Abstract
Context:
Using single-nucleotide polymorphism analysis, we observed allelic loss of the gene for serum glucocorticoid (GC) kinase 1 (SGK1), a GC-responsive kinase involved in multiple cellular functions, in a subset of cortisol-secreting adenomas.
Objective:
Our objective was to analyze SGK1 expression in adrenocortical tumors and to further characterize its role in ACTH-independent cortisol secretion, tumor progression, and prognosis.
Design and Setting:
Gene expression levels of SGK1, SGK3, and CTNNB1 (coding for β-catenin) and protein expression levels of SGK1, nuclear β-catenin, and phosphorylated AKT were determined in adrenocortical tumors and normal adrenal glands.
Patients:
A total of 227 adrenocortical tumors (40 adenomas and 187 carcinomas) and 25 normal adrenal tissues were included. Among them, 62 frozen tumor samples were used for mRNA analysis and 203 tumors were investigated on tissue microarrays or full standard slides by immunohistochemistry.
Main Outcome Measures:
We evaluated the relationship between SGK1 mRNA and/or protein levels and clinical parameters.
Results:
SGK1 mRNA levels were lower in cortisol-secreting than in nonsecreting tumors (P < 0.005). Nonsecreting neoplasias showed a significant correlation between SGK1 and CTNNB1 mRNA levels (P < 0.001; r = 0.57). Low SGK1 protein levels, but not nuclear β-catenin and phosphorylated AKT, were associated with poor overall survival in patients with adrenocortical carcinoma (P < 0.005; hazard ratio = 2.0; 95% confidence interval = 1.24–3.24), independent of tumor stage and GC secretion.
Conclusion:
Low SGK1 expression is related to ACTH-independent cortisol secretion in adrenocortical tumors and is a new prognostic factor in adrenocortical carcinoma.
Adrenal tumors have a high overall prevalence of 2% in the general population (1). They mostly consist of adrenocortical adenomas (ACAs), whereas adrenal carcinomas (ACCs) are rare aggressive cancers with an incompletely understood pathogenesis (2). Adrenocortical tumors can be either endocrinologically silent or hormonally active, with steroid hormone production being present in about 60% of ACCs (3). However, the molecular mechanisms responsible for ACTH-independent glucocorticoid (GC) secretion, which is per se associated with serious morbidity and increased mortality (4, 5), are still unclear. Thus, a better understanding of the genetic mechanisms underlying adrenocortical tumor development and abnormal GC secretion might lead to new treatment strategies.
In a previous study using high-resolution single-nucleotide polymorphism (SNP) microarray analysis, we observed a copy number microdeletion at 6q23 region involving the serum GC kinase 1 (SGK1) gene in two of 15 cortisol-secreting ACAs (6). The mRNA expression evaluated by quantitative real-time PCR analysis in this series of tumors showed low SGK1 levels not only in cases with losses but also in many other tumors, indicating that additional factors could affect SGK1 expression. We confirmed by further SNP array analysis frequent microdeletions at the SGK1 locus in a larger series of 46 tumors (two of 15 cortisol-secreting ACAs, zero of nine non-cortisol-secreting ACAs, three of 14 cortisol-secreting ACCs, and zero of eight non-cortisol-secreting ACCs) (Ronchi, C. L., S. Sbiera, E. Leich, M. Fassnacht, B. Allolio, unpublished data). Because SGK1 is an important downstream effector of both the GC receptor and the Wnt/β-catenin signaling pathway, we reasoned that SGK1 might be of importance in adrenocortical tumor pathogenesis.
SGK1 is a ubiquitously expressed serine/threonine kinase, which is up-regulated by multiple factors including GC, mineralocorticoids, androgens, growth factors, p53 (7), and mammalian target of rapamycin complex-2 (mTORC2) (8). SGK1 and its two related isoforms (SGK2 and -3) share their molecular structure and targets for phosphorylation with the protein kinase B (PKB)/AKT isoforms (8). SGK1 is also involved in steroid-dependent cell survival signals and cell cycle progression, acting as an antiapoptotic factor (9, 10). It mainly works through the phosphorylation and inhibition of glycogen synthase kinase-3, in combination with an activated PKB/AKT pathway, which in turn inhibits the degradation of oncogenic β-catenin and leads to its translocation to the nucleus (8, 11).
However, the role of SGK1 for tumor growth is conflicting, because its expression is up-regulated in some tumors, such as breast cancer (12), cholangiocarcinoma (8), multiple myeloma (13), kidney (7), and non-small-cell lung cancer (14), and down-regulated in others (e.g. prostate, hepatocellular, and colorectal cancer) (8, 15, 16). The impact of SGK1 expression on clinical outcome has been evaluated only in a small number of studies, again with contradictory results in different cancers (14, 17). However, expression of SGK1 in adrenocortical tumors has not yet been investigated, and its clinical significance remains unknown.
Here we have determined the mRNA and protein expression of SGK1 in a large group of benign and malignant adrenocortical tumors, aiming to elucidate its role in both cancer progression and cortisol hypersecretion. Moreover, β-catenin and phosphorylated AKT levels were assessed to investigate their relationship with SGK1. Finally, we investigated the possible role of SGK1 as prognostic factor in ACC.
Patients and Methods
Patients and clinical data collection
A total of 227 adrenocortical tumors (40 ACAs and 187 ACCs) and 25 normal adrenal (NA) tissues (15 derived from the area surrounding the adrenocortical tumor and 10 derived from adrenalectomies performed during surgery for renal carcinoma) were included. In particular, 62 tumors with available fresh-frozen tumor material (30 ACAs, 14 males and 16 females, mean age 49.7 ± 13.3 yr; and 32 ACCs, 12 males and 21 females, mean age 48.1 ± 13.8 yr), including the 46 tumors used for the previous SNP array analysis (6) (Ronchi, C. L., S. Sbiera, E. Leich, M. Fassnacht, B. Allolio, unpublished data), were used for evaluation of mRNA levels. Thirty-nine of them were cortisol-secreting (CS) tumors (11 with subclinical and 28 with overt hypersecretion), and 23 were non-CS (NCS) tumors. In addition, 203 tumors (29 ACAs and 174 ACCs, including 38 of those evaluated for gene expression) were available for immunohistochemistry.
Clinical parameters, such as sex, age at diagnosis, date of surgery, tumor size, pathological classification, and results of hormone analysis, were collected from patient records. Malignancy was defined according to recognized clinical, biochemical, and morphological criteria, whereas the biochemical diagnosis of cortisol hypersecretion was made according to established criteria (18). Subclinical hypercortisolism was defined as follows: post-dexamethasone cortisol levels over 5 μg/dl (>138 nmol/liter), ACTH levels below 25 pg/ml (5.5 pmol/liter), and at least one additional pathological test among 24-h urinary free cortisol, and midnight cortisol levels in the absence of typical clinical features of Cushing's syndrome (19). For ACC patients, additional data, such as tumor stage according to the European Network for the Study of Adrenal Tumors (ENSAT) classification (20), Weiss score, Ki67 index, presence and number of distant metastases, and a detailed follow-up, were collected through the German ACC Registry (21). Different treatment modalities were also recorded, and the response to therapy was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria (22). In particular, 39 ACC patients had been treated with mitotane and 46 with different chemotherapeutic drugs as first-line therapy. Among those, 17 received an etoposide/doxorubicin/cisplatin scheme, five received other platinum-based regimens, 10 received streptozotocin, and the last 13 received other citotoxic drugs combinations including gemcitabine, fluorouracil, ifosfamide, or trofosfamide.
The study was approved by the ethical committee of the University of Wuerzburg, and written informed consent for collecting tissue and clinical data were obtained from all patients.
Gene expression analysis
SGK1 and SGK3 mRNA expression levels were investigated by real-time quantitative PCR. To evaluate the relationship between SGK1 and β-catenin at the transcription level, also CTNNB1 (i.e. gene coding for β-catenin) expression was determined.
RNA was isolated from fresh-frozen tissue samples using the RNeasy lipid tissue mini kit (QIAGEN, Valencia, CA) and reverse transcribed using the QuantiTect reverse transcription kit (QIAGEN). Predesigned TaqMan gene expression assays for SGK1 (Hs00178612_m1), SGK3 (Hs00179430_m1), and CTNNB1 (Hs00994404_g1) were purchased from Applied Biosystems (Darmstadt, Germany). Endogenously expressed β-actin (Hs9999903_m1) was used for normalization. Forty nanograms of cDNA was used for each PCR, and each sample was performed in duplicate. Transcript levels were determined by using the TaqMan gene expression master mix (Applied Biosystems), the C1000 thermal cycler (CFX96 real-time system; Bio-Rad, Hercules, CA) and the Bio-Rad CFX Manager version 2.0 software. Cycling conditions were 95 C for 3 min followed by 50 cycles of 95 C for 30 sec, 60 C for 30 sec, and 72 C for 30 sec. Using the delta cross-over threshold method (23), the gene expression levels were normalized to those of β-actin, as previously described (6).
Protein expression analysis
Tissue samples
Samples from 182 tumors (15 ACAs and 167 ACCs) and five NAs were assembled into three tissue microarrays (TMAs) (24, 25), whereas 25 additional samples (14 ACAs, seven ACCs, and four NAs) were placed on standard full slides and were used to confirm homogeneous staining of SGK1 and to increase the number of samples. TMA samples were included in the analysis only if two or more evaluable cores per patient were available after the staining procedure.
SGK1 immunostaining
TMA and full sections were deparaffinized, and immunohistochemical detection was performed using an indirect immunoperoxidase technique after high-temperature antigen retrieval in 10 mm citric acid monohydrate buffer (pH 6.5) in a water bath for 13 min. Blocking of unspecific protein-antibody interactions was performed with 20% human AB serum in PBS for 1 h at room temperature. Primary antibody was a monoclonal rabbit antihuman against SGK1 (ab32374; Abcam, Cambridge, UK) used at a dilution of 1:50 at 4 C overnight together with the N-Universal negative control antirabbit (Dako, Glostrup, Denmark). Signal amplification was achieved by En-Vision system-labeled polymer-horseradish peroxidase antirabbit (Dako) for 40 min and developed for 10 min with diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. Nuclei were counterstained with Mayer's hemalaun for 2 min. For positive controls, sections with NA and kidney, pancreas, and prostate cancer were chosen, whereas cells of the tumor stroma served as internal negative control.
Microscopic analysis of SGK1 expression
All slides were analyzed independently by two investigators blinded to clinical information (C.L.R. and S.Sb.). Eleven ACC cases among those assembled in the TMAs with fewer than two evaluable cores were excluded from the series. Both nuclear and cytoplasmic staining was evaluated, and staining intensity was graded as negative (0), low (1), medium (2), or strong (3). The percentage of positive tumor cells was calculated for each specimen and scored 0 if 0% were positive, 0.1 if 1–9% were positive, 0.5 if 10–49% were positive, and 1 if 50% or more were positive. A semiquantitative H-score was then calculated by multiplying the staining-intensity grading score with the proportion score as described previously (24). Where discrepancies were observed, results were jointly assessed by both investigators and the final score was formed by consensus. Interobserver agreement was strong with a Fleiss k-coefficient of 0.878 and a Pearson's correlation coefficient of 0.70 [95% confidence interval (CI) = 0.58–0.78].
Nuclear β-catenin immunostaining and microscopic analysis
Immunohistochemistry for β-catenin (BD Science, San Jose, CA, 1:400) had been previously performed in our TMAs, and the results have been already published elsewhere (26). Briefly, staining was assessed by a single pathologist experienced in β-catenin immunohistochemistry reading (F.T.), and nuclear staining (representative of β-catenin pathway activation) was quantitatively assessed and defined as negative or positive. A total of 59 cases among those assembled in the TMAs with fewer than two evaluable cores were excluded from the final series (seven ACAs, 50 ACCs, and two NAs). For this study, the clinical data have been updated.
Phospho-AKT (pAKT) immunostaining and microscopic analysis
The immunohistochemistry for pAKT (Ser473) (Cell Signaling Technology, Danvers, MA) had been already previously established in standard full slides of adrenal tumors (27). The same procedure was then applied on TMA. A total of 14 ACC cases with fewer than two evaluable cores were excluded from the final series.
Statistical analysis
The comparison of clinical and histopathological characteristics was performed by appropriate statistical methods. The Fisher's exact test or the χ2 test was used to investigate dichotomous variables, whereas a one-way ANOVA model, eventually followed by Bonferroni post hoc test, or a two-sided t test (or nonparametric test) was used to test continuous variables. Correlations between different parameters were evaluated by linear regression analysis and 95% CI are also shown. Overall survival (OS) was defined as the time from the date of primary diagnosis to death or last follow-up. Disease-free survival (DFS) was defined as the time from the date of complete tumor resection to the first radiological evidence of disease relapse or death. All the survival curves were obtained by Kaplan-Meier estimates, and the differences between survival curves were assessed by the log-rank (Mantel-Cox) test. In this context, the RNA expression was considered as a categorical value (cutoff value for this data set was median value + 2 sd). A multivariate regression analysis was performed by Cox proportional hazard regression model to identify those factors that might independently influence survival. Statistical analyses were made using GraphPad Prism version 5.0 (GraphPad, La Jolla, CA) and SPSS Software (PASW version 19.0; SPSS Inc., Chicago, IL). P values <0.05 were considered as statistically significant.
Results
SGK1 gene expression in adrenocortical tumors: relationship with cortisol secretion and CTNNB1
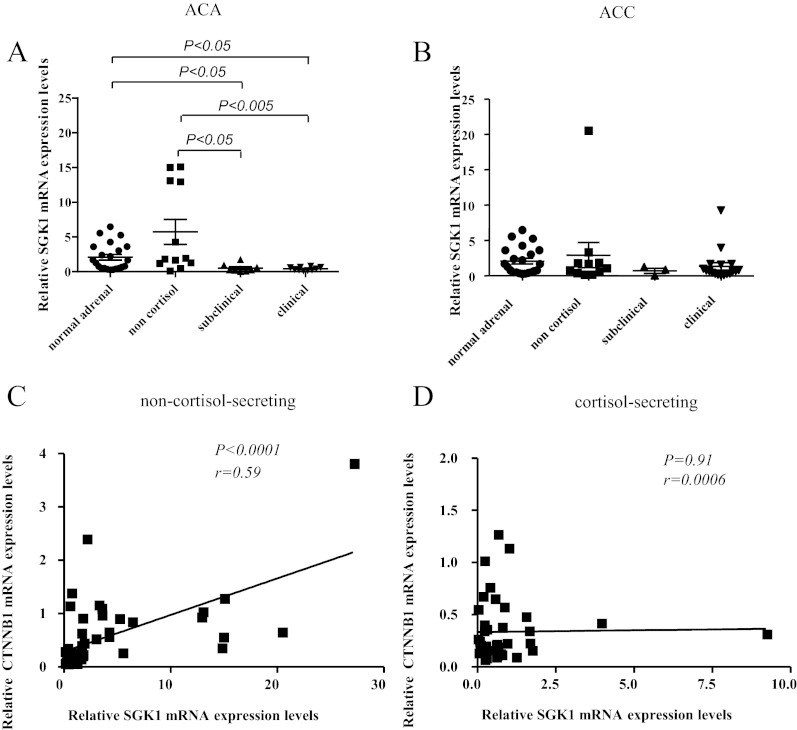
The mean SGK1 expression at the mRNA level was similar in ACAs (n = 30) and ACCs (n = 32) but was lower in adrenocortical tumors compared with NAs (n = 25; P < 0.05). This difference was due to the very low mRNA levels observed in CS tumors compared with NCS neoplasias (P < 0.005, Fig. 1, A and B). These findings were even more striking regarding only the ACAs (P < 0.0005, Fig. 1A). Accordingly, a negative correlation was observed between SGK1 expression and cortisol levels after dexamethasone in ACAs, although it did not reach significance (P = 0.11; r = −0.44; 95% CI = −1.7–0.29). No other correlation with clinical parameters was found.
Fig. 1.
A and B, Relative SGK1 gene expression levels evaluated by real-time quantitative PCR in 62 adrenocortical tumors and 25 NA glands. The gene β-actin was used as a loading control (reference gene). A, Thirty ACAs according to the cortisol secretion status (NCS, n = 12; subclinical CS, n = 8; overt CS, n = 10; P < 0.0005 by one-way ANOVA). B, Thirty-two ACCs according to the cortisol secretion status (NCS, n = 11; subclinical CS, n = 3; overt CS, n = 18; P = 0.069 by one-way ANOVA). C and D, Correlation between relative SGK1 and CTNNB1 (β-catenin) gene expression levels evaluated by real-time quantitative PCR in adrenocortical tumors and NA glands (linear regression analysis) with β-actin used as a loading control (reference gene); C, NCS tumors (n = 22) and NA glands (n = 25); D, CS tumors (n = 40).
In contrast to SGK1, SGK3 mRNA levels were comparable between CS and NCS tumors and were similar in NA, ACA, and ACC. SGK3 expression showed some association with SGK1 expression (P = 0.11; r = 0.10; 95% CI = −2.0–19.2).
Because SGK1 is a target gene of the Wnt/β-catenin pathway and it has also been reported to activate β-catenin (11), we also analyzed the CTNNB1 mRNA expression level in the same samples. There was no difference in CTNNB1 expression between NA, ACA, and ACC. A positive correlation was found between CTNNB1 and both SGK1 (P < 0.001; r = 0.57; 95% CI = 3.47–6.58) and SGK3 gene expression levels (P < 0.01; r = 0.14; 95% CI = 0.4–2.9). However, subgroup analysis revealed that the correlation between CTNNB1 and SGK1 was restricted to NCS tumors (P < 0.0001; r = 0.59; Fig. 1, C and D).
All these findings remained unaltered when considering as a single criterion for ACTH-independent cortisol secretion a post-dexamethasone cortisol cutoff of 1.8 μg/dl (50 nmol/liter).
SGK1 protein expression in adrenocortical tumors: relationship with malignancy
The SGK1 protein was detectable in all nine evaluable NAs, in all 29 ACAs, and in 161 of 163 ACCs (Table 1). In the NAs and in benign tumor tissues, SGK1 staining was usually localized both in the nucleus and the cytoplasm being homogeneously distributed both in standard tissue slides (Fig. 2, A and B) and among the different cores from the same tissue sample in TMAs. Within the adrenal gland, its expression was strong in the cortex, being higher in the zona glomerulosa and zona fasciculata and lower in the zona reticularis, whereas it was weak in the medulla (Fig. 2A). In ACCs, SGK1 staining was more inhomogeneous and often restricted to the cytoplasm (Fig. 2, C and D).
Table 1.
Serum SGK1 protein expression in 201 evaluable samples from adrenocortical tissues (176 from tissue microarrays and 25 from standard full slides)
| SGK1 expression H-score | n | Low expression |
High expression |
P vs. NA | P vs. ACA | P | ||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||||
| NA | 9 | 0 | 3 (33%) | 4 (44%) | 2 (22%) | |||
| ACA | 29 | 0 | 10 (34.5%) | 18 (62%) | 1 (3.5%) | NS | ||
| CS adenoma | 16 | 0 | 3 (19%) | 12 (75%) | 1 (6%) | NS | NSa | |
| NCS adenoma | 13 | 0 | 7 (54%) | 6 (46%) | 0 | NS | ||
| ACC | 163 | 2 (1%) | 61 (37%) | 75 (46%) | 23 (14%) | NS | NS | |
| CS carcinoma | 52 | 0 | 23 (44%) | 25 (48%) | 4 (8%) | NS | NS | 0.09a |
| NCS carcinoma | 27 | 0 | 6 (22%) | 15 (56%) | 6 (22%) | NS | 0.07 | |
| Primary tumor | 132 | 1 (1%) | 47 (36%) | 63 (48%) | 19 (14%) | NSb | ||
| Local recurrence | 19 | 1 (5%) | 8 (42%) | 8 (42%) | 2 (10%) | |||
| Distant metastasis | 12 | 0 | 6 (50%) | 4 (33%) | 2 (17%) | |||
Semiquantitative H-score was calculated by multiplying the staining intensity grading score (negative = 0, low = 1, medium = 2, strong = 3) with the proportion score (0 = 0% positive, 0.1 = 1–9% positive, 0.5 = 10–49% positive, 1 = 50% or more positive) as described previously (24). NS, Not significant.
P value between CS and NCS.
P value among primary tumor, local recurrence, and distant metastasis.
Fig. 2.
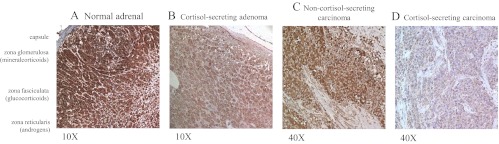
SGK1 protein expression in the adrenal tissue with a specific monoclonal antibody against SGK1. Immunohistochemical staining intensity was quantified on a scale from 0 (absent) to 3 (strong expression). Representative staining of a NA gland with the identification of the three different adrenal parts (A, strong intensity), a CS adenoma (B, moderate intensity), a NCS carcinoma (C, moderate intensity), and a CS carcinoma (D, low intensity). Original magnification, ×100 (A and B) or ×400 (C and D).
SGK1 expression was considered low with an H-score of 0 or 1 and high with an H-score of 2 or 3. The SGK1 levels were similar among NAs, ACAs, and ACCs (Table 1). Taken together, tumors with cortisol excess (n = 68) had a similar SGK1 protein expression compared with those with normal cortisol secretion (n = 42). There was also no significant difference between ACC samples derived from primary surgery (n = 132) and from local recurrences (n = 19) or distant metastases (n = 12). However, considering only NCS tumors, ACCs (n = 27) showed a trend to higher SGK1 expression compared with ACAs (n = 13) (P = 0.07, Table 1).
To evaluate the relationship between SGK1 and histopathological and clinical parameters in ACC, patients were included in the analysis only when tumor from the primary surgery and complete clinical data were available (n = 126). There was no significant correlation between the SGK1 protein expression and age, tumor size, ENSAT stage, Weiss score, Ki67 index, presence of distant metastasis, and duration of follow-up. Cortisol-secreting ACCs (n = 47) had a significantly lower SGK1 protein expression than the other ACCs (P < 0.05, Table 2). In addition, six of seven ACCs without any hormonal hypersecretion showed high SGK1 expression.
Table 2.
Relationship between serum SGK1 protein expression and baseline clinical or pathological characteristics of 126 patients with ACC (only tumor samples derived from primary surgery)
| Low SGK1 expression (H-score 0–1) | High SGK1 expression (H-score 2–3) | P | |
|---|---|---|---|
| n (%) | 47 (37) | 79 (63) | |
| Age, median (yr) | 47 | 49.4 | NS |
| Sex [n male (%)] | 12 (25.5) | 47 (59.5) | <0.05 |
| Tumor size, median (cm) | 13 | 12 | NS |
| Tumor stage (ENSAT) [n (%)] | |||
| 1 | 3 (60) | 2 (40) | NS |
| 2 | 15 (32) | 32 (68) | |
| 3 | 12 (31) | 27 (69) | |
| 4 | 15 (52) | 17 (48) | |
| Hormonal status [n (%)] | |||
| Known | 39 | 60 | |
| CS tumors | 21 (45) | 26 (55) | <0.05 |
| NCS tumors | 5 (18) | 23 (82) | |
| Weiss score, median | 5 | 5 | NS |
| Ki67 index, median (%) | 10 | 9 | NS |
| β-Catenin nuclear staining [n (%)] | |||
| Known | 36 | 45 | |
| Yes | 17 (57) | 13 (43) | 0.11 |
| No | 19 (37) | 32 (63) | |
| pAKT staining [n (%)] | |||
| Known | 43 | 75 | |
| 0–1 | 31 (40) | 47 (60) | NS |
| 2 | 12 (30) | 28 (70) | |
| Distant metastasis [n (%)] | |||
| Known | 44 | 75 | |
| Affected patients | 15 (47) | 17 (53) | NS |
| Nonaffected patients | 29 (33) | 58 (67) | |
| Medical treatment [n (%)] | |||
| Mitotane | |||
| Yes | 33 (41) | 47 (59) | NS |
| No | 14 (30) | 32 (70) | |
| Cytotoxic drugs | |||
| Yes | 20 (48) | 22 (52) | NS |
| No | 27 (32) | 57 (68) | |
| Chemoresistant | 17 (53) | 15 (47) | NS |
| Chemoresponsive | 3 (30) | 7 (70) | |
| Follow-up duration, median (months) | |||
| Still alive patients | 66 | 68 | NS |
Semiquantitative H-score was calculated by multiplying the staining intensity grading score (negative = 0, low = 1, medium = 2, strong = 3) with the proportion score (0 = 0% positive, 0.1 = 1–9% positive, 0.5 = 10–49% positive, 1 = 50% or more positive) as described previously (24). P values were calculated by Fisher exact test or χ 2 test. NS, Not significant.
A positive β-catenin nuclear staining was observed in 45% of evaluable ACCs and in 75% of ACAs (P < 0.05), without any relationship with tumor stage. No difference was found between CS and NCS tumors. Beta-catenin-positive ACCs tended to have a lower SGK1 expression than beta-catenin-negative ACCs (57 vs. 37%, P = 0.11, Table 2). This was particularly evident considering only CS tumors (P < 0.05).
Because SGK1 activity is related to the activation of PKB/AKT isoforms, we also evaluated the pAKT protein expression. A strong pAKT staining was detected in 40 of 121 ACCs (33%) but not in NAs or ACAs (P < 0.0005). However, a similar percentage of cases with weak or strong pAKT expression showed a low SGK1 expression (30 vs. 40%), and no correlation was observed between nuclear β-catenin and pAKT staining.
Impact of SGK1 expression levels on survival in ACC
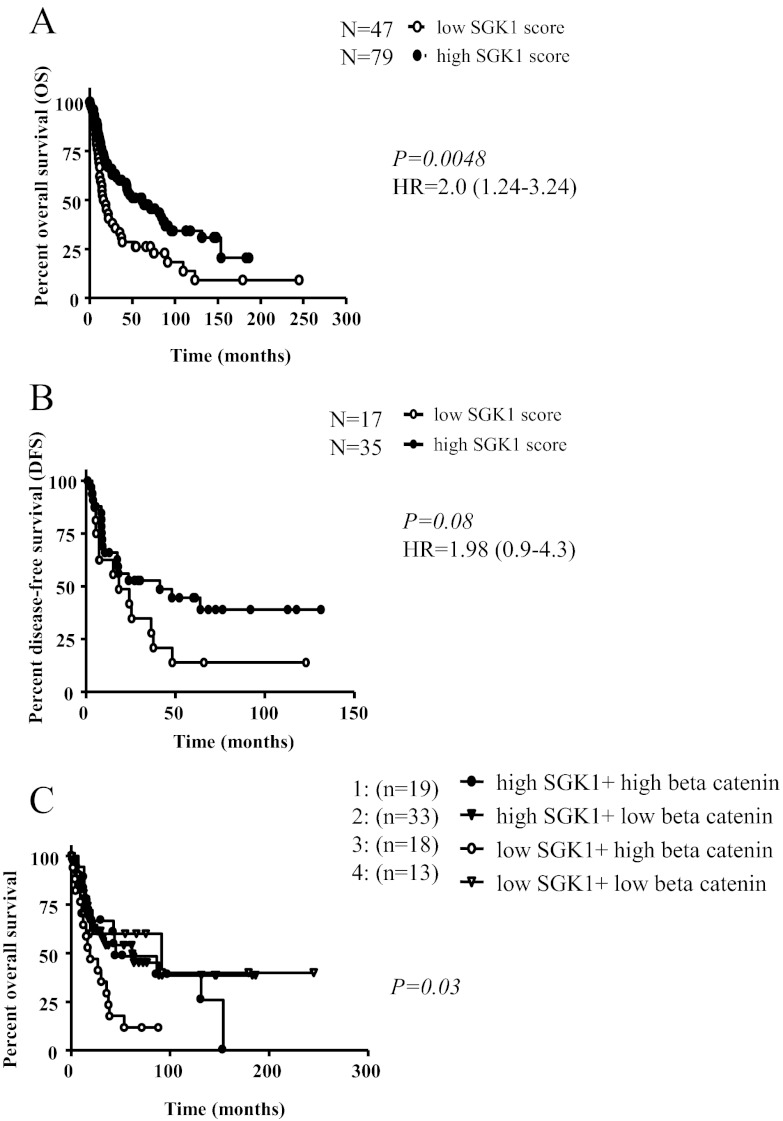
OS was shorter in patients with low SGK1 expression. For these patients, the risk of death was about 2-fold increased in comparison with patients with high SGK1 expression, both at the mRNA and at the protein level. However, using the mRNA data with only 15 cases in the low-SGK1 group and 17 in the high-SGK1 group, this difference failed significance with a median OS of 40 vs. 123 months [hazard ratio (HR) = 1.97; 95% CI = 0.69–5.64; P = 0.20]. Analyzing the protein expression, we observed a significantly shorter OS in cases with low SGK1 levels (n = 126; median survival, 19 vs. 62 months; HR = 2.0; 95% CI = 1.24–3.24; P < 0.005; Table 3 and Fig. 3A). Similar results were obtained also for DFS (n = 52; median survival, 18 vs. 41 months; HR = 1.98; 95% CI = 0.9–4.3; P = 0.08; Fig. 3B). The statistical significance of these findings remained unaltered when adjusted for tumor stage or cortisol excess, even if they were more evident considering only NCS tumors (data not shown). Interestingly, in a multivariate Cox regression analysis, including the SGK1 protein levels together with tumor size, tumor stage, Ki67 index, and cortisol excess, SGK1 was confirmed as an independent prognostic factor for OS (HR = 2.4; 95% CI = 1.15–4.61; P < 0.05; Table 3). Similarly, 38% of patients were alive without tumor recurrence at 2 yr in the high-SGK1 group vs. 17% in the low-SGK1 group (P < 0.01; odds ratio = 3.50; 95% CI = 1.39–8.80).
Table 3.
Prognostic factors for OS in patients with ACC (only tumor samples derived from primary surgery)
| n | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| Median survival (months) | HR | 95% CI | P | HR | P | ||
| All patients | 133 | ||||||
| Tumor size (cm) | 1.64 | 1.05–2.55 | 0.029 | 1.36 | NS | ||
| <12 | 80 | 47 | |||||
| >12 | 53 | 19 | |||||
| Tumor stage (ENSAT) | 5.0 | 2.8–8.9 | 0.0001 | 3.41 | 0.001 | ||
| 1–3 | 96 | 63 | |||||
| 4 | 37 | 12 | |||||
| Weiss score | 1.32 | 0.85–2.05 | NS | 1.61 | NS | ||
| <5 | 80 | 44 | |||||
| >5 | 53 | 24 | |||||
| Ki67 index (%) | 2.88 | 1.55–5.34 | 0.0008 | 3.65 | 0.0001 | ||
| <10 | 55 | 89 | |||||
| >10 | 30 | 24 | |||||
| Cortisol hypersecretion | 1.24 | 0.72–2.14 | NS | 1.11 | NS | ||
| No | 32 | 26 | |||||
| Yes | 52 | 30 | |||||
| SGK1 protein | 2.0 | 1.24–3.24 | 0.0048 | 2.40 | 0.017 | ||
| Low expression | 47 | 19 | |||||
| High expression | 79 | 62 | |||||
| Patients treated with cytotoxic drugsa | 33 | ||||||
| Tumor size (cm) | 2.34 | 1.1–5.0 | 0.027 | 1.47 | NS | ||
| <12 | 14 | 43 | |||||
| >12 | 19 | 18 | |||||
| Tumor stage (ENSAT) | 1.55 | 0.70–3.40 | NS | 1.50 | NS | ||
| 1–3 | 16 | 26 | |||||
| 4 | 16 | 17 | |||||
| Ki67 index (%) | 0.71 | 0.29–1.76 | NS | 1.79 | NS | ||
| <10 | 12 | 22 | |||||
| >10 | 12 | 24 | |||||
| Cortisol hypersecretion | 4.77 | 1.73–13.1 | 0.0024 | 1.58 | NS | ||
| No | 11 | 154 | |||||
| Yes | 12 | 13 | |||||
| SGK1 protein | 2.60 | 1.16–5.80 | 0.011 | 4.31 | 0.088 | ||
| Low expression | 16 | 13 | |||||
| High expression | 17 | 43 | |||||
P was evaluated by log-rank (Mantel-Cox) test (univariate analysis) or Cox proportional hazard regression model (multivariate analysis including SGK1 protein levels, tumor size, tumor stage, Ki67 index, and cortisol excess). NS, Not significant.
Patients treated with the two most frequent cytotoxic drugs, such as platinum compounds (n = 23) and streptozotocin (n = 10).
Fig. 3.
A and B, Impact of SGK1 protein expression on OS (A) and DFS (B) in patients with adrenocortical carcinoma; n = 126 tumor samples derived from primary surgery with complete clinical data. For DFS, only patients with complete resection have been analyzed. C, Impact of combined SGK1 and nuclear β-catenin protein expression on OS in patients with adrenocortical carcinoma; n = 85 tumor samples derived from primary surgery and available nuclear β-catenin staining (26).
In the present series of ACC samples with available nuclear β-catenin protein staining (n = 85), we observed an impact of β-catenin expression levels on OS in univariate analysis (median survival, 35 months in positive vs. 63 months in negative tumors; HR = 1.8; 95% CI = 0.96–2.95; P = 0.07). Moreover, we recognized a subgroup of patients with a combination of low SGK1 and high nuclear β-catenin protein expression showing a very poor prognosis (median survival, 19 months; HR = 3.3 when compared with the other groups; 95% CI = 0.5–7.3; P < 0.05; Fig. 3C). Again, pAKT protein expression did not affect prognosis in ACC, but in a combined analysis with SGK1, a subgroup with very poor prognosis was recognized (low SGK1 and strong pAKT expression; median survival, 16.3 months; HR = 2.7; 95% CI = 2.2–3.2; P < 0.05).
Finally, we analyzed separately the 46 ACC patients treated with different chemotherapeutic drugs. We observed that SGK1 expression was inversely correlated with OS after initiation of first-line cytotoxic drug administration (median survival, 7 months in 22 patients with low SGK1 vs. 22 months in 24 patients with high SGK1; HR = 2.56; 95% CI = 1.24–5.29; P < 0.05). The negative impact of low SGK1 expression and cortisol hypersecretion was confirmed considering only the 33 patients receiving the two most frequent cytotoxic drugs, such as platinum compounds or streptozotocin, without any specific difference between the two drugs (Table 3). In this subgroup, cortisol excess was also correlated with a worse prognosis. However, in multivariate analysis, only SGK1 remained a strong prognostic factor.
Discussion
Our research was triggered by the observation of frequent microdeletion in the SGK1 gene and low mRNA expression in CS adenomas (6). The major findings of this more extensive investigation are the close relationship between low SGK1 expression and ACTH-independent cortisol secretion and the profound prognostic impact of low SGK1 protein expression on survival in ACC.
The negative relationship of low SGK1 levels with poor prognosis was unexpected and is, at a first glance, counterintuitive. SGK1 is a serine/threonine kinase with a 50% homology to PKB/AKT isoforms and is involved in cellular proliferation and apoptosis protection. SGK1 is regulated by growth factors, steroid hormones, and a multitude of other stimulators (28) and exerts antiapoptotic activity through forkhead family members and nuclear factor-κB (9). It has been assumed that activation of AKT and SGK1 coordinately regulates cell growth and survival (13). Accordingly, high SGK1 expression has been implicated in cell survival in different neoplasias (7, 8, 12–14). However, the role of SGK1 in cancer cells may be rather complex and largely dependent on the cellular context, because in some cancers SGK1 expression is down-regulated (8, 15, 16). The negative predictive role of low SGK1 expression in ACC in our study was robust and remained evident also after adjustment for multiple prognostic factors including GC secretion and tumor stage. In addition, SGK1 and cortisol excess were the only two prognostic factors found also in patients with more severe disease undergoing cytotoxic therapy, although a protective role of high SGK1 expression against cytotoxic drugs has been reported (8, 9, 29). It has been suggested that low SGK1 expression in some cancers may indicate a feedback inhibition by strong activation of the PKB/AKT pathway or may be compensated by high SGK3 expression (8, 9). However, in our series, we did not find evidence for such mechanisms because SGK1 was only weakly correlated with SGK3 and not at all with pAKT.
SGK1 is a downstream target of the Wnt/β-catenin signaling pathway (8, 11) but also positively regulates its activation. However, although we were able to confirm a negative impact of high nuclear β-catenin protein expression on OS in ACC (26), we observed the negative prognostic value of low SGK1 particularly in the presence of high nuclear β-catenin expression, indicating that the interaction between SGK1 and Wnt/β-catenin pathway is disrupted in ACC patients with poor prognosis.
Of great interest in the context of our findings is the recently described interaction between SGK1 and the Notch signaling pathway (30). It was demonstrated that SGK1 reduced the protein stability of active Notch through the Fbw7 ubiquitin ligase. Accordingly, the transcriptional activity of the Notch1 intracellular domain was increased in SGK1-deficient cells. Activation of the Notch1 pathway has been demonstrated in the majority of patients with T-lineage acute lymphoblastic leukemia (31), but it is most likely also implicated in a variety of other cancers (32). Using SNP array analysis, we have recently demonstrated that Notch1 signaling is the most frequently altered pathway in benign adrenal neoplasia (6). Importantly, overexpression of Jagged 1, an endogenous ligand of Notch1, has recently been reported in ACC, confirming activation of the Notch1 pathway. New evidence suggests that SGK1 is an inhibitor of γ-secretase (33), a key player in the Notch1 pathway, which represents a promising target for tumor therapy (34). Of note, the down-regulation of Notch1 protein stability is dependent on the kinase activity of SGK1 but independent of glycogen synthase kinase-3β (30), a major component of the Wnt/β-catenin pathway, allowing for the discordant findings regarding SGK1 and β-catenin expression.
An intriguing result of our investigation is the inverse relationship between SGK1 expression and cortisol secretion, especially in ACA, suggesting a role of SGK1 expression on adrenal GC secretion or vice versa. It has been reported that in rat brain chronic cortisol-excess can inhibit SGK1 expression through a down-regulation of GC receptors in brain (35). However, a similar mechanism in adrenocortical tumor cells is unlikely, because their steroidogenesis is usually less efficient than normal adrenocortical cells. On the other hand, SGK1 may play a role in the feedback loop whereby intraadrenal GCs inhibit steroidogenesis (36, 37). In this case, low SGK1 levels would reduce the negative feedback leading to uninhibited cortisol secretion and potentially also increased proliferation (38). In favor of this hypothesis is the evidence of genetic microdeletion in some cortisol secreting adrenocortical tumors, suggesting a primary defect rather than a secondary down-regulation. Furthermore, although the expected correlation between SGK1 and CTNNB1 mRNA expression was evident in NCS tumors, it was disrupted in CS ones, suggesting a specific alteration of the interaction of these pathways in presence of chronic GC excess. Finally, in SGK1-knockout mice a renal aldosterone resistance has been observed (39). Thus, it is conceivable that lack of intraadrenal SGK1 may induce a state of local GC resistance contributing to abnormal cortisol secretion.
In conclusion, low SGK1 protein expression is associated with ACTH-independent cortisol secretion in ACA, suggesting a potential role for SGK1 in intraadrenal GC feedback, and with poor prognosis in ACC, possibly through complex interactions with other factors and pathways, such as the Notch1 pathway.
Supplementary Material
Acknowledgments
We are grateful to all the technical assistants for expert and kind help. We are grateful to all colleagues who provided tumor material and clinical data for the German ACC registry.
C.L.R. is receiving a Postdoc Researchers Fellowship from the Alexander von Humboldt Foundation. This work was partially supported by grants of the FIRM-ACT (01KG0501 to B.A. and M.F.) and of the Bundesministerium für Bildung und Forschung (BMBF01 EO1004).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACA
- Adrenocortical adenoma
- ACC
- adrenocortical carcinoma
- CI
- confidence interval
- CS
- cortisol-secreting
- DFS
- disease-free survival
- ENSAT
- European Network for the Study of Adrenal Tumors
- GC
- glucocorticoid
- HR
- hazard ratio
- NA
- normal adrenal
- NCS
- non-CS
- OS
- overall survival
- pAKT
- phospho-AKT
- PKB
- protein kinase B
- SGK1
- serum GC kinase 1
- SNP
- single-nucleotide polymorphism
- TMA
- tissue microarrays.
References
- 1. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. 2004. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 25:309–340 [DOI] [PubMed] [Google Scholar]
- 2. Fassnacht M, Libé R, Kroiss M, Allolio B. 2011. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol 7:323–335 [DOI] [PubMed] [Google Scholar]
- 3. Fassnacht M, Allolio B. 2009. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab 23:273–289 [DOI] [PubMed] [Google Scholar]
- 4. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jørgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. 2001. Incidence and late prognosis of Cushing's syndrome: a population-based study. J Clin Endocrinol Metab 86:117–123 [DOI] [PubMed] [Google Scholar]
- 5. Hassan-Smith ZK, Sherlock M, Reulen RC, Arlt W, Ayuk J, Toogood AA, Cooper MS, Johnson AP, Stewart PM. 2012. Outcome of Cushing's disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab 97:1194–1201 [DOI] [PubMed] [Google Scholar]
- 6. Ronchi CL, Leich E, Sbiera S, Weismann D, Rosenwald A, Allolio B, Fassnacht M. 2012. Single nucleotide polymorphism microarray analysis in cortisol-secreting adrenocortical adenomas identifies new candidate genes and pathways. Neoplasia 14:206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amato R, D'Antona L, Porciatti G, Agosti V, Menniti M, Rinaldo C, Costa N, Bellacchio E, Mattarocci S, Fuiano G, Soddu S, Paggi MG, Lang F, Perrotti N. 2009. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J Mol Med (Berl) 87:1221–1239 [DOI] [PubMed] [Google Scholar]
- 8. Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. 2006. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86:1151–1178 [DOI] [PubMed] [Google Scholar]
- 9. Lang F, Perrotti N, Stournaras C. 2010. Colorectal carcinoma cells–regulation of survival and growth by SGK1. Int J Biochem Cell Biol 42:1571–1575 [DOI] [PubMed] [Google Scholar]
- 10. Lang F, Artunc F, Vallon V. 2009. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens 18:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Failor KL, Desyatnikov Y, Finger LA, Firestone GL. 2007. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid-induced protein kinase and Akt signaling and controls β-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol Endocrinol 21:2403–2415 [DOI] [PubMed] [Google Scholar]
- 12. Tangir J, Bonafé N, Gilmore-Hebert M, Henegariu O, Chambers SK. 2004. SGK1, a potential regulator of c-fms related breast cancer aggressiveness. Clin Exp Metastasis 21:477–483 [DOI] [PubMed] [Google Scholar]
- 13. Fagerli UM, Ullrich K, Stühmer T, Holien T, Köchert K, Holt RU, Bruland O, Chatterjee M, Nogai H, Lenz G, Shaughnessy JD, Jr, Mathas S, Sundan A, Bargou RC, Dörken B, Børset M, Janz M. 2011. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene 30:3198–3206 [DOI] [PubMed] [Google Scholar]
- 14. Abbruzzese C, Mattarocci S, Pizzuti L, Mileo AM, Visca P, Antoniani B, Alessandrini G, Facciolo F, Amato R, D'Antona L, Rinaldi M, Felsani A, Perrotti N, Paggi MG. 2012. Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J Exp Clin Cancer Res 31:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lessi F, Beggs A, de Palo M, Anti M, Macarone Palmieri R, Francesconi S, Gomes V, Bevilacqua G, Tomlinson I, Segditsas S. 2010. Down-regulation of serum/glucocorticoid regulated kinase 1 in colorectal tumours is largely independent of promoter hypermethylation. PLoS One 5:e13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rauhala HE, Porkka KP, Tolonen TT, Martikainen PM, Tammela TL, Visakorpi T. 2005. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int J Cancer 117:738–745 [DOI] [PubMed] [Google Scholar]
- 17. Szmulewitz RZ, Chung E, Al-Ahmadie H, Daniel S, Kocherginsky M, Razmaria A, Zagaja GP, Brendler CB, Stadler WM, Conzen SD. 2012. Serum/glucocorticoid-regulated kinase 1 expression in primary human prostate cancers. Prostate 72:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. 2008. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fassnacht M, Beuschlein F, Quinkler M, Petersenn S. 2010. [Arterial hypertension and subclinical Cushing's syndrome]. MMW Fortschr Med 152:39–41 (German) [PubMed] [Google Scholar]
- 20. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, Mueller HH, Hahner S, Allolio B. 2009. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115:243–250 [DOI] [PubMed] [Google Scholar]
- 21. Fassnacht M, Johanssen S, Fenske W, Weismann D, Agha A, Beuschlein F, Führer D, Jurowich C, Quinkler M, Petersenn S, Spahn M, Hahner S, Allolio B. 2010. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J Clin Endocrinol Metab 95:4925–4932 [DOI] [PubMed] [Google Scholar]
- 22. Therasse P, Eisenhauer EA, Verweij J. 2006. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 42:1031–1039 [DOI] [PubMed] [Google Scholar]
- 23. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ronchi CL, Sbiera S, Kraus L, Wortmann S, Johanssen S, Adam P, Willenberg HS, Hahner S, Allolio B, Fassnacht M. 2009. Expression of excision repair cross complementing group 1 and prognosis in adrenocortical carcinoma patients treated with platinum-based chemotherapy. Endocr Relat Cancer 16:907–918 [DOI] [PubMed] [Google Scholar]
- 25. Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, Ragazzon B, Beuschlein F, Willenberg HS, Hahner S, Saeger W, Bertherat J, Allolio B, Fassnacht M. 2010. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab 95:E161–E171 [DOI] [PubMed] [Google Scholar]
- 26. Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, Libé R, Chokri I, Audebourg A, Royer B, Sbiera S, Vacher-Lavenu MC, Dousset B, Bertagna X, Allolio B, Bertherat J, Tissier F. 2011. β-Catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res 17:328–336 [DOI] [PubMed] [Google Scholar]
- 27. Fassnacht M, Weismann D, Ebert S, Adam P, Zink M, Beuschlein F, Hahner S, Allolio B. 2005. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. J Clin Endocrinol Metab 90:4366–4370 [DOI] [PubMed] [Google Scholar]
- 28. Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. 1993. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amato R, Menniti M, Agosti V, Boito R, Costa N, Bond HM, Barbieri V, Tagliaferri P, Venuta S, Perrotti N. 2007. IL-2 signals through Sgk1 and inhibits proliferation and apoptosis in kidney cancer cells. J Mol Med (Berl) 85:707–721 [DOI] [PubMed] [Google Scholar]
- 30. Mo JS, Ann EJ, Yoon JH, Jung J, Choi YH, Kim HY, Ahn JS, Kim SM, Kim MY, Hong JA, Seo MS, Lang F, Choi EJ, Park HS. 2011. Serum- and glucocorticoid-inducible kinase 1 (SGK1) controls Notch1 signaling by downregulation of protein stability through Fbw7 ubiquitin ligase. J Cell Sci 124:100–112 [DOI] [PubMed] [Google Scholar]
- 31. Jundt F, Schwarzer R, Dörken B. 2008. Notch signaling in leukemias and lymphomas. Curr Mol Med 8:51–59 [DOI] [PubMed] [Google Scholar]
- 32. Maliekal TT, Bajaj J, Giri V, Subramanyam D, Krishna S. 2008. The role of Notch signaling in human cervical cancer: implications for solid tumors. Oncogene 27:5110–5114 [DOI] [PubMed] [Google Scholar]
- 33. Mo JS, Yoon JH, Hong JA, Kim MY, Ann EJ, Ahn JS, Kim SM, Baek HJ, Lang F, Choi EJ, Park HS. 2012. Phosphorylation of nicastrin by SGK1 leads to its degradation through lysosomal and proteasomal pathways. PLoS One 7:e37111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Purow B. 2012. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol 727:305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. 2010. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 151:1177–1186 [DOI] [PubMed] [Google Scholar]
- 36. Gummow BM, Scheys JO, Cancelli VR, Hammer GD. 2006. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol 20:2711–2723 [DOI] [PubMed] [Google Scholar]
- 37. Briassoulis G, Damjanovic S, Xekouki P, Lefebvre H, Stratakis CA. 2011. The glucocorticoid receptor and its expression in the anterior pituitary and the adrenal cortex: a source of variation in hypothalamic-pituitary-adrenal axis function; implications for pituitary and andrenal tumors. Endocr Pract 17:941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlossmacher G, Stevens A, White A. 2011. Glucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cells. J Endocrinol 211:17–25 [DOI] [PubMed] [Google Scholar]
- 39. Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Náray-Fejes-Tóth A, Staub O. 2012. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol 302:F977–F985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.