Over many years, recombinant protein biologics developers have addressed product immunogenicity with a focus on the active pharmaceutical ingredient. Recently, immune responses to the native host cell proteins (HCP) have gained attention, as they too may have an effect on the immune response to the formulated drug, namely diminished drug safety and efficacy. The recent suspension of two clinical trials due to the presence of antibodies to Chinese Hamster Ovary (CHO) HCPs in subjects treated with a recombinant biologic clearly reveals the serious concern regulatory agencies attribute to contaminating HCPs. It appears that even minor amounts of CHO-derived HCP in the final formulation of therapeutics can potentially stimulate an immune response to these contaminants; of even greater concern are immune responses that may be cross-reactive with human proteins. Publication of the CHO-K1 genome and transcriptome provides an opportunity to gain insight into one of the most commonly used expression systems in recombinant protein production. We recently applied immunoinformatics tools to evaluate the immunogenic potential of CHO HCP. Rather than evaluate HCP for their intrinsic potential immunogenicity, we suggest that we should estimate their immunogenicity on a fine-tuned scale that accounts for regions that are homologous to human sequences. As more information on the exact identity of the HCP that drive immunogenicity emerges, the accuracy of this approach is likely to improve.
Bio-process engineers are scrambling to identify means for reducing host cell protein (HCP) content and ways to identify HCP that have the potential to raise antibody responses following the cancellation of two phase III clinical trials. The trials were evaluating the safety and efficacy of Inspiration’s IB1001, a recombinant factor IX produced in CHO cells; the development of anti-Chinese hamster ovary (CHO) antibodies at higher levels than expected in patients treated with IB1001 triggered the FDA ruling.1 Anti-CHO antibodies did not reduce F.IX efficacy, thus the ruling was presumably not related to drug efficacy, but rather to drug safety.
The discovery of anti-CHO antibodies in F.IX-treated patients and the FDA ruling is likely to have a chilling effect on the recombinant protein industry. Fortunately, a number of tools have been developed in the past decade that dramatically accelerate immunogenicity screening, whether for the “protein of interest” (POI) or for HCP. Here we describe the recent application of existing immunogenicity screening tools to evaluate the potential immunogenicity of CHO proteins. The availability of the CHO genome2 and transcriptome3 has made it possible to apply these validated immunoinformatics tools to HCP analysis, significantly accelerating research on the impact of HCP on immunogenicity.
Recombinant protein therapeutics have revolutionized present day medicine. Currently, more than 165 biotherapeutic agents are licensed for treatment of a wide variety of illnesses and generate over $99 billion in global sales.4,5 These proteins are generally produced by expressing the gene in mammalian cell lines, which can be cultured to high density in large bioreactors (Fig. 1). Since the early days of recombinant protein production in the 1980s, protein engineers have expanded the number of CHO-derived and other mammalian cell lines that are available for protein expression. In the vaccine context, animal cells such as chicken embryo fibroblasts, dog kidney cells, monkey kidney cells, rabbits and hamsters have been used for production of poliovirus, mumps virus, rubella virus, measles virus, influenza and many others.6,7 In the biologics field, Chinese hamster ovary (CHO) cells are the most commonly used expression systems.8 About 70% of recently approved recombinant proteins are expressed in CHO cells. CHO cell lines are the preferred choice for recombinant protein expression due to their capacity to tolerate genetic engineering and their ability to produce complex therapeutics.9 A key advantage of CHO cells is their ability to perform human-compatible post-translational modifications (e.g., glycosylation), an aspect of protein production that is believed to be relevant to the efficacy of the protein product.10,11 Methods for cell transfection, gene amplification and clone selection are also well characterized in CHO cells, further adding to their value in the biopharmaceuticals market.
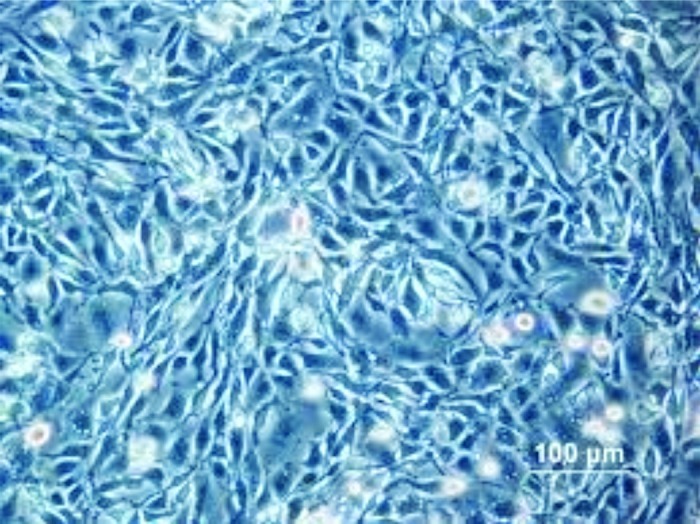
Figure 1. CHO K1 (Chinese hamster ovary) cells at 17 h post plating (image is from the Health Protection Agency Culture Collections website: www.hpacultures.org.uk/products/celllines/generalcell/detail.jsp?refId = 85051005&collection = ecacc_gc
The field of CHO-based protein production has been marked by several major milestones. For example, CHO cells have been subjected to genetic engineering, improving their ability to grow to high density in culture;8 RNA silencing has also been used in CHO cells,12 as has treatment with drugs that are normally intended for the treatment of human diseases.13 Industry-wide conferences such as “Cell Culture Engineering” (run by Engineering Conferences International) currently devote several entire days to CHO engineering. Thus, it is not surprising that CHO was one of the first cell lines for which the entire genome was sequenced2,14 and for which the proteome is also being determined.3
The widespread use of CHO cells for recombinant protein production has not escaped the attention of regulatory bodies such as the FDA. The presence of cell-culture-derived “process impurities” such as “Host Cell Proteins” (HCP) is discussed in European Medicines Agency (EMA) and US Food and Drug Agency (FDA) guidances.15,16 One issue that is receiving increased attention is the potential for HCP to contribute to the immunogenicity of biologics.17,18
Like other process-related contaminants, HCP are readily found in final products, even at levels as low as 1–100 ppm, regardless of the close monitoring and purification standards throughout downstream processing.19 At the same time, HCP comprise a unique and complex group of impurities. Their composition and abundance is dependent on factors that include not only host expression system, but also subcellular localization of expression, culture conditions, the purification process, and the protein produced.
One of the driving forces behind concern about HCP immunogenicity is similarity with human proteins, and the inherent risk of anti-self (auto-immune) responses. There are a number of animal models in which imperfect homology between an antigen and the host-origin protein contributes to the development of antibody responses to the protein (human TSH-R,20 to human diabetes antigens21); epitope spreading from the original non-homologous epitope to other conserved epitopes has been described.22 Regulatory guidelines from the FDA and European Commission regulations require that the level of HCP in protein therapeutics be identified and quantified during manufacturing and before approval.23 Extremely low levels of HCP may still be present, especially in final bioproducts, due to the propensity of some HCP to ‘hitchhike’ on the protein of interest. Currently, the most common techniques for HCP detection and quantification involve protein separation techniques followed by analytical assays, such as enzyme-linked immunosorbent assay (ELISA), western blotting, and mass spectrometry.24 However, detecting nanograms of HCP in milligrams of protein therapeutics may require use of even more sensitive methods. It is also worth noting that the HCP content is highly dependent ELISA assay system. For example, a commercial assay (multiproduct immunoassay) may estimate low HCP content. But product-specific immunoassays may estimate high HCP content. CHO cell culture supernatant and partially purified HCP are used as the antigen, respectively.19 Establishment of precise analytical methods that can detect all or the majority of the HCP in the biologic represents a challenge, due to the vast molecular divergence and complexity of HCP.
So as to be able to help the biologics (and vaccine) industry reduce immunogenicity related to HCP, our immunoinformatics research team has begun to examine constitutively expressed CHO proteins as described in the CHO-K1 genome (and transcriptome) and to evaluate their T-cell epitope content using EpiMatrix.25 This algorithm takes overlapping 9-mer frames derived from CHO protein sequences and scores them for potential binding affinity against a panel of class II HLA alleles; each frame-by-allele assessment that scores highly and is predicted to bind HLA is a putative T cell epitope. Immunogenic potential is assessed by epitope density and ranked on an immunogenicity scale, which was developed to contrast therapeutic proteins of interest against recognized immunogenic and non-immunogenic proteins. In parallel, EpiMatrix evaluates the aggregate epitope density of a given protein with respect to the aggregate epitope density of a set of randomly-generated pseudo-protein sequences of similar size.26 Correcting for size and expected epitope density allows the potential immunogenicity of a candidate protein to be determined. The epitope prediction tools have been benchmarked, and we have observed that both pathogen and biologic proteins (such as proteins used in coagulation factor deficiencies) that have higher epitope densities as predicted by EpiMatrix tend to be more immunogenic (EpiMatrix whole protein score > 20), while low-density proteins tend to be immunologically inert.27 High-scoring proteins may stimulate unwanted immune responses.
The observed correlation between pathogen proteins and immunogenicity score has been helpful for vaccine development. However, we believe that the overall HCP immunogenicity score for individual CHO proteins may need to be adjusted for regional homology to human proteins, because immune responses to these homologous regions may be muted or absent due to endogenous tolerance. In general, the identity of human proteins to CHO-expressed proteins is greater than 77%, thus CHO HCP do not pose as great an immunological threat of foreignness as would E. coli HCP. However, the high similarity with self does contribute to concern that epitope cross-reactivity with a human homolog may stimulate autoreactive T cells to break tolerance and lead to autoimmunity. On the other hand, sequence differences on the 9-mer level that are HLA-restricted require careful consideration because of their potential for immunogenicity. Immunogenicity may initially arise in response to the foreign epitope, which then leads to epitope spreading, involving autoreactive T cells, or autoimmunity. Thus once immunogenicity is established, epitope spreading may lead to more serious sequelae.28,29
When identifying CHO proteins to evaluate, one of the problems that protein manufacturers face is the variability of protein expression from run to run and product to product. The range of proteins produced by CHO cells depends entirely on the cell line, conditions for growth, and growth factors that are administered to the culture or engineered into the host cell.30 In a pilot project to be published separately, we have initially focused on secreted proteins (Gutierrez et al., manuscript in revision31), but, as CHO cells may be apoptotic in the final stages of protein production, non-secreted internal proteins should also be evaluated for potential immunogenicity. While the relative abundance of secreted and intracellular CHO proteins in growth medium is not known, the importance of intracellular proteins to potential immunogenicity is underscored by a study showing that intracellular human proteins, as a group, contain greater numbers of putative HLA class II restricted epitopes in comparison with extracellular proteins.32
Notably, it is not the mere presence of T-cell epitopes in HCP that poses a risk. A combination of factors associated with the drug therapy, including route of delivery, vehicle, the presence of aggregates, the presence of innate immune system triggers, frequency of dosing and the ability of the protein to interface with the humoral (B cell) and cellular (T cell) immune systems, all impact potential immunogenicity. Even if not sufficient, T cell epitopes are necessary for stimulation of pro-inflammatory T cells and are certainly present in HCP.
While some level of HCP contamination may be inevitable, it is nonetheless crucial to minimize its impact. Products that were previously thought to contain “undetectable” amounts of HCP have been shown to contain contaminants using new analytical technologies. In general, however, immune response to HCP, as compared with immune response to the therapeutic drug are not considered to be critical safety concerns. There has yet to be any evidence of “anti-self” immune response or autoimmunity caused by HCP contaminants, however, based on ample evidence of anti-self immune responses in the literature (Fig. 2), the presence of these HCP contaminants may lead to autoreactivity. Thus, it is important to consider the potential for HCP immunogenicity, and new immunoinformatics tools, such as those described here, make it both feasible and relatively easy to evaluate HCP for immunogenicity.
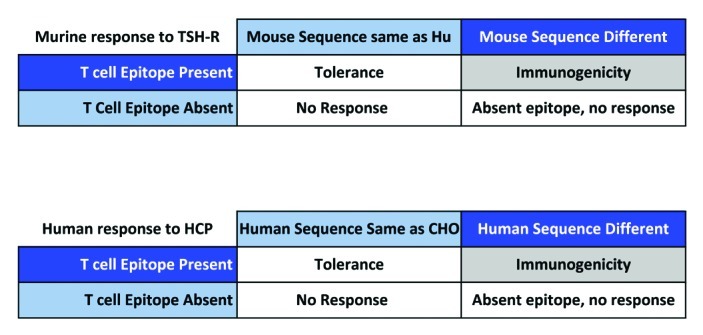
Figure 2. Anti-self immune responses in the literature.
The propensity for CHO proteins to induce anti-autologous protein responses may be directly related to the presence of epitopes that are significantly different from human. Examples can be abundant in the literature. Given any human protein, injected to an animal that has a similar autologous protein, any epitope that is significantly different in terms of its T-cell-receptor facing residues but still is able to bind to the animal’s MHC will induce an immune response particularly when a “danger signal” is present, immune response to the “foreign” epitope then leads to the spread of immune responses to other epitopes that may be homologous, in the same protein, to host T cell epitopes.17,18 One example illustrated above comes from the Graves’ disease literature (see Inaba H. et al.).20,21 Murine immune response to human FVIII (used to study Hemophilia A in vivo) also illustrates this principle.33
Figure A
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/22378
References
- 1.Ipsen. Ipsen’s partner Inspiration Biopharmaceuticals announces hold of phase III clinical trials evaluating IB1001 for the treatment and prevention of hemophilia B 2012. www.ipsen.com/sites/default/files/communiques-presse/PR_IB1001%20Clinical%20Hold_ EN_0.pdf Visited 4 August 2012.
- 2.Xu X, et al. Nat Biotechnol. 2011;29:735–41. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker J, et al. J Biotechnol. 2011;156:227–35. doi: 10.1016/j.jbiotec.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Strohl WR, et al. Curr Opin Biotechnol. 2009;20:668–72. doi: 10.1016/j.copbio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Walsh G. Nat Biotechnol. 2010;28:917–24. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Requirements for the use of animal cells as in vitro substrates for the production of biologicals. www.who.int/biologicals/publications/trs/areas/ vaccines/cells/WHO_TRS_878_A1Animalcells.pdf
- 7.Hess RD, et al. Vaccine. 2012;30:2715–27. doi: 10.1016/j.vaccine.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Puck TT, et al. J Exp Med. 1958;108:945–56. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ifandi V, et al. Cytotechnology. 2003;41:1–10. doi: 10.1023/A:1024203518501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins E. Glycoconj J. 2010;27:211–25. doi: 10.1007/s10719-009-9261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morfini M, et al. Haemophilia. 2012;18:431–6. doi: 10.1111/j.1365-2516.2011.02674.x. [DOI] [PubMed] [Google Scholar]
- 12.Malphettes L, et al. Biotechnol Bioeng. 2004;88:417–25. doi: 10.1002/bit.20230. [DOI] [PubMed] [Google Scholar]
- 13.Liu CH, et al. J Biosci Bioeng. 2009;107:312–7. doi: 10.1016/j.jbiosc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Hammond S, et al. Biotechnol Bioeng. 2012;109:1353–6. doi: 10.1002/bit.24374. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency. www.ema.europa.eu/ema/ index.jsp?curl=pages/regulation/general/general_content_000431.jsp&mid=WC0b01ac0580029593 Visited 5 August 2012.
- 16.FDA. Draft Guidance for Industry Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product. www.fda.gov/Drugs/GuidanceComplianceRegulatory Information/Guidances/ucm259797.htm Visited 5 August 2012.
- 17.Bianchi ME. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 18.van Eden W, et al. Cell Stress Chaperones. 2012;17:281–92. doi: 10.1007/s12192-011-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Champion K, et al. BioProcess International. 2005;3:52–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba H, et al. Thyroid. 2009;19:1271–80. doi: 10.1089/thy.2008.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad S, et al. J Autoimmun 2012; PMID:22647732.
- 22.Grosenbaugh DA, et al. Am J Vet Res. 2011;72:1631–8. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 23.ICH Harmonised Tripartite Guideline. Specifications: Test procedures and acceptance criteria for biotechno -logical/biological products Q6B 1999.
- 24.Wang X, et al. Biotechnol Bioeng. 2009;103:446–58. doi: 10.1002/bit.22304. [DOI] [PubMed] [Google Scholar]
- 25.De Groot AS, et al. AIDS Res Hum Retroviruses. 1997;13:529–31. doi: 10.1089/aid.1997.13.529. [DOI] [PubMed] [Google Scholar]
- 26.Diamond B. Speculations on the immunogenicity of self proteins. Dev Biol (Basel) 2003;112:29–34. [PubMed] [Google Scholar]
- 27.De Groot AS, et al. Clin Immunol. 2009;131:189–201. doi: 10.1016/j.clim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Chen CR, et al. Thyroid. 2011;21:773–81. doi: 10.1089/thy.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moise L, et al. Clin Immunol. 2012;142:320–31. doi: 10.1016/j.clim.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogwood CE, et al. Biotechnol Bioeng 2012; PMID:22806637.
- 31.Ferrando-Martínez S, et al. J Immunol Methods. 2010;352:111–7. doi: 10.1016/j.jim.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 32.De Groot AS, et al. Cell Immunol. 2006;244:148–53. doi: 10.1016/j.cellimm.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Moise L, et al. Clin Immunol. 2012;142:320–31. doi: 10.1016/j.clim.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


