Summary
Ciliary compartmentalization plays pivotal roles in ciliogenesis and in various signaling pathways. Here we describe a structure at the ciliary base that appears to have all the features required for compartmentalization and which we thus call the “ciliary partitioning system” (CPS). This complex consists of the terminal plate, which serves as a cytosolic “ciliary pore complex” (CPC), and a membrane region well suited to serve as a diffusion barrier. The CPC is a plate-shaped structure containing 9 pores through which the microtubule doublets of the basal body pass. Each pore expands from the doublet B-tubule into an opening well-suited for the passage of intraflagellar transport particles. The membrane diffusion barrier encompasses an extended region of detergent-resistant periciliary membrane (ciliary pocket) and a ring complex that connects the CPC to the membrane. Proteomics analysis shows involvement of the ciliary pocket in vesicle trafficking, suggesting that this region plays an active role in membrane transport. The CPC and the ring together form a complete partition defining the ciliary boundary.
Results and Discussion
The composition of cilia is distinct from that of the rest of the cell in terms of both membrane and axoneme structure. The mechanisms that establish and maintain this separation involves selective recruitment of components and transport into the cilium. Ciliary trafficking is facilitated by the specialized, microtubule-based “intraflagellar transport system” (IFT) [1]. IFT complexes bind selectively to cargo, and motor proteins that travel on microtubule doublets shuttle the cargo between cytoplasm and cilia [1–2]. Various structures have been proposed to perform as gatekeepers that limit cilia access to IFT and possibly other specific transport systems [3–7], but how non-ciliary components are blocked from freely diffusing into cilia and ciliary components are retained, what structures contribute to this process and how the sorting machinery works with the IFT particles remain open questions. One consistent idea about the ciliary gatekeeper is that, in order to regulate permeability to the cilia during early ciliogenesis, it must be located in the transition zone associated with the basal body [3; 5].
To pursue the identification and characterization of a putative ciliary pore system, we isolated basal bodies within the pellicle of Tetrahymena pyriformis (Figs. 1a, S1). Electron microscopy of stained samples confirms the retention of many of the basal body-associated structures. One striking feature associated with the basal bodies, which appears to have all the necessary characteristics to compartmentalize the cilium, is the terminal plate (TP) [8] (also identified earlier as the basal plate [9]). To better visualize structural details of the TP, we solubilized the microtubules and further isolated the complex by gradient and differential centrifugation. Isolation of the TP revealed that it is part of a larger complex that includes a detergent-resistant membrane region and a ring connecting the TP to the membrane (Fig. 1b-g). Our data reveal that the TP is roughly 340 nm in diameter with a 9-fold symmetric, disc-shaped architecture. Distinctly shaped pores of the TP match the microtubule doublet shape at their inner region, while the outer portion of the pore has a size (~53 nm) similar to that of the nuclear pore complex (NPC) [10].
Fig. 1. The CPC is a prominent feature in ciliate basal bodies.
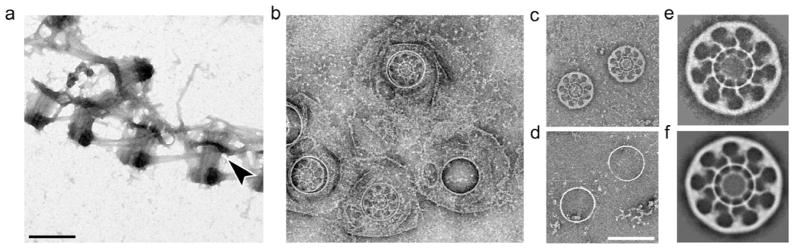
(a) Image of negatively stained, isolated pellicular basal bodies indicates that the TP (arrow) is the only basal body-associated structure that can function as a physical barrier to the cilia. The TP is functionally identified as the CPC. (b) EM of negatively stained, isolated CPS from pellicular basal bodies showing partially disassembled CPS with periciliary membrane. (c) Isolated CPCs. (d) Isolated ring complexes. (e) average of 10 images of individual CPCs. (f) 9-fold rotational average of (e). Scale bars in a-d 500 nm.
The similarity of the TP and ring sizes to that of the cilia opening (~400 nm) and the tight connection the ring makes between the TP and the periciliary membrane domain suggest the function of the TP as a physical barrier that filters the passage of cytosolic material into and out of the cilia while the ring and periciliary membrane act as a membrane diffusion barrier. Thus we identify the terminal plate as the ciliary pore complex (CPC) that limits cytosolic movement. This organization is in excellent agreement with the proposal that the physical ciliary barrier would comprise 2 major parts, the cytosolic and membrane diffusion barriers [11]. We thus propose to term this arrangement “the ciliary partitioning system” (CPS).
Rings formed by septin are found at the base of both primary cilia [12] and motile cilia [13] in mice and in Xenopus, respectively, and may play a role in the membrane diffusion barrier. In yeast, septin forms a diffusion barrier separating mother and daughter cells during cell division [14]. Notably, the diameter of the CPS ring is comparable to the size of rings formed in vitro by purified yeast septins (~350–500 nm) [15]. Although we have not yet identified the protein(s) that comprise the ring, it is plausible that the ring complex provides a selective mechanism for membrane traffic similar to that of a septin ring in addition to anchoring the CPC to the membrane.
Fluorescence microscopy has demonstrated the existence of a lipid barrier 0.8–1.8 μm in diameter at the base of mammalian cilia [16], nearly the same size as the CPS detergent-resistant membrane domain (0.7–1.3 μm). The detergent resistance of this structure indicates that it has a substantial protein content that is likely to inhibit diffusion within the membrane [17]. The membrane raft may be part of the Tetrahymena alveolar membrane rather than the plasma membrane, and in either case this structure appears to tightly seal the ciliary membrane compartment. It should be noted that the alveolar membrane has been shown to directly connect with the plasma membrane and is also involved in the lipid recycling pathway [18–19].
Electron cryotomography (cryo-ET) of isolated basal body arrays from the Tetrahymena oral apparatus reveals the relation between microtubule doublets and the CPC. Array that contain 3 rows of basal bodies tend to stand upright on the EM grid while those that contain 2 roes lie on their side, thus providing two orthogonal views. Figures 2a,b show slices through 3-D reconstructions of basal body arrays with the microtubules arranged parallel or perpendicular to the specimen plane, respectively, and thus with cross section or en face views of the CPC. An averaged view of the plate region from the tomograms not only confirms close interaction between the microtubule doublets and the CPC but allows identification of the position of the A- and B-tubules of the doublets as well.
Fig. 2. Cryo-ET of the CPC and interaction with microtubules.
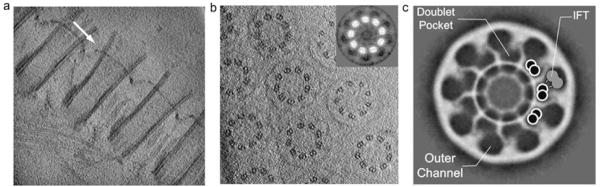
(a) Section through cryo-tomogram of basal bodies lying sideways on grid (arrow indicates position of CPC near distal end). (b) Section through tomogram of basal bodies standing upright on grid, at the position of the CPC. Inset shows symmetrized projection of the CPC region averaged from tomograms of intact basal bodies, with contrast inverted to match that in images of the stained samples. (c) Proposed model showing how the outer channel may serve as a tunnel for IFT trains (gray) moving along the microtubule doublets (white circles).
The larger section of the CPC pore is adjacent to the B-tubule of the doublet. The pore size is well suited for accommodating the IFT particles [2] and the position of the pore is consistent with the movement of the IFT trains along the B-tubule [1–2]. The diameter of each pore, about 53 nm, is a little larger than the size of the IFT particle itself (~37.5 nm) [2]. While IFT particles of molecular weight in the megadalton range are permitted access to cilia [1], passive diffusion of such large structures is blocked. Recent permeation experiments have demonstrated a size cutoff between 10 and 40 kD for diffusion of dextran into the cilia [6], implying the existence of a size exclusion filter along with a mechanism to usher cargo through the CPC to the cilia, perhaps similar to the mechanism of the NPC [1; 6; 11].
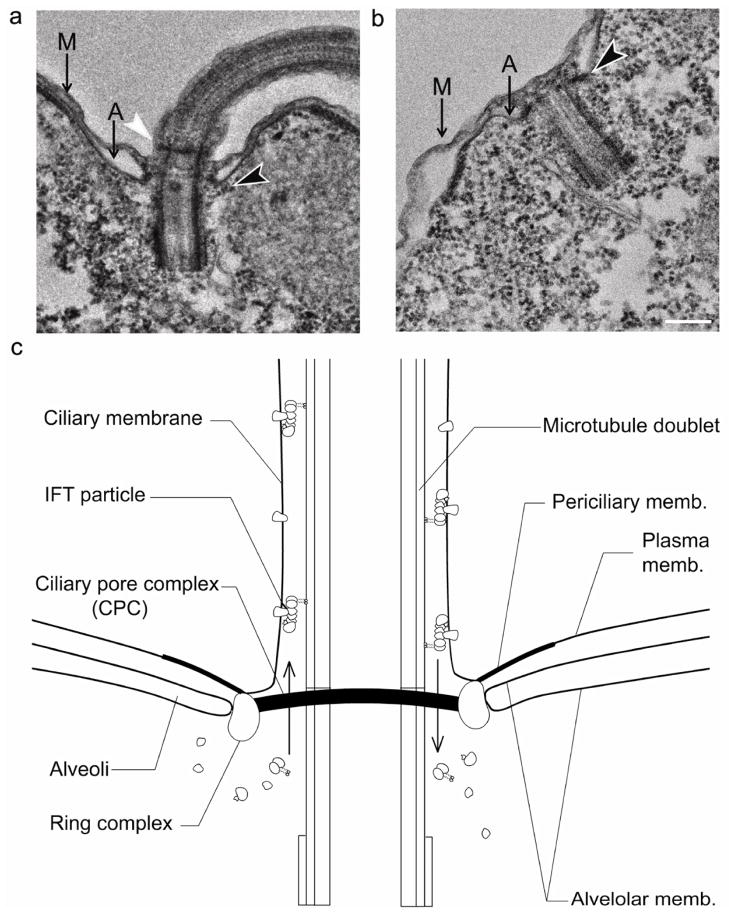
The CPS must be present before ciliogenesis can take place in order to regulate new cilia formation and also must remain in place following deciliation to limit permeability during autotomy. It has been shown that a structure most easily interpreted as the Tetrahymena CPS appears at the time that a nascent basal body re-orients and joins the membrane and is thus in place at the onset of ciliogenesis [20]. To confirm its presence after deciliation, we characterized the morphology of both intact and deciliated cells in thin sections of resin-embedded samples (Fig. 3). As expected, the CPC remains associated with the basal bodies at the interface where the cilia were shed. Another electron dense disc shape, previously identified as the “axosome”, was also observed in the cilium portion distal to the TP [20]. However, this second structure is lost upon deciliation and is thus unlikely to constitute a vital part of a pore complex.
Fig. 3. Ciliary partitioning system (CPS) localizes at the deciliation site after cellular autotomy, suggesting a role in ciliary homeostasis and early ciliogenesis.
Black arrows indicate the location of the TP (CPC) of pellicular basal bodies in resin embedded sections of (a) intact and (b) deciliated cells. White arrow indicates axosome. A, Alveoli; M, cell membrane; scale bar = 200 nm. The connection of this complex to the cell membrane indicates that it serves as a physical barrier at the base of the cilia. (c) Schematic representation of the ciliary base showing location of the CPS components including ciliary pore complex (CPC) and membrane diffusion barrier, comprising the ring complex and periciliary membrane. This cartoon reflects the understanding that IFT particles remodel near the basal bodies prior to transporting cargo into cilia. To facilitate ciliogenesis and maintenance of ciliary function, an IFT particle has to pass through the physical barrier of the CPS (10). It is also proposed that the periciliary membrane raft at the base of the cilia is implicated in membrane filtering (3); this domain may actually be part of the alveolar membrane.
To further verify the functionality of the CPS and to gain insight on its components, we used two mass spectrometry techniques to obtain a preliminary characterization of its proteome. We identified 290 candidate proteins (summarized in Table S1, details in Table S2). Included in these are subsets of proteins found in proteomes of the basal body, cilia and pellicle [21–23] in addition to a number of other proteins. The overlap with both cilia and basal body proteins is surprisingly small, while there is a large overlap with the pellicular proteins. The lack of proteins strongly represented in cilia, such as dyneins and radial spoke components, and absence of almost all of the basal body’s Bbc family proteins, is an indication of the relative purity of the samples. Among the many pellicular proteins not found in our analysis, three of the four pellicle proteins selected by Gould et al. [21] for validation of their results were not detected in our sample. These are similar to viral A-type inclusion proteins, highly represented in the pellicle MS data, and their absence indicates that the lipid region included in our sample is distinct from the full cell membrane. Over 50% of the identified proteins are ribosomal components and abundant proteins involved in transcription, translation, metabolism and mitochondria, which are common contaminants in MS analyses and thus unlikely to be actually part of the CPS. While our MS experiments were not aimed at quantitative results, we have obtained estimates of the relative protein abundances using methods described in the Supplemental Materials. We presume that most of the CPS components will be represented at relatively high abundance. Thus the MS analysis gives some constraints on which proteins are most likely candidates for CPS components.
Of the few proteins found in our CPS analysis as well as basal bodies, cilia and pellicles, alpha and beta tubulins as well as ribosomal proteins are likely contaminants due to their high abundance. Eighty proteins previously identified in the basal body analysis were present in the CPS, including Bbc49, Bbc73 and Bbc82 [23]. Some of these proteins are likely candidates for the CPC and ring complex, although many in this set are still currently described as hypothetical with no functional characterization. Of the other cytoskeletal proteins found, tetrins are specific to the Tetrahymena oral apparatus [24], while actin has been suggested to be part of the membrane diffusion barrier [25]. Bug22p, a conserved centrosomal/ciliary protein that has been seen localized at the TP [26], is also found in our candidate list. This protein has been shown to have an important role in cilia motility and function [26] and, to our knowledge, is the only protein that has been clearly identified in the location of the CPS. However, it was also observed in the basal body and cilia, suggesting that it may be involved in transport rather than structure. As expected, a large fraction of the remainder, including those found in the pellicle list, which would be expected to be mainly proteins in the periciliary membrane domain, are associated with the endomembrane system. These include several active vesicle trafficking proteins, for example Arl, Sar-1 and several members of RabGTPase family – Rab1, Rab7 and Rab11E. The presence of these proteins is in good agreement with the recent findings demonstrating the role of Rab11 in the initiation of primary cilia membrane assembly [27–28]. Arf and Arl proteins have also been shown to be involved in ciliogenesis [28–30]. Our results thus confirm that this periciliary region is an active site for ciliary membrane regulation [31–32].
A surprising degree of homology between ciliary transport and nuclear transport has recently been recognized, favoring the hypothesis that an equivalent of the NPC must exist in cilia [6; 33–34]. This evidence includes: (i) the localization sequences for these two processes are almost identical [34–35]; (ii) ciliary kinesin KIF17 is translocated into the cilioplasm via importin proteins in a RanGTP-dependent manner [33–34]; (iii) nucleoporins have been found in cilia [6]; and (iv) centriolar proteins such as centrin (CEN-2) have been found to play a role in mRNA and protein transport through the NPC [36]. We identified 7 centrin-family proteins localized in the CPS, including a CEN-2 homolog, which might contribute to ciliary transport similarly to CEN-2 in the NPC [36]. Ndc-1, a nuclear envelope-anchored protein that links the NPC to the nuclear membrane [37], is present in the CPS inventory as well as in isolated cilia [22]. Although no membrane anchoring nucleoporins were found in the basal body [6], it is quite possible that Ndc-1 plays a critical role of anchoring the CPC into the periciliary membrane of Tetrahymena. Additionally, Ran proteins are found in our candidate list, in good agreement with recent work demonstrating the involvement of Ran in cilia transport [33–34; 38]. Although the Ran proteins might be just transiently associated with the CPS, the existence of Ran in our list further strengthens the notion that the CPS is involved in ciliary permeation in a way related to the NPC. Just as visualization and proteomics characterization of the NPC have set the stage for understanding nuclear trafficking mechanisms [10], understanding the structure and composition of the CPS should provide novel insights on ciliary transport mechanisms [11; 39–40].
Figure 3C shows a schematic of the Tetrahymena CPS. Despite the almost universal conservation of the “9+2” and “9+0” architectures of motile and non-motile axonemes, respectively, there is significant diversity of structure in the transition zone [7; 32]. One can thus expect that there may be some variety in CPS structure. However, the high degree of overall similarity in axoneme and basal body architectures, along with strong homology from protists to higher animals in many of the proteins involved in these structures, suggests common structural principles. Comparisons among axonemes from different sources should help to define the underlying mechanisms of partitioning the ciliary compartment. Identification of the CPS composition and mapping individual components into the structure will be of fundamental importance. This study offers a structural indication of how the cilia maintain and control their tight compartmentalization and thus provides valuable physiological and therapeutic insights into ciliary trafficking and regulation, as well as ciliogenesis and cilia dysfunction.
Experimental procedures
Tetrahymena cell culture and cellular fractionation
Tetrahymena pyriformis were grown in 1.2% PPYS media at room temperature. The growth of cells was monitored by light microscopy. At stationary phase, cells were harvested by centrifugation and washed twice in 0.12 M sucrose solution. Tetrahymena cytoskeletons were prepared following the protocols of Wolfe [9] with some modifications. Briefly, cells were lysed by osmotic shock in a solution of 1 M sucrose, 10 mM Tris pH 9.0, 1mM EDTA and 0.1 % 2-mercaptoethanol. Triton X100 or NP40 was added subsequently to a final concentration of 2% and the sample was incubated on ice for 1 hour. Extracted cytoskeletons were harvested by centrifugation at 13,000g for 30 min. Samples were resuspended and washed several times in TE buffer (10 mM Tris pH 8.0, 0.01% β-mercaptoethanol, 0.1 mM EDTA). Basal bodies were separated by sonication at 4°C and were isolated by centrifugation on a step gradient of 40% and 50% sucrose at 16,000g for 30 min. Fractions were collected, pelleted and resuspended in TE buffer with no sucrose. To remove microtubules, samples were treated with 2% potassium phosphotungstate pH 8.0 at 25 °C for 2 min and subsequently diluted 10 fold with TE buffer. Samples were centrifuged at 16,000g for 30 min. The pellets were washed several times and resuspended in TE buffer.
Protein isolation and sample preparation for mass spectrometry analysis
The same sample preparation procedures were used for protein identification studies in MS Experiments 1 and 2. Briefly, Tetrahymena basal bodies were fractionated by gradient centrifugation and the CPS were extracted as described above. CPS proteins were separated by SDS PAGE (NuPAGE, 1 mm, 10 well, 4–12 % Bis-Tris gradient mini gels, Invitrogen NP0001) and stained with colloidal Coomassie blue (PageBlue Protein Staining Solution, Thermo Scientific). Visible gel spots were excised and manually digested according to the standard protocol [41]. Briefly, the gel bands were stripped of Coomassie blue stain and dehydrated with acetonitrile, the proteins were reduced with 10 mM DTT (55°C, 45 min), and the reduced cysteine residues were then alkylated with 100 mM iodoacetamide (25°C, 30 min, in the dark). Prior to enzymatic digestion excess reagents were removed and the gel pieces were washed twice with 25 mM ammonium bicarbonate, dehydrated, and incubated with 250 ng sequencing grade trypsin (37°C overnight). The resulting tryptic peptides were extracted from the gel with 50% acetonitrile/0.1% trifluoroacetic acid (TFA).
Mass spectrometry and protein identification
To maximize the number of identified proteins, MS analysis employed both MALDI and ESI ionization modes: samples derived from Experiments 1 and 2 were analyzed using LC MALDI MS/MS and LC ESI MS/MS platforms, respectively. For LC MALDI MS/MS the peptide mixtures were fractionated utilizing a dual column HPLC system (Ultimate 3000, Dionex, Sunnyvale, CA). Samples were desalted on-line using a monolithic reversed phase column (Monolithic PepSwift-DVB column, 200 mm × 1 cm) followed by an analytical column (Monolithic PepSwift-DVB column, 200 mm × 5 cm), both from Dionex. Peptides were separated at a flow rate of 2.5 μl/min using an acetonitrile gradient (isocratic at 0% B for 5 min followed by a linear rise from 0%B to 60% B in 14 min; buffer A: 0.1% TFA in water; buffer B, 80% acetonitrile/0.1% TFA). Starting from 9.7 min, eluates were mixed with the matrix (α-cyano-4-hydroxy-cinnamic acid, 6 mg/ml in 10 mM ammonium phosphate/80% acetonitrile containing 20 pmol/ml [Glu1]-fibrinopeptide B, m/z = 1570.677, as an internal standard to facilitate one-point internal calibration) which was pumped at a flow rate of 0.9 μl/min and spotted onto the MALDI target using a fraction collector/spotter (SunChrom, Friedrichsdorf, Germany). Each sample was separated into 129 fractions, i.e., spotted over an 8 min collection window with a frequency of 3.66 s per spot. Data were acquired on a 4800 MALDI TOF/TOF mass spectrometer (AB Sciex, Framingham, MA) in batch mode utilizing an automated data-dependent selection of 12 precursor ions per spot under the control of the 4000 Series Explorer software (AB Sciex). For LC ESI MS/MS the resulting peptide mixtures were analyzed by nanoLC ESI MS/MS utilizing a 2D LC NanoLC system (Eksigent, Dublin, CA) interfaced with the LTQ XL linear ion trap mass spectrometer (Thermo Fisher, Glen Burnie, MD) equipped with the Advance CaptiveSpray source (Michrom Bioresources). Automated external calibration was performed by the LTQ Tune program, using caffeine, peptide MRFA and reserpine as standards. Five μL of sample was introduced by a direct 10 μl metered injection onto a monolithic C18 column (Onyx 100 μm i.d. × 150 mm, Phenomenex) prior to peptide separation using a 14-minute linear gradient from 2% B to 50% B at a flow rate of 800 nL/min [A: 2% acetonitrile (HPLC grade, Burdick and Jackson)/0.1% formic acid (Pierce Scientific)]; B: 80% acetonitrile/0.1% formic acid). Data-dependent precursor ion selection employed an automated routine (data dependent MS/MS, Excalibur 2.0.7, Thermo Fisher) that consisted of six MS/MS scans (m/z 100–1500) for every survey MS scan (m/z 400–1700). An ESI voltage of 1.8 kV was applied directly to the HPLC mobile phase distal to the chromatography column by the Advance source. The ion transfer tube temperature on the LTQ XL ion trap was set to 200°C. The tube lens in the LTQ XL was set to 100 V. The automatic gain control target values (the approximate number of accumulated ions) for full MS and MS/MS were 10000 and 8000, respectively. Normalized collision energy was set to 35% with activation times of 30 ms. The MS/MS triggering threshold was set to 5000 counts, with a default charge state of 3+. Dynamic exclusion was set to 40 s. A single repeat count and an exclusion list size of 50 were used. The maximum ion injection time was 20 ms for full MS and 100 ms for MS/MS scans.
Details of peptide analysis are provided in Supplementary Materials.
Supplementary Material
Highlights.
We identify a structure comprising a pore complex and membrane diffusion barrier that may serve as a partitioning system
Direct association of the pore complex and membrane domain seals the cilia opening
The pore complex contains doublet-associated pores likely involved in intraflagellar transport
Proteomics reveals the involvement of the periciliary membrane domain in trafficking
Acknowledgments
We are grateful to Dr. R. Csencsits, Dr. E. D. Szakal, Ms. A. Lo and Ms. A. Rector for technical support. HL, MD and HEW acknowledge Drs. S. Fisher (UCSF) and M. Biggin (LBNL) for support, encouragement and discussion. This research is supported by National Institute of Health Grant No. GM051487. The UCSF Sandler-Moore Mass Spectrometry Core Facility acknowledges support from the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Materials - Summary:
Supplemental information includes details of methods, two figures, one movie and two tables as follows:
Materials and Methods; supplementary references.
Figure S1 Electron micrographs of the oral apparatus CPS.
Figure S2 Coomassie-stained SDS gel of samples used for MS analysis.
Movie S1 Tomographic reconstruction of basal bodies
Table S1 Summary of MS analysis of the CPS proteome
Table S2 Detailed listing of proteins identified by MS
References
- 1.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 2.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 6.Kee HL, Dishinger JF, Lynne Blasius T, Liu CJ, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 2012;14:431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella - back to the future! Biol Cell. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- 8.Munn EA. Fine structure of basal bodies (kinetosomes) and associated components of Tetrahymena. Tissue Cell. 1970;2:499–512. doi: 10.1016/s0040-8166(70)80026-x. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe J. Structural analysis of basal bodies of the isolated oral apparatus of Tetrahymena pyriformis. J Cell Sci. 1970;6:679–700. doi: 10.1242/jcs.6.3.679. [DOI] [PubMed] [Google Scholar]
- 10.Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, Baumeister W, Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. doi: 10.1126/science.1104808. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 12.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray MA, Bertin A, Garcia G, 3rd, Lam L, Nogales E, Thorner J. Septin filament formation is essential in budding yeast. Dev Cell. 2011;20:540–549. doi: 10.1016/j.devcel.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia G, 3rd, Bertin A, Li Z, Song Y, McMurray MA, Thorner J, Nogales E. Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J Cell Biol. 2011;195:993–1004. doi: 10.1083/jcb.201107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams NE, Honts JE, Kaczanowska J. The formation of basal body domains in the membrane skeleton of Tetrahymena. Development. 1990;109:935–942. doi: 10.1242/dev.109.4.935. [DOI] [PubMed] [Google Scholar]
- 18.Williams NE. Surface membrane regeneration in deciliated Tetrahymena. J Cell Sci. 1983;62:407–417. doi: 10.1242/jcs.62.1.407. [DOI] [PubMed] [Google Scholar]
- 19.Satir BH, Wissig SL. Alveolar sacs of Tetrahymena: ultrastructural characteristics and similarities to subsurface cisterns of muscle and nerve. J Cell Sci. 1982;55:13–33. doi: 10.1242/jcs.55.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Allen RD. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J Cell Biol. 1969;40:716–733. doi: 10.1083/jcb.40.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould SB, Kraft LG, van Dooren GG, Goodman CD, Ford KL, Cassin AM, Bacic A, McFadden GI, Waller RF. Ciliate pellicular proteome identifies novel protein families with characteristic repeat motifs that are common to alveolates. Mol Biol Evol. 2011;28:1319–1331. doi: 10.1093/molbev/msq321. [DOI] [PubMed] [Google Scholar]
- 22.Smith JC, Northey JG, Garg J, Pearlman RE, Siu KW. Robust method for proteome analysis by MS/MS using an entire translated genome: demonstration on the ciliome of Tetrahymena thermophila. J Proteome Res. 2005;4:909–919. doi: 10.1021/pr050013h. [DOI] [PubMed] [Google Scholar]
- 23.Kilburn CL, Pearson CG, Romijn EP, Meehl JB, Giddings TH, Jr, Culver BP, Yates JR, 3rd, Winey M. New Tetrahymena basal body protein components identify basal body domain structure. J Cell Biol. 2007;178:905–912. doi: 10.1083/jcb.200703109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honts JE, Williams NE. Tetrins: polypeptides that form bundled filaments in Tetrahymena. J Cell Sci. 1990;96(Pt 2):293–302. doi: 10.1242/jcs.96.2.293. [DOI] [PubMed] [Google Scholar]
- 25.Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193:219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laligne C, Klotz C, de Loubresse NG, Lemullois M, Hori M, Laurent FX, Papon JF, Louis B, Cohen J, Koll F. Bug22p, a conserved centrosomal/ciliary protein also present in higher plants, is required for an effective ciliary stroke in Paramecium. Eukaryot Cell. 2010;9:645–655. doi: 10.1128/EC.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–221. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- 29.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 32.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan S, Whiteman EL, Hurd TW, McIntyre JC, Dishinger JF, Liu CJ, Martens JR, Verhey KJ, Sajjan U, Margolis B. Induction of Ran GTP drives ciliogenesis. Mol Biol Cell. 2011;22:4539–4548. doi: 10.1091/mbc.E11-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12:703–710. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurd TW, Fan S, Margolis BL. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J Cell Sci. 2011;124:718–726. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Resendes KK, Rasala BA, Forbes DJ. Centrin 2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. Mol Cell Biol. 2008;28:1755–1769. doi: 10.1128/MCB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Fan S, Margolis B. The Ran importin system in cilia trafficking. Organogenesis. 2011;7:147–153. doi: 10.4161/org.7.3.17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Q, Nelson WJ. Ciliary diffusion barrier: the gatekeeper for the primary cilium compartment. Cytoskeleton (Hoboken) 2011;68:313–324. doi: 10.1002/cm.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garczarek F, Dong M, Typke D, Witkowska HE, Hazen TC, Nogales E, Biggin MD, Glaeser RM. Octomeric pyruvate-ferredoxin oxidoreductase from Desulfovibrio vulgaris. J Struct Biol. 2007;159:9–18. doi: 10.1016/j.jsb.2007.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.