Abstract
Background:
In contrast to the many studies in females, there are few data in males on the relationships between childhood growth and weight gain and the timing of pubertal maturation and its relevance to adult body mass index (BMI) and body composition.
Methods:
A total of 2008 males in the 1946 British Birth Cohort Study had assessment of pubertal status including voice-breaking status (no change, starting, or complete) at age 14 yr. These responses were related to growth measurements at birth (weight only) and at 2, 4, 6, 7, 11, 14, 20, 26, 36, 43, 53, and 60–64 yr. Body composition was assessed by dual-energy x-ray absorptiometry at 60–64 yr.
Results:
Males with more advanced voice-breaking status at age 14 yr had similar birth weights compared with other males; they showed faster weight gain from 0–2 yr and had higher mean weight and BMI at age 2 yr. Subsequently, they continued to accelerate in weight and BMI, and also in height, and maximum differences in body size were seen at age 14 yr. Adult height did not differ between groups, but males with advanced voice breaking had higher adult BMI and greater whole-body lean mass and greater android fat mass at 60–64 yr.
Conclusion:
Similar to females with earlier menarche, the trajectory to earlier sexual maturation in males is manifested by faster early postnatal growth and weight gain and leads to higher adult BMI. Timing of pubertal maturation has potential relevance to adult disease risks in males. We also describe conditional height difference in sd score as a proxy marker of pubertal timing in males.
There is a wide variation in the timing of puberty in boys and girls. In girls, age at menarche, the onset of the first menstrual bleed during late pubertal development, can be self-recalled in women many years later and has been widely used in epidemiological studies despite only moderate accuracy (1–3). Earlier menarche has been related to low birth weight and faster postnatal weight gain, which is apparent even within the first few weeks of life (4–6). In adult life, earlier menarche is associated with shorter height, higher body mass index (BMI), increased risks for type 2 diabetes and cardiovascular disease (CVD), higher all-cause mortality, and higher mortality from CVD and cancer (7, 8). In boys, more advanced pubertal maturation has been associated with taller childhood height, larger childhood and adult BMI, and higher adult systolic and diastolic blood pressure in one study (9). The lack of additional evidence in boys is largely due to the lack of easily recordable and validated measures of pubertal timing.
Male pubertal onset and progression are manifested by the gradual enlargement of genital size and spread of pubic and axillary hair, as opposed to any distinct changes in appearance (10). The five Tanner stages of sexual maturation in boys were originally described in 1969 (11) and are universally regarded as the convention. However, these stages are difficult to assign accurately without direct observation and palpation of testicular size by trained observers, which has become increasingly unacceptable by many participants in research studies. Self-reported pubertal stage in boys is prone to large errors. Other physical measures have been proposed, such as the timing of the adolescent growth acceleration, which occurs in boys in late puberty; however, this requires frequent growth measures.
Voice breaking in boys usually occurs as a distinct event during late puberty, due to the increased length of the vocal cords that follows the growth spurt of the larynx and represents a further noninvasive measure of pubertal timing, akin to age at menarche in girls (12). In Danish choir boys, data on weekly voice assessments supplemented by fiberoptic laryngoscopy demonstrated a secular trend to earlier puberty associated with increasing BMI (13). In the National Survey of Health and Development (NSHD), voice-breaking status was more simply recorded on one occasion at age 14 yr (14). It was used in a composite measure of pubertal maturation related to growth and blood pressure (9) and was more strongly associated than other parameters of male puberty with the genetic locus for age at menarche at LIN28B (15).
Wehkalampi et al. (16) recently described height difference (HD) in sd scores (SDS) (between ages 11.5–17.5 yr in girls and 14.0–17.5 yr in boys) as a proxy measure of pubertal timing, because it showed strong correlations with age at peak height velocity in boys (r = 0.84) and girls (r = 0.78) (16). However, simple differences between any two repeated measurements may be prone to regression to the mean bias, where more extreme initial values tend to be less extreme at follow-up simply by chance, and vice versa. Cole (17) described conditional change in SDS to correct for regression to the mean in infant weight monitoring.
We therefore assessed voice-breaking status at age 14 yr and also a newly derived parameter, conditional HD-SDS, as noninvasive markers of timing of pubertal maturation in boys and related these and other available measures of pubertal timing in the NSHD to childhood growth and weight gain and also to adult BMI and body composition at age 60–64 yr.
Materials and Methods
Study sample
The NSHD is a socially stratified birth cohort of 2547 men and 2815 women of white European descent born during 1 wk in 1946 who have been followed with repeated data collections since then (14). Between 2006 and 2010, at 60–64 yr, contact was made with 2661 (84%) of 3164 eligible study members still alive and living in England, Scotland, or Wales. No contact was attempted for those who had died (n = 717), were living abroad (n = 567), had previously withdrawn from the study (n = 595), or had been lost to follow-up for more than 10 yr (n = 319). Of the 2661 who provided information, 1690 (63.5%) attended one of six regional clinical research facilities; the remainder were visited at home or completed a postal questionnaire only (18). Inclusion criteria for the current analysis was men with data on birth weight and voice breaking at age 14 yr (n = 2001). The study received Multi-Centre Research Ethics Committee approval, and informed consent was given by cohort participants.
Anthropometry
Birth weight to the nearest quarter pound (113 g) was extracted from medical records and converted to kilograms. Heights and weights were measured using standard protocols at ages 2, 4, 6, 7, 11, 15, 36, 43, 53, and 60–64 yr and were self-reported at ages 20 and 26 yr.
Growth and pubertal assessment
Pubertal characteristics were described by the child's school doctor (medically qualified practitioners who regularly attended schools as part of routine primary care), at a special clinic examination in 1961 when the boys were 14 yr old (mean 14.5, range 14.3–15.2 yr). In boys, the school doctor was asked to rate appearance of the genitals as infantile, early, or advanced. Pubic hair was rated as none, yes sparse, or yes profuse. Axillary hair was rated as no or yes. Voice-breaking status was rated as no, starting to break, or completely broken.
Body composition
During clinic visits at 60–64 yr, measures of body composition were obtained in the supine position using a QDR 4500 Discovery scanner (Hologic Inc., Bedford, MA); the same software (Hologic APEX version 3.1), hardware, and procedures were used in each clinic, and all measures were calibrated internally and across clinics using a European spine phantom. From these scans, whole-body fat and lean mass, android (abdominal) region fat mass and gynoid region fat mass, and appendicular lean mass were obtained and converted into kilograms. The ratio of android to gynoid fat mass was derived, with higher values indicating greater fat distribution in the abdomen than hips. Lean mass was defined as mass excluding fat and bone mass, and in all measures, data from the head were excluded due to the high proportion of bone mass known to affect the accuracy of soft-tissue measures (19). Routine anthropometric measures were taken during the clinic visit using standardized protocols by trained nurses (18). In total, 746 male participants had data available for all body composition outcomes, with missing data mostly due to artifacts such as pacemakers obstructing the x-rays.
Calculations
BMI at each measurement and maternal BMI were calculated as weight in kilograms divided by height in meters squared. Measures of BMI, weight, and height were converted into SDs using internally generated growth charts, which is preferable for historical cohorts, and were constructed using the LMS method (where L is skewness, M is median, and S is coefficient) (20).
HD-SDS was originally described as the difference between adolescent height SDS (at age 14 yr in males) and adult height SDS (16), and we calculated this parameter as height SDS at 36 yr minus height SDS at 14 yr because 36 yr was the first time adult height was measured in the NSHD. HD-SDS was then categorized into three groups for display purposes. Lower HD-SDS values indicate those males with less growth occurring after age 14 yr and who therefore had earlier pubertal growth acceleration and more advanced pubertal maturation at age 14 yr than those with higher HD-SDS values (16).
Conditional HD-SDS was calculated as adolescent height SDS − (r × adult height SDS)/(√1 − r2), where r is the Pearson correlation coefficient for the association between adolescent height SDS and adult height SDS. This equation was as previously described for conditional weight gain (17), but we arranged the variables in the equation to design a parameter that is independent of adult height. Note that higher conditional HD-SDS values indicate males with more advanced puberty at age 14 yr.
Analyses
Linear regression was initially performed to test the cross-sectional linear trends in body size across groups of voice breaking at age 14 yr, adjusted for precise age at pubertal assessment.
Random intercept mixed models were used to test the longitudinal associations between voice-breaking status at age 14 yr and changes in weight, BMI, and height SDS with age, using the xtmixed command in Stata. This approach takes correlations between repeated measures on the same individual into account and allows for missing measurement data assuming that data are missing at random. The linear change in outcome with age was allowed to vary by voice-breaking status by adding a voice-breaking group by age interaction term. We used Wald test statistics to compare models that included linear, quadratic, and cubic functions of age to test whether changes in body size by voice-breaking status were nonlinear between birth to 60–64 yr. To estimate linear coefficients between growth and age during specific developmental periods, we fitted linear piecewise models with knots at 2 (weight only), 7, 14, and 20 yr to allow different linear coefficients for 0–2 yr (infancy; data available for weight only), 2–7 yr (prepubertal childhood), 7–14 yr (early adolescence), 14–20 yr (late adolescence), and 20 to 60–64 yr (adult). All analyses were performed using Stata version 11.0.
Results
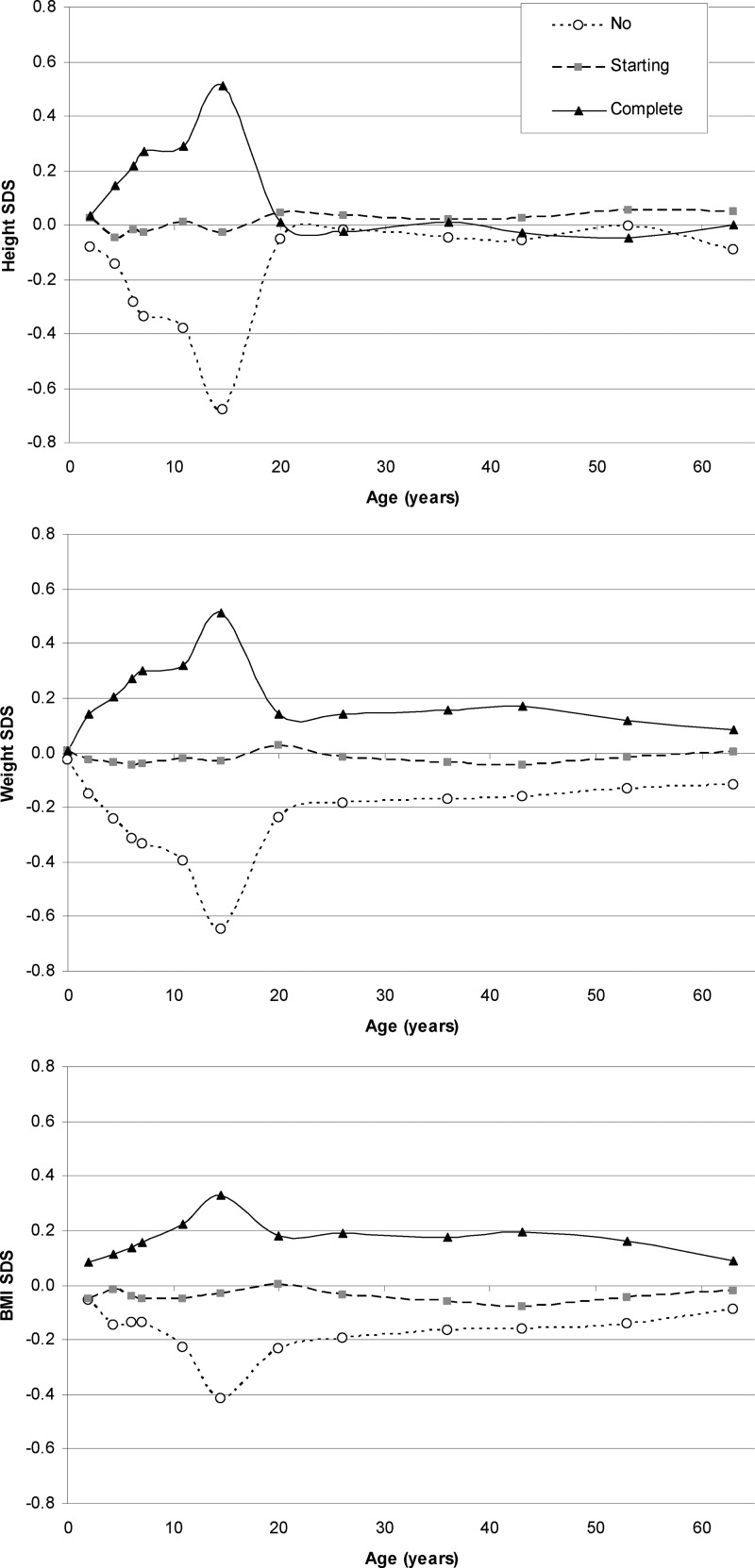
At age 14 yr, 537 (26.8%) of 2001 males had no voice breaking, 743 (37.1%) were starting, and 721 (36.0%) had complete voice breaking. Age at the time of pubertal assessment was slightly later in those with complete (14.57 yr) vs. starting (14.54) or no voice breaking (14.50; P trend < 0.0001), and this was adjusted for in all subsequent analyses. Males with different voice-breaking status at age 14 yr showed divergent patterns of growth in weight, height, and BMI during childhood and adulthood (Fig. 1); longitudinal models confirmed nonlinear associations with age (quadratic age terms in models for weight, height, and BMI SDS were all P < 0.001, not shown). Separate analyses were subsequently performed in the prespecified age groups.
Fig. 1.
Mean height, weight, and BMI SDS from birth to age 60–64 yr by voice-breaking status at age 14 yr.
Infancy weight 0–2 yr
There was no difference in birth weight between groups of voice-breaking status (P trend = 0.7; Table 1); thereafter, the groups diverged in weight (Fig. 1). More advanced voice breaking at age 14 yr was associated with heavier weight and BMI at age 2 yr and with a trend to taller height (Table 1). In longitudinal analyses, compared with males with no voice breaking, those with starting to break (P = 0.04) and complete voice breaking at age 14 yr (P < 0.0005) showed faster weight gain between birth and 2 yr (Table 2).
Table 1.
Childhood and adult weight, height, and BMI in males by voice-breaking status at age 14 yr
| Voice broken at age 14 yr |
Total | P trend | |||
|---|---|---|---|---|---|
| No | Starting | Complete | |||
| n | 537 | 743 | 721 | 2001 | |
| Weight (kg) at age (yr): | |||||
| 0 | 3.47, 3.42–3.51 | 3.49, 3.45–3.52 | 3.48, 3.44–3.52 | 2001 | 0.6 |
| 2 | 13.0, 12.9–13.1 | 13.2, 13.1–13.3 | 13.4, 13.3–13.5 | 1696 | <0.0001 |
| 4 | 17.0, 16.8–17.1 | 17.4, 17.2–17.6 | 17.9, 17.8–18.1 | 1863 | <0.0001 |
| 6 | 20.1, 19.9–20.3 | 20.7, 20.6–20.9 | 21.5, 21.3–21.7 | 1814 | <0.0001 |
| 7 | 22.1, 21.9–22.4 | 22.9, 22.7–23.1 | 23.9, 23.7–24.2 | 1789 | <0.0001 |
| 11 | 32.3, 31.8–32.8 | 34.1, 33.7–34.5 | 36.0, 35.6–36.5 | 1845 | <0.0001 |
| 14 | 45.8, 45.0–46.6 | 51.3, 50.6–51.9 | 56.7, 56.1–57.3 | 1883 | <0.0001 |
| 20 | 68.7, 67.9–69.5 | 71.1, 70.3–71.8 | 72.1, 71.4–72.8 | 1619 | <0.0001 |
| 26 | 72.0, 71.0–72.9 | 73.6, 72.8–74.4 | 75.1, 74.3–75.9 | 1541 | <0.0001 |
| 36 | 74.9, 73.7–76.1 | 76.3, 75.3–77.3 | 78.3, 77.4–79.3 | 1364 | <0.0001 |
| 43 | 77.5, 76.2–78.7 | 78.8, 77.8–79.9 | 81.4, 80.3–82.4 | 1338 | <0.0001 |
| 53 | 82.3, 80.9–83.8 | 83.8, 82.5–85.0 | 85.6, 84.4–86.9 | 1193 | 0.0008 |
| 60–64 | 84.1, 82.4–85.8 | 85.8, 84.3–87.3 | 87.0, 85.6–88.5 | 886 | 0.01 |
| Height (cm) at age (yr) | |||||
| 2 | 85.6, 85.1–86.0 | 86.1, 85.7–86.5 | 86.1, 85.6–86.5 | 1667 | 0.1 |
| 4 | 102.9, 102.4–103.3 | 103.4, 103.0–103.7 | 104.4, 104.0–104.8 | 1819 | <0.0001 |
| 6 | 113.1, 112.6–113.6 | 114.5, 114.1–114.8 | 115.7, 115.3–116.1 | 1799 | <0.0001 |
| 7 | 118.7, 118.2–119.1 | 120.3, 119.9–120.7 | 122.0, 121.6–122.4 | 1854 | <0.0001 |
| 11 | 138.2, 137.6–138.8 | 140.8, 140.4–141.3 | 142.7, 142.2–143.2 | 1862 | <0.0001 |
| 14 | 155.8, 155.1–156.5 | 161.9, 161.3–162.4 | 166.8, 166.2–167.3 | 1899 | <0.0001 |
| 20 | 176.5, 175.9–177.2 | 177.2, 176.7–177.7 | 177.0, 176.5–177.5 | 1629 | 0.7 |
| 26 | 177.1, 176.5–177.8 | 177.5, 177.0–178.0 | 177.1, 176.6–177.7 | 1543 | 0.8 |
| 36 | 175.1, 174.4–175.8 | 175.5, 175.0–176.1 | 175.5, 174.9–176.1 | 1360 | 0.6 |
| 43 | 175.0, 174.2–175.7 | 175.5, 174.9–176.1 | 175.1, 174.6–175.7 | 1337 | 1.0 |
| 53 | 174.6, 173.9–175.3 | 175.0, 174.4–175.6 | 174.3, 173.7–174.9 | 1193 | 0.3 |
| 60–64 | 174.2, 173.4–175.1 | 175.1, 174.4–175.8 | 174.7, 174.0–175.5 | 886 | 0.4 |
| BMI (kg/m2) at age (yr) | |||||
| 2 | 17.9, 17.6–18.1 | 17.9, 17.7–18.1 | 18.2, 18.0–18.4 | 1600 | 0.03 |
| 4 | 16.1, 15.9–16.2 | 16.3, 16.1–16.4 | 16.5, 16.3–16.6 | 1785 | <0.0001 |
| 6 | 15.7, 15.6–15.8 | 15.8, 15.7–15.9 | 16.1, 16.0–16.2 | 1728 | <0.0001 |
| 7 | 15.7, 15.6–15.8 | 15.8, 15.7–15.9 | 16.1, 16.0–16.2 | 1780 | <0.0001 |
| 11 | 16.8, 16.7–17.0 | 17.1, 17.0–17.3 | 17.6, 17.5–17.8 | 1831 | <0.0001 |
| 14 | 18.7, 18.5–19.0 | 19.5, 19.3–19.7 | 20.3, 20.2–20.5 | 1867 | <0.0001 |
| 20 | 22.0, 21.8–22.2 | 22.6, 22.4–22.8 | 23.0, 22.8–23.2 | 1591 | <0.0001 |
| 26 | 22.9, 22.6–23.2 | 23.3, 23.1–23.6 | 23.9, 23.7–24.1 | 1541 | <0.0001 |
| 36 | 24.4, 24.1–24.7 | 24.8, 24.5–25.0 | 25.4, 25.1–25.7 | 1357 | <0.0001 |
| 43 | 25.3, 24.9–25.6 | 25.6, 25.3–25.9 | 26.5, 26.2–26.8 | 1337 | <0.0001 |
| 53 | 27.0, 26.5–27.4 | 27.4, 27.0–27.7 | 28.2, 27.8–28.5 | 1193 | <0.0001 |
| 60–64 | 27.7, 27.2–28.2 | 28.0, 27.5–28.4 | 28.5, 28.0–28.9 | 885 | 0.02 |
Results are shown as means, 95% confidence interval. P values for linear trend were adjusted for precise age at pubertal assessment.
Table 2.
Longitudinal analyses of voice-breaking status at 14 yr and growth and weight gain
| Age period (yr) | Starting to break |
Complete voice breaking |
||||
|---|---|---|---|---|---|---|
| Weight | BMI | Height | Weight | BMI | Height | |
| 0–2 | 0.054 ± 0.026 | NA | NA | 0.150 ± 0.026 | NA | NA |
| 2–7 | 0.028 ± 0.010 | 0.009 ± 0.012 | 0.035 ± 0.008 | 0.053 ± 0.010 | 0.016 ± 0.012 | 0.080 ± 0.008 |
| 7–14 | 0.048 ± 0.008 | 0.037 ± 0.009 | 0.058 ± 0.006 | 0.081 ± 0.008 | 0.067 ± 0.009 | 0.095 ± 0.006 |
| 14–20 | −0.063 ± 0.009 | −0.026 ± 0.010 | −0.095 ± 0.007 | −0.123 ± 0.009 | −0.052 ± 0.010 | −0.186 ± 0.007 |
| 20 to 60–64 | −0.003 ± 0.001 | −0.003 ± 0.002 | −0.001 ± 0.001 | −0.003 ± 0.001 | −0.003 ± 0.002 | −0.002 ± 0.001 |
Regression coefficients ± se, estimated from random intercept mixed models with piecewise specification of age periods, represent changes in SDS per year during the specific age periods for males in the starting to break or complete voice breaking groups compared with those with no voice breaking as the reference group. NA, Not available.
Coefficients with P < 0.05 are shown in bold.
Childhood
Males with complete or starting voice breaking at 14 yr showed faster gains in weight and also in height during childhood compared with those with no voice breaking, during both prepubertal childhood (2–7 yr) and early adolescence (7–14 yr) (Table 2 and Fig. 1). Differences in BMI were maintained during the prepubertal years (2–7 yr; Table 1) and widened further during early adolescence (7–14 yr; Table 2). Differences in weight, height, and BMI were all maximal at age 14 yr and reduced during late adolescence (14–20 yr; Tables 1 and 2 and Fig. 1).
Adult size and body composition
Differences in weight and BMI, but not height, by voice-breaking status at age 14 yr were apparent at all time points between 20 and 60–64 yr, with only modest attenuation in the association with weight with increasing age (Table 2). At age 60–64 yr, when body composition was assessed by dual-energy x-ray absorptiometry (DXA), more advanced voice breaking at age 14 yr was associated with greater whole-body lean body mass (P = 0.002) and a trend toward greater appendicular lean mass (P = 0.07; Table 3). Males with more advanced voice breaking at age 14 yr also showed a trend toward greater whole-body fat mass (P = 0.1), specifically greater android fat mass (P = 0.02), and a greater android to gynoid fat mass ratio (P = 0.02); they also had greater waist circumferences at age 53 yr (P = 0.002; Table 3).
Table 3.
Body composition outcomes at 60–64 yr by voice-breaking status at 14 yr
| Voice broken at age 14 yr? |
P trend | |||
|---|---|---|---|---|
| No | Starting | Complete | ||
| DXA at 60–64 yr (n) | ||||
| n | 167 | 221 | 236 | |
| Whole-body fat mass (kg) | 23.5, 22.4–24.6 | 23.8, 22.8–24.8 | 24.7, 23.8–25.6 | 0.1 |
| Whole-body lean mass (kg) | 52.7, 51.6–53.9 | 53.4, 52.5–54.3 | 55.0, 54.1–55.9 | 0.002 |
| Appendicular fat mass (kg) | 10.1, 9.6–10.6 | 10.2, 9.7–10.6 | 10.3, 9.9–10.7 | 0.7 |
| Appendicular lean mass (kg) | 24.4, 23.8–25.0 | 24.5, 24.1–24.9 | 25.1, 24.6–25.5 | 0.07 |
| Android fat mass (kg) | 2.4, 2.3–2.6 | 2.5, 2.3–2.6 | 2.7, 2.5–2.8 | 0.02 |
| Gynoid fat mass (kg) | 3.7, 3.6–3.9 | 3.8, 3.6–3.9 | 3.9, 3.7–4.0 | 0.2 |
| Android to gynoid fat mass ratio | 0.65, 0.63–0.67 | 0.65, 0.63–0.67 | 0.68, 0.66–0.70 | 0.02 |
| Fat mass (%) | 29.5, 28.8–30.3 | 29.4, 28.7–30.1 | 29.7, 29.0–30.4 | 0.6 |
| Waist circumference at 53 (cm) | 96.9, 95.7–98.1 | 97.7, 96.7–98.7 | 99.3, 98.3–100.2 | 0.002 |
| n | 321 | 439 | 436 | |
Results are shown as means, 95% confidence interval; n = 624 for all variables. P values for linear trend were adjusted for age at pubertal assessment and height at 60–64 yr.
Comparison with other markers of pubertal maturation at age 14
There were moderate intercorrelations between voice-breaking status and other markers of pubertal status at age 14 yr, with r2 values for linear associations ranging from 22.1–32.7% (Table 4). All markers of pubertal maturation at age 14 yr showed consistent associations with childhood weight and growth, with the exception of HD-SDS, which was the only marker of pubertal maturation that was associated with birth weight and adult height (Table 5). Lower HD-SDS values, which indicate earlier pubertal maturation, were associated with lower birth weight and shorter adult height, and HD-SDS also showed different patterns of association with childhood size compared with the other markers (Table 5).
Table 4.
Intercorrelations between voice breaking and other markers of pubertal maturation at age 14 yr
| Genital development (%) | Pubic hair (%) | Axillary hair (%) | HD-SDS (%) | |
|---|---|---|---|---|
| Voice breaking (%) | 28.1 | 32.7 | 22.4 | 28.2 |
| Genital development (%) | 46.5 | 21.3 | 22.4 | |
| Pubic hair (%) | 23.2 | 19.2 | ||
| Axillary hair (%) | 15.0 |
Values are r2 from linear associations between each pair of variables. HD-SDS is difference in height SDS between age 14 yr and adult (continuous variable).
Table 5.
Comparison of associations between different markers of pubertal maturation at age 14 yr and childhood and adult size
| Marker | Child-like | Intermediate | Advanced | Total | P trend |
|---|---|---|---|---|---|
| Voice breaking | No change | Starting | Complete | ||
| n | 537 | 743 | 721 | ||
| Birth weight SDS | 0.00, −0.09–0.08 | 0.02, −0.06–0.09 | 0.01, −0.07–0.08 | 2001 | 0.9 |
| Weight SDS at 2 yr | −0.15, −0.24 to −0.06 | −0.03, −0.11–0.05 | 0.14, 0.07–0.22 | 1696 | <0.0001 |
| Weight SDS at 36 yr | −0.17, −0.28 to −0.06 | −0.04, −0.13–0.05 | 0.16, 0.07–0.24 | 1364 | <0.0001 |
| Height SDS at 7 yr | −0.34, −0.42 to −0.25 | −0.03, −0.10–0.04 | 0.27, 0.19–0.35 | 1854 | <0.0001 |
| Height SDS at 36 yr | −0.05, −0.16–0.06 | 0.02, −0.07–0.10 | 0.01, −0.07–0.10 | 1360 | 0.6 |
| Genital development | Infantile | Early | Advanced | ||
| n | 87 | 804 | 1085 | ||
| Birth weight SDS | −0.09, −0.28–0.11 | 0.01, −0.06–0.07 | 0.00, −0.05–0.06 | 1976 | 0.7 |
| Weight SDS at 2 yr | −0.15, −0.39–0.10 | −0.10, −0.17 to −0.03 | 0.10, 0.03–0.16 | 1676 | <0.0001 |
| Weight SDS at 36 yr | −0.26, −0.57–0.05 | −0.10, −0.19 to −0.01 | 0.09, 0.02–0.17 | 1345 | <0.0001 |
| Height SDS at 7 yr | −0.43, −0.67 to −0.19 | −0.20, −0.27 to −0.13 | 0.18, 0.12–0.24 | 1833 | <0.0001 |
| Height SDS at 36 yr | −0.14, −0.44–0.16 | −0.02, −0.11–0.06 | 0.03, −0.04–0.10 | 1341 | 0.2 |
| Pubic hair | No | Yes sparse | Yes profuse | ||
| n | 245 | 796 | 947 | ||
| Birth weight SDS | −0.03, −0.15–0.10 | 0.01, −0.05–0.08 | −0.01, −0.07–0.05 | 1988 | 0.8 |
| Weight SDS at 2 yr | −0.27, −0.42 to −0.12 | 0.00, −0.08–0.07 | 0.08, 0.01–0.14 | 1684 | <0.0001 |
| Weight SDS at 36 yr | −0.23, −0.39 to −0.06 | −0.02, −0.11–0.07 | 0.06, −0.01–0.14 | 1353 | <0.0001 |
| Height SDS at 7 yr | −0.49, −0.62 to −0.37 | −0.11, −0.18 to −0.04 | 0.21, 0.14–0.28 | 1842 | <0.0001 |
| Height SDS at 36 yr | −0.13, −0.27–0.02 | 0.03, −0.05–0.12 | 0.00, −0.07–0.08 | 1349 | 0.2 |
| Axillary hair | No | Yes | |||
| n | 821 | 1154 | |||
| Birth weight SDS | 0.01, −0.05–0.08 | −0.02, −0.07–0.04 | 1975 | 0.5 | |
| Weight SDS at 2 yr | −0.12, −0.19 to −0.05 | 0.09, 0.03–0.15 | 1672 | <0.0001 | |
| Weight SDS at 36 yr | −0.15, −0.24 to −0.07 | 0.11, 0.05–0.18 | 1346 | <0.0001 | |
| Height SDS at 7 yr | −0.25, −0.32 to −0.18 | 0.17, 0.11–0.23 | 1830 | <0.0001 | |
| Height SDS at 36 yr | −0.01, −0.09–0.08 | 0.00, −0.06–0.07 | 1342 | 0.9 | |
| HD–SDS | >0.67 | −0.67–0.67 | <−0.67 | ||
| n | 430 | 432 | 430 | ||
| Birth weight SDS | 0.07, −0.02–0.16 | −0.01, −0.10–0.08 | −0.11, −0.20 to −0.03 | 1292 | 0.008 |
| Weight SDS at 2 yr | 0.00, −0.10–0.11 | 0.00, −0.10–0.10 | 0.04, −0.06–0.14 | 1105 | 0.8 |
| Weight SDS at 36 yr | 0.12, −0.01–0.24 | −0.01, −0.09–0.06 | −0.06, −0.17–0.05 | 1289 | 0.04 |
| Height SDS at 7 yr | −0.06, −0.15–0.04 | −0.03, −0.13–0.07 | 0.08, −0.02–0.17 | 1201 | 0.2 |
| Height SDS at 36 yr | 0.43, 0.33–0.52 | −0.05, −0.13–0.04 | −0.38, −0.46 to −0.30 | 1292 | <0.0001 |
| Conditional HD–SDS | <−0.67 | −0.67–0.67 | >0.67 | ||
| n | 355 | 577 | 360 | ||
| Birth weight SDS | −0.02, −0.12–0.08 | −0.02, −0.10–0.06 | −0.01, −0.11–0.08 | 1292 | 0.9 |
| Weight SDS at 2 yr | −0.15, −0.27–0.04 | −0.01, −0.10–0.08 | 0.22, 0.12–0.32 | 1105 | <0.0001 |
| Weight SDS at 36 yr | −0.18, −0.29–0.07 | 0.02, −0.07–0.10 | 0.16, 0.06–0.26 | 1289 | <0.0001 |
| Height SDS at 7 yr | −0.40, −0.51–0.30 | −0.06, −0.14–0.02 | 0.48, 0.38–0.58 | 1201 | <0.0001 |
| Height SDS at 36 yr | 0.03, −0.08–0.14 | −0.05, −0.13–0.03 | 0.05, −0.04–0.14 | 1292 | 0.8 |
HD-SDS is the difference in height SDS between age 14 yr and adult (lower HD-SDS values indicate more advanced pubertal maturation at age 14 yr). Conditional HD-SDS is the conditional difference in height SDS between adult and age 14 yr (higher HD-SDS values indicate more advanced pubertal maturation at age 14 yr).
Our new parameter, conditional HD-SDS, designed to correct the bias inherent in (unconditional) HD-SDS (17), showed a pattern of associations with childhood and adult weight and height that was more consistent with the other physical markers of pubertal maturation at age 14 yr (Table 5).
Discussion
Similar to females with earlier menarche, the trajectory to earlier sexual maturation in males, as indicated by earlier voice breaking, is manifested by faster weight gain and growth in infancy and early childhood and leads to persistently higher adult BMI. Unlike findings in females, timing of sexual maturation in males was not associated with birth weight or with adult height (9).
It is well established that earlier pubertal maturation is linked to a faster tempo of growth, that is to say a faster growth trajectory that approaches the adult height target at an earlier age. Such differences in tempo are particularly marked during adolescence and are consequent to the pubertal rise in sex steroids (21). Our current findings support the concept that the trajectory to earlier sexual maturation in males, as well as in females, is manifested as a faster tempo of growth throughout infancy and childhood, rather than being confined to the pubertal years. Such early life processes could potentially include the transient pituitary-gonadal activation that has been described in both male (22) and female (23) infants. Alternatively, it could reflect mechanisms independent of sex hormones, such LIN28 and LIN28B, regulators of microRNA preprocessing that have been linked to childhood growth and pubertal timing in human genetics studies (15, 24) and in mouse models (25). Males with earlier voice breaking had higher BMI than other males, and this was already apparent from ages 2 yr onward. Higher childhood body weight and BMI could also directly promote earlier pubertal onset and progression through leptin, insulin, and/or other hormonal mechanisms (26).
In adult life, males with earlier voice breaking continued to have higher BMI than other males throughout the duration of follow-up with no detectable attenuation up to age 60–64 yr. The differences in BMI at age 60–64 yr by timing of voice breaking were accompanied by differences in whole-body lean mass and in regional fat mass, such that males with earlier voice breaking had greater total lean mass and greater abdominal fat mass. In females, puberty is associated with a rise in percent body fat, relative to body weight, from around 23–29% between early to late puberty (27), and females with earlier timing of menarche have greater fat mass in late adolescence (27) and as adults (28). In contrast, puberty in males is characterized by greater gains in fat-free mass than in fat mass (29) and by greater gains in central fat relative to total body fat (30); we now show that these pubertal changes appear to be exacerbated in those males with earlier timing of sexual maturation.
Our findings are consistent with data from the few other studies in males, which invariably estimated the timing of puberty by analysis of adolescent height growth. Earlier age at peak height velocity has been associated with greater central but not peripheral fat mass by DXA at age 18 yr (31); greater central to peripheral skinfold ratio at age 30 yr (32); greater BMI, waist circumference, and percent body fat at age 27–35 yr (33); and higher BMI at age 63 yr (34). Only one other study used a non-growth-based measure of pubertal timing; in the 1958 British birth cohort, more advanced axillary hair stage at age 16 yr was associated with greater BMI at 23 and 33 yr old (35).
Elevated lifetime BMI and adult abdominal fat in males with earlier voice breaking may have important implications for increased CVD and type 2 diabetes risks, although conversely, their greater lean body mass may be protective. In the NSHD, we previously reported that male early developers, defined by a composite of pubertal measures at age 14 yr, had substantially higher blood pressure (BP) at age 53 yr; mean systolic BP was 6.4 mm Hg higher in the earliest puberty group compared with the latest, and for diastolic BP, the difference was 4.6 mm Hg (9). Other studies have associated earlier age at peak height velocity to higher fasting plasma triglyceride and insulin levels (33). Earlier pubertal maturation might also have adverse consequences for insulin sensitivity and glucose metabolism through mechanisms other than BMI and body composition, such as the regulation of microRNA (36). Additional studies are needed to confirm these differences, to understand their mechanisms and to identify their consequences for disease and mortality as have been reported in women with early menarche (7).
A key limitation in characterizing such associations in males is the difficulty in accurately assessing the timing of puberty in cohort studies. Although our findings show that the timing of voice breaking appears to be a noninvasive marker, additional studies should assess the risks of bias in observed, self-reported, or even recalled timing of voice breaking. Alternatively, where archival data are available, the difference between height at age 14 yr and adult height may provide a reasonable proxy (16). However, we found that when using HD-SDS, the early maturers showed lower birth weights and lower adult heights, which was at odds with our direct markers of puberty (Table 5). Our earlier analysis in NSHD also reported no association between pubertal timing and adult height in men, although earlier puberty was associated with greater trunk to leg length ratio (9). It is therefore highly likely that the positive association between HD-SDS and adult height is spurious due to regression to the mean inherent in the formula (HD-SDS = adult height SDS − adolescent height SDS) and that this bias led to a spurious association with birth weight and masked the true differences in infancy and early childhood size.
We propose a simple modification of HD-SDS to make this value independent of adult height. Conditional change in SDS was originally described for weight gain from birth where it was designed to make that value independent of birth weight (17). We confirmed that conditional HD-SDS was indeed independent of adult height and shows a similar pattern of associations with weight and height with the direct markers of pubertal timing (Table 5). However, in females, in whom earlier timing of puberty is associated with modest reductions in adult height (7), neither HD-SDS (which is strongly related to adult height) nor conditional HD-SDS (which is unrelated to adult height) may be appropriate alternatives for pubertal timing, and the best noninvasive measure remains age at menarche.
Strengths of our study include the direct assessment of pubertal timing during adolescence by medically qualified school doctors, combined with unique very-long-term follow-up over the life course and adult body composition assessed by whole-body DXA. However, our study has several limitations. Pubertal maturation was assessed by a large number of school doctors working in routine practice throughout England, Scotland, and Wales in 1961, before publication of the current Tanner stage classification (11), and the categories used provided relatively limited discrimination between groups. However, these categories still provided sufficient power to detect highly significant associations with childhood and adult growth and weight gain, with strong consistency in these associations between the various measures of pubertal maturation. Adult height was not measured until age 36 yr, and attrition in follow-up meant that available numbers were smaller for HD-SDS than for other markers of pubertal timing; however, highly significant associations were still observed. Unfortunately, body composition was not assessed before age 60–64 yr of age, and it would be interesting to monitor the effects of pubertal timing on adult changes in body composition with aging. Finally, although the NSHD cohort remains broadly representative of the national population of a similar age (37), in both males and females, there has been a secular trend to earlier puberty in more recent birth cohorts (38).
In conclusion, similar to females with earlier menarche, the trajectory to earlier sexual maturation in males is manifested by faster weight gain from birth and faster growth from early childhood and leads to higher adult BMI, greater lean mass, and greater abdominal fat mass, with potential relevance for adult health. In studies where direct assessments of pubertal development are not available, conditional HD-SDS is an appropriate proxy marker for pubertal timing in males.
Supplementary Material
Acknowledgments
We thank the NSHD study members as well as the staff involved in data collection for this cohort over the last 65 yr. We also thank the other members of NSHD's Bone and Muscle Ageing Project Management Group (Dr. Ann Prentice, Prof. Cyrus Cooper, Prof. Judith Adams, and Prof. Avan Ahie Sayer) for their helpful advice and comments.
This work was supported by the United Kingdom Medical Research Council (U120063239, U123092720, and U105960371).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- CVD
- cardiovascular disease
- DXA
- dual-energy x-ray absorptiometry
- HD
- height difference
- NSHD
- National Survey of Health and Development
- SDS
- sd score.
References
- 1. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. 2003. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 24:668–693 [DOI] [PubMed] [Google Scholar]
- 2. Cairns BJ, Liu B, Clennell S, Cooper R, Reeves GK, Beral V, Kuh D. 2011. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med Res Methodol 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME, Pearce MS, Kuh D. 2006. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 60:993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth ME. 2002. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol 31:405–412 [DOI] [PubMed] [Google Scholar]
- 5. Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, Wells JC, Dunger DB. 2009. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab 94:1527–1532 [DOI] [PubMed] [Google Scholar]
- 6. Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. 1996. Childhood growth and age at menarche. Br J Obstet Gynaecol 103:814–817 [DOI] [PubMed] [Google Scholar]
- 7. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. 2009. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 94:4953–4960 [DOI] [PubMed] [Google Scholar]
- 8. He C, Zhang C, Hunter DJ, Hankinson SE, Buck Louis GM, Hediger ML, Hu FB. 2010. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol 171:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardy R, Kuh D, Whincup PH, Wadsworth ME. 2006. Age at puberty and adult blood pressure and body size in a British birth cohort study. J Hypertens 24:59–66 [DOI] [PubMed] [Google Scholar]
- 10. Marshall WA, Tanner JM. 1970. Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tanner JM. 1969. Growth and endocrinology in the adolescent. In: Gardner L, ed. Endocrine and genetic diseases of childhood. Philadelphia: Saunders [Google Scholar]
- 12. Harries ML, Walker JM, Williams DM, Hawkins S, Hughes IA. 1997. Changes in the male voice at puberty. Arch Dis Child 77:445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE. 2007. Age at voice break in Danish boys: effects of pre-pubertal body mass index and secular trend. Int J Androl 30:537–542 [DOI] [PubMed] [Google Scholar]
- 14. Wadsworth M, Kuh D, Richards M, Hardy R. 2006. Cohort Profile: The 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 35:49–54 [DOI] [PubMed] [Google Scholar]
- 15. Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U, Gillson CJ, Glaser B, Golding J, Hardy R, Khaw KT, Kuh D, Luben R, Marcus M, McGeehin MA, Ness AR, Northstone K, Ring SM, Rubin C, Sims MA, Song K, et al. 2009. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet 41:729–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wehkalampi K, Silventoinen K, Kaprio J, Dick DM, Rose RJ, Pulkkinen L, Dunkel L. 2008. Genetic and environmental influences on pubertal timing assessed by height growth. Am J Hum Biol 20:417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole TJ. 1995. Conditional reference charts to assess weight gain in British infants. Arch Dis Child 73:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, Ghosh AK, Harwood N, Hughes A, Macfarlane PW, Mishra G, Pellerin D, Wong A, Stephen AM, Richards M, Hardy R. 2011. Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol 40:e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plank LD. 2005. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care 8:305–309 [DOI] [PubMed] [Google Scholar]
- 20. Cole TJ, Green PJ. 1992. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11:1305–1319 [DOI] [PubMed] [Google Scholar]
- 21. Tanner JM. 1987. Issues and advances in adolescent growth and development. J Adolesc Health Care 8:470–478 [DOI] [PubMed] [Google Scholar]
- 22. Kuiri-Hänninen T, Seuri R, Tyrväinen E, Turpeinen U, Hämäläinen E, Stenman UH, Dunkel L, Sankilampi U. 2011. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. J Clin Endocrinol Metab 96:98–105 [DOI] [PubMed] [Google Scholar]
- 23. Kuiri-Hanninen T, Kallio S, Seuri R, Tyrvainen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L. 2011. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab 96:3432–3439 [DOI] [PubMed] [Google Scholar]
- 24. Widén E, Ripatti S, Cousminer DL, Surakka I, Lappalainen T, Järvelin MR, Eriksson JG, Raitakari O, Salomaa V, Sovio U, Hartikainen AL, Pouta A, McCarthy MI, Osmond C, Kajantie E, Lehtimäki T, Viikari J, Kähönen M, Tyler-Smith C, Freimer N, Hirschhorn JN, Peltonen L, Palotie A. 2010. Distinct variants at LIN28B influence growth in height from birth to adulthood. Am J Hum Genet 86:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, Takeuchi A, Grasemann C, Rinn JL, Lopez MF, Hirschhorn JN, Palmert MR, Daley GQ. 2010. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet 42:626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmed ML, Ong KK, Dunger DB. 2009. Childhood obesity and the timing of puberty. Trends Endocrinol Metab 20:237–242 [DOI] [PubMed] [Google Scholar]
- 27. Vink EE, van Coeverden SC, van Mil EG, Felius BA, van Leerdam FJ, Delemarre-van de Waal HA. 2010. Changes and tracking of fat mass in pubertal girls. Obesity (Silver Spring) 18:1247–1251 [DOI] [PubMed] [Google Scholar]
- 28. Chen L, Zhang C, Yeung E, Ye A, Mumford SL, Wactawski-Wende J, Schisterman EF. 2011. Age at menarche and metabolic markers for type 2 diabetes in premenopausal women: the BioCycle Study. J Clin Endocrinol Metab 96:E1007–E1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, Preece MA, Dunger DB. 1999. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab 84:899–905 [DOI] [PubMed] [Google Scholar]
- 30. Taylor RW, Grant AM, Williams SM, Goulding A. 2010. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 18:1410–1416 [DOI] [PubMed] [Google Scholar]
- 31. Kindblom JM, Lorentzon M, Norjavaara E, Lönn L, Brandberg J, Angelhed JE, Hellqvist A, Nilsson S, Ohlsson C. 2006. Pubertal timing is an independent predictor of central adiposity in young adult males: the Gothenburg osteoporosis and obesity determinants study. Diabetes 55:3047–3052 [DOI] [PubMed] [Google Scholar]
- 32. Beunen G, Malina RM, Lefevre J, Claessens AL, Renson R, Simons J, Maes H, Vanreusel B, Lysens R. 1994. Size, fatness and relative fat distribution of males of contrasting maturity status during adolescence and as adults. Int J Obes Relat Metab Disord 18:670–678 [PubMed] [Google Scholar]
- 33. Sun SS, Schubert CM. 2009. Prolonged juvenile states and delay of cardiovascular and metabolic risk factors: the Fels Longitudinal study. J Pediatr 155:S7.e1–S7.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandhu J, Ben-Shlomo Y, Cole TJ, Holly J, Davey Smith G. 2006. The impact of childhood body mass index on timing of puberty, adult stature and obesity: a follow-up study based on adolescent anthropometry recorded at Christ's Hospital (1936–1964). Int J Obes (Lond) 30:14–22 [DOI] [PubMed] [Google Scholar]
- 35. Power C, Lake JK, Cole TJ. 1997. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am J Clin Nutr 66:1094–1101 [DOI] [PubMed] [Google Scholar]
- 36. Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ. 2011. The Lin28/let-7 axis regulates glucose metabolism. Cell 147:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C, Hilder WS, Connor M. 2003. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med 57:2193–2205 [DOI] [PubMed] [Google Scholar]
- 38. Li L, Hardy R, Kuh D, Lo Conte R, Power C. 2008. Child-to-adult body mass index and height trajectories: a comparison of 2 British birth cohorts. Am J Epidemiol 168:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.