1R signaling in the same model system. We have shown that loss of one CSF-1R allele affords substantial protection from colon damage in male mice receiving a single challenge with DSS. In contrast, both WT and Csf1r +/− female mice were minimally affected by the same DSS treatment regime. Interestingly the same gender difference has been reported for colitis in man [42] and in there is indirect evidence that estrogen treatment might be protective in female patients with inflammatory bowel disease [43], [44]. In contrast to the protection we observed in male Csf1r +/− mice, heterozygous loss of PPRAγ gene in female mice exacerbated DSS-induced colitis [45]. The fact that Csf1r+/− mice are indistinguishable from WT litter-mates when unchallenged, but protected when stressed indicates the importance of Csf1r gene dosage in DSS-induced colitis. Humans with hypomorphic mutations that dampen CSF-1R signaling might be protected from IBD and, as has been suggested by others [31], therapies inhibiting CSF-1 or CSF-1R may be beneficial in the treatment of this disease. However, additional studies are needed to determine whether DSS treatment affects the colon directly, or if the acute damage response is mediated by macrophages.
CSF-1 is the primary regulator of monocyte proliferation and differentiation [46], but more broadly it has also been implicated in trophoblastic implantation [47] and mammary gland development particularly during pregnancy [48]. The CSF-1R is expressed in normal intestinal [49] and upper airway [50] epithelia and lung, ovarian, breast and prostate cancers, or epithelial cell lines derived from them [51], [52], [53], [54]. Furthermore normal and adenomatous polyps and colon tumors express the CSF-1R [52], [53] and its expression is evident in the lamina propria macrophages of the SI [55]. CSF-1R signaling also plays an augmenting role in the expansion of hematopoietic stem and progenitor cells [14], [56], [57], an indispensible role in osteoclastogenesis [58] and regulates both microglia and neural progenitors in brain [59]. Our previous observations [9] in SI, coupled with these studies in colon, showing that multiple epithelial lineages are influenced by CSF-1R ablation, clearly indicate that this receptor plays an important role in the development and function of the gastrointestinal tract.
Methods
Human Colon Crypts
Normal human mucosa was obtained from a surgical resection for colon cancer as tissue adjacent, but separate from the tumor, with institutional human ethics approval. Crypts were released as described elsewhere [60] and subjected to confocal microscopy as reported previously [19] using anti-CD115/c-FMS/CSF-1R (abm-77 Rat anti-human monoclonal RDI-Fitzgerald) at 1∶250 and secondary anti-Rat FITC antibody.
Mice
Csf1 op/+ [10] and Csf1r−/ + [15] mice, backcrossed on the FVB/NJ background for at least 10 generations [16], were used to generate homozygous mutant and WT (+/+) control mice. Mice were housed under SPF conditions and all experimentation was carried out with approval of the Peter MacCallum Cancer Centre institutional animal ethic committee (Project #E389).
Histochemistry, Immunohistochemistry and Immunofluorescence
Post-natal day 14 mouse pups were perfused at atmospheric pressure with periodate-lysine-2% paraformaldehyde-0.05% glutaraldehyde, pH 7.4 (PLPG) [61], their intestines from the anus to stomach removed and opened longitudinally by incision along the length of the intestine, the contents removed by rinsing in PBS and the intestines fixed in PLPG overnight, prior to immersion in 70% ethanol. Paraffin embedding of the tissues was arranged such that tissue orientation could be determined. Sections were treated with periodic acid, then stained in Schiff’s reagent (0.5% pararosanaline, 1% sodium metabisulfite; PAS staining) and counterstained with hematoxylin. Some sections were also stained with Alcian Blue. The mouse anti-PCNA antibody, PC-10 (1∶800) (Dako, #M0879) was used to identify PCNA followed by processing with the Dako Envison+ mouse detection kit and photographed using a Olympus Bx51 upright microscope. For Myb and hematoxylin and eosin (H & E) staining, colon sections were fixed in methacarn (60% methanol, 30% chloroform, 10% acetic acid) for 2 h and transferred to 70% ethanol, embedded, sectioned and stained with H & E. Full crypts, exposing a lumen and identifiable base, were scored. Crypt cells per 40–50 longitudinal sections were scored as previously described [62]. Sample groups were subjected to one-way analysis of variance (ANOVA) using OriginR software. c-Myb was visualized using Mab1.1 and processed as described previously [63] using antigen retrieval by boiling slides in 1 mM EDTA in a pressure cooker for 3 min. Rabbit anti-Chromogranin A was used at 1∶100 (SC-13090-Santa Cruz) and anti-Phospho-histone 3 at a final titer of 1∶200 (Upstate Biotech) following citrate buffer antigen retrieval. Donkey anti-goat-HRP at 1∶250 (Santa Cruz) was used as a secondary antibody. Crypt cell counting was performed on longitudinal sections, blinded for genotype where nuclei were used to identify each cell. Crypts were identified at 100X magnification, whereby the lumen could be seen to traverse the crypt, then counted at 400X magnification. At least 40 crypts were examined per genotype with 3–5 mice per genotype.
Dextran Sulfate Sodium (DSS) Studies
Female and male FVB/n mice aged from 8–10 weeks were fed water with 2% w/v DSS ad libitum for 8 days and observed for inflammatory responses as well as changes in body weight. Clinical symptoms were assessed based on the modified criteria reported by Marshall et al., 2007 [31] to define the Animal Disease Activity Index, in which a higher score correlates with increasing severity: 0 = Healthy; 1 = Appearance of diarrhoea; 2 = Sign of fecal blood, 3 = Bloody diarrhoea; 4 = Bleeding from the anus. For histology, colons were separated into proximal and distal regions and the regions were then further divided into 600 µm sections. Colonic crypt damage in each section was graded according to the modified criteria published by Dieleman et al (1998) [34], which we describe as the Tissue Disease Activity Index: 0 = No damage; 1 = Basal 1/3 damaged; 2 = Basal 2/3 damaged; 3 = Only surface epithelium intact; 4 = Entire crypt and epithelium lost.
Quantitative RT-PCR Analysis of Crypt Epithelium RNA
Crypt epithelium was prepared and its purity confirmed as described [64]. Real-time RT-PCR reactions were conducted on genomic DNA-depleted RNA using the Bio-Rad iQ-5 i-cycler system (Bio-Rad Laboratories, CA) and the appropriate primers. Gene expression was normalized to gapdh.
Cell Culture
YAMC [26] and IM-5 [19] cells are grown in RPMI-1640 plus HEPES medium with 10% FCS (fetal calf serum). For the growth factor stimulated proliferation studies, cells were plated in 12 well plates at low density and allowed to attach for 24 hr in FCS-containing medium. The wells were washed three times with PBS and the cells incubated for an additional for 24 hr in FCS-free medium, followed by further incubation in FCS-free medium containing different concentrations of pregnant mouse uterine extract (PMUE), as a source of CSF-1 [19], or purified growth factors. Viable cells were scored at 3–5 days post-growth factor addition by incubation in MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), followed by cell lysis and reading of the optical density at 570 nm. The growth factors, recombinant murine CSF-1 (Peprotech), murine interleukin 34 (IL-34), Wnt3a and R-Spondin (500 ng/ml; R&D Systems), were diluted to their final working concentrations in PBS immediately prior to addition to cell cultures. For mRNA analyses, cells were washed with PBS and then lysed in Tryzol. RNAse-free DNase1-treated total RNA was then processed for RT-PCR. List of primers used in Q-RT-PCR analyses is provided in Table 1 .
Table 1. List of primers used in Q-RT-PCR analyses.
| Gene | Forward Primer | Reverse Primer |
| c-myc | 5′-AAGGCCCCCAAGGTAGTGA-3′ | 5′-TCCATTCAAGCAGACGAGCA-3′ |
| c-myb | 5′-AATTATCTGCCCAACCGG-3′ | 5′- AGACCAACGCTTCGGACC-3′ |
| Csf1r | 5′-CCTCCTCTGGTCCTGCTG-3′ | 5′-CATTCCACACTGCCATTGC-3′ |
| cyclinD1 | 5′-AGGCTACAGAAGAGTATTTATGGGAAA-3′ | 5′-TGCGTTTGAATCAAGGGAGAT-3′ |
| lgr5 | 5′-CAAGCCATGACCTTGGCCCTG-3′ | 5′-TTTCCCAGGGAGTGGATTCTATT-3′ |
| c-fos | 5′-CCGATGACCTTGGCTTCC-3′ | 5′-TGCTGATGCTCTTGACTGG-3′ |
| c-jun | 5′-GCAGACAGACAGACAGAC-3′ | 5′-GAAGACAAACGGATGAACAG-3′ |
| ets-2 | 5′-GATCGCGCACTTCCGCTCTC-3′ | 5′-GATCGAGAGCGGAAGTGCGC-3′ |
| R-spondin-1 | 5′-CAAGGGCAAGAGACAGAG-3′ | 5′-TCCAGCAGAATGAAGAGC-3′ |
| CD24a | 5′ GGCAACCACAAGTCCAATG-3′ | 5′-AACTCCAGCAGATTCAATAGC-3′ |
| Gapdh | 5′-CAACTACATGGTCTACATGTTCCAGTATG-3′ | 5′-CTCCCTAGGCCCCTCCTGTTATTAT-3′ |
Cytokine Array
RayBioR Cytokine antibody Array 3 was used to assess the presence of CSF-1 and other factors present in PMUE according to manufacturer instructions.
Statistics
Data were analyzed using GraphPad Prism5 statistical package where p<0.05 was considered to be statistically significant.
Supporting Information
Alcian blue (AB) staining shows increased acidic mucin production in Csf1r−/− and Csf1op/op colonic epithelium. Similar to, but more extensive than observed with PAS staining ( Figure 1 ), AB staining shows aberrant goblet cell localization and mucin deposition in both the proximal and distal colon. Mucin deposition was most pronounced in the Csf1r−/− colonic epithelium. Bar = 50 µm.
(PDF)
PMUE-stimulation of immortalized colonic epithelial cells YAMC cells following serum starvation. (A) Five×104 YAMC cells were cultured in fetal calf serum-free media with and without increasing amounts of PMUE (a source of CSF-1) prior to assessment of viable cell numbers by MTT assay at day 5. Upper panel: Representative images of wells incubated without or with the indicated concentrations of PMUE. Lower panel: Quantitation of data from multiple plates (n = 4, *P<0.05; **0.01; ***0.001; ANOVA with Bonferroni’s multiple comparison Testing). (B) Time course of Immorto-5 (IM-5) cells Erk1/2 phosphorylation status in response to PMUE. Cytosolic fractions of cells grown with fetal calf serum (FCS), or serum-starved, or serum-starved and then incubated with 5 µl/ml PMUE for the indicated times were subjected to SDS-PAGE and western blotted for phospho-ERK 1/2 and total ERK 1/2. (C) Cytokine antibody arrays show that CSF-1 is the predominant, but not the only growth factor/cytokine in PMUE (red ellipse). The next most abundant factors identified were IGFBP-3 and MIP-2 (black rectangles). (D–E) IM-5 (like YAMC, data not shown) cells show robust proliferation by MTT assay in response to PMUE but and slight stimulation by purified the CSF-1R ligands, CSF-1 or IL-34. (F) Immorto-5 cells respond strongly to R-spondin in the presence or absence of CSF-1, while Wnt3a has no stimulatory effect alone or in combination with R-spondin or CSF-1, (*P<0.05; **0.01. *P<0.001, analysed using one-tailed t-tests).
(PDF)
Cell cycle and immediate gene expression induction in colonic epithelial cells following PMUE stimulation. IM-5 cells were cultured in the presence of FCS (+FCS), or serum-starved (-FCS), or serum-starved and incubated with 5 µl/ml PMUE for the indicated times prior to extraction of RNA for analysis of gene expression by qRT-PCR. Results show induction of immediate early genes (ets-2 & c-Jun) and cell cycle genes (c-myc, c-myb & cyclinD1) following PMUE stimulation, (Means ± SEM, 6 replicates. **P<0.01; one way ANOVA with Bonferroni’s multiple comparison testing).
(PDF)
Absence of obvious differences between male FVB/NJ Csf1r+/− and WT small intestines following DSS-induced colitis. As it has been reported that an increase in villus height and crypt depth may occur in response to DSS-induced colitis [65], the number of cell nuclei in the small intestinal villus and crypt of male mice was determined (25 crypt & villi per region, n = 4). No morphological (duodenum, jejunum or ileum) or numerically significant differences in cells per crypts (bottom left panel) or villi (bottom middle panel) between WT and Csf1r+/− mice was observed. In addition, no significant differences in intestinal length between the DSS-treated WT and Csf1r+/− mice were detected in small intestines or colons (bottom right panel). Images are representative H&E stained section of small intestine, (Bar = 100 µm).
(PDF)
CD24a and R-spondin mRNA expression are not significantly altered in Csf1r−/− colonic crypts. (A) Absence of Csf1r mRNA in Csf1r−/− crypts. No significant change in CD24a mRNA (B) or R-spondin-1 mRNA (C) expression in Csf1r−/− compared with WT crypts was observed, (Means ± SEM, 4 replicates. **P<0.05; one-tailed t-test).
(PDF)
Acknowledgments
We thank Ms Sally Lightowler and Ms Shienny Sampurno for expert research assistance and Dr Markus Germann for reading and making comments on the manuscript.
Funding Statement
This work was supported by the National Health and Medical Research Council of Australia Program # 487922, the Cancer Council of Victoria project grant #400217 (R.R.), National Institutes of Health grant CA32551 (E.R.S), the Albert Einstein College of Medicine Cancer Center grant 5P30-CA13330, an American Society of Hematology Fellow Scholar Award (X-M. D.) and a Leukaemia and Lymphoma Society Special Fellow Award (X-M.D). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cheng H, Leblond CP (1974) Origin, Differentiation And Renewal Of 4 Main Epithelial-Cell Types In Mouse Small Intestine.1. Columnar Cell. American Journal Of Anatomy 141: 461–479. [DOI] [PubMed] [Google Scholar]
- 2. Cheng H (1974) Origin, Differentiation And Renewal Of 4 Main Epithelial-Cell Types In Mouse Small Intestine.2. Mucous Cells. American Journal Of Anatomy 141: 481–501. [DOI] [PubMed] [Google Scholar]
- 3. Sancho E, Batlle E, Clevers H (2004) Signaling pathways in intestinal development and cancer. Annual Review Of Cell And Developmental Biology 20: 695–723. [DOI] [PubMed] [Google Scholar]
- 4. Cheng H, Leblond CP (1974) Origin, Differentiation And Renewal Of 4 Main Epithelial-Cell Types In Mouse Small Intestine.3. Entero-Endocrine Cells. American Journal Of Anatomy 141: 503–519. [DOI] [PubMed] [Google Scholar]
- 5.Hocker M, Wiedenmann B (1998) Molecular mechanisms of enteroendocrine differentiation. Annals of the New York Academy of Sciences; Intestinal plasticity in health and disease: 160–174. [DOI] [PubMed]
- 6. Gregorieff A, Clevers H (2005) Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890. [DOI] [PubMed] [Google Scholar]
- 7. Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, et al. (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144. [DOI] [PubMed] [Google Scholar]
- 8. Potten CS (1992) The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 11: 179–195. [DOI] [PubMed] [Google Scholar]
- 9.Huynh D, Dai XM, Nandi S, Lightowler S, Trivett M, et al.. (2009) Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine. Gastroenterology 137: 136–144, 144 e131–133. [DOI] [PMC free article] [PubMed]
- 10. Marks SC, Lane PW (1976) Osteopetrosis, A New Recessive Skeletal Mutation On Chromosome 12 Of Mouse. Journal Of Heredity 67: 11–18. [DOI] [PubMed] [Google Scholar]
- 11. Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, et al. (1994) Role Of Colony-Stimulating Factor-I In The Establishment And Regulation Of Tissue Macrophages During Postnatal-Development Of The Mouse. Development 120: 1357–1372. [DOI] [PubMed] [Google Scholar]
- 12. Pollard JW, Stanley ER (1996) Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Advances in Developmental Biochemistry 4: 153–193. [Google Scholar]
- 13. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, et al. (1990) Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A 87: 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, et al. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345: 442–444. [DOI] [PubMed] [Google Scholar]
- 15. Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, et al. (2002) Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99: 111–120. [DOI] [PubMed] [Google Scholar]
- 16. Dai XM, Zong XH, Akhter MP, Stanley ER (2004) Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res 19: 1441–1451. [DOI] [PubMed] [Google Scholar]
- 17. Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, et al. (1994) Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120: 1357–1372. [DOI] [PubMed] [Google Scholar]
- 18. Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, et al. (2006) Langerhans cells arise from monocytes in vivo. Nat Immunol 7: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramsay RG, Micallef SJ, Williams B, Lightowler S, Vincan E, et al. (2004) Colony-stimulating factor-1 promotes clonogenic growth of normal murine colonic crypt epithelial cells in vitro. J Interferon Cytokine Res 24: 416–427. [DOI] [PubMed] [Google Scholar]
- 20.Roussel MF (1994) Signal transduction by the macrophage-colony-stimulating factor receptor (CSF-1R). J Cell Sci Suppl 18: 105–108. [DOI] [PubMed]
- 21. Roussel MF (1997) Regulation of cell cycle entry and G1 progression by CSF-1. Mol Reprod Dev 46: 11–18. [DOI] [PubMed] [Google Scholar]
- 22. Orlofsky A, Stanley ER (1987) CSF-1-induced gene expression in macrophages: dissociation from the mitogenic response. EMBO J 6: 2947–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malaterre J, Carpinelli M, Ernst M, Alexander W, Cooke M, et al. (2007) c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci U S A 104: 3829–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 25.Cheasley D, Pereira L, Lightowler S, Vincan E, Malaterre J, et al.. (2011) Myb Controls Intestinal Stem Cell Genes and Self-Renewal. Stem Cells. [DOI] [PubMed]
- 26. Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS (1993) Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A 90: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bertoncello I, Bartelmez SH, Bradley TR, Stanley ER, Harris RA, et al. (1986) Isolation and analysis of primitive hemopoietic progenitor cells on the basis of differential expression of Qa-m7 antigen. J Immunol 136: 3219–3224. [PubMed] [Google Scholar]
- 28. Bertoncello I, Bradley TR, Hodgson GS (1981) Characterization and enrichment of macrophage progenitor cells from normal and 5-fluorouracil treated mouse bone marrow by unit gravity sedimentation. Exp Hematol 9: 604–610. [PubMed] [Google Scholar]
- 29. Wei S, Nandi S, Chitu V, Yeung YG, Yu W, et al. (2010) Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol 88: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, et al. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marshall D, Cameron J, Lightwood D, Lawson AD (2007) Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm Bowel Dis 13: 219–224. [DOI] [PubMed] [Google Scholar]
- 32. Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, et al. (1998) Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol 274: G544–551. [DOI] [PubMed] [Google Scholar]
- 33. Staley EM, Schoeb TR, Lorenz RG (2009) Differential susceptibility of P-glycoprotein deficient mice to colitis induction by environmental insults. Inflamm Bowel Dis 15: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, et al. (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260. [DOI] [PubMed] [Google Scholar]
- 36. Martin GR, Wallace JL (2006) Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp Biol Med (Maywood) 231: 130–137. [DOI] [PubMed] [Google Scholar]
- 37. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. [DOI] [PubMed] [Google Scholar]
- 38. Bjerknes M, Cheng H (1981) The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat 160: 51–63. [DOI] [PubMed] [Google Scholar]
- 39. Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, et al. (2012) Paneth cells in intestinal homeostasis and tissue injury. PLoS One 7: e38965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altmann GG (1983) Morphological observations on mucus-secreting nongoblet cells in the deep crypts of the rat ascending colon. Am J Anat 167: 95–117. [DOI] [PubMed] [Google Scholar]
- 41. Chitu V, Stanley ER (2006) Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 18: 39–48. [DOI] [PubMed] [Google Scholar]
- 42. Romberg-Camps M, Kuiper E, Schouten L, Kester A, Hesselink-van de Kruijs M, et al. (2010) Mortality in inflammatory bowel disease in the Netherlands 1991–2002: results of a population-based study: the IBD South-Limburg cohort. Inflamm Bowel Dis 16: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 43. Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, et al. (2004) Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 286: G118–125. [DOI] [PubMed] [Google Scholar]
- 44. Saleiro D, Murillo G, Benya RV, Bissonnette M, Hart J, et al. (2012) Estrogen receptor-beta protects against colitis-associated neoplasia in mice. Int J Cancer 131: 2553–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, et al. (2006) Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 55: 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bradley TR, Hodgson GS (1979) Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood 54: 1446–1450. [PubMed] [Google Scholar]
- 47.Pollard JW (1997) Role of colony-stimulating factor-1 in reproduction and development. Mol Reprod Dev 46: 54–60; discussion 60–61. [DOI] [PubMed]
- 48. Sapi E, Kacinski BM (1999) The role of CSF-1 in normal and neoplastic breast physiology. Proc Soc Exp Biol Med 220: 1–8. [DOI] [PubMed] [Google Scholar]
- 49. Alexander RJ, Panja A, Kaplan-Liss E, Mayer L, Raicht RF (1995) Expression of growth factor receptor-encoded mRNA by colonic epithelial cells is altered in inflammatory bowel disease. Dig Dis Sci 40: 485–494. [DOI] [PubMed] [Google Scholar]
- 50. Bedard M, McClure CD, Schiller NL, Francoeur C, Cantin A, et al. (1993) Release of interleukin-8, interleukin-6, and colony-stimulating factors by upper airway epithelial cells: implications for cystic fibrosis. Am J Respir Cell Mol Biol 9: 455–462. [DOI] [PubMed] [Google Scholar]
- 51. Horiguchi J, Sherman ML, Sampson-Johannes A, Weber BL, Kufe DW (1988) CSF-1 and C-FMS gene expression in human carcinoma cell lines. Biochem Biophys Res Commun 157: 395–401. [DOI] [PubMed] [Google Scholar]
- 52. Chen WS, Kung HJ, Yang WK, Lin W (1999) Comparative tyrosine-kinase profiles in colorectal cancers: enhanced arg expression in carcinoma as compared with adenoma and normal mucosa. Int J Cancer 83: 579–584. [DOI] [PubMed] [Google Scholar]
- 53. Calatayud S, Warner TD, Breese EJ, Mitchell JA (2002) Modulation by colony stimulating factors of human epithelial colon cancer cell apoptosis. Cytokine 20: 163–167. [DOI] [PubMed] [Google Scholar]
- 54. Ide H, Seligson DB, Memarzadeh S, Xin L, Horvath S, et al. (2002) Expression of colony-stimulating factor 1 receptor during prostate development and prostate cancer progression. Proc Natl Acad Sci U S A 99: 14404–14409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, et al. (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 56. Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, et al. (1992) CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol 20: 1004–1010. [PubMed] [Google Scholar]
- 57. Begg SK, Radley JM, Pollard JW, Chisholm OT, Stanley ER, et al. (1993) Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med 177: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stanley ER, Berg KL, Einstein DB, Lee PS, Pixley FJ, et al. (1997) Biology and action of colony–stimulating factor-1. Mol Reprod Dev 46: 4–10. [DOI] [PubMed] [Google Scholar]
- 59. Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, et al. (2012) The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol 367: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whitehead RH, Brown A, Bhathal PS (1987) A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol 23: 436–442. [DOI] [PubMed] [Google Scholar]
- 61. McLean IW, Nakane PK (1974) Periodate-Lysine-Paraformaldehyde Fixative - New Fixative For Immunoelectron Microscopy. Journal Of Histochemistry & Cytochemistry 22: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 62. Ramsay RG, Micallef S, Lightowler S, Mucenski ML, Mantamadiotis T, et al. (2004) c-myb Heterozygous mice are hypersensitive to 5-fluorouracil and ionizing radiation. Mol Cancer Res 2: 354–361. [PubMed] [Google Scholar]
- 63. Rosenthal MA, Thompson MA, Ellis S, Whitehead RH, Ramsay RG (1996) Colonic expression of c-myb is initiated in utero and continues throughout adult life. Cell Growth Differ 7: 961–967. [PubMed] [Google Scholar]
- 64. Whitehead RH, Demmler K, Rockman SP, Watson NK (1999) Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology 117: 858–865. [DOI] [PubMed] [Google Scholar]
- 65. Yazbeck R, Howarth GS, Geier MS, Demuth HU, Abbott CA (2008) Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci 13: 6850–6858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alcian blue (AB) staining shows increased acidic mucin production in Csf1r−/− and Csf1op/op colonic epithelium. Similar to, but more extensive than observed with PAS staining ( Figure 1 ), AB staining shows aberrant goblet cell localization and mucin deposition in both the proximal and distal colon. Mucin deposition was most pronounced in the Csf1r−/− colonic epithelium. Bar = 50 µm.
(PDF)
PMUE-stimulation of immortalized colonic epithelial cells YAMC cells following serum starvation. (A) Five×104 YAMC cells were cultured in fetal calf serum-free media with and without increasing amounts of PMUE (a source of CSF-1) prior to assessment of viable cell numbers by MTT assay at day 5. Upper panel: Representative images of wells incubated without or with the indicated concentrations of PMUE. Lower panel: Quantitation of data from multiple plates (n = 4, *P<0.05; **0.01; ***0.001; ANOVA with Bonferroni’s multiple comparison Testing). (B) Time course of Immorto-5 (IM-5) cells Erk1/2 phosphorylation status in response to PMUE. Cytosolic fractions of cells grown with fetal calf serum (FCS), or serum-starved, or serum-starved and then incubated with 5 µl/ml PMUE for the indicated times were subjected to SDS-PAGE and western blotted for phospho-ERK 1/2 and total ERK 1/2. (C) Cytokine antibody arrays show that CSF-1 is the predominant, but not the only growth factor/cytokine in PMUE (red ellipse). The next most abundant factors identified were IGFBP-3 and MIP-2 (black rectangles). (D–E) IM-5 (like YAMC, data not shown) cells show robust proliferation by MTT assay in response to PMUE but and slight stimulation by purified the CSF-1R ligands, CSF-1 or IL-34. (F) Immorto-5 cells respond strongly to R-spondin in the presence or absence of CSF-1, while Wnt3a has no stimulatory effect alone or in combination with R-spondin or CSF-1, (*P<0.05; **0.01. *P<0.001, analysed using one-tailed t-tests).
(PDF)
Cell cycle and immediate gene expression induction in colonic epithelial cells following PMUE stimulation. IM-5 cells were cultured in the presence of FCS (+FCS), or serum-starved (-FCS), or serum-starved and incubated with 5 µl/ml PMUE for the indicated times prior to extraction of RNA for analysis of gene expression by qRT-PCR. Results show induction of immediate early genes (ets-2 & c-Jun) and cell cycle genes (c-myc, c-myb & cyclinD1) following PMUE stimulation, (Means ± SEM, 6 replicates. **P<0.01; one way ANOVA with Bonferroni’s multiple comparison testing).
(PDF)
Absence of obvious differences between male FVB/NJ Csf1r+/− and WT small intestines following DSS-induced colitis. As it has been reported that an increase in villus height and crypt depth may occur in response to DSS-induced colitis [65], the number of cell nuclei in the small intestinal villus and crypt of male mice was determined (25 crypt & villi per region, n = 4). No morphological (duodenum, jejunum or ileum) or numerically significant differences in cells per crypts (bottom left panel) or villi (bottom middle panel) between WT and Csf1r+/− mice was observed. In addition, no significant differences in intestinal length between the DSS-treated WT and Csf1r+/− mice were detected in small intestines or colons (bottom right panel). Images are representative H&E stained section of small intestine, (Bar = 100 µm).
(PDF)
CD24a and R-spondin mRNA expression are not significantly altered in Csf1r−/− colonic crypts. (A) Absence of Csf1r mRNA in Csf1r−/− crypts. No significant change in CD24a mRNA (B) or R-spondin-1 mRNA (C) expression in Csf1r−/− compared with WT crypts was observed, (Means ± SEM, 4 replicates. **P<0.05; one-tailed t-test).
(PDF)