Abstract
Since both tumor cells and immune cell repertoires are diverse and heterogeneous, immune responses against tumor-associated antigens might be substantially different among individual patients. Personalized selection of right peptides for individuals could thus be an appropriate strategy for cancer vaccines. We have developed a novel immunotherapeutic approach, personalized peptide vaccination (PPV), in which HLA-matched peptides are selected and administered, based on the pre-existing host immunity before vaccination. Recent clinical trials of PPV have demonstrated a feasibility of this new therapeutic approach in various types of advanced cancers. For example, a randomized phase II trial for patients with castration resistant prostate cancer showed a possible clinical benefit in the PPV group. In the patients undergoing PPV, lymphocyte counts, increased IgG responses to the vaccine peptides, and inflammatory factors in pre-vaccination peripheral blood might be potential biomarkers for prognosis. Further randomized phase III trials would be recommended to prove clinical benefits of PPV.
Keywords: peptide vaccine, personalized vaccine, cytotoxic T lymphocytes, advanced cancer, biomarker, inflammation
Introduction
The field of cancer immunotherapy has drastically moved forward during these two decades since Boon and his colleagues reported for the first time a tumor-associated antigen, MAGE-A1, recognized by cytotoxic T lymphocyte (CTL) in 1991.1 In particular, there have recently been noteworthy advances in the clinical application of cancer immunotherapy.2,3 In 2010, sipuleucel-T (Provenge; Dendreon Corporation), an autologous cellular immunotherapy product designed to stimulate T cell immune responses against human prostatic acid phosphatase (PAP), was first approved for patients with castration-resistant prostate cancer (CRPC) by the US. Food and Drug Administration (FDA).4 In addition, another immunotherapeutic agent, ipilimumab, an anti–cytotoxic T lymphocyte antigen (CTLA)-4 monoclonal antibody, was also approved for melanoma patients by the FDA in 2011.5 Despite these significant advances, however, most of other randomized clinical trials in cancer immunotherapy have so far failed to show beneficial therapeutic effects compared with existing treatments.6,7 The failure of recent clinical trials has raised several issues to be addressed for development of cancer vaccines. Here, we have proposed a novel immunotherapeutic approach, “personalized peptide vaccination (PPV)” for advanced cancer patients.
Rationale for Personalized Selection of Vaccine Antigens in Individual Cancer Patients
A large number of tumor-associated antigens have been identified by several different approaches, including cDNA expression cloning, serologic analysis of recombinant cDNA expression libraries (SEREX), and reverse immunological approach.8 Although the number of cancer vaccine candidates is becoming almost limitless, antigens currently employed for vaccination against individual cancer patients might not always be appropriate. In general, anti-tumor immunity is known to be dependent on both immunological characters of tumor cells and immune cell repertoires. Since immune cell repertoires are quite diverse and heterogeneous, anti-tumor immunity might be substantially different among individuals. Therefore, it is likely that vaccine antigens that are selected and administered without considering the immune cell repertoires of the hosts could not efficiently induce beneficial anti-tumor immune responses. To increase the clinical benefits from cancer vaccines, particular attentions should be paid to immunological status of each patient by characterizing the pre-existing immune responses to vaccine antigens before vaccination.
Nevertheless, in most of current clinical trials of therapeutic cancer vaccines, common antigens are employed for vaccination independently of immunological status of patients. Patients, who have immunological memory to vaccine antigens, are expected to show quick and strong immune responses to them. In contrast, patients with no immunological memory against vaccine antigens would take more time for development of effective anti-tumor immune responses, because several rounds of repeated vaccinations might be required to prime antigen-specific naive T cells to functional effector cells (Fig. 1). In such situations, vaccinations could not easily provide clinical benefits, especially in advanced cancer patients, who show a relatively quick disease progression. Moreover, immune responses induced by inadequate vaccines that are non-specific to tumor cells may not only be ineffective for tumor control, but also erode pre-existing immunity.9 Based on the current paradigm that the size and composition of the adaptive immune system are limited and that individual immune cells are constantly competing each other in the limited space, inadequate vaccination may have negative consequences for the hosts by suppressing pre-existing beneficial memory cells specific to tumors and/or infections, which might result in acceleration of cancer progression or early death in vaccinated patients.10 Considering these issues, it would be quite reasonable that vaccine antigens should be selected based on the pre-existing immunological status in each patient.
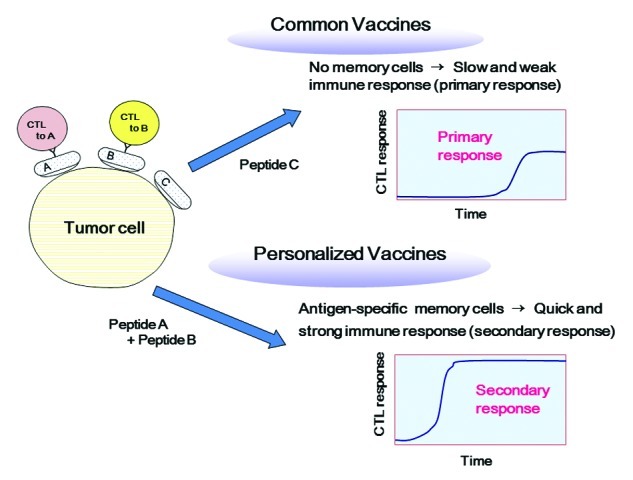
Figure 1. Personalized vaccines are more promising than common vaccines. Personalized antigens can induce quick and strong secondary immune responses, whereas common antigens without immunological memory induce slow and weak primary immune responses.
In addition, it should be noted that cancer cells possess or develop a variety of mechanisms to maintain their malignant behavior. For example, it has been well recognized that cancer cells escape from host immunological surveillance.11 Through the interaction between host immune system and tumor cells at the equilibrium phase, immunological pressure often produces tumor cell variants that decrease or lose tumor-associated antigens. Therefore, to better control cancer cells, it would be recommended to target multiple tumor-associated antigens to reduce the risk of outgrowth of antigen-loss variants.
PPV as a Novel Immunotherapeutic Approach
In view of complexity and diversity of immunological characters of tumors and immune cell repertoires, we have developed a new concept of PPV.12 In this “personalized” cancer vaccine formulation, appropriate peptide antigens for vaccination are screened and selected from a list of vaccine candidates in each patient, based on pre-existing host immunity. Currently, we employ 31 HLA class I-restricted peptide candidates, which were identified from a variety of tumor-associated antigens mainly through cDNA expression cloning method with tumor-infiltrating lymphocyte clones/lines; 12 peptides for HLA-A2, 14 peptides for HLA-A24, 9 peptides for HLA-A3 supertype (A3, A11, A31, or A33), and 4 peptides for HLA-A26. The safety and potential immunological effects of these vaccine candidates have been shown in previously conducted clinical studies.12-14 A maximum of 4 peptides, which are selected based on the results of HLA typing and the pre-existing immune responses specific to each of the 31 different vaccine candidates, are subcutaneously administered in complex with incomplete Freund’s adjuvant weekly or bi-weekly.
Currently, we evaluate the pre-existing immune responses to vaccine candidates by B cell responses, but not by T cell responses, since the performance characteristics, such as sensitivity and reproducibility, of current T cell assays are unsatisfactory.3,15 In contrast to these drawbacks inherent to T cell assays, B cell assays have more potential for screening and/or monitoring antigen-specific immune responses even to MHC class I-restricted peptides. Indeed, we have recently published several papers describing the clear correlations between clinical benefits and antigen-specific B cell responses measured by IgG antibody production in patient plasma after vaccination.16 Notably, the multiplex bead-based LUMINEX technology that we have developed for monitoring B cell responses allows simple, quick and highly reproducible high-throughput screening of IgG responses specific to large numbers of peptide antigens with a tiny amount of plasma.17
In the clinical trials of PPV conducted during the past several years, we have shown promising results in various types of cancers.12,13,16,18,19Table 1 shows the clinical responses in 500 advanced cancer patients who received PPV from October 2000 to October 2008.16 The best clinical response assessed in 436 evaluable patients were partial response (PR) in 43 patients (10%), stable disease (SD) in 144 patients (33%) and progressive disease (PD) in 249 patients (57%), with a median overall survival of 9.9 mo. Of note, as shown in Figure 2, a recently conducted phase II randomized clinical trial of PPV for 57 CRPC patients demonstrated that patients receiving PPV in combination with low-dose estramustine phosphate (EMP) showed a significantly longer progression-free [median survival time (MST), 8.5 vs 2.8 mo; hazard ratio (HR), 0.28 (95% confidence interval (CI), 0.14–0.61); p = 0.0012] and overall survival [MST, undefined vs 16.1 mo; HR, 0.30 (95% CI, 0.10–0.91); p = 0.0328] than those receiving standard-dose EMP alone.18 In addition, PPV was also conducted in an early phase clinical trial of patients with recurrent or progressive glioblastoma multiforme, one of the most aggressive brain tumors, with median overall survival of 10.6 mo.19 Based on these promising results, randomized phase III trials are currently underway in CRPC and glioblastoma. To prove clinical benefits of PPV for accelerating cancer vaccine development, further randomized phase III trials would also be recommended in other different types of cancers.
Table 1. Clinical responses of advanced cancer patients treated with PPV.
| |
Patient (n) | Evaluable patient (n) | |
Best clinical response (n) |
|
Response rate (%) | Disease control rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| PR | SD | PD | |||||||
| Total |
500 |
436 |
|
43 |
144 |
249 |
|
9.9 |
42.9 |
| |
|
|
|
|
|
|
|
|
|
| Prostatic |
174 |
155 |
|
29 |
36 |
90 |
|
18.7 |
41.9 |
| Colorectal |
74 |
68 |
|
1 |
23 |
44 |
|
1.5 |
35.3 |
| Pancreatic |
50 |
41 |
|
4 |
23 |
14 |
|
9.8 |
65.9 |
| Gastric |
42 |
35 |
|
0 |
8 |
27 |
|
0 |
22.9 |
| Brain |
33 |
30 |
|
5 |
11 |
14 |
|
16.7 |
53.3 |
| Cervical |
28 |
23 |
|
3 |
7 |
13 |
|
13.0 |
43.5 |
| Non-small cell lung |
22 |
21 |
|
0 |
11 |
10 |
|
0 |
52.4 |
| Renal cell |
13 |
12 |
|
0 |
9 |
3 |
|
0 |
75.0 |
| Melanoma |
12 |
11 |
|
0 |
5 |
6 |
|
0 |
45.5 |
| Breast |
11 |
10 |
|
0 |
1 |
9 |
|
0 |
10.0 |
| Uroepithelial |
10 |
7 |
|
1 |
2 |
4 |
|
14.3 |
42.9 |
| Others | 31 | 23 | 0 | 8 | 15 | 0 | 34.8 | ||
Best clinical responses were evaluated by RECIST criteria (or PSA values in prostatic cancer). PR, partial response; SD, stable disease; PD, progressive disease
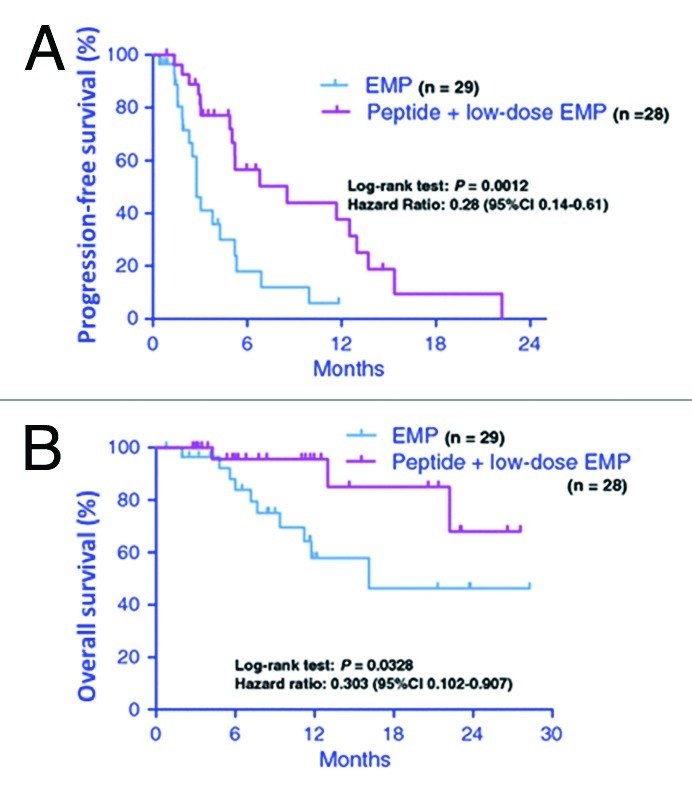
Figure 2. Progression-free and overall survival in patients with castration-resistant prostate cancer using personalized peptide vaccination. Kaplan-Meier curves of progression-free (A) and overall survival (B) in patients treated with personalized peptide vaccination plus low-dose estramustine phosphate (EMP) or standard-dose EMP. Adapted from Noguchi et al.18
Lymphocyte Counts, Increased Humoral Responses to the Vaccine Antigens, and Inflammatory Factors as a Biomarker for PPV
Only a subset of patients show clinical benefits from cancer immunotherapy, including peptide-based cancer vaccines. In addition, even worse, some large clinical trials in the past several years have demonstrated that cancer vaccines might sometimes show worse clinical outcomes.6,7 Therefore, it would be critical to identify biomarkers that accurately portray anti-tumor immune responses and predict prognosis in treated patients.3,6 With regard to post-vaccination biomarkers, several factors, including CTL responses, Th1 responses, delayed-type hypersensitivity (DTH) and autoimmunity, have been reported to be associated with clinical responses in some clinical trials.20-23 However, as they have not been always reproducible in other studies, there are currently no validated prognostic or predictive biomarkers in widespread use.
We also investigated immunological biomarkers in 500 advanced cancer patients who received PPV from October 2000 to October 2008.16 By the statistical analysis in this patient population, both lymphocyte counts prior to the vaccination (p = 0.0095) and increased IgG responses (p = 0.0116) to the vaccine peptides, along with performance status (p < 0.0001), were well correlated with overall survival.
To identify biomarkers useful for selecting appropriate patients before vaccination, we further addressed pre-vaccination prognostic markers in patients with several different types of advanced cancers who underwent PPV. In CRPC treated with PPV (n = 40), a comprehensive study of soluble factors and gene expression profiles by microarray analysis demonstrated that higher IL-6 level and granulocytic myeloid-derived suppressor cells (MDSC) in the peripheral blood before vaccination were closely associated with poorer prognosis.24 In patients with refractory non-small cell lung cancer (n = 41), multivariate Cox regression analyses showed that higher C-reactive protein (CRP) level before vaccination was a significant predictor of unfavorable overall survival (HR = 10.115, 95% CI = 2.447 – 41.806, p = 0.001).25 In addition, in refractory biliary tract cancer patients (n = 25), higher IL-6 and lower albumin levels before vaccination were significantly unfavorable factors for overall survival [HR = 1.123, 95% CI = 1.008 - 1.252, p = 0.035; HR = 0.158, 95% CI = 0.029 - 0.860, p = 0.033; respectively].26 Collectively, these findings have demonstrated that less inflammation may contribute to better responses to PPV, suggesting that evaluation of the inflammatory factors before vaccination could be useful for selecting appropriate cancer patients for PPV. Based on these findings, an early phase clinical trial is currently underway to show whether the blockage of IL-6-mediated inflammatory signaling with a humanized anti-IL-6 receptor monoclonal antibody, tocilizumab, would be beneficial for enhancing the immune and/or clinical responses of PPV.27
Conclusions
The field of cancer immunotherapy has drastically moved forward during the past 20 years, but there have been several issues to be addressed for success of cancer vaccine development. In view of complexity and diversity of immunological characters of tumors and immune cell repertoires, we have developed a new concept of PPV. In the clinical trials conducted during the past several years, we have shown promising results of PPV as a new treatment modality for patients with various types of advanced cancers. Further randomized phase III clinical trials would be essential to prove clinical benefits of PPV. In addition, novel biomarkers for selecting patients who would most benefit from PPV remain to be identified.
Acknowledgments
This study was supported by the grants from the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, from the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct) of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Sendai Kousei Hospital, Japan.
Glossary
Abbreviations:
- PPV
personalized peptide vaccination
- CTL
cytotoxic T lymphocytes
- CRPC
castration-resistant prostate cancer
- FDA
Food and Drug Administration
- MST
median survival time
- HR, hazard ratio
CI, confidence interval
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest and financial relationships to disclose.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/20988
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasada T, Komatsu N, Suekane S, Yamada A, Noguchi M, Itoh K. Overcoming the hurdles of randomised clinical trials of therapeutic cancer vaccines. Eur J Cancer. 2010;46:1514–9. doi: 10.1016/j.ejca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer. 2009;45:2087–90. doi: 10.1016/j.ejca.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, McCluskey J. Immunodominance and immunodomination: critical factors in developing effective CD8+ T-cell-based cancer vaccines. Adv Cancer Res. 2006;95:203–47. doi: 10.1016/S0065-230X(06)95006-4. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki K, Sato Y, Tsuda N, Shomura H, Sakamoto M, Matsuura K, et al. Immunological evaluation of vaccination with pre-designated peptides frequently selected as vaccine candidates in an individualized peptide vaccination regimen. Int J Oncol. 2004;25:121–31. [PubMed] [Google Scholar]
- 11.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 12.Itoh K, Yamada A. Personalized peptide vaccines: a new therapeutic modality for cancer. Cancer Sci. 2006;97:970–6. doi: 10.1111/j.1349-7006.2006.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol. 2009;39:73–80. doi: 10.1093/jjco/hyn132. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K, Noguchi M, Mine T, Komatsu N, Yutani S, Ueno T, et al. Characteristics of severe adverse events after peptide vaccination for advanced cancer patients: Analysis of 500 cases. Oncol Rep. 2011;25:57–62. [PubMed] [Google Scholar]
- 15.Whiteside TL. Immune monitoring of clinical trials with biotherapies. Adv Clin Chem. 2008;45:75–97. doi: 10.1016/S0065-2423(07)00004-2. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M, Mine T, Komatsu N, Suekane S, Moriya F, Matsuoka K, et al. Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol Ther. 2011;10:1266–79. doi: 10.4161/cbt.10.12.13448. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu N, Shichijo S, Nakagawa M, Itoh K. New multiplexed flow cytometric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Invest. 2004;64:535–45. doi: 10.1080/00365510410007008. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi M, Kakuma T, Uemura H, Nasu Y, Kumon H, Hirao Y, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59:1001–9. doi: 10.1007/s00262-010-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen--A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol. 2011;29:337–44. doi: 10.1200/JCO.2010.29.7499. [DOI] [PubMed] [Google Scholar]
- 20.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60:433–42. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499–509. doi: 10.1182/blood-2011-01-325266. [DOI] [PubMed] [Google Scholar]
- 23.López MN, Pereda C, Segal G, Muñoz L, Aguilera R, González FE, et al. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27:945–52. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu N, Matsueda S, Tashiro K, Ioji T, Shichijo S, Noguchi M, et al. Gene expression profiles in peripheral blood as a biomarker in cancer patients receiving peptide vaccination. Cancer. 2011 doi: 10.1002/cncr.26636. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiyama K, Terazaki Y, Matsueda S, Shichijo S, Noguchi M, Yamada A, et al. Personalized peptide vaccination in patients with refractory non-small cell lung cancer. Int J Oncol. 2012;40:1492–500. doi: 10.3892/ijo.2012.1351. [DOI] [PubMed] [Google Scholar]
- 26.Yoshitomi M, Yutani S, Matsueda S, Ioji T, Komatsu N, Shichijo S, et al. Personalized peptide vaccination for advanced biliary tract cancer: IL-6, nutritional status, and pre-existing antigen-specific immunity as possible biomarkers for patient prognosis. Exp Ther Med. 2012;3:463–9. doi: 10.3892/etm.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]


