Abstract
BACKGROUND
Improving care coordination is a national priority and a key focus of health care reforms. However, its measurement and ultimate achievement is challenging.
OBJECTIVE
To test whether patients whose providers frequently share patients with one another—what we term ‘care density’—tend to have lower costs of care and likelihood of hospitalization.
DESIGN
Cohort study
PARTICIPANTS
9,596 patients with congestive heart failure (CHF) and 52,688 with diabetes who received care during 2009. Patients were enrolled in five large, private insurance plans across the US covering employer-sponsored and Medicare Advantage enrollees
MAIN MEASURES
Costs of care, rates of hospitalizations
KEY RESULTS
The average total annual health care cost for patients with CHF was $29,456, and $14,921 for those with diabetes. In risk adjusted analyses, patients with the highest tertile of care density, indicating the highest level of overlap among a patient’s providers, had lower total costs compared to patients in the lowest tertile ($3,310 lower for CHF and $1,502 lower for diabetes, p < 0.001). Lower inpatient costs and rates of hospitalization were found for patients with CHF and diabetes with the highest care density. Additionally, lower outpatient costs and higher pharmacy costs were found for patients with diabetes with the highest care density.
CONCLUSION
Patients treated by sets of physicians who share high numbers of patients tend to have lower costs. Future work is necessary to validate care density as a tool to evaluate care coordination and track the performance of health care systems.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2104-7) contains supplementary material, which is available to authorized users.
KEY WORDS: care coordination, performance measure, provider social networks, care density
BACKGROUND
Care coordination has been identified as a priority area for the nation.1–3 Leading payment and delivery reform proposals—including bundled payment, accountable care organizations (ACOs), and patient-centered medical homes (PCMHs)—are expected to reduce costs and increase quality through mechanisms that improve harmonization of care across multiple providers.4–7 These policies would be facilitated by practical approaches that can document different aspects of coordination. While, in the future, it may be possible to use interoperable cross-provider electronic health records to measure coordination, health insurance claims are currently the only source of digital data that can practically be used for this purpose on a large scale.
Coordination is a multidimensional concept, encompassing the ways in which information is shared “across people, functions, and sites” and “over time”.8 Previous claims-based measurement approaches have emphasized continuity indices, such as percent of visits with the same provider, and dispersion and fragmentation metrics, which consider the number and types of providers seen in a given timeframe.9–12 These types of measures document the availability of longitudinal care and the potential challenges of having multiple providers. Such indicators are unable to account for relationships that exist between a patient’s providers that serve to facilitate key domains of coordination including communication among team members and appropriate follow-up.
In this paper, we attempt to fill this gap by examining whether patients whose providers frequently share patients with one another tend to have lower costs of care. The approach is built on a network analytic approach—the analysis of relationships among people.13–16 It is based on the premise that certain aspects of coordination may be reflected and/or facilitated by patients seeing physicians whose patient panels significantly overlap. Barnett and colleagues found that physicians “sharing” more patients (with “sharing” measured as claims for a common patient) are more likely to know one another through referrals and advice seeking.13 These physicians may be more likely to communicate and work with one another, potentially leading to better coordination for their patients.
To implement this approach, we constructed physician networks based on the observed patient sharing within health plans. For patients with congestive heart failure (CHF) and diabetes—two clinical conditions for which coordination is likely important17—we calculated the extent of patient sharing among their doctors—a measure we term ‘care density’. We tested the association between patients’ care density and their health care costs.
METHODS
Data Sources
Administrative databases from five large commercial insurance plans were the primary data source. Plans ranged in size from 460,000 to 890,000 members and represented all four Census regions. All plans were obtained from the IMS Health Plan Claims Database and represented a range of different product types including employer-sponsored insurance and Medicare Advantage. Plan membership files from 2009 were used to assign age, sex, and months of enrollment. Inpatient, outpatient, and outpatient pharmacy claims data were employed.
Cohort Definition
We generated a nested cohort for the two clinical conditions. The larger cohorts of patients with cardiac and endocrine diseases were used to define the amount of patient sharing between physicians. The decision to use the larger cohorts was based on the premise that physicians may share patients for a host of clinical conditions (e.g. CHF, angina, arrhythmias) and that the patient sharing across conditions reflects the extent to which doctors know one another. The amount of patient sharing among physicians was then applied to the physicians seen by patients in the smaller cohort. The smaller cohorts (i.e. the CHF and diabetes cohorts) were subsets of the larger ones and provided a more homogeneous sample with which to determine the association between patient sharing and costs.
The larger cohorts included all patients age 40 and over with a cardiac or endocrine condition, respectively, as defined by expanded diagnostic clusters (EDCs). EDCs are part of the Johns Hopkins Adjusted Clinical Group (ACG) Case-Mix Assessment System (version 10.0) and are groupings of diagnostic codes that describe the same or related conditions.18 EDCs are applied to all visits, and a visit may have multiple EDCs.
For the larger cardiac cohort, patients were included if they had outpatient visits (either office-based or outpatient hospital visits) with at least two different cardiac providers during 2009. Cardiac providers were defined as either primary care providers (internal medicine doctor without subspecialty training, family practitioner, or general practitioner) and/or cardiologists. Visits to these types of physicians represented 82.6% of all cardiac-related outpatient visits; the remaining specialty types each billed for less than 5% of visits. Due to the high prevalence of hypertension and relatively low proportion of patients who saw multiple providers for this condition, hypertension was not included with the other cardiac EDCs. The cardiac cohort had 86,987 patients.
Similarly, the endocrine cohort included 80,804 patients who had outpatient visits with at least two endocrine providers. Endocrine providers included primary care doctors (57.9% of all endocrine-related outpatient visits), endocrinologists (9.3%), cardiologists (7.8%), ophthalmologists (7.5%), and podiatrists (7.2%). The remaining specialty types each billed for less than 3% of visits.
For the smaller cohorts, we included all patients over age 40 with CHF/diabetes who were enrolled in the health plans for a full year. For the CHF cohort, patients were required to have outpatient visits with at least two different cardiac providers. The CHF cohort included 9,596 patients.
For diabetes, patients were required to have diabetes EDC and outpatient visits with at least two different endocrine providers. The diabetes cohort included 52,688 patients.
Calculating Care Density
To determine the extent of patient sharing, we calculated a measure we term ‘care density’. This is a patient-level measure that quantifies the amount of patient sharing among his or her providers. The numerator is the total number of instances of patient sharing over a time period (e.g. a year) among a patient’s doctors. The denominator is the total number of pairs of doctors for that patient. Specifically, the care density (Cp) for a given patient p is represented by the formula:
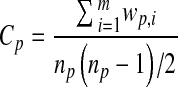 |
Where n is the number of distinct doctors that patient p saw, m is the total number of possible pairs of doctors, and w is the number of shared patients for each pair of doctors.
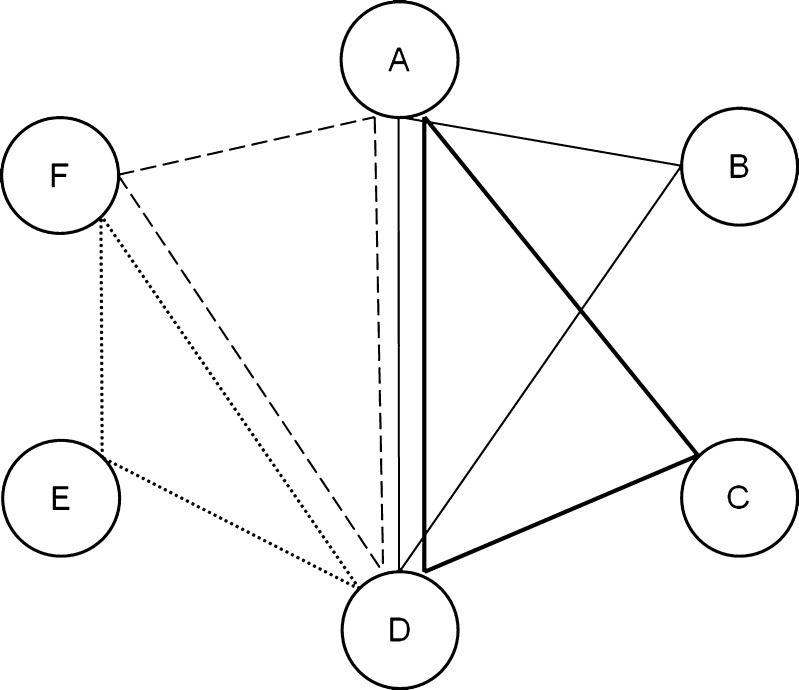
An example is given in Figure 1. The patient represented by the thick solid line saw 3 doctors (A, C, and D). There are three possible pairs of doctors (AC, AD, and CD). The denominator is 3. Doctors AB share 1 patient, AD share 3, and CD share 1 (each of these sums include patient under consideration). The numerator is 5. The patient’s care density would therefore be Cp = 1.67.
Figure 1.
Simplified care density network map. Doctors are represented by circles (A to F). Four patients are represented by different types of lines (solid light, solid heavy, large dashed, and small dashed lines). Each patient saw 3 doctors (and thus 3 pairs of doctors). Doctors are connected to one another if they shared in the care of a patient. The weight (strength) of connections between pairs of doctors represents the number of shared patients: the doctor pair AD is given a weight of 3, pair DF a weight of 2, and pairs AB, BD, CD, DE, EF, and AF a weight of 1. For each patient, the care density represents the sum of the weights of his/her doctor pairs divided by the number of doctor pairs he/she sees.
We calculated care density based on outpatient visits among cardiac/endocrine providers. Because provider identification numbers were unique to each commercial health plan, care density was calculated separately for each plan using the igraph software package in R version 2.14.1.19
Outcome Measures
Outcomes were total, outpatient, inpatient, outpatient and pharmacy costs during the 2009 calendar year. Costs were measured as the sum of allowed charges which include the paid amount plus any member liability (e.g., co-pay, deductible and co-insurance). Costs were not specific to cardiac/endocrine EDCs. We also calculated the total number of inpatient admissions per patient.
Patient and Health Plan Characteristics
Age and gender were determined from the plan membership files. Comorbidity was assessed using the Johns Hopkins ACG Case-Mix Assessment System.20 Diagnostic codes were assigned to 32 different Aggregated Diagnostic Groups (ADGs). Each ADG is a morbidity grouping with clinically homogeneous diagnosis codes that have similar expected need for health care resources. The count of different ADGs (morbidity types) for each patient was used as a measure of comorbidity.
Products in each insurance plan were classified as commercial, Medicare Advantage, and other/unknown payer. We also designated each plan type as HMO, PPO, and other/unknown.
Coordination Measures
We assessed multiple measures of care coordination used in previous studies: whether the patient saw a PCP;21 the total number of different cardiac or endocrine providers; the usual provider of care (UPC) index, which reflects the proportion of visits to the doctor that the patient saw most frequently;12 and the Bice Boxerman Continuity of Care (COC) index, which represents the dispersion of visits across providers.11
Statistical Analyses
We used descriptive statistics to examine patient characteristics, care density, total costs, and hospitalizations among CHF and diabetes patients. Bivariate analyses were used to examine the association between covariates and care density. Care density was categorized in plan-specific tertiles due to its non-normal, skewed distribution and the hypothesized non-linear association with costs. Multivariable linear regression models were constructed to assess whether care density was correlated with total costs of care as well as with inpatient, outpatient, and pharmacy costs separately. A negative binomial model with quadratic variance function was used for counts of hospitalization.
In sensitivity analyses we constructed sets of nested cohorts of patients with CHF and diabetes including both inpatient and outpatient visits. Second, we included patients who received CHF/diabetes care from a single provider. In order to keep the inclusion criteria parallel to the main analytic sample, we required that patients have at least two CHF/diabetes visits during the study period. Third, we tested whether adjusting for UPC or COC indices changed the association between care density and outcomes. Due to collinearity between UPC and COC, the two were assessed in separate models. Fourth, in the diabetes cohort, we calculated care density using only primary care doctors and endocrinologists, because it was hypothesized that these doctors may be most central to coordinating diabetes care. This study was exempted by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
RESULTS
Our sample included 9,596 patients with CHF, of whom 44.7% were female and the mean age was 72.5 years (Table 1). They saw a mean of 2.86 different outpatient cardiac providers (range 2–17). The average total costs of care were $29,456, and 41.8% of the sample had been hospitalized at least once during 2009. For the diabetes cohort, there were 52,668 patients. Over half (51.4%) were female and the mean age was 63.7 years. They saw an average of 2.5 different endocrine providers. Their average total costs of care were $14,921 and 14.4% had been hospitalized. Patients in both cohorts represented a range of insurance payers and product types (Supplemental Table 1 presents the range of values across the 5 insurance plans, available online). Care density ranged from 1 to 100 in the CHF cohort with a mean of 4.09 and from 1 to 120 in the diabetes cohort with a mean of 3.16.
Table 1.
Demographic and Health Care Characteristics of Study Populations
| CHF | Diabetes | |
|---|---|---|
| Total | Total | |
| N | 9,596 | 52,668 |
| Gender, female (%) | 4,287 (44.7%) | 27,051 (51.4%) |
| Age, mean (SD) | 72.5 (11.1) | 63.7 (11.3) |
| Number of ADGs, mean (SD) | 11.3 (3.9) | 9.2 (3.9) |
| Payer type distribution | ||
| Commercial | 2,619 (27.3%) | 28,245 (53.6%) |
| Medicare | 5,809 (60.5%) | 17,429 (33.1%) |
| other/unknown | 1,168 (12.2%) | 6,994 (13.3%) |
| Product type distribution | ||
| HMO | 3,336 (34.8%) | 22,626 (43.0%) |
| PPO | 5,268 (54.9%) | 24,025 (45.6%) |
| other/unknown | 992 (10.3%) | 6,017 (11.4%) |
| Mean number of providers seen (SD)* | 2.86 (1.37) | 2.50 (0.83) |
| PCP seen (%) | 8,201 (85.5%) | 45,293 (86.0%) |
| Usual provider of care, mean (SD) | 61.3 (15.9) | 61.3 (15.0) |
| Continuity of care, mean (SD) | 0.42 (0.19) | 0.34 (0.22) |
| Total costs, mean (SD) | $29,456 ($42,252) | $14,921 ($27,177) |
| Inpatient costs, mean (SD) | $14,255 ($32,636) | $4,278 ($16,657) |
| Outpatient costs, mean (SD) | $12,219 ($18,384) | $7,421 ($16,344) |
| Pharmacy costs, mean (SD) | $2,983 ($4,795) | $3,222 ($5,165) |
| Hospitalization (%) | 4,012 (41.8%) | 7,562 (14.4%) |
| Number of hospitalizations, mean (SD)† | 1.74 (1.24) | 1.47 (1.03) |
*PCPs and cardiologists were providers for the CHF cohort. PCPs, cardiologists, endocrinologists, ophthalmologists and podiatrists were providers for the diabetes cohort
†Among patients who were hospitalized
Table 2 presents comparisons of care density with patient and plan characteristics, coordination measures, and outcomes. In both cohorts, patients with higher care densities (defined as the top tertile and indicating the highest level of patient sharing among a patient’s providers) had the lowest average total, inpatient, and outpatient costs. There were no clear patterns of association between care density and measures of care coordination. The strength of the association was generally low, and insignificant for some measures (see Supplemental Table 2, available online).
Table 2.
Characteristics of CHF and Diabetes Cohorts, Stratified by the Care Densities
| CHF cohort | Diabetes cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Care Densities* | Care Densities* | |||||||
| Lower | Middle | Upper | p-value† | Lower | Middle | Upper | p-value† | |
| N | 3,437 | 2,844 | 3,315 | 24,396 | 10,268 | 18,004 | ||
| Mean care density | 1.05 (0.21) | 2.17 (1.01) | 8.88 (8.82) | 1.01 (0.06) | 2.04 (0.84) | 6.70 (10.51) | ||
| Gender, % female | 44.90% | 44.20% | 44.90% | 0.853 | 49.60% | 53.60% | 52.50% | < 0.001 |
| Age, mean (SD) | 70.7 (11.7) | 72.6 (10.9) | 74.3 (10.5) | < 0.001 | 63.3 (11.1) | 63.6 (11.3) | 64.3 (11.5) | < 0.001 |
| ADGs, mean (SD) | 11.0 (4.0) | 11.6 (3.9) | 11.2 (3.9) | < 0.001 | 9.1 (3.9) | 9.9 (4.0) | 8.9 (3.9) | < 0.001 |
| Payer type distribution | ||||||||
| Commercial | 31.10% | 27.20% | 23.40% | 50.80% | 58.80% | 54.50% | ||
| Medicare | 52 | 61 | 69 | 31.3 | 33.1 | 35.5 | ||
| other/unknown | 16.9 | 11.8 | 7.6 | < 0.001 | 17.9 | 8.1 | 10 | < 0.001 |
| Product type distribution | ||||||||
| HMO | 31.80% | 33.60% | 38.90% | 34.20% | 52.80% | 49.30% | ||
| PPO | 54.4 | 56.2 | 54.2 | 51 | 40.7 | 41.2 | ||
| other/unknown | 13.8 | 10.2 | 6.9 | < 0.001 | 14.8 | 6.5 | 9.5 | < 0.001 |
| Number of providers, mean (SD)‡ | 2.4 (0.8) | 3.2 (1.4) | 3.1 (1.7) | < 0.001 | 2.3 (0.6) | 3.0 (1.1) | 2.5 (0.8) | < 0.001 |
| PCP seen (Yes) | 87.00% | 89.50% | 80.40% | < 0.001 | 87.70% | 88.30% | 82.30% | < 0.001 |
| UPC, mean (SD) | 64.3 (14.9) | 58.3 (16.3) | 60.7 (15.9) | < 0.001 | 63.7 (14.3) | 56.7 (15.7) | 60.7 (14.9) | < 0.001 |
| COC, mean (SD) | .44 (.20) | .39 (.19) | .42 (.19) | < 0.001 | .36 (.22) | .31 (.20) | .34 (.21) | < 0.001 |
| Total costs, mean (SD) | $30,076 ($46,129) | $32,602 ($43,471) | $26,115 ($36,350) | < 0.001 | $15,403 | $16,748 | $13,225 | < 0.001 |
| ($29,112) | ($30,129) | ($22,125) | ||||||
| Inpatient costs, mean (SD) | $14,851 ($35,403) | $16,182 ($33,847) | $11,983 ($28,158) | < 0.001 | $4,689 | $4,965 | $3,329 | < 0.001 |
| ($18,151) | ($18,114) | ($13,296) | ||||||
| Outpatient costs, mean (SD) | $12,297 ($20,166) | $13,226 ($18,431) | $11,274 ($16,239) | < 0.001 | $7,617 | $8,406 | $6,593 | < 0.001 |
| ($17,492) | ($18,635) | ($12,943) | ||||||
| Pharmacy costs, mean (SD) | $2,928 ($4,936) | $3,194 | $2,859 ($4,400) | 0.017 | $3,097 | $3,377 | $3,303 | < 0.001 |
| ($5,051) | ($5,441) | ($4,879) | ($4,932) | |||||
| Hospitalization, % | 42.20% | 45.30% | 38.40% | < 0.001 | 15.20% | 14.90% | 12.90% | < 0.001 |
| Number of hospitalizations, mean (SD) § | 1.72 (1.27) | 1.79 (1.28) | 1.69 (1.16) | < 0.001 | 1.46 (1.01) | 1.56 (1.15) | 1.42 (0.97) | < 0.001 |
*Density tertiles are plan-specific
†One-way ANOVA F tests and Pearson Chi-squared tests
‡Includes primary care doctors and cardiologists (CHF) / cardiologists, endocrinologists, ophthalmologists, podiatrists (Diabetes)
§Among patients who were hospitalized
Figure 2 presents the differences in annual costs among the middle and high care density groups, relative to the low density group and adjusting for patient and plan characteristics (see Supplemental Table 3 for complete model, available online). For patients with CHF, high care density was associated with a $3,310 reduction in total costs compared to the lowest tertile (p < 0.001). High care density was associated with significantly lower inpatient costs ($2,563, p = 0.001) but not lower outpatient or pharmacy costs. The adjusted annual rate of hospitalization for the high care density group was 83.4% of the rate in the low density group (p < 0.001).
Figure 2.
Regression adjusted estimates of the impact of medium and high care density compared to low density on annual costs of care for patients with CHF and diabetes.
Similar results were found for patients with diabetes (see Fig. 2 and Supplemental Table 4, available online). Patients with high care density had, on average, $1,502 lower total costs compared to patients in the lowest tertile (p < 0.001). High care density was associated with lower inpatient ($992) and outpatient ($670) costs. The adjusted annual rate of hospitalization for the high care density group was 87.9% of the rate in the low density group (p < 0.001). Higher pharmacy costs ($160) were associated with having a high compared to a low care density (p < 0.001).
In sensitivity analyses, qualitatively similar results were found for patients when constructing care density using both inpatient and outpatient providers (Supplemental Tables 5 and 6, available online). For each cohort, higher care density was associated with lower total costs. Including patients who saw only a single physician did not significantly alter the associations between care density and costs/hospitalization (Supplemental Tables 7 and 8, available online). Having a single provider was associated with lower costs (total and outpatient costs for CHF; total, inpatient, outpatient, and pharmacy costs for diabetes) and lower rates of hospitalization compared to patients with more than one provider in the lowest care density tertile. Neither UPC nor COC were significantly associated with costs/hospitalizations and including them in regression models did not change the associations between care density and outcomes. Constructing care density with only primary care doctors and endocrinologists in the diabetes cohort revealed similar patterns of results (Supplemental Table 9, available online).
DISCUSSION
We found that CHF and diabetes patients receiving care from doctors with higher levels of shared patients (i.e. higher care density) had significantly lower total and inpatient costs and rates of hospitalization.
Prior work has validated that physicians with higher levels of patient-sharing in claims data are more likely to seek advice from one another, have referral relationships, and work in the same practice.13 In this context, patient sharing is likely to reflect higher levels of information exchange and interactive communication.13,22,23 This exchange may occur through formal (e.g., shared medical records, case conferences) or informal mechanisms (e.g., curbside consults, hallway conversations) and would be enhanced by the structural capabilities that allow doctors within and between practices to more effectively communicate with one another (e.g. health information technology, electronic referrals).24–26 It is therefore plausible that patients who see doctors with higher number of shared patients may receive better coordinated care. However, caution must be taken when interpreting the results—having a high number of shared patients does not indicate that two physicians are necessarily exchanging information about a particular patient. Further validation of the current approach is warranted.
Administrative and claims data may sometimes contain information about practice structure27,28 and methods have been developed to link physicians to hospitals where they are most likely to be affiliated.29 However, grouping physicians into practices networks and by hospital affiliation is usually challenging with standard data. Moreover, these groupings do not necessarily capture advice seeking or informal modes of communication that may be an important component of coordinated care, especially given the large proportion of PCPs that practice in solo- and small-group practices.30 The current approach may help address these shortcomings by providing a reasonably straightforward way to measure the strength of linkages between doctors. It is also possible that care density may be useful in describing differential levels of coordination that patients may receive within ACOs and PCMHs and between different health care delivery systems.
The majority of existing coordination and fragmentation measures use surveys to determine the patient and/or family perspective on their satisfaction with care coordination.8 The patient-centeredness of care has been postulated to be a key component of care integration.31 It is unknown whether patients with higher care densities perceive higher levels of coordination and have greater satisfaction. Further, the current approach does not account for other dimensions of care coordination that may impact outcomes, such as longitudinal continuity of care. We observed a low correlation between care density and existing measures that assess visit concentration and fragmentation. This suggests that care density may measure a unique aspect of care that is not captured by existing measures. However, further research is necessary to determine to what extent care density may be used in conjunction with these measures to more completely characterize the multiple facets of coordination. Additionally, given the exponential increase in the availability of EHRs and other HIT, the degree to which care density can be integrated into electronically derived measures of coordination attainment (e.g. electronic exchange of consult information) also represents a fertile area for future metric development.
The study was specifically designed to use claims data to assess care coordination. However, the use of claims data is associated with multiple limitations and suggests potential extensions of this work. First, as with all claims-based measures of coordination, we are unable to evaluate whether care was truly coordinated for a given patient. Instead, we present an approach that may suggest conditions that are more or less favorable towards coordinated care. Second, although we applied a well-tested case-mix adjustment methodology, we were unable to assess the severity of the clinical conditions (e.g. ejection fraction in CHF and hemoglobin A1c in diabetes). Third, we were unable to determine structural features of the relationships between physicians (e.g. being members of a group practice or IPA). To the extent that this correlates with higher levels of patient sharing, the proposed measure of care coordination captures this information. Fourth, we relied on total costs as the primary outcome measure as claims data are limited in their ability to attribute costs to particular conditions.32 Additional analyses are necessary to examine services that may be directly on the causal pathway (e.g. overuse of imaging and tests) and which may be markers for quality of care. Fifth, we construct our network measures for specific diseases, limiting it to doctors who we believe are most likely involved in care coordination. It is possible to consider alternative specifications, especially given that patients frequently have multiple comorbid illnesses. Sixth, our analyses are limited to patients from larger insurers rather than the universe of all patients within a given geographic area. We therefore underestimate the number of shared patients between doctors. Finally, we were unable to examine how care density varies according to patient race/ethnicity and socioeconomic status, and whether care density mediates potential disparities in costs and outcomes.
Care coordination is postulated to be a key mechanism in improving health care quality and reducing costs. It is therefore critical to measure care coordination at a population-level using existing data sources. The current study suggests a novel approach that uses patient sharing to assess potential aspects of coordination, is based on claims data, and finds significant associations with costs of care and hospitalizations.
Electronic Supplementary Material
(DOCX 47 kb)
Acknowledgments
Contributors
The authors thank Donniell Fishkind for his careful review of the manuscript. He did not receive compensation for his effort.
Funders
Dr. Pollack’s salary was supported by a career development award from the NIH National Cancer Institute and Office of Behavioral and Social Sciences Research (1K07CA151910-01A1). The funders had no role in the design and conduct off the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of Interest
This work was performed with support by faculty and staff at The Johns Hopkins University, where the ACG method was developed and is maintained. The Johns Hopkins University holds the copyright to the ACG software. To help support research and development, The Johns Hopkins University receives royalties from health plans and other organizations that use the ACG software. The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Priority Areas for National Action: Transforming Health Care Quality. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.National Priorities Partnership. National Priorities and Goals: Aligning Our Efforts to Transform America's Healthcare. Washington, DC: National Quality Forum; 2008: http://www.nationalprioritiespartnership.org/uploadedFiles/NPP/08-253-NQF%20ReportLo%5B6%5D.pdf.
- 3.National Strategy for Quality Improvement in Health Care. Washington, DC: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 4.Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28:1418–1428. doi: 10.1377/hlthaff.28.5.1418. [DOI] [PubMed] [Google Scholar]
- 5.Rittenhouse DR, Shortell SM, Fisher E. Primary care and accountable care–two essential elements of delivery-system reform. N Engl J Med. 2009;361:2301–2303. doi: 10.1056/NEJMp0909327. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in Medicare. Health Aff. 2009;28(2):w219–w231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal MB. Beyond pay for performance–emerging models of provider-payment reform. N Engl J Med. 2008;359:1197–1200. doi: 10.1056/NEJMp0804658. [DOI] [PubMed] [Google Scholar]
- 8.National Quality Forum, NQF-Endorsed Definition and Framework for Measuring Care Coordination, http://www.qualityforum.org/projects/care_coordination.aspx, Accessed April 24, 2012.
- 9.McDonald K, Schultz E, Albin L, et al. Care Coordination Measures Atlas. Vol No. 11-0023-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2011: http://www.ahrq.gov/qual/careatlas/. Accessed April 24, 2012.
- 10.Saultz JW. Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–143. doi: 10.1370/afm.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care. 1977;15:347–349. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63:158–188. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 13.Barnett ML, Landon BE, O'Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self-reported and administrative data. Health Serv Res. 2011;46:1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke DA, Harris JK. Network analysis in public health: history, methods, and applications. Ann Rev Pub Health. 2007;28:69–93. doi: 10.1146/annurev.publhealth.28.021406.144132. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman S, Faust K. Social Network Analysis: Methods and Applications. New York: Cambridge University Press; 1999. [Google Scholar]
- 16.Smith KP, Christakis NA. Social networks and health. Ann Rev Sociol. 2008;34:405–429. doi: 10.1146/annurev.soc.34.040507.134601. [DOI] [Google Scholar]
- 17.2010 National Healthcare Quality Report. 2010; http://www.ahrq.gov/qual/qrdr10.htm. Accessed April 24, 2012.
- 18.The Johns Hopkins ACG System Reference Manual Version 9.0. 2009; www.acg.jhsph.org. Accessed April 24, 2012.
- 19.Csardi G, Nepusz T. The igraph software package for complex network research. Paper presented at: International Conference on Complex Systems 2006; Boston, MA.
- 20.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. Br Med J. 2003;327:1219–1221. doi: 10.1136/bmj.327.7425.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foy R, Hempel S, Rubenstein L, et al. Meta-analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152:247–258. doi: 10.7326/0003-4819-152-4-201002160-00010. [DOI] [PubMed] [Google Scholar]
- 24.O'Malley AS, Reschovsky JD. Referral and consultation communication between primary care and specialist physicians. Arch Intern Med. 2011;171:56–65. doi: 10.1001/archinternmed.2010.480. [DOI] [PubMed] [Google Scholar]
- 25.Chen AH, Kushel MB, Grumbach K, Yee HF. A safety-net systems gains efficiencies through 'eReferrals' to specialists. Health Aff. 2010;29:969–971. doi: 10.1377/hlthaff.2010.0027. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Chen AH, Keith E, Yee HF, Kushel MB. Not perfect, but better: primary care providers' experiences with electronic referrals in a safety net health system. J Gen Intern Med. 2009;24:614–619. doi: 10.1007/s11606-009-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham HH, O'Malley AS, Bach PB, Saiontz-Martinez C, Schrag D. Primary care physicians' links to other physicians through Medicare patients: the scope of care coordination. Ann Intern Med. 2009;150(4):236–242. doi: 10.7326/0003-4819-150-4-200902170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 29.Bynum JPW, Bernal-Delgado E, Gottlieb D, Fisher E. Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health Serv Res. 2007;42:45–62. doi: 10.1111/j.1475-6773.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebhaber A, Grossman JM. Physicians Moving to Mid-Sized, Single-Specialty Practices. Tracking Report No.18. 2007. http://www.hschange.com/CONTENT/941/941.pdf. Accessed April 24, 2012. [PubMed]
- 31.Singer SJ, Burgers J, Friedberg M, Rosenthal MB, Leape L, Schneider E. Defining and measuring integrated patient care: promoting the next frontier in health care delivery. Med Care Res Rev. 2011;68:112–127. doi: 10.1177/1077558710371485. [DOI] [PubMed] [Google Scholar]
- 32.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med. 1997;127:666–674. doi: 10.7326/0003-4819-127-8_part_2-199710151-00048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 47 kb)