Abstract
The aim of this investigation is to look at whether MENK could improve antitumor effect of CD8+T cell elicited by BMDCs. We investigated the effects of MENK on the differentiation, maturation, and functions of murine BMDC loaded with Rac-1 antigens (RG) and CTL of tumor speci□c immune response elicited by the BMDC in vitro and in vivo. The production of cytokine IL-12 and TNF-αsecreted by BMDCs in the presence of MENK was assayed with ELISA and key surface markers of CD40,CD86, CD83 and MHC-II on the BMDCs were analyzed with use of flow cytometry (FCM). In addition, the activities to induce CD8+ T cell proliferation, along with displayed cytotoxicity of the CD8+T cells(CTL) by the BMDCs after treatment with MENK were determined with use of FCM as well as MTS. Our results indicated that MENK induced phenotypic and functional maturation of BMDC loaded with RG antigen, as evidenced by higher level of expression of key surface markers and more production of cytokines. Subsequently, the BMDC activated by MENK intensified immune responses mounted by CTL, resulting in stronger antitumor activity. Our results suggest that MENK could be working as an effective immune adjuvant in vaccine preparation for cancer fight and other immune related diseases. We concluded that MENK could be a positive immune modulator in the improved functions of BMDCs loaded with antigen as well as in CD8+T cell mediated anti-tumor responses.
Keywords: adjuvant, bone marrow derived dendritic cells, c cytotoxic T lymphocytes, methionine enkephalin, tumor immunity, tumor specific cytotoxic T lymphocytes
Introduction
MENK, an endogenous opioid peptide composing of five amino acids with the sequence: Tyr-Gly-Gly-Phe-Met, generated in adrenal gland and derived from proenkephalin1 is an important agent to be involved in the regulatory loop between the neuroendocrine and immune systems, and participates in functional regulation of the cells of both the innate and adaptive immune systems. Subsequent findings were that the MENK at a suitable dose indeed activated T cells,NK cells, LAK cells and TIL cells2,3as well as antitumor effect.4-6
Dendritic cells (DCs) functioned as highly efficient professional antigen-presenting cells (APCs) with a crucial role in both innate immunity and adaptive immunity7,8via antigen capture, processing, and presentation to initiate T cell response. Immature DCs (iDCs), with high activities of antigen uptaking and processing, but with low activity of stimulating T-cell proliferation, can develop further into mature DCs (mDCs), with signi□cantly increased induction to naïve T cell into CTL due to higher expression of MHC and costimulatory molecules.9,10
The Rac-1 cell line was established C57 T lymphoma, which is a stable cell line in vitro for numerous studies. In pre-studies we proved that BMDCs was sensitive to the Rac-1 cell antigen.
However, DCs loaded with relevant antigens had been used as therapeutic cellular vaccines for different tumors, but DCs only pulsed with tumor cell fragments or tumor lysates were usually not very effective.11
Our previous work proved that MENK played an12 important role in the differentiation, maturation, and function of dendritic cells, through up-regulating the expression of major costimulatory molecules such as CD40, CD83, CD86 and production of cytokine IL-12.3,11 So far there is little report on MENK as an adjuvant in combination with DCs loaded with tumor antigen to drive CD8+T cell mediated response and we are curious about the concrete mechanism of this process. Due the great importance of DC in cancer fight therefore we conducted the following research to try to dig the mechanisms.
Results
Purification of BMDCs by FCM.
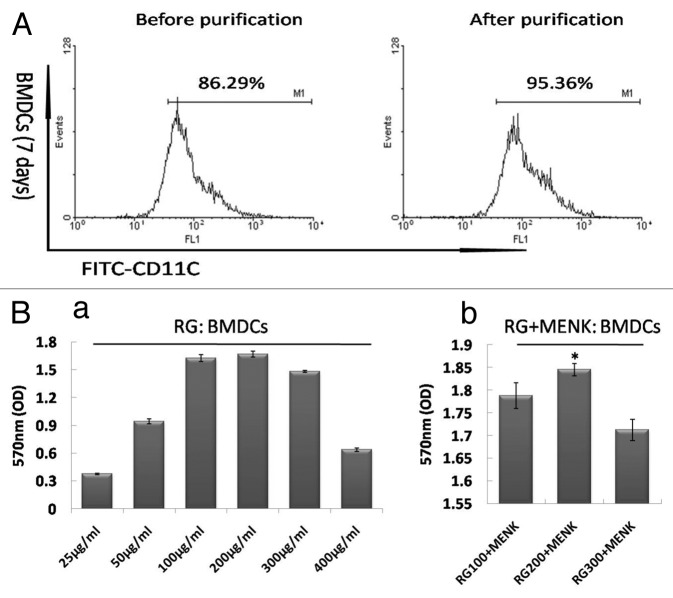
After co-culture for 7d, the purity of CD11c+ cells was examined and the percentage was over 85%. Followed by puri□cation with MACS, the CD11c+ cells were enriched and the results of FCM showed that the purity CD11c+ cells approached 95% (Fig. 1A).
Figure 1. The CD11c+ cell purification with MACS. (A) We can see easily that the BMDCs have been purified significantly and approached 95%. (B) BMDCs proliferation under a range of RG doses and BMDCs proliferation under a range of RG+MENK doses.
BMDCs response to a range of RG doses.
Under the influence of a range of concentrations of RG, BMDCs in wells grew into differential numbers. The data showed that the concentration of RG from 100μg/ml to 300μg/ml could induce cell proliferation. Subsequently, we found that RG in 200μg/ml plus MENK (10−12M) exerted significant effect on proliferation of BMDCs (Fig. 1B).
Morphologic characteristics of the BMDCs by FCM.
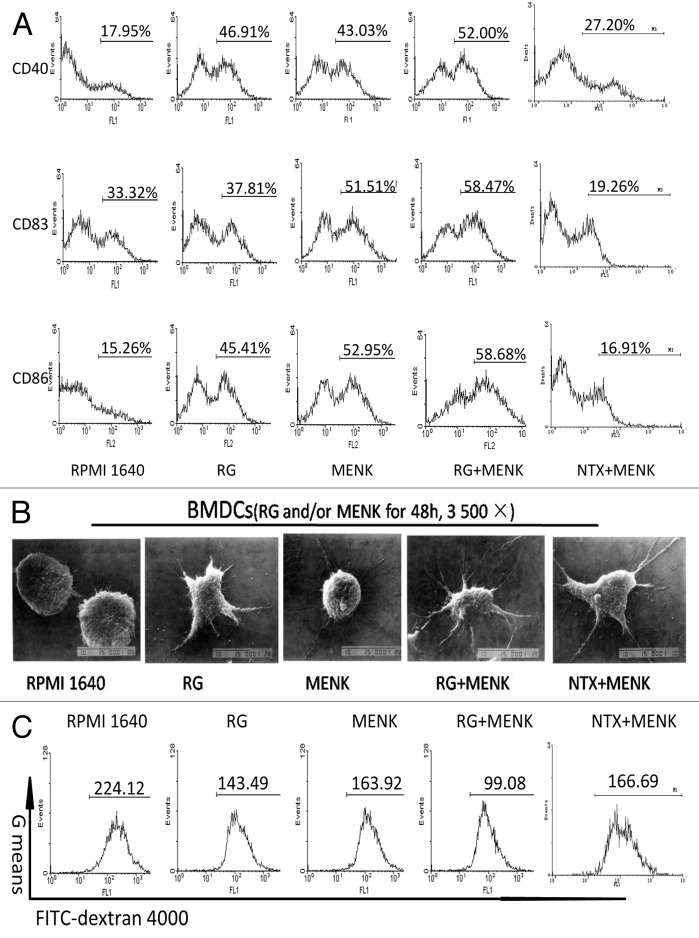
FCM data revealed that CD83 expression increased, which is commonly an indicator for mature DCs. There were also various degrees of increments of molecules CD86 and CD40 (Fig. 2A). Namely, CD83 yielded 58.17±1.616% in the RG+MENK group (p < 0.01) vs. 32.59±2.168% in the RPMI 1640 group,38.13±0.433% in the RG group,52.36±0.876% in the MENK group and vs.19.26±0.634% in the NTX+MENK group. Similarly, CD86 yielded 56.28±1.774% in the MENK group (p < 0.05) vs.17.07±1.852% in the RPMI 1640 group, vs.16.91±2.052% in the NTX+MENK (p < 0.05), and vs. 48.25±3.645% in the RG group, and (p < 0.05) vs.52.52±1.357% in the MENK group. CD40 yielded 51.21±2.246% in the RG+MENK group (p < 0.01) vs. 33.74±0.44% in the RPMI 1640 group and (p < 0.05)vs. 45.50±2.687% in the RG group as reflected in Fig. 2A.
Figure 2. Up-regulation of key surface molecules on BMDCs after treatment with RG and/or MENK for 48h. (A) The cells were respectively collected and stained with mAbs to CD40, CD83, and CD86. Expression of surface markers was analyzed by FCM and was displayed respectively by the single parameter diagram. The values shown in the profiles were the gated %. Results represent the mean ±SD of three independent samples. The images of BMDCs before and after treatment with RG and or MENK (B) by SEM (B) (×3500).
Morphology of BMDCs.
The morphology of the BMDCs from secured SEM photos was compared before and after treatment. The cells in the RG+MENK group exhibited more matured shape with more protrusions and more cascading folds than untreated BMDCs did (Fig. 2B).
Phagocytosis study of BMDCs by FCM.
The data of phagocyting FITC-conjugated dextran by BMDCs indicated that the ability of the BMDCs to phagocyte the dextran,in the RG+MENK group have reduced significantly as compared with that in RPMI1640 group. G means yielded 99.24±3.018 in the RG+MENK group (p < 0.01) vs. 219.93±5.051 in the RPMI 1640 group, 138.55±8.531 in the RG group, 165.37±6.610 in the MENK group, and 166.96±4.378 in the NTX+MENK group respectively (Fig.2C).
Cytokine secretion by BMDCs.
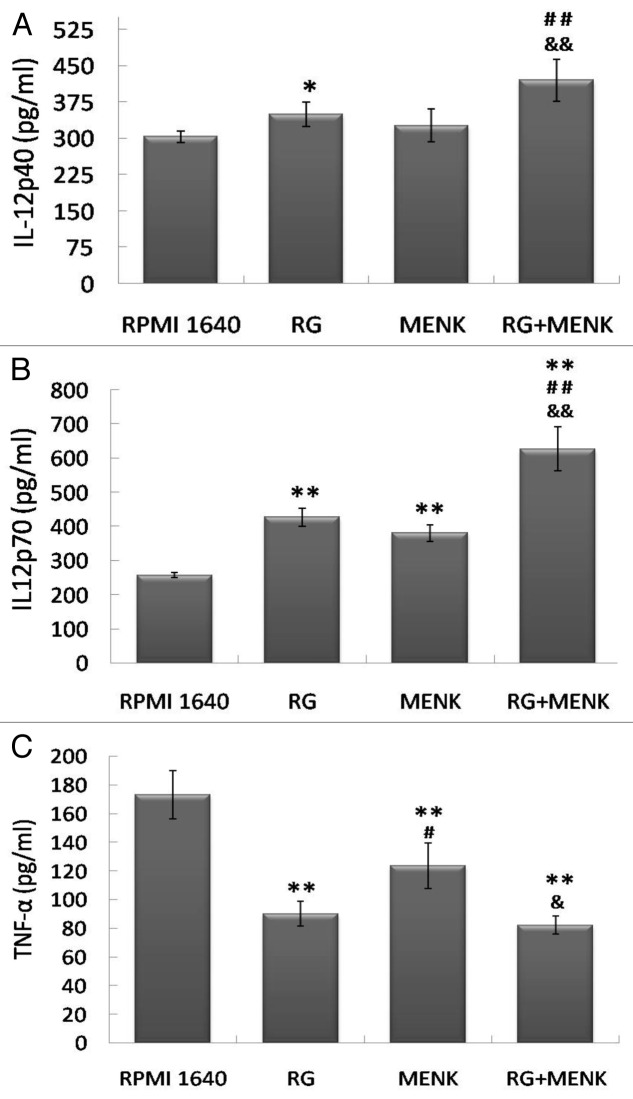
DCs play a driving role in T cell-mediated immune response via secretion of different cytokines. In particular we focused on the production of IL-12p40, IL-12p70 and TNF-α. After the BMDCs were treated with RG and/or MENK for 48h, cytokines IL-12p40, IL-12p70 and TNF-α were assayed by ELISA. The results demonstrated that the levels of cytokines were consistent to corresponding maturity of DCs. The ELISA results showed that, the IL-12p40 secreted by BMDCs yielded 427.6±8.429 pg/ml in the RG+MENK group (p < 0.01) vs. 303.74±16.039 pg/ml in the RPMI 1640 group, 345.71±13.557 pg/ml in the RG group and 324.73±8.342 pg/ml in the MENK group. Similarly, IL-12p70 yielded 627.15±65.03 pg/ml in the RG+MENK group (p < 0.01) vs. 256.83±7.234 pg/ml in the RPMI 1640 group, 427.53±26.332 pg/ml in the RG group and 380.67±23.995 pg/ml in the MENK group in the RG+MENK group; Also TNF-α yielded 627.15±65.03pg/ml in the RG+MENK group (p < 0.01) vs. 256.83±7.234 pg/ml in the RPMI 1640 group and (p < 0.05) vs. 123.17±15.821pg/ml in the MENK group, as shown in Fig. 3.
Figure 3. The production of IL-12p40 (a), IL-12p70 (b) and TNF-α (c) by BMDCs after treatment with RG and/or MENK for 48hr by ELISA. After treatment with RG and/or MENK the supernatant from cell cultures was collected and the secreted cytokines were quantified by ELISA. The histograms above showed the levels of IL-12p40, IL-12p70 and TNF-α production .Results represented the mean±SD of three independent experiments samples.
MLR test.
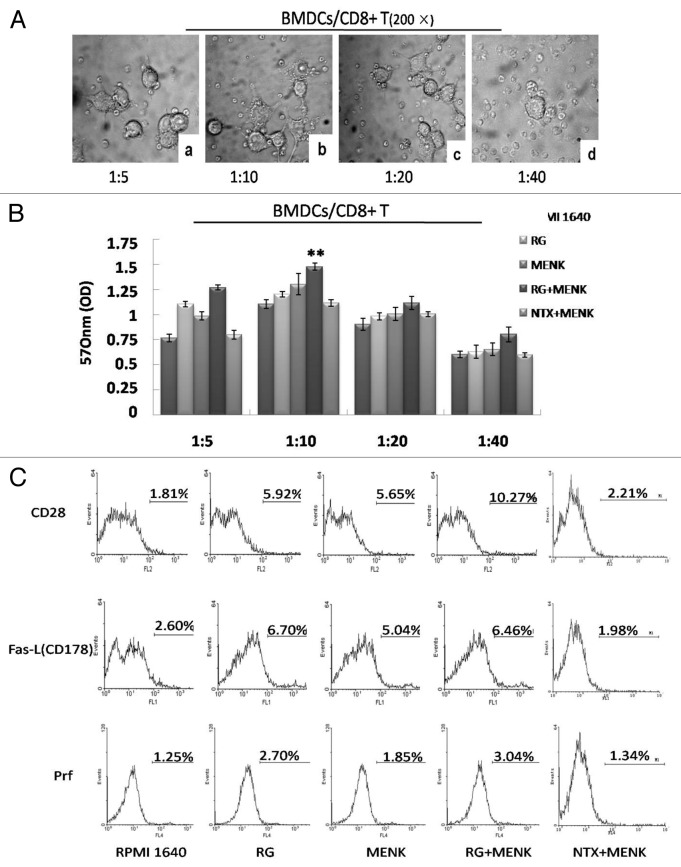
A functional characteristic of DCs was their ability to induce primary MLR. The purified CD8+ T cells (Fig. 4) displayed a significant proliferation enlicited by BMDCs treated with RG and MENK at the ratio of DC: CD8+ T 1:10 and (Fig. 5A and Fig. 5B).
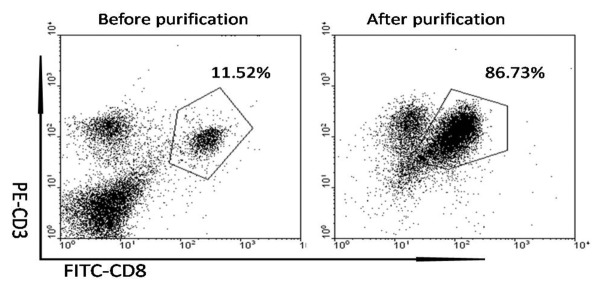
Figure 4. The CD3+ CD8+ T cell purification with MACS. The CD8+T cells from the splenocytes were separated and purified with magnetic beads under sterile condition t. The purity of CD11c+ cells were examined and the percentage were approached 10% to 12%. Followed by purification with MACS, the CD3+ CD8+ T cells were enriched and the results of FCM showed that the purity approached 85%.
Figure 5. The co-cultured BMDCs and CD8+ T cells (A) in a range of different ratio under a light microscope (200 ×). The purified CD8+T cells were incubated with BMDCs (BMDCs/CD8+ T cells in a ratio of 1:5, 1:10, 1:20 and 1:40) for 5d (B). The BMDCs/CD8+T cells at ratio 1:10 showed the best effect on driving CD8+ T cells proliferation. Up-regulation of CD28, FasL and Prf on CD8+ T cells after treatment with RG and/or MENK BMDCs for 5d (C). The expression of markers was analyzed by FCM and was displayed respectively by the single parameter diagram. The values shown in the profiles were the gated %. Results represent the mean ±SD of three independent samples.
CTL cytotoxicity.
The co-culture purified CD8+T cells were collected on 5d as effector cells and were checked by FCM. FCM data revealed that CD28 expression increased, which is an important marker for activated CD8+T cells. Correspondingly, the expression of either FasL (CD178) or Prf increased significantly. The number for CD28 yielded 10.29±0.212% in the MENK+RG group (p < 0.01) vs.1.790±0.063% in the RPMI 1640 group,5.94±0.076% in the RG group, 5.75±0.095% in the MENK group, and 2.21±0.102% in the NTX+MENK group; FasL (CD178) yielded 6.37±0.086% in the RG+MENK group (p < 0.05) vs. 6.71±0.125% in the RG group, and (p < 0.01)vs. 2.67±0.145% in the RPMI 1640 group, 5.19±0.141% in the MENK group and1.98±0.317% in the NTX+MENK group; Prf yielded 3.06±0.764% in the MENK+RG group (p < 0.01) vs.1.32±0.586% in the RPMI 1640 group, 2.75±0.896% in the RG group, 1.88±0.049% in the MENK group and1.34±0.980% in the NTX+MENK group (Fig. 5C).
The cytotoxicity of CTL was determined by MTS and compared within the five groups. The number in the RG + MENK group indicated that activity to kill Rac-1 was significantly up-regulated (Fig. 6).
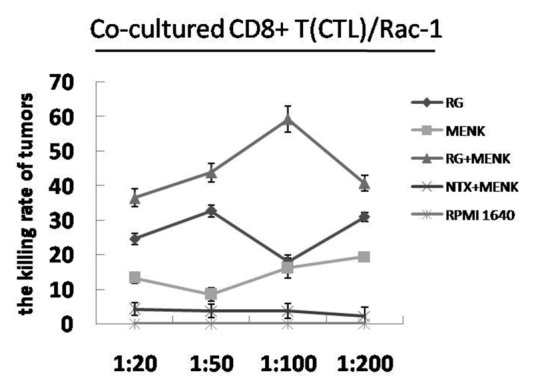
Figure 6. CTL cytotoxicity. The CTL cells were incubated with Rac-1cells (in a ratio of 1:20, 1:50, 1:100 and 1: 200) for 5d. At the ratio 1:100, The CTL cells treated with RG + MENK showed the best cytotoxicity to kill tumor cells( p <0.01).
Discussion
Many strategies exist for initiating antitumor immune response of CTL, including peptide loading, freeze-thaw lysis, DC/tumor cell fusion, and DC priming with tumor RNA.13-17 Tumor antigen loading is one of the effective methods in cancer therapy, including the whole tumor antigen loading and the single tumor antigen loading. Whole tumor antigen loading strategy is advantageous in providing many tumor-specific or tumor-associated antigens, which will theoretically decrease the ability of tumors to evade the immune response elicited by the latter Kazama18,19
Although the whole tumor antigens can be presented by DCs and recognized by CTLs, the responses are ineffective in eradicating tumor cells due to immune tolerance because of the absence of essential co-stimulatory signals necessary for the priming of T cells responses.20
MENK, the endogenous neuropeptide, could be involved in immune responses via inducing the secretion of various cytokines and the expression of key surface molecules such as B7(CD80,86) molecules in multiple types of immune cells.21,22 Recently our team has demonstrated that BMDCs treated with MENK at the concentration of 10−12M expressed expanded CD83, CD40 and CD80 molecules.3,11,23
In this study, BMDCs loaded with RG and MENK were employed for initiating antitumor immune response of CTL. We demonstrated that under influence of MENK, the BMDCs loaded with RG showed more maturation both phenotypically and functionally, as evidenced by the our data above. The concrete findings from our study are: (1) FCM analysis showed MENK could markedly enhance the expression of CD86, CD83 and CD40 molecules on BMDCs loaded with RG. (2) SEM and FCM data suggested that under influence of MENK, the BMDCs loaded with RG exhibited maturation with more protrusions and stronger phagocytosis than the BMDCs dad in other groups. (3) ELISA test showed that MENK could induce maturation of BMDCs loaded with RG via the enhanced secretion of IL-12p40 and IL-12p70 and the diminished secretion of TNF-α. (4) With the purified CD8+ T cells we tested DC driven cytotoxicity of CD8+T cells and found that the BMDCs in RG+MENK group increased markedly the expression of CD28, which is the most important marker for CTL. Meanwhile the higher expression of FasL (CD178) and Prf were observed with consistent trend. (5) Finally we analyzed CTL cytotoxicity by detecting the killing effect of CTL on Rac-1 cells. The CTL activated by BMDCs in RG+MENK group possessed more potent cytotoxicity than CTL activity in other group.
Moreover, the p40 and p70 are two subtypes of IL-12. When we look at mRNA we usually detect p40 and p35, however, when we detect IL-12 proteins we measure p40 and p70. Functionally, in some studies of infection model p70 was quantified and in some cancer model p40 and p70 were quantified.24-26 So we tested tow to dig the mechanism
Despite the meaningful results we got above there are still more approaches needed to be done in depth, such as the signal mechanisms on MENK such as precise signal pathway via which MENK exerts effect on DC-CD8+T cell route as well as the connection between opioid receptors and immune receptors.
We also should pay attention to that dendritic cells have emerged as the most potent and professional antigen presenting cells that initiate activation of naïve T cells to trigger T cell responses, acting as mediator between the innate and adaptive immunities. They can thus potentially be used in therapeutic vaccines in cancer fight and for other human threatening diseases.1
This contribution could therefore help the better understanding of MENK's modulating effects on immune system, and antitumor mechanism via which, MENK is working. Furthermore, this work also provides a meaningful mode of action for potential application of Rac-1 antigen alone, or in combination with MENK and highlights the clinical significance of MENK as an immune stimulator or adjuvant, which may play a critical role in fighting cancer. Likewise, we may consider MENK as a possible adjuvant to be used in vaccine preparations against immune handicapped diseases.27
We believe it is the first ever to publish the article to push the possibility that DC loaded with Rac-1 antigen under influence of MENK can become more matured and drive antitumor effect of .CD8+T cell.
Materials and Methods
Mice
Female 6- to 8-week-old C57BL/6 mice were obtained from Harlan Slac Laboratory animals Co. Ltd (Shanghai, China), and were housed in the pathogen-free animal house at China medical university. All experiments with animals were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the China National Institutes of Health.
Reagents
MENK was provided by Penta Biotech. Inc. USA (≥ 97% purity). The mAbs used in this study were purchased from eBioscience (San Diego, CA) and BD Pharmingen (San Jose, CA) respectively. IL-4 and GM-CSF were obtained from PeproTech Inc (Rocky Hill, NJ, USA), ELISA assay kits for IL-12p40, IL-12p70and TNF-α analysis were purchased from eBioscience (San Diego, CA). Other chemicals frequently used in our laboratory were all products from Sigma-Aldrich or BD Pharmingen.
Preparation of Rac-1 cells freeze-thaw antigens (RG)
A total of 3×108 Rac-1cells were collected, washed 3 times with cold PBS washing buffer, and resuspended in 5ml of RPMI 1640 medium. The cells were kept in -80°C refrigerator for 4h and thawed at room temperature. The cycle was repeated 3 times at same condition of centrifugation and the supernatants were stored for further use.
BMDCs culture and loaded with antigen in vitro
The preparation of BMDCs was modified by method as previously described.27 Briefly, bone marrow cells from the femurs and tibias of female C57BL/6 mice (4-6 weeks old) were □rst depleted of red cells with lyses buffer and subsequently, 107/ml cells were placed in 24-well plates in 2 ml of RPMI 1640 supplemented with 10% fetal bovine serum, recombinant murine granulocyte macrophage colony stimulating factor (GM-CSF) (10ng/ml), interleukin (IL)-4 (10 ng/ml), 2 mM L-glutamine, 100units/ml penicillin, 100μg/ml streptomycin. After incubation for 4h the medium containing non-adherent cells was removed and replaced with fresh medium as described above. After 7d of culture, the cells were kept overnight with LPS (1µg/ml) to get more number of cells before purification with CD11c-MACS magnet beads (Miltenyi Biotec). CD11c+DCs were purified by immunomagnetic sorter, using anti-CD11c-coated magnetic beads and the auto- MACS system according to the manufacturer’s instructions (Miltenyi Biotec, CA, USA). Purity of the sorted cells was determined by FCM analysis (more than 90% for CD11c+ cells).
Preparation for conventional SEM
The cultured BMDCs cells incubated with or without MENK (10−12M), plus RG (200μg/ml) for 48h were collected and checked for morphology by SEM (JEOL, JSM-7300). The cell preparation steps included glutaraldehyde fixation, cleaning, osmium tetroxide post-fixation, cleaning, replacement, critical point drying, and gold spraying etc. Finally, the prepared samples were analyzed with SEM.
Analysis of key surface molecules by FCM
The cultured BMDCs cells incubated with or without MENK (10−12M), plus RG (200μg/ml) for 48h were collected for analysis of key surface molecules. The BMDCs were washed in PBS and incubated at4°C for 20 min with combination of anti-CD40, anti-CD86, anti-CD83 and anti-MHC II antibodies. The specificity of the primary mAbs was established with appropriate isotype-matched controls. After extensive washing, the stained cells were analyzed using FCM (BD Biosciences). The data, obtained from the analysis of the fixed cells by FCM, were then analyzed using WinMDI2.9 (Joseph Trotter, BD Biosciences).
Phagocytosis study
The cultured BMDCs cells incubated with or without MENK (10−12M), plus RG (200μg/ml) for 48h were collected for the study of phagocytic activity. 100μl FITC-Dextran(40,000 D)28-30 were added to the BMDCs culture, incubated at 4°Cfor 2h, subsequently at 37°Cfor 1h and finally samples were checked by FACS Calibur (Becton Dickinson, San Diego, CA).
Cytokine assay of IL-12p40, IL-12p70 and TNF-α
The cultured BMDCs cells incubated with or without MENK (10−12M), plus RG (200μg/ml) for 48h were collected for the assay of production of IL-12p40, IL-12p70 and TNF-α respectively per instruction included in the ELISA kit. The absorbance at 450 nm (A450) was determined using a bichromatic microplate reader (BIO-TEK, USA).
Mixed lymphocyte reaction(MLR)
For the mixed lymphocyte reaction (MLR) the BMDCs were harvested on 7d. The CD8+T cells from the splenocytes were separated and purified with magnetic beads under sterile condition and subsequently the purified CD8+T cells were grown with ConA 10μg/ml for 48h to a concentration of 1×107/ml. The purified CD8+T cells (2-5×105/well) were incubated in 96-well cell-culture plates (Corning-Costar) with graded numbers of BMDCs (at a ratio of 1:5, 1:10, 1:20 and 1:40) for 5d. The proliferation of T cells was monitored by measuring the absorbance in each well at 570nm (A570) using a bichromatic microplate reader. Results were expressed as the mean±sd from triplicate wells.
MTS assay for effect of CTL cytotoxicity
The co-cultured CD8+T cells were collected after stimulation for 5d as effector cells and then anti-CD8, anti-CD28, anti-Prf and anti-FasL antibodies were used to check corresponding changes on CTL cells with FASC. At same time Rac-1 cells were collected as target cells. The co-cultured CD8+T cells (2×105/well) were incubated in 96-well plates (Corning-Costar) with Rac-1 cells for 4h at a ratio of 1:20, 1:50, 1:100 and 1:200. The CTL cytotoxicity in each well was determined by the absorbance at 570nm (A570) by MTS on bichromatic microplate reader.
Statistical analysis
Statistical analysis was performed using the statistical program SPSS (Statistical Package for Social Sciences, Version 16.0) for Windows. All variables are presented as mean± sd. Differences were evaluated by ANOVA for multiple groups and by the student t-test for two groups using the Prism (Graph Pad Software). Turkey test used for post hoc analysis indicated significance when p<0.05 by ANOVA.
Acknowledgments
This work was supported financially by China Liaoning provincial foundation for international collaboration, No.2006305007 (to Fengping Shan). We apologize to the researchers whose works could not be discussed here due to space limitations.
Glossary
Abbreviations:
- MENK
methionine enkephalin
- DC
dendritic cell
- BMDC
bone marrow-derived dendritic cell
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL-4
interleukin-4
- IL-3
interleukin-3
- LPS
lipopolysaccharide
- RG
Rac-1antigen
- FCM
flow cytometry
- CTL
cytotoxic T lymphocytes
- SEM
scanning electronic microscopy
- NTX
Naloxone
- MTS
5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazoly)-3-(4-sulfophenyl)tetrazolium
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/21128
References
- 1.Marcotte I, Dufourc EJ, Ouellet M, Auger M. Interaction of the neuropeptide met-enkephalin with zwitterionic and negatively charged bicelles as viewed by 31P and 2H solid-state NMR. Biophys J. 2003;85:328–39. doi: 10.1016/S0006-3495(03)74477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotnikoff GA. Spirituality, religion, and the physician: new ethical challenges in patient care. Bioethics Forum. 1997;13:25–30. [PubMed] [Google Scholar]
- 3.Shan F, Xia Y, Wang N, Meng J, Lu C, Meng Y, et al. Functional modulation of the pathway between dendritic cells (DCs) and CD4+T cells by the neuropeptide: methionine enkephalin (MENK) Peptides. 2011;32:929–37. doi: 10.1016/j.peptides.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin PJ, Stack BC, Jr., Braine KM, Ruda JD, Zagon IS. Opioid growth factor inhibition of a human squamous cell carcinoma of the head and neck in nude mice: dependency on the route of administration. Int J Oncol. 2004;24:227–32. [PubMed] [Google Scholar]
- 5.Zagon IS, McLaughlin PJ. Opioids and differentiation in human cancer cells. Neuropeptides. 2005;39:495–505. doi: 10.1016/j.npep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Blüml S, Zupkovitz G, Kirchberger S, Seyerl M, Bochkov VN, Stuhlmeier K, et al. Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood. 2009;114:5481–9. doi: 10.1182/blood-2008-11-191429. [DOI] [PubMed] [Google Scholar]
- 7.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Kuriyama H, Kjaergaard J, Lee W, Tanaka H, Shu S. Comparative analysis of antigen loading strategies of dendritic cells for tumor immunotherapy. J Immunother. 2004;27:265–72. doi: 10.1097/00002371-200407000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Chen W, Meng J, Lu C, Wang E, Shan F. Induction on differentiation and modulation of bone marrow progenitor of dendritic cell by methionine enkephalin (MENK) Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suen JL, Wu CH, Chen YY, Wu WM, Chiang BL. Characterization of self-T-cell response and antigenic determinants of U1A protein with bone marrow-derived dendritic cells in NZB x NZW F1 mice. Immunology. 2001;103:301–9. doi: 10.1046/j.1365-2567.2001.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubsky P, Ueno H, Piqueras B, Connolly J, Banchereau J, Palucka AK. Human dendritic cell subsets for vaccination. J Clin Immunol. 2005;25:551–72. doi: 10.1007/s10875-005-8216-7. [DOI] [PubMed] [Google Scholar]
- 14.Fry TJ, Shand JL, Milliron M, Tasian SK, Mackall CL. Antigen loading of DCs with irradiated apoptotic tumor cells induces improved anti-tumor immunity compared to other approaches. Cancer Immunol Immunother. 2009;58:1257–64. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 16.Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. doi: 10.1080/08830180600992456. [DOI] [PubMed] [Google Scholar]
- 17.Van Nuffel AM, Benteyn D, Wilgenhof S, Pierret L, Corthals J, Heirman C, et al. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4+ and CD8+ T cells in melanoma patients. Mol Ther. 2012;20:1063–74. doi: 10.1038/mt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson TA, Stuart PM, Herndon JM, Griffith TS. Apoptosis, tolerance, and regulatory T cells--old wine, new wineskins. Immunol Rev. 2003;193:111–23. doi: 10.1034/j.1600-065X.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Kraus J. Regulation of mu-opioid receptors by cytokines. Front Biosci (Schol Ed) 2009;1:164–70. doi: 10.2741/s16. [Schol Ed] [DOI] [PubMed] [Google Scholar]
- 23.Goel C, Govindaraj D, Singh BP, Farooque A, Kalra N, Arora N. Serine protease Per a 10 from Periplaneta americana bias dendritic cells towards type 2 by upregulating CD86 and low IL-12 secretions. Clin Exp Allergy. 2012;42:412–22. doi: 10.1111/j.1365-2222.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci. 2008;28:2814–9. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marotti T, Haberstok H. Naloxone is an inappropriate antagonist of met-enkephalin-modulated superoxide anion release. Brain Behav Immun. 1992;6:223–33. doi: 10.1016/0889-1591(92)90045-P. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilovac J, Marotti T. Gender-related differences in murine T- and B-lymphocyte proliferative ability in response to in vivo [Met(5)]enkephalin administration. Eur J Pharmacol. 2000;392:101–8. doi: 10.1016/S0014-2999(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Lichtman AH. T cell costimulatory and coinhibitory pathways in vascular inflammatory diseases. Front Physiol. 2012;3:18. doi: 10.3389/fphys.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Stockhammer F, Schmitt A, Casalegno-Garduno R, Enders A, Mani J, et al. Therapeutical doses of temozolomide do not impair the function of dendritic cells and CD8+ T cells. Int J Oncol. 2012;40:764–72. doi: 10.3892/ijo.2011.1269. [DOI] [PubMed] [Google Scholar]
- 29.Cirone M, Lucania G, Aleandri S, Borgia G, Trivedi P, Cuomo L, et al. Suppression of dendritic cell differentiation through cytokines released by Primary Effusion Lymphoma cells. Immunol Lett. 2008;120:37–41. doi: 10.1016/j.imlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Wang JC, Kobie JJ, Zhang L, Cochran M, Mosmann TR, Ritchlin CT, et al. An 11-color flow cytometric assay for identifying, phenotyping, and assessing endocytic ability of peripheral blood dendritic cell subsets in a single platform. J Immunol Methods. 2009;341:106–16. doi: 10.1016/j.jim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]