Abstract
Background
Recent population studies have suggested that children with multiple exposures to anesthesia and surgery at an early age are at an increased risk of cognitive impairment. We therefore have established an animal model with multiple versus single exposures of anesthetic(s) in young versus adult mice, aiming to distinguish the role of different anesthesia in cognitive impairment.
Methods
Six day and 60 day-old mice were exposed to various anesthesia regimen. We then determined the effects of the anesthesia on learning and memory function, levels of pro-inflammatory cytokine interleukin-6 and tumor necrosis factor-α in brain tissues, and the amount of ionized calcium binding adaptor molecule 1 positive cells, the marker of microglia activation, in the hippocampus.
Results
Here we show that anesthesia with 3% sevoflurane two hours daily for three days induced cognitive impairment and neuroinflammation [e.g., increased interleukin-6 levels: 151% ± 2.3 (mean ± SD) versus 100% ± 9.0, P = 0.035, n = 6] in young, but not adult, mice. Anesthesia with 3% sevoflurane two hours daily for one day and 9% desflurane two hours daily for three days induced neither cognitive impairment nor neuroinflammation. Finally, an enriched environment and anti-inflammation treatment (ketorolac) ameliorated the sevoflurane anesthesia-induced cognitive impairment.
Conclusions
Anesthesia-induced cognitive impairment may depend on developmental stage, anesthetic agent, and the number of exposures. These findings also suggest the cellular basis and the potential prevention and treatment strategies for the anesthesia-induced cognitive impairment, which may ultimately lead to safer anesthesia care and better postoperative outcomes for children.
Introduction
An estimated six million children undergo surgical care each year in America alone 1. The widespread and prevalent use of anesthesia in children makes its safety a major health issue of interest [2, reviewed in 3]. Recent population studies have suggested that anesthesia and surgery could be risk factors for subsequent cognitive impairment [reviewed in 3]. Specifically, children who have multiple exposures (e.g., three times) to anesthesia and surgery at an early age (e.g., before age 4) are at an increased risk to develop learning disabilities [4,5, reviewed in 3]. These data suggest that children may not reach cognitive potentials compared to their peers who have not undergone anesthesia and surgery. These findings have become a major public health issue 2.
However, not every child develops cognitive impairment after having anesthesia and surgery, and older children may be less susceptible to this phenomenon 4. Therefore, we have hypothesized that there is a multifactorial model of the cognitive impairment such that the combination of an environmental insult (precipitating factors, e.g., selective anesthesia) with an age vulnerability (predisposing factors, e.g., certain age groups) is needed to cause the cognitive impairment. In the present studies, we have tested this hypothesis by identifying the selective effects of anesthetics (sevoflurane versus desflurane) and anesthesia regimen (one versus three times) on cognitive impairment in different age groups (six versus 60 days) of mice.
Neuroinflammation, including microglia activation and increases in the levels of pro-inflammatory cytokines in the brain, may lead to cognitive impairment 6–10. Specifically, pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), can be released by the microglia during its activation, fueling brain inflammation and leading to cognitive impairment in humans 11–14 and in animals 15–17. We therefore assessed the effects of different anesthetics and anesthesia regimen on the levels of IL-6 and TNF-α, and ionized calcium binding adaptor molecule 1 (IBA1), the marker of microglia activation 18,19, in the brain tissues of mice.
Finally, enriched environment (EE) has been shown to improve brain function. Delayed EE mitigates anesthesia-induced learning and memory impairment in rats 20. We therefore determined whether EE, which occurred immediately after the anesthesia, could attenuate the anesthesia-induced cognitive impairment in mice in the present experiments.
Materials and Methods
Mice anesthesia and treatment
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, Massachusetts. Postnatal day (P) 6 or P60 C57BL/6J (Jackson Lab, Bar Harbor, ME) mice (both male and female) received either anesthetic sevoflurane or desflurane plus 60% oxygen (balanced with nitrogen) to maintain sufficient partial pressure of oxygen levels in the mice during anesthesia, as performed in our previous studies 21. Control groups received 60% oxygen at an identical flow rate in similar chambers. There was no significant difference in learning and memory function between the mice that received 60% oxygen and the mice that received 21% oxygen (data not shown). The anesthetic and oxygen concentrations were measured continuously (Ohmeda, GE Healthcare, Tewksbury, MA). The temperature of the anesthetizing chamber was controlled to maintain a 37 ± 0.5°C rectal temperature in the mice. We determined pH, partial pressure of oxygen and partial pressure of carbon dioxidein the neonatal mice using the methods described by Satomoto et al. 22. Specifically, the young mice had a quick arterial blood sampling from femoral arterial at the end of two hours anesthesia and the samples were transferred into heparinized glass capillary tubes. A single sample (100 ul) was analyzed immediately after blood collection by blood gas analyzer (ITC, Edison, NJ). Anesthesia with 3% sevoflurane 21,22 for two hours did not significantly change the values of pH, partial pressure of oxygen, or partial pressure of carbon dioxide as compared to control group. Anesthesia with 9% desflurane for two hours did not significantly change the values of pH, partial pressure of oxygen, or partial pressure of carbon dioxide as compared to control group: pH: 7.33 ± 0.05 versus 7.41 ± 0.14 (control versus desflurane anesthesia); partial pressure of oxygen: 174 ± 12.4 mmHg versus 142 ± 27.0 mmHg (control versus desflurane anesthesia); partial pressure of carbon dioxide: 48 ± 5.1 mmHg versus 41 ± 9.9 mmHg (control versus desflurane anesthesia). Furthermore, as compared to the control mice, the anesthetized mice did not show significant changes in behavior (e.g., eating, drinking, general activity, and body weight) after the anesthesia. Mortality rate of mice in these studies was less than 1%. For the intervention studies, ketorolac (1 mg/kg) 23, one of the nonsteroidal anti-inflammatory drugs, was given to mice 30 minutes through intraperitoneal injection before each of the three day sevoflurane anesthesia.
Morris Water Maze
A round steel pool, 150 cm in diameter and 60 cm in height, was filled with water to a height of 1.0 cm above the top of a 15-centimeter diameter platform. The pool was covered with a black curtain and was located in an isolated room with four visual cues on the wall of pool. Water was kept at 20 ° C and opacified with titanium dioxide. The P30 or P84 mice were tested in the Morris Water Maze (MWM) four trials per day for 7 days. Each mouse was placed in the pool to search for the platform. The starting points were random for each mouse. Mice that found the platform were allowed to stay on it for 15 seconds. If a mouse did not find the platform within a 90 second period, it was gently guided to the platform and allowed to stay on it for 15 seconds. A video tracking system recorded the swimming motions of the animals, and the data were analyzed using motion-detection software for the MWM (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P.R. China). At the end of the reference training (P36 or P90), the platform was removed from the pool and the mouse was placed in the opposite quadrant. Each mouse was allowed to swim for 90 seconds and the times the mouse swam to cross the platform area was recorded (platform crossing times). Mouse body temperature was maintained by active heating as described by Bianchi et al. 24. Specifically, after every trial, each mouse was placed in a holding cage under a heat lamp for one to two minutes to dry before returning to its regular cage.
Brain tissue harvest and protein level quantification
Different groups of mice in both the control and anesthesia conditions were used for biochemistry studies. Immediately following the anesthesia, the mice were killed by decapitation (for Western blot analysis) or transcardial perfusion (for immunohistochemistry studies). Separate groups of mice were used for the Western blot analysis and the immunohistochemistry studies. For the Western blot analysis, the harvested brain tissues were homogenized on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A). The lysates were collected, centrifuged at 12,000 rpm for 10 minutes, and quantified for total proteins with bicinchoninic acid protein assay kit (Pierce, Iselin, NJ).
Western blot analysis
IL-6 antibody (1:1,000 dilution, Abcam, Cambridge, MA) was used to recognize IL-6 (24 kDa). TNF-α antibody (1:1,000 dilution; Abcam) was used to recognize TNF-α (17 kDa). Western blot quantification was performed as described by Xie et al. 25. Briefly, signal intensity was analyzed using image analysis program Quantity One (Bio-Rad, Hercules, CA). We quantified the Western blots in two steps, first using β-Actin levels to normalize protein levels (e.g., determining the ratio of IL-6 to β-Actin amount) and control for loading differences in the total protein amount. Second, we presented protein level changes in mice undergoing anesthesia as a percentage of those in the control group. 100% of protein level changes refer to control levels for the purpose of comparison to experimental conditions.
Immunohistochemistry
Mice were anesthetized with isoflurane briefly (1.4% isoflurane for five minutes) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.4. The anesthesia with 1.4% isoflurane for five minutes in mice provided adequate anesthesia for the perfusion procedure without causing significant changes in blood pressure and blood gas according to our previous studies 25. Mouse brain tissues were removed and kept at 4 ° C in paraformaldehyde. Five μm frozen sections from the mouse brain hemispheres were used for the immunohistochemistry staining. The sections were incubated with IBA1 antibody (ab5076, 1:100, Abcam) and secondary antibody (Alexa 568, 1:500, Invitrogen, Carlsbad, CA). Finally, the sections were analyzed in mounting medium under a 20 X objective lens confocal microscope and photos of the sections were taken. An investigator who was blind to the experimental design counted the number of IBA1 positive cells using Image J Version 1.38 (National Institutes of Health, Bethesda, MD).
Enriched Environment
The EE in the current experiment was performed as described in previous studies with modifications 26–28. Specifically, an EE was created in a large cage (70 × 70 × 46 centimeter) that included 5 – 6 toys (e.g., wheels, ladders, and small mazes). The mice were put in the EE everyday for two hours from P8 to P30. The objects were changed two to three times a week to provide a novel and challenging environment.
Statistics
Data in biochemistry changes were expressed as mean ± SD. Data in changes of escape latency were expressed as mean ± SEM. The data for platform crossing time were not normally distributed, thus were expressed as median and interquartile range. The number of samples varied from 6 to 16, and the samples were normally distributed except platform crossing time (tested by normality test, data not shown). Interaction between time and group factors in a two way ANOVA with repeated measurements was used to analyze the difference of learning curves (based on escape latency) between mice in the control group and mice treated with anesthesia in the MWM. Post-hoc Bonferroni test was used to compare the difference in escape latency between the control and anesthesia group in each day of the MWM. The Mann-Whitney test was used to determine the difference between the sevoflurane and control conditions on platform crossing times. There were no missing data for the variables of MWM (escape latency and platform crossing time) during the data analysis. Finally, a student two sample t-test was used to determine the difference between the anesthesia and control groups in levels of IL-6, TNF-α and IBA1 positive cells. P values less than 0.05 were considered statistically significant. SAS software version 9.2 (Cary, NC) was used to analyze the data.
Results
Multiple exposures of sevoflurane in young mice induced cognitive impairment and accumulation of brain IL-6 and TNF-α in mice
Given the clinical observation that three, but not one, exposures to anesthesia and surgery increased the risk of cognitive impairment in children 4,5, we have established an animal model in which mice were treated with sevoflurane two hours daily for one or three days. This animal model conceptually mimics the single versus multiple exposures of anesthesia and allows us to study the anesthesia-induced developmental neurotoxicity.
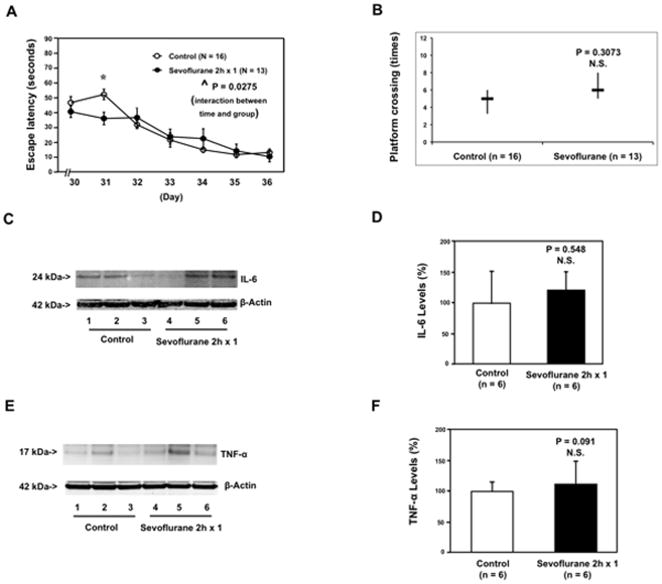
The mice were treated with 3% sevoflurane anesthesia two hours daily for three days from P6 to P8. The mice were tested in the MWM from P30 to P36. A comparison of the time that each mouse took to reach the platform during reference training (escape latency) showed that there was a statistically significant interaction of time and group based on escape latency in the MWM between mice following the control condition and mice following the sevoflurane anesthesia (Figure 1A, ^^ P = 0.0063, two-way ANOVA with repeated measurement). Post-hoc Bonferroni test showed that the mice that received the sevoflurane anesthesia had a longer escape latency than the mice following the control condition on P33, P34, P35 and P36. A comparison of the number of times that each mouse crossed the location of the absent platform at the end of reference training (platform crossing times) indicated that the sevoflurane anesthesia decreased the platform crossing times as compared to the control condition: sevoflurane (median = 3 and interquartile range = 2 to 4) versus control (median = 6 and interquartile range = 4 to 7), Figure 1B, * P = 0.0129, n = 13 (control), n = 15 (sevoflurane), Mann-Whitney test. There was no significant difference in mouse swimming speed between the mice in the sevoflurane anesthesia group and the mice in the control group (data not shown). These data suggest that multiple exposures of sevoflurane in young mice may induce cognitive impairment in the mice approximately one month post-anesthesia.
Figure 1. Anesthesia with 3% sevoflurane two hours daily for three days in P6 mice induces cognitive impairment in the mice tested from P30 to P36, and increases IL-6 and TNF-α levels in the brain tissues of the mice.
A. Anesthesia with 3% sevoflurane two hours daily for three days in P6 mice increases the escape latency of mice swimming in the Morris Water Maze (MWM) as compared to the control condition (tested from P30 to P36) (control: n = 13, sevoflurane: n = 15). Two way ANOVA with repeated measurement analysis shows that there is a statistically significant interaction of time and group based on escape latency of MWM between mice following the control condition and mice following the sevoflurane anesthesia in the MWM. Specifically, mice that received the sevoflurane anesthesia had a longer escape latency time at P33, P34, P35 and P36 as compared to the control condition. B. Anesthesia with 3% sevoflurane two hours daily for three days in P6 mice reduces the platform crossing times of mice swimming in the MWM as compared to the control condition tested at P36 (control: n = 13, sevoflurane: n = 15). C. The sevoflurane anesthesia increases IL-6 levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the sevoflurane anesthesia and control conditions. D. Quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia increases IL-6 levels in the brain tissues of the mice as compared to the control condition. E. Sevoflurane anesthesia increases TNF-α levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in brain tissues of the mice between the sevoflurane anesthesia and control groups. F. Quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia increases TNF-α levels in the brain tissues of the mice as compared to the control group. P, postnatal day; MWM, Morris Water Maze; ANOVA, analysis of variance. IL, interleukin; TNF, tumor necrosis factor.
Given the findings that multiple exposures to sevoflurane in young mice might induce cognitive impairment, next we investigated the underlying mechanisms. Pro-inflammatory cytokines, particularly IL-6 and TNF-α, are associated with cognitive impairment 11–17. We therefore assessed the effects of the sevoflurane anesthesia on the levels of IL-6 and TNF-α in brain tissues of the mice. The brain tissues of the mice were harvested at the end of the anesthesia (P8) and were subjected to Western blot analysis to determine levels of IL-6 and TNF-α. Immunoblotting of IL-6 showed that the sevoflurane anesthesia led to a more visible band representing IL-6 as compared to the control condition (Figure 1C). There was no significant difference in β-Actin levels between the mice that received the sevoflurane anesthesia and the mice following the control condition (Figure 1C). The quantification of the Western blot illustrated that the sevoflurane anesthesia increased IL-6 levels in brain tissues of the mice: 151% ± 2.3 versus 100% ± 9.0, n = 6, * P = 0.035 (Figure 1D). Immunoblotting of TNF-α showed that the sevoflurane anesthesia caused a more visible band representing TNF-α as compared to the control group (Figure 1E). There was no significant difference in β-Actin levels between the mice that received the sevoflurane anesthesia and the mice following the control condition (Figure 1E). The quantification of the Western blot revealed that the sevoflurane anesthesia increased TNF-α levels in the brain tissues of the mice: 178% ± 24 versus 100% ± 72.2, n = 6, * P = 0.013 (Figure 1F). Taken together, these results suggest that multiple exposures of sevoflurane in young mice may increase pro-inflammatory cytokine levels in brain tissues, ultimately leading to cognitive impairment.
Single exposure of sevoflurane in young mice induced neither cognitive impairment nor accumulation of brain IL-6 and TNF-α in mice
Next, we asked whether a single exposure to sevoflurane anesthesia in young (P6) mice could induce cognitive impairment and accumulation of brain IL-6 and TNF-α in mice. The six day-old mice were treated with 3% sevoflurane anesthesia for two hours at P6 only. The mice were tested in the MWM from P30 to P36. Although there was a statistically significant interaction of time and group based on escape latency in the MWM between the mice in the control group and the mice in the sevoflurane anesthesia group, the mice that received sevoflurane anesthesia had faster, rather slower, escape latency than the mice following the control condition on P31 (Figure 2A). There was no significant difference in the platform crossing times between the mice in the control group and the mice in the sevoflurane anesthesia group (Figure 2B). Western blot analysis showed that the anesthesia with 3% sevoflurane anesthesia two hours daily for one day at P6 did not increase levels of IL-6 (Figure 2C and 2D) or TNF-α (Figure 2E and 2F) in the brain tissues of the mice. These data suggest that a single exposure of sevoflurane anesthesia in young mice may not induce neuroinflammation or cognitive impairment in the mice.
Figure 2. Anesthesia with 3% sevoflurane two hours daily for one day in P6 mice does not induce cognitive impairment in the mice tested from P30 to P36, and does not increase IL-6 and TNF-α levels in the brain tissues of the mice.
A. Anesthesia with 3% sevoflurane two hours daily for one day in P6 mice does not increase escape latency of mice swimming in the MWM as compared to the control condition (tested from P30 to P36). Two way ANOVA with repeated measurement analysis shows that there is a statistically significant interaction of time and group based on escape latency of MWM between the mice following the control condition and mice following the sevoflurane anesthesia in the MWM (control: n = 16, sevoflurane: n = 13). Specifically, mice that received the sevoflurane anesthesia have a shorter escape latency time at P31 as compared to the control condition. B. Anesthesia with 3% sevoflurane two hours daily for one day in P6 mice does not reduce the platform crossing times of mice swimming in the MWM as compared to the control condition tested at P36 (control: n = 16, sevoflurane: n = 13). C. The sevoflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control group. There is no significant difference in β-Actin levels in the brain tissues of the mice between the sevoflurane anesthesia and control groups. D. The quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control group. E. The sevoflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the sevoflurane anesthesia and control conditions. F. The quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to control condition. P, postnatal day; MWM, Morris Water Maze; ANOVA, analysis of variance. IL, interleukin; TNF, tumor necrosis factor.
Multiple exposures of desflurane in young mice induced neither cognitive impairment nor accumulation of brain IL-6 and TNF-α in mice
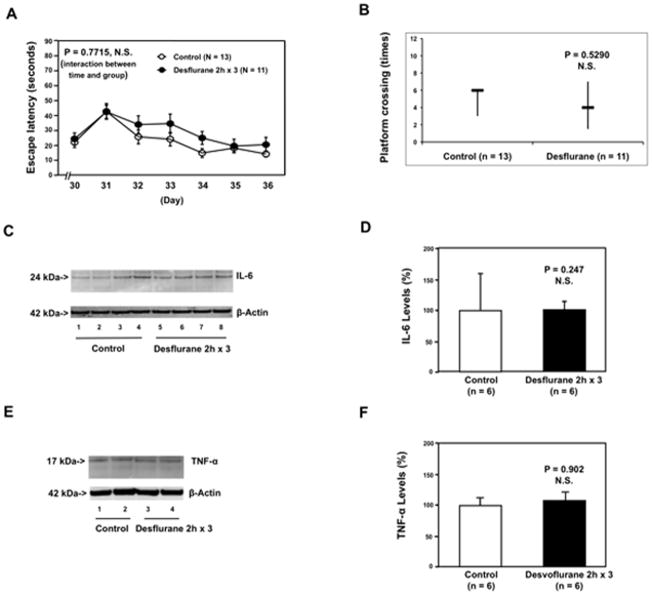
It has been reported that sevoflurane can induce apoptosis and Aβ accumulation 21,22,29, whereas desflurane does not 30–32. Therefore, we assessed the effects of multiple exposures of 9% desflurane [equivalent minimum alveolar concentration as 3% sevoflurane] on the function of learning and memory and brain levels of pro-inflammatory cytokines in mice. The six day-old mice were treated with 9% desflurane anesthesia two hours daily for three days from P6 to P8. The mice were tested in the MWM from P30 to P36. MWM studies showed that the desflurane anesthesia did not increase escape latency (Figure 3A) and did not reduce platform crossing times as compared to the control group (Figure 3B). Moreover, the desflurane anesthesia did not increase IL-6 (Figure 3C and 3D) or TNF-α (Figure 3E and 3F) levels in the brain tissues of the mice. These data suggest that desflurane may not lead to cognitive impairment and neuroinflammation in the developing brain.
Figure 3. Anesthesia with 9% desflurane two hours daily for three days in P6 mice does not induce cognitive impairment in the mice tested from P30 to P36, and does not increase IL-6 and TNF-α levels in the brain tissues of the mice.
A. Anesthesia with 9% desflurane two hours daily for three days in P6 mice dose not increase escape latency of mice swimming in the MWM as compared to the control condition (tested from P30 to P36). Two way ANOVA with repeated measurement analysis shows that there is no statistically significant interaction of time and group based on escape latency of MWM between mice following the control condition and mice following the desflurane anesthesia in the MWM (control: n = 13, sevoflurane: n = 11). B. Anesthesia with 9% desflurane two hours daily for three days in P6 mice does not reduce the platform crossing times of mice swimming in the MWM as compared to the control condition tested at P36 (control: n = 13, sevoflurane: n = 11). C. The desflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the desflurane anesthesia and control groups. D. Quantification of the Western blot (n = 6) shows that the desflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control condition. E. The desflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the desflurane anesthesia and control groups. F. Quantification of the Western blot (n = 6) shows that the desflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to the control condition. P, postnatal day; MWM, Morris Water Maze; ANOVA, analysis of variance. IL, interleukin; TNF, tumor necrosis factor.
Multiple exposures of sevoflurane in adult mice induced neither cognitive impairment nor neuroinflammation in the mice
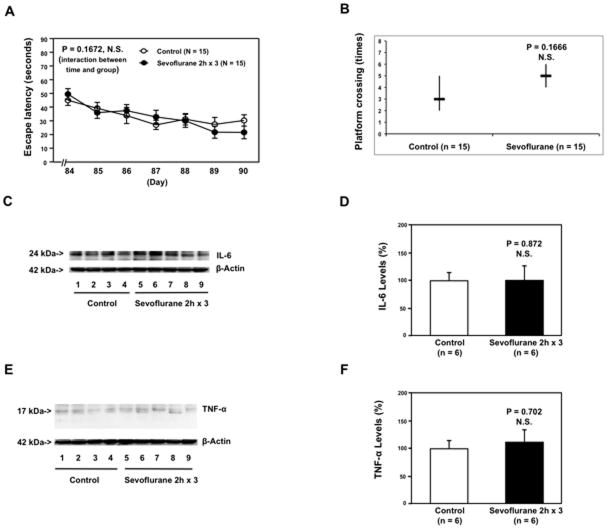
There is a critical period of vulnerability for the developing brain, also known as the brain growth spurt period, in humans (up to 36 months), and in rodents (up to three weeks) 33,34. In this period, the brain is susceptible to develop acute neural injuries. Moreover, older children may have less of a risk to develop the cognitive impairment following anesthesia and surgery 4. We therefore asked whether the same multiple exposures of sevoflurane anesthesia could induce cognitive impairment and neuroinflammation in adult mice. The 60 day-old mice were treated with 3% sevoflurane two hours daily for three days at P60. The mice were tested in the MWM approximately one month post-anesthesia (from P84 to P90). MWM studies showed that the sevoflurane anesthesia did not increase escape latency (Figure 4A) and did not reduce platform crossing times (Figure 4B) in the adult (P60) mice. Moreover, the sevoflurane anesthesia did not increase IL-6 (Figure 4C and 4D) or TNF-α (Figure 4E and 4F) levels in the brain tissues of the adult mice.
Figure 4. Anesthesia with 3% sevoflurane two hours daily for three days in P60 mice induce neither cognitive impairment in the mice tested from P84 to P90 nor increases in levels of IL-6 and TNF-α in the brain tissues of the mice.
A. Anesthesia with 3% sevoflurane two hours daily for three days in P60 mice does not increase escape latency of mice swimming in the MWM as compared to the control condition (tested from P84 to P90). Two way ANOVA with repeated measurement analysis (control: n = 15, sevoflurane: n = 15) shows that there is no significant difference in the learning curve based on escape latency between mice following the control condition and mice following the sevoflurane anesthesia in the MWM. B. Anesthesia with 3% sevoflurane two hours daily for three days in P60 mice does not reduce the platform crossing times of mice swimming in the MWM as compared to the control condition tested at P90 (control: n = 15, sevoflurane: n = 15). C. The sevoflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the sevoflurane anesthesia and control groups. D. Quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia does not increase IL-6 levels in the brain tissues of the mice as compared to the control condition. E. The sevoflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to the control condition. There is no significant difference in β-Actin levels in the brain tissues of the mice between the sevoflurane anesthesia and control conditions. F. Quantification of the Western blot (n = 6) shows that the sevoflurane anesthesia does not increase TNF-α levels in the brain tissues of the mice as compared to the control group. IL, interleukin; TNF, tumor necrosis factor; P, postnatal day; MWM, Morris Water Maze; ANOVA, analysis of variance.
Neuroinflammation includes an increase in pro-inflammatory cytokines and microglia activation 6–10. We therefore assessed and compared the effects of the multiple exposures of sevoflurane on microglia activation in the hippocampus between young and adult mice. Immuonhistochemistry image analysis showed that the anesthesia with 3% sevoflurane two hours daily for three days increased the density of IBA1 positive cells, the marker of microglia activation 18,19, as compared to the control group in the hippocampus of young (P8), but not adult (P62), mice (Figure 5A). Quantification of the images illustrated that the sevoflurane anesthesia increased the amount of IBA1 positive cells in the hippocampus of young mice: 128% ± 16.6 versus 100% ± 13.5, n = 6, * P = 0.036 (Figure 5B), but not adult mice: 95% ± 13.9 versus 100% ± 12.7, n = 6, P = 0.378, N.S. The two-way ANOVA demonstrated that there was a significant interaction and young age potentiated the sevoflurane anesthesia-induced increases in the amount of IBA1 positive cells (# P = 0.027). Collectively, these data suggest that multiple exposures of sevoflurane may induce neuroinflammation, which includes increases in the levels of pro-inflammatory cytokines and microglia activation, leading to cognitive impairment in young mice (P6, developing brain), but not adult mice (P60). However, the increases in the amount of IBA1 positive cells disappeared at about one month post-anesthesia in the hippocampus of young mice (detected at P30, data not shown). Taken together, these data suggest that the sevoflurane anesthesia-induced neuroinflammation may trigger other neuropathological events, ultimately leading to cognitive impairment.
Figure 5. Anesthesia with 3% sevoflurane two hours daily for three days increases the amount of IBA1 positive cells in the hippocampus of P6, but not P60, mice.
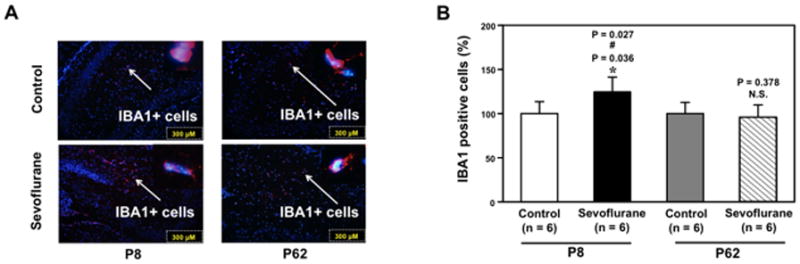
A. The sevoflurane anesthesia increases the amount of IBA1 positive cells in the hippocampus of P6 mice (the left column), but not P60 mice (right column), harvested at the end of the three-day anesthesia. B. Quantification of the immunohistochemistry image (n = 6) shows that the sevoflurane anesthesia increases the amount of IBA1 positive cells in the hippocampus of P6 mice (black bar versus white bar), but not in P60 mice (net bar versus gray bar). The two-way ANOVA shows that there is a significant interaction and that young age potentiates the sevoflurane anesthesia-induced increases in the amount of IBA1 positive cells. P, postnatal day; IBA1, ionized calcium binding adaptor molecule 1. ANOVA, analysis of variance.
Enriched environment and anti-inflammation treatment attenuated the sevoflurane-induced cognitive impairment
EE has been shown to improve brain function 26–28. We therefore asked whether EE could ameliorate the sevoflurane-induced cognitive impairment in young mice. The mice were treated with 3% sevoflurane two hours daily for three days (from P6 to P8). Then, the mice were exposed to either EE or standard environment from P8 to P30 (the weaning started at P26) (Figure 6A). Finally, the mice were tested in the MWM from P30 to P36. Two-way ANOVA with repeated measurement showed that there was a statistically significant interaction of time and group based on escape latency of MWM between mice following the sevoflurane anesthesia plus standard environment and those following the sevoflurane anesthesia plus EE (Figure 6B, ^^ P = 0.001). Post-hoc Bonferroni test showed that the mice that received sevoflurane plus EE had faster escape latency as compared to the mice that received sevoflurane plus standard environment at P32 and P33. There was no significant difference in platform crossing times between the mice that received sevoflurane plus standard environment and the mice that received sevoflurane plus EE (Figure 6C). Two-way ANOVA with repeated measurement showed that there was no statistically significant interaction of time and group based on escape latency of MWM between mice following the control condition plus standard environment and those following the control condition plus EE (Figure 6D, P = 0.0524, N.S.). There was no significant difference in platform crossing times between the mice following the control condition plus standard environment and the mice following the control condition plus EE (Figure 6E). Finally, EE did not affect the amount of IBA1 positive cells in the mouse hippocampus (data not shown). These results suggest that EE may ameliorate the sevoflurane-induced cognitive impairment in young mice, which is supported by the results from a recent study that a delayed EE (four weeks after the anesthesia) can attenuate the sevoflurane anesthesia-induced learning and memory impairment in rats tested at 8 weeks after the anesthesia 20.
Figure 6. EE attenuates the sevoflurane-induced cognitive impairment in mice.
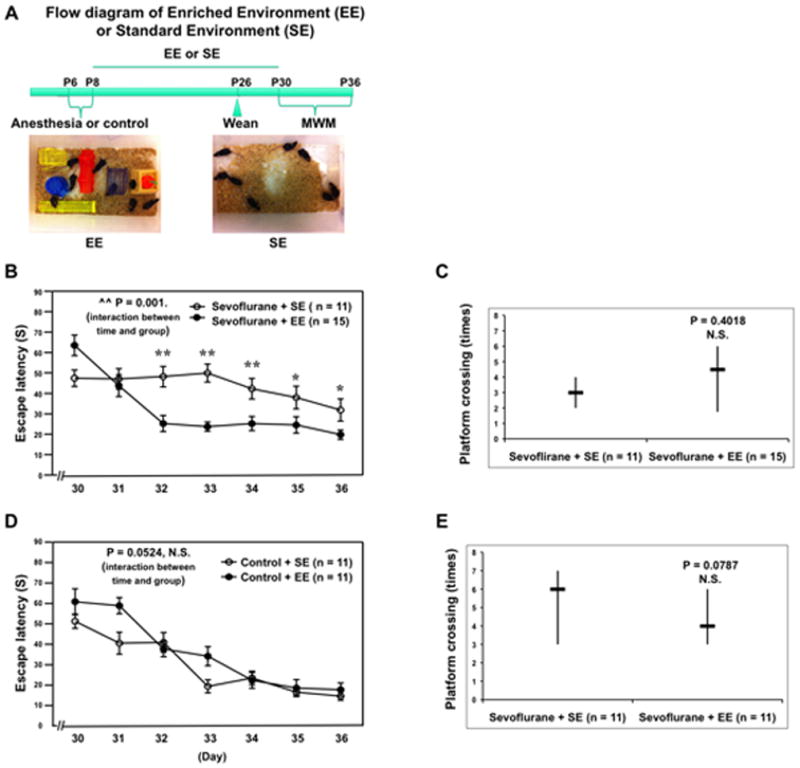
A. The flow diagram and pictures of EE and SE. B. Two-way ANOVA with repeated measurement analysis shows that there is a statistically significant interaction of time and group based on escape latency in MWM between mice following sevoflurane anesthesia plus SE and sevoflurane anesthesia plus EE (control: n = 11, sevoflurane: n = 15). C. The Mann-Whitney test shows that the platform crossing times of mice swimming in the MWM following the sevoflurane anesthesia plus EE is not significantly different from that of mice following the sevoflurane anesthesia plus SE (control: n = 11, sevoflurane: n = 15). D. Two-way ANOVA with repeated measurement analysis shows that there is no statistically significant interaction of time and group based on escape latency of MWM between the control condition plus SE and the control condition plus EE (control: n = 11, sevoflurane: n = 11). E. The Mann-Whitney test shows that there is no significant difference in platform crossing times of mice swimming in the MWM between the control condition plus SE and the control condition plus EE (control: n = 11, sevoflurane: n = 11). MWM, Morris Water Maze; ANOVA, analysis of variance; SE, standard environment; EE, enriched environment.
Moreover, nonsteroidal anti-inflammatory drug ketorolac also ameliorated the sevoflurane anesthesia-induced cognitive impairment (Figure 7). Specifically, the sevoflurane anesthesia did not increase the escape latency of MWM (Figure 7A) and did not reduce platform crossing times (Figure 7B) as compared to control condition in the mice pretreated with ketorolac. These data potentially suggest the causal relationship of the sevoflurane-induced neuroinflammation and sevoflurane-induced cognitive impairment.
Figure 7. Ketorolac attenuates the sevoflurane-induced cognitive impairment in mice.
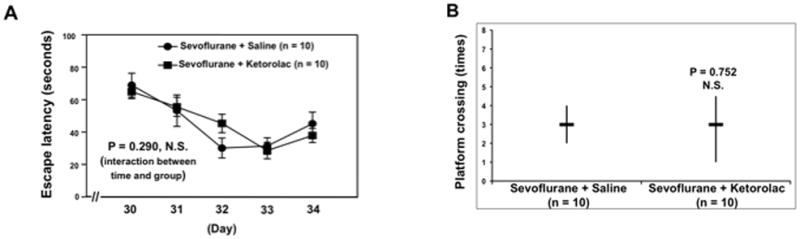
A. Two-way ANOVA with repeated measurement analysis shows that there is no statistically significant interaction of time and group based on escape latency of MWM between the sevoflurane plus saline and sevoflurane plus ketorolac treated mice (control: n = 10, sevoflurane: n = 10). B. The Mann-Whitney test shows that there is no significant difference in platform crossing times of mice swimming in the MWM between the sevoflurane plus saline and sevoflurane plus ketorolac (control: n = 10, sevoflurane: n = 10). MWM, Morris Water Maze; ANOVA, analysis of variance.
Discussion
Sevoflurane is the most commonly used anesthetic in children. We first found that anesthesia with 3% sevoflurane two hours daily for three days in young (P6) mice induced cognitive impairment detected at a later time (P30 to P36) (Figure 1). Moreover, we found that anesthesia with 3% sevoflurane two hours daily for only one day in young (P6) mice did not induce cognitive impairment in the mice (Figure 2). These results suggest the selectivity of anesthesia-induced cognitive impairment such that only specific anesthesia regimen(s) (e.g., multiple exposures) may induce detrimental effects. These findings would support the clinical observation that three, but not one, exposures to anesthesia and surgery increased the risk of cognitive impairment in children 4,5, and suggest that anesthesia may contribute to the clinically observed cognitive impairment in children following anesthesia and surgery.
In addition, anesthesia with 9% desflurane (equivalent minimal alveolar concentration with 3% sevoflurane) two hours daily for three days in young (P6) mice did not induce cognitive impairment in the mice (Figure 3). These findings may suggest that desflurane could induce a lesser degree of insults in the developing brain, which would promote clinical studies to further determine whether desflurane is a safer anesthetic for children. The findings have also established a system to investigate the difference between sevoflurane and desflurane in brain function.
Brain in the growth spurt period (up to 36 months in humans and up to three weeks in rodents) 33,34 is susceptible to acute neural injuries including anesthetics 35. We therefore compared the effects of the same sevoflurane anesthesia on cognitive function between young (P6) and adult (P60) mice, and found that the anesthesia with 3% sevoflurane two hours daily for three days did not lead to cognitive impairment in adult (P60) mice. These findings are supported by the results from the clinical studies that early exposure to anesthesia/surgery may lead to an increased risk to develop cognitive impairment in children 4,5, and from the pre-clinical animal studies that isoflurane anesthesia only induced cognitive impairment in young rats 35.
Neuroinflammation, including pro-inflammatory cytokine accumulation and microglia activation, is associated with cognitive impairment in humans and animals 11,13,17. Pro-inflammatory cytokines, particularly TNF-α, IL-6, and IL-1β, can be released by the microglia during its activation, fueling brain inflammation and leading to cognitive dysfunction in humans 11 and animals 16,17. Pro-inflammatory cytokines can induce microglia activation in discrete brain regions, leading to the production of the same pro-inflammatory cytokines 36,37. These pro-inflammatory cytokines inhibit long-term potentiation 38,39 and induce neurobehavioral deficits 40–46. We found that anesthesia with 3% sevoflurane two hours daily for three days (from P6 to P8) in young mice was able to increase the levels of pro-inflammatory cytokine IL-6 and TNF-α in brain tissues (Figure 1) and the amount of IBA1 positive cells, the marker of microglia activation, in the hippocampus (Figure 5) of the mice at P8. The anesthesia with 3% sevoflurane two hours daily for one day in P6 mice (Figure 2) and 3% sevoflurane two hours daily for three days in adult (P60) mice (Figure 4) did not increase levels of IL-6, TNF-α, or IBA1 positive cells in the brain tissues of the mice. Collectively, these data suggest that the multiple exposures of sevoflurane anesthesia in young mice may cause cognitive impairment via inducing neuroinflammation. The findings that the same sevoflurane anesthesia increased the amount of IBA1 positive cells in the hippocampus of young (P6) mice but not adult (P60) mice further suggest that mice at different ages may have different neuroinflammation reactions to anesthesia.
The mechanisms by which anesthesia induces neuroinflammation remain to be determined. Anesthetics have been shown to increase cytosolic calcium levels 47–50. The elevation of cytosolic calcium is associated with increased levels of pro-inflammatory cytokines 51, potentially through activation of nuclear factor-κB signaling 52–57. Therefore, we postulate that anesthetics can increase calcium levels to trigger generation of TNF-α and IL-6 via nuclear factor-κB signaling, and the cytokines then induce microglia activation to generate more pro-inflammatory cytokines, ultimately leading to neuroinflammation.
Finally, 9% desflurane two hours daily for three days in P6 mice did not increase the levels of IL-6 and TNF-α in the brain tissues of mice (Figure 3). These different effects between sevoflurane and desflurane are consistent with the previous findings that sevoflurane can induce apoptosis and Aβ accumulation 21,22,29, whereas desflurane does not 30,31. The exact mechanisms by which sevoflurane and desflurane have different effects on neurotoxicity and cognitive impairment remain to be determined. Our recent studies suggest that sevoflurane may affect Wnt pathway (unpublished data, Dr. Yiying Zhang, M.D., M.S., Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, most recent communication: January 27, 2012). Therefore, in the future studies, we may also compare the effects of the sevoflurane and desflurane anesthesia on Wnt pathway to determine the underlying mechanisms of different effects of sevoflurane and desflurane on neurotoxicity and cognitive impairment.
Interestingly, there was no significant increase in the amount of IBA1 positive cells in the mouse hippocampus at P30 (data not shown), the time when the mice developed cognitive impairment (P30 to P36). These results suggest the acute sevoflurane anesthesia-induced neuroinflammation may not last long enough to directly cause the lately occurred cognitive impairment. Moreover, the findings suggest that there should be other pathological event(s), e.g., synaptic dysfunction, between the sevoflurane anesthesia-induced neuroinflammation and the sevoflurane anesthesia-induced cognitive impairment. Future studies to test this hypothesis are warranted.
In the current studies, we have not found that EE can affect the amount of IBA1 positive cells in the mouse hippocampus. Thus, it is unlikely that EE attenuates the sevoflurane anesthesia-induced cognitive impairment through directly mitigating the sevoflurane anesthesia-induced neuroinflammation. EE may improve brain function through various mechanisms, e.g., promotion of neurogenesis and synapse function 26–28,58,59. Our current findings that EE can ameliorate the sevoflurane-induced cognitive impairment suggest that the sevoflurane anesthesia could affect neurogenesis, synapse and others, leading to cognitive impairment. These findings have established a system for us to perform additional studies to determine the underlying mechanisms (e.g., neurogenesis and synapse function) of the sevoflurane-induced cognitive impairment. Moreover, pending further studies, these findings may imply that susceptible children should have more exposures to EE after anesthesia and surgery.
A recent study shows that nociceptive stimulation can potentiate the anesthesia-induced neurotoxicity in the rat developing brain 60. It is therefore possible that surgery and other perioperative factors may worsen the anesthesia-induced neuroinflammation in mouse developing brain and cognitive impairment. Other studies suggest that there is no significant difference in the incidence of postoperative cognitive dysfunction between surgery with general anesthesia and surgery without it (with epidural, spinal or local anesthesia) [61–66; reviewed in 67,68]. Taken together, it is important to perform both human and animal studies to determine the interaction of anesthesia and surgery on potential neurotoxicity and postoperative cognitive dysfunction.
The studies have several limitations. First, we did not investigate the effects of anesthesia on other domains of cognitive impairment (e.g., executive function), instead focusing on learning and memory function because it is the major domain of cognitive impairment. However, the current data have suggested that multiple exposures of sevoflurane anesthesia in young mice may cause neuroinflammation and cognitive impairment, which will allow us to further study the up-stream mechanisms of the anesthesia-induced cognitive impairment and down-stream consequences of the anesthesia-induced neuroinflammation. Second, we only assessed the effects of anesthesia in P6 mice. It is unknown whether anesthesia-induced neuroinflammation and cognitive impairment can also occur in other ages of young mice and the developing brain. Therefore, future studies will include an assessment of the effects of different anesthetic(s) and anesthesia regimen on varied ages of young mice, e.g., P2, P14 and P21, to further test the hypothesis that the developing brain is more susceptible to anesthesia neurotoxicity. Third, it is unknown whether 3% sevoflurane has equal potency of 9% desflurane in mice. We chose 3% sevoflurane and 9% desflurane in the current studies because the minimum alveolar concentration of sevoflurane and desflurane in children is 2.5 and 8.72, respectively 69. Finally, brain and blood cytokines could not be easily separated at the time of sacrifice. However, the desflurane anesthesia and anesthesia with sevoflurane for one day neither increased brain levels of cytokines nor induced cognitive impairment in the mice; the anesthesia with sevoflurane for three days increased number of Iba1 positive cells in mouse hippocampus; finally, anti-inflammatory treatment ketorolac (Figure 7) ameliorated the sevoflurane anesthesia-induced cognitive impairment in the mice. Taken together, these findings support the conclusion that neuroinflammation may, at least partially, contribute to the sevoflurane anesthesia-induced cognitive impairment.
In conclusion, we have found the selectivity of anesthetics and anesthesia regimen neurotoxicity and age-dependent vulnerability. The findings suggest the combination of an environmental insult (precipitating factors, e.g., multiple exposures to a specific anesthetic) with age vulnerability (predisposing factors, e.g., young age) plays a role in cognitive impairment. Finally, EE and anti-inflammation treatment could be strategies used to prevent and treat anesthesia-induced cognitive impairment. These findings would promote more studies to investigate anesthesia neurotoxicity in the developing brain, ultimately leading to safer anesthesia care and better postoperative outcomes for children who could be vulnerable to brain damage.
Summary Statement.
Anesthesia with sevoflurane, but not desflurane, two hours daily for three, but not one, days induced cognitive impairment and neuroinflammation in young, but not adult, mice. An enriched environment and anti-inflammation treatment ameliorated the sevoflurane anesthesia-induced cognitive impairment.
Acknowledgments
Funding: This research was supported by R21AG029856, R21AG038994, R01 GM088801 and R01 AG041274 from National Institutes of Health, Bethesda, Maryland, Investigator-initiated Research grant from Alzheimer’s Association, Chicago, Illinois, and Cure Alzheimer’s Fund, Wellesley, Massachusetts to Zhongcong Xie. Anesthetic sevoflurane and desflurane were generously provided by the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Footnotes
These studies are attributed to the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School.
References
- 1.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13:1–209. [PubMed] [Google Scholar]
- 2.Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364:1387–90. doi: 10.1056/NEJMp1102155. [DOI] [PubMed] [Google Scholar]
- 3.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105:61–8. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, Khabbaz K, Levkoff SE, Marcantonio ER. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci. 2008;63:184–9. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalman J, Juhasz A, Bogats G, Babik B, Rimanoczy A, Janka Z, Penke B, Palotas A. Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem Int. 2006;48:177–80. doi: 10.1016/j.neuint.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Ramlawi B, Rudolph JL, Mieno S, Feng J, Boodhwani M, Khabbaz K, Levkoff SE, Marcantonio ER, Bianchi C, Sellke FW. C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006;140:221–6. doi: 10.1016/j.surg.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Ramlawi B, Rudolph JL, Mieno S, Khabbaz K, Sodha NR, Boodhwani M, Levkoff SE, Marcantonio ER, Sellke FW. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006;244:593–601. doi: 10.1097/01.sla.0000239087.00826.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, Gainotti G, Tonali PA, Batocchi AP, Marra C. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. 2010;88:1106–12. doi: 10.1002/jnr.22276. [DOI] [PubMed] [Google Scholar]
- 12.Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA, Jonker C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–9. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Tan EK, Chan LL. Neurovascular compression syndromes and hypertension: Clinical relevance. Nat Clin Pract Neurol. 2007;3:416–7. doi: 10.1038/ncpneuro0558. [DOI] [PubMed] [Google Scholar]
- 14.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–15. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–73. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 17.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 18.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 19.Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–15. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- 20.Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z. Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology. 2010;112:1404–16. doi: 10.1097/ALN.0b013e3181d94de1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 23.Ulugol A, Ozyigit F, Yesilyurt O, Dogrul A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth Analg. 2006;102:443–7. doi: 10.1213/01.ane.0000194587.94260.1d. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–10. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 27.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 28.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66:620–31. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Dong Y, Zhang G, Moir RD, Xia W, Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE, Xie Z. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J Biol Chem. 2008;283:11866–75. doi: 10.1074/jbc.M800199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–37. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 35.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 36.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 37.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–9. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 38.Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–26. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 39.Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–70. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–52. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, Nau R. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333–45. doi: 10.1093/brain/awh711. [DOI] [PubMed] [Google Scholar]
- 42.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn J Pharmacol. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- 44.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005;86:244–51. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–16. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G, Dong Y, Zhang B, Ichinose F, Wu X, Culley DJ, Crosby G, Tanzi RE, Xie Z. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–60. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Dong Y, Xu Z, Zhang Y, Pan C, McAuliffe S, Ichinose F, Yue Y, Liang W, Xie Z. 2-deoxy-D-glucose attenuates isoflurane-induced cytotoxicity in an in vitro cell culture model of H4 human neuroglioma cells. Anesth Analg. 2011;113:1468–75. doi: 10.1213/ANE.0b013e31822e913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim D, Cho SH, Kim JS, Jo SH, Lee SJ, Kim KT, Choi SY. Human astrocytic bradykinin B(2) receptor modulates zymosan-induced cytokine expression in 1321N1 cells. Peptides. 2010;31:101–7. doi: 10.1016/j.peptides.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–8. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 53.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31:378–93. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Z, Yenari MA. Post-ischemic inflammation: Molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–92. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 56.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–9. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 58.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 59.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shu Y, Zhou Z, Wan Y, Sanders RD, Li M, Pac-Soo CK, Maze M, Ma D. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–50. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–6. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 62.Williams-Russo P, Sharrock NE, Mattis S, Szatrowski TP, Charlson ME. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 63.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 64.Berant A, Kaufman V, Leibovitz A, Habot B, Bahar M. Effects of anesthesia in elective surgery on the memory of the elderly. Arch Gerontol Geriatr. 1995;20:205–13. doi: 10.1016/0167-4943(94)00618-h. [DOI] [PubMed] [Google Scholar]
- 65.Ghoneim MM, Hinrichs JV, O’Hara MW, Mehta MP, Pathak D, Kumar V, Clark CR. Comparison of psychologic and cognitive functions after general or regional anesthesia. Anesthesiology. 1988;69:507–15. doi: 10.1097/00000542-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Karhunen U, Jonn G. A comparison of memory function following local and general anaesthesia for extraction of senile cataract. Acta Anaesthesiol Scand. 1982;26:291–6. doi: 10.1111/j.1399-6576.1982.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 67.Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22:67–79. doi: 10.3233/JAD-2010-101086. [DOI] [PubMed] [Google Scholar]
- 68.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: A systematic review. Anesthesiology. 2007;106:572–90. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 69.Cote CJ, Lerman J, Ward RM, Lugo RA, Goudsouzian N. Pharmacokinetics and Pharmacology of Drug Used in Children. In: Cote CJ, Lerman J, Todres ID, editors. A Practice of Anesthesia for Infants and Children. 2008. pp. 108–9. [Google Scholar]