Abstract
Considerable progress has been recently made in understanding the brain mechanisms underlying speech and language control. However, the neurochemical underpinnings of normal speech production remain largely unknown. We investigated the extent of striatal endogenous dopamine release and its influences on the organization of functional striatal speech networks during production of meaningful English sentences using a combination of positron emission tomography (PET) with the dopamine D2/D3 receptor radioligand [11C]raclopride and functional MRI (fMRI). In addition, we used diffusion tensor tractography (DTI) to examine the extent of dopaminergic modulatory influences on striatal structural network organization. We found that, during sentence production, endogenous dopamine was released in the ventromedial portion of the dorsal striatum, in its both associative and sensorimotor functional divisions. In the associative striatum, speech-induced dopamine release established a significant relationship with neural activity and influenced the left-hemispheric lateralization of striatal functional networks. In contrast, there were no significant effects of endogenous dopamine release on the lateralization of striatal structural networks. Our data provide the first evidence for endogenous dopamine release in the dorsal striatum during normal speaking and point to the possible mechanisms behind the modulatory influences of dopamine on the organization of functional brain circuits controlling normal human speech.
Keywords: dopamine, striatum, speech functional networks, structural networks
1. INTRODUCTION
Human speech, one of the most complex and rapid motor behaviors, relies on multi-level integration between brain networks regulating speech perception, processing and production. Although our knowledge of the organization and function of these sensorimotor and higher-order cognitive speech controlling systems has evolved considerably over the last decade (Price, 2012), the neuromodulatory bases of normal human speech and language control remain poorly understood (Simonyan et al., 2011).
Since the discovery of dopamine as a major neurotransmitter in the brain in 1950s (Carlsson, 1959), its modulatory effects on neural activity and networks have been shown to influence a wide range of human behaviors (Montague et al., 2004; Redgrave et al., 2010; Salimpoor et al., 2011; van Schouwenburg et al., 2010). In the speech control system, recent studies have shown that dopaminergic transmission may influence phonological speech processing (Tettamanti et al., 2005), verbal episodic memory and word generation (Cervenka et al., 2008). On the other hand, the role of dopaminergic neuromodulation in normal speech motor control remains unknown and is based largely on indirect clinical evidence. Case reports in patients treated with dopamine D2 receptor antagonists have demonstrated that drug-induced alterations of dopaminergic transmission may lead to the development of uncontrolled laryngeal muscle spasms due to the transient disruption of sensorimotor control of speech production (Newton-John, 1988; Warren and Thompson, 1998). The other well-known clinical illustrations of dopaminergic deficits affecting speech and language control in patients with neurological and psychiatric problems range from a fundamental inability to control motor aspects of speech production (e.g., monotone, hypotonic speech with reduced loudness and pitch, decreased accuracy of articulation and a raspy voice in Parkinson’s disease; involuntary voice breaks in spasmodic dysphonia; frequent repetitions of syllables in stuttering; and vocal tics in Tourette’s syndrome) to profound speech-associated cognitive impairments (e.g., difficulties with phonological processing, syntactic complexity and language comprehension in Parkinson’s disease; and auditory verbal hallucinations in schizophrenia) (Heinz et al., 1998; Kataoka and Ueno, 2010; Ludlow et al., 2008; Thompson et al., 2009; Walsh and Smith, 2011; Wu et al., 1997). Interestingly, in Parkinson’s disease, in contrast to the main motor impairments of the extremities, speech motor changes, occurring in nearly 90% of all patients (Aronson, 1980), do not respond to traditional dopamine replacement therapy and usually require additional treatment protocols, such as behavioral therapy, in order to manage the symptoms (Ciucci et al., 2010; Ramig et al., 2001). In addition, patients with advanced Parkinson’s disease may occasionally develop compulsive involuntary singing and humming while receiving high-dose dopamine replacement therapy (Bonvin et al., 2007; Friedman, 1993; Kataoka and Ueno, 2010). Moreover, while deep brain stimulation of the subthalamic nucleus (STN DBS) is used to successfully improve the motor symptoms affecting extremities in Parkinson’s disease (Fasano et al., 2012), it is less effective, if at all, in the management of speech impairments (Karlsson et al., 2012; Tripoliti et al., 2011; Van Lancker Sidtis et al., 2010; Xie et al., 2011). Such differential outcome of pharmacological and surgical treatments may, in part, be dependent on different mechanisms of dopaminergic transmission for speech compared to limb control. Thus, despite the lack of direct knowledge about the dopaminergic function underlying normal speech production, clinical evidence strongly suggests a particular role for central dopamine modulation of both speech motor commands and higher-order cognitive processes during speaking.
In this study, we investigated the mechanisms of dopaminergic influences on the central control of normal human speech production by examining the extent of speech-induced striatal endogenous dopamine release and its relationship with striatal neural activity and the organization of functional and structural speech networks. We used a combination of three different neuroimaging methodologies (positron emission tomography with the dopamine D2/D3 receptor radioligand [11C]raclopride (RAC PET), event-related sparse-sampling functional MRI (fMRI) with striatal functional connectivity analysis, and diffusion tensor imaging (DTI) with striatal probabilistic tractography) in 20 healthy subjects. The experimental condition during RAC PET and fMRI studies involved production of meaningful English sentences with correct grammatical and lexical structure and resting as a baseline. The experimental condition (i.e., sentence production) was chosen to represent human speech and language as complex behaviors used in everyday communication as closely as possible. RAC PET enabled detection of changes in striatal dopamine release between speaking and resting due to the binding competition for D2/D3 dopamine receptors between endogenous dopamine and the radiolabeled D2/D3 dopamine receptor antagonist, [11C]raclopride, with a decrease in RAC binding during speaking being interpreted as a speech-induced increase in dopamine release (Laruelle, 2000). In addition, the use of fMRI in the same subjects undergoing RAC PET allowed for characterization of striatal activity associated with dopamine release during speech production and mapping of striatal functional networks, while DTI tractography determined the underlying striatal structural connections.
Based on the previous neuroanatomical tract tracing studies (Jurgens, 1976; Simonyan and Jurgens, 2003), we hypothesized that significant dopamine release during speech production would be found in the ventromedial portion of the dorsal striatum consistent with the striatal projection fields of the laryngeal motor cortex. We also predicted that speech-induced striatal dopaminergic release would be associated with BOLD signal change during speech production to influence the organization of striatal functional networks controlling normal speech production. We expected to find minimal modulatory effects of dopamine release on the striatal structural network involved in speech control.
2. MATERIALS AND METHODS
2.1 Subjects
Twenty healthy subjects participated in each of RAC PET, fMRI and DTI study. The same 20 subjects (age 53.2 ± 10.1 years, mean ± s.d., 13 females/7 males) underwent both the PET and fMRI studies. Eight subjects from this group participated in the DTI study, while 12 additional age- and gender-matched subjects (age 53.8 ± 10.9 years, mean ± s.d., 13 females/7 males) were scanned with the same DTI sequence to match the total number of 20 subjects, who participated in the PET and fMRI studies.
All subjects were right-handed on the Edinburgh Handedness Inventory (Oldfield, 1971) and monolingual native English speakers. No participant had any neurological, psychiatric or laryngeal problems based on history and physical examination. All subjects underwent fiberoptic nasolaryngoscopy to confirm normal anatomy and function of the larynx during rest and different laryngeal tasks. Routine neuroradiological evaluation of subjects’ high-resolution MRI found normal brain structure without any gross abnormalities.
All participants provided written informed consent before participation in the study, which was approved by the Institutional Review Boards of the Mount Sinai School of Medicine, National Institute of Neurological Disorders and Stroke, and the NIH Radiation Safety Committee.
2.2 Data acquisition
The scanning sessions were randomized between the subjects. All subjects completed the fMRI/DTI scans in the afternoon (between 2:00 PM and 5:00 PM) and the PET scan in the morning or early afternoon (18 subjects between 8:30 AM and 11:30 AM, 2 subjects between 12:45 PM and 2:00 PM) to control for possible diurnal variations in dopamine transmission. Participants were instructed to abstain from alcohol one week prior to the PET scanning, not to drink any beverages containing caffeine within 24 hours and not to eat 3 hours before the PET study.
RAC PET data were acquired on a GE Advance PET scanner (GE Medical Systems, Milwaukee, WI). An 8-min transmission scan was first obtained using a 68Ge source for attenuation correction of emission data. Through a catheter placed in the antecubital vein, RAC was administered as a 1-min bolus followed by a constant infusion over 99 min (bolus-plus-infusion (B/I) method) (Watabe et al., 2000) using a computer-operated pump (Harvard Instruments, Natick, MA) (Fig. 1A). Accounting for decay, the effectively injected RAC activity over 100-min PET was 19.7 ± 1.4 mCi (i.e., 728.9 ± 51.8 MBq) (mean ± s.d.) with specific activity at the time of injection 2,484.5 ± 1,164.7 mCi/umol (i.e., 91.9 ± 43.1 GBq/umol) (mean ± s.d.). The practical advantages of the B/I method included the requirement for only a single radiochemical synthesis and a single administration of the radiotracer, which significantly minimized the levels of radiation exposure to the study participant. Importantly, the B/I method allowed for the avoidance of potential physiological effects of different scanning sessions, helped the maintenance of RAC equilibrium throughout the entire study (Carson et al., 1997; Slifstein and Laruelle, 2001; Watabe et al., 2000), and avoided the confounding effects of behavior-induced regional cerebral blood flow (rCBF) changes on specific binding values (Carson, 2000; Carson et al., 1997; Carson et al., 1993; Endres and Carson, 1998; Endres et al., 1997).
Figure 1.
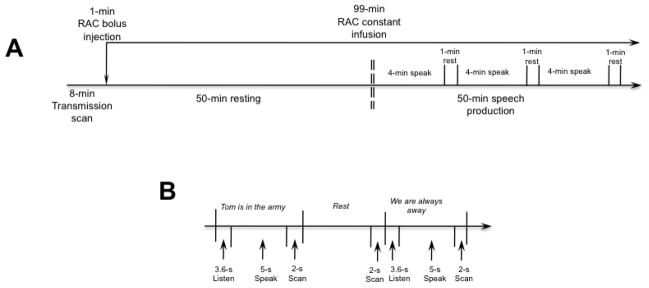
Schematic illustration of the experimental design during RAC PET (A) and speech-production fMRI (B) studies.
A dynamic RAC PET scan in the 3D scanning mode with septa retracted was initiated at the start of RAC injection, generating 27 time frames of 30 s to 5 min epochs over 100 min (FOV 148 mm, reconstructed resolution 6 mm in all direction). The 100-min PET scan included two conditions: an initial 50-min resting state, during which the subjects were asked to relax with their eyes closed in an environment with dimmed light and reduced ambient noise; and 50-min speaking, during which the subjects listened to the auditory sample of meaningful English sentences with correct grammatical and lexical structure (e.g., “Tom is in the army”, “We are always away”) and repeated it at a convenient conversational speech level. Subjects produced different sentences for 4 minutes and rested for 1 min in order to avoid boredom (Fig. 1A). Ten different sentences were presented one at a time and were pseudorandomized between subjects. Auditory stimuli were recorded from a native English female speaker for the purpose of this study. No auditory stimulus was presented during the resting condition. Subjects were trained to speak without head movements, which were additionally minimized by using a comfortably tight thermoplastic mask molded around the subject’s head.
Functional MRI
Whole-brain functional images were acquired with a gradient-weighted EPI pulse sequence using BOLD contrast and a sparse-sampling event-related design on a 3.0 Tesla GE scanner equipped with a quadrature birdcage radio frequency head coil (Fig. 1B). The EPI sequence included TE 30 ms, TR 10.6 s with 8.6 s for task production and 2 s for image acquisition, FA 90 degrees, FOV 240 × 240 mm, matrix 64 × 64 mm, in-plane resolution 3.75 mm, 33 sagittal slices, and slice thickness 4.0 mm. Experimental tasks included 1) production of the same sentences as during the RAC PET study (one sentence was acoustically presented and reproduced by the subjects per acquisition volume); 2) whimper (not reported here), and 3) rest as silent fixation at a cross that appeared on the screen in front of subjects’ eyes. During scanning, subjects were asked first to listen to the auditory sample of the sentence delivered through MR-compatible headphones (SilentScan Audio System, Avotec Inc., Stuart, FL) for a 3.6-s period (Fig. 1B). A visual cue (arrow) then instructed the subjects to speak the sentence within the 5-s interval followed by a 2-s images acquisition. All tasks were pseudorandomized between scanning sessions and subjects. Five scanning sessions were acquired with a total of 36 trials per task.
The use of a sparse-sampling event-related fMRI design helped reduce the effect of self-speech perception on data acquisition during speech production; this design, however, was impossible to implement during the PET scan. Thus, while fMRI acquisition may have been less influenced by perception of each subject’s own speech, both fMRI and PET contained, to some extent, the effects of self-produced sentences on brain activity and neurotransmission, respectively. This, however, did not present a major problem for our current study because our aim was to examine speech production as a complex human behavior, in which speech perception for production represents one of the integral components.
DTI
Whole-brain diffusion-weighted images were acquired using a single-shot spin-echo EPI sequence and an array spatial sensitivity encoding (ASSET) factor of 2 (TE 73.4 ms, TR 13 s, FOV 240 mm, matrix 256 × 256 mm, in-plane resolution 0.9375 mm, 54 contiguous axial slices, slice thickness 2.4 mm) on the same scanner. Diffusion was measured along 33 noncollinear directions (b 1000 s/mm2); three reference images were collected with no diffusion gradients applied.
High-resolution MRI
In each subject, a high-resolution T1-weighted image was collected as an anatomical reference for the PET and fMRI data using 3D magnetization prepared rapid acquisition gradient echo (MPRAGE: TI 450 ms; TE 3.0 ms; FA 10 degrees; bandwidth 31.25 mm; FOV 240 mm; matrix 256 × 256 mm; 128 contiguous axial slices; slice thickness 1.2 mm).
2.3 Data analysis
2.3.1
PET data analysis was performed to determine the extent of dopamine release during sentence production. Initially, correction for subject motion during the dynamic PET scan was performed using the registered attenuation correction method. After reconstruction of emission images with filtered backprojection with no attenuation correction, all emission frames were registered with mutual information to the first emission frame with sufficient counts (i.e., prime emission image) (FLIRT, FSL, FMRIB Software Library). The transmission image was registered to the same prime emission image with the same algorithm. For each emission frame, the transmission image was resliced taking into account the motion between transmission and each frame. The emission frame was reconstructed with filtered backprojection, and the newly synthesized transmission scan was used for attenuation correction, after which the emission image was resliced back to the transmission position, thus correcting for motion.
Taking into the account the pharmacokinetics of the RAC (Laruelle, 2000), the motion- and decay-corrected PET frames (Woods et al., 1993) were averaged over 40–50 min of baseline resting and over 60–100 min of speech production once equilibrium was achieved (PMOD Technologies, Zurich, Switzerland), as reported earlier in a similar experimental design (Garraux et al., 2007). The two PET datasets per subject (i.e., resting and speech production) were aligned to each individual’s T1-weighted MR image using Hellinger distance and two-pass alignment strategy, normalized to the standard Talairach space and spatially smoothed with a 6-mm Gaussian filter (PMOD Technologies and AFNI software (Cox, 1996)). All transformed images were visually inspected for alignment errors.
Detection of dopamine release was based on estimation of changes in the concentration of available receptor sites (Bavail) in response to the associated changes in dopamine concentration. The kinetic behavior of RAC is known to be linear at tracer concentrations and dependent on Bavail, which enables determination of binding potential (BP) (Laruelle, 2000). BP equals the equilibrium ratio of bound ligand to free and non-specifically bound tracer under the assumption that non-specific binding is uniform throughout the brain (Innis et al., 2007). The parametric voxelwise RAC BP maps during each condition (i.e., resting and speech production) were calculated in each subject using an equation BP = (C-C′)/C′ based on the radioactivity concentration in the striatum (C) as a region with the highest density of dopamine D2/D3 receptors and the cerebellum (C′) as a region devoid of D2/3 receptors (Hall et al., 1996). The striatal and cerebellar regions were defined using the probabilistic macrolables atlas of the Anatomy Toolbox (Eickhoff et al., 2005) similar to previously described methods (Del Campo et al., 2010; Salimpoor et al., 2011). Striatal regions-of-interest (ROIs) were sampled at the entire rostro-caudal and dorso-ventral extents; the cerebellar ROI included the gray matter defined on the five consecutive slices of both hemispheres. Radioactivity in the cerebellar gray matter did not show significant differences (two-sample independent t-test, p = 0.81) between resting (BP = 9.6 ± 2.1 nCi/ml/mCi injected) and speech production (BP = 9.5 ± 1.9 nCi/ml/mCi injected).
Next, derived statistical parametric t maps of RAC BP for each condition were used to calculate percentage change in RAC BP (ΔBP) caused by speech production in each subject (ΔBP = (BPspeech − BPresting)/BPresting × 100% (Watabe et al., 2000)). The group statistical significance of speech-induced changes on RAC BP was assessed with a voxelwise paired t-test at a corrected p ≤ 0.01. The anatomical location of significant striatal RAC ΔBP in striatal functional divisions was determined as described earlier (Mawlawi et al., 2001).
2.3.2 fMRI
To estimate the extent and intensity of brain activity during sentence production, we conducted univariate analysis of functional imaging data using AFNI software. Each subject’s first two volumes in each series, collected before magnetization equilibrium was reached, were discarded. The EPI volumes from all runs were registered to the single EPI volume collected closest in time to the high-resolution anatomical scan using heptic polynomial interpolation and spatially smoothed with a 6-mm Gaussian filter. Voxelwise normalization to percent signal change was applied to each voxel in the whole brain. The task-related responses were analyzed using multiple linear regression with a single regressor for each task convolved with a canonical hemodynamic response function. Baseline drifts were modeled using quadratic polynomials in time for each separate imaging run, and motion parameter estimates were used as additional regressors of no interest in the multiple regression analysis. The correction for multiple comparisons was performed using Monte-Carlo simulations that identified a minimum cluster size of 808 mm3 at a voxelwise threshold of 0.001 to achieve overall significance level of a corrected p ≤ 0.01. For group analysis, the anatomical datasets of each subject were spatially normalized and converted to the standard Talairach space. The resulting normalization parameters were applied to the 4-D time series datasets, which were transformed into the standard space. To estimate the main effect of speech production, the group analysis was carried out using a two-way within-subject mixed effect design analysis of variance (ANOVA) with subject as a random factor and the task as the fixed factor (p ≤ 0.01, corrected).
2.3.4 Relationships between striatal dopamine release and neural activation
Using AFNI software, voxelwise striatal Spearman’s rank correlation coefficients were computed to estimate the statistical dependence between speech-induced dopamine release and striatal neural activity. The normalized PET and fMRI datasets were first resampled to match the same orientation and grid spacing. To create a single 3D+subjects volume for each of the fMRI and PET datasets, the respective images were concatenated across all subjects. Voxelwise Spearman’s correlation coefficients were computed between the corresponding voxels of RAC ΔBP and speech-induced BOLD signal change. The resultant maps were thresholded at a corrected p ≤ 0.05.
To account for possible influences on correlation coefficients by a few points, a jackknife procedure was performed to eliminate any correlation coefficients that are statistically significant due to the presence of an outlier. Further, a bootstrap procedure with replacement in 1000 samples was performed to obtain the estimates of statistical accuracy of correlation coefficients (Horwitz et al., 1986; Horwitz et al., 1991).
2.3.5 Striatal network analyses
To examine the influences of speech-induced dopamine release on striatal functional networks, we used psychophysiological interactions (PPI) analysis (Friston et al., 1997) of fMRI data during sentence production. A 4-mm spherical seed region were placed in the left dorsal anterior putamen (APU) (x,y,z = ±23,4,6), which showed a statistically significant relationship between speech-related BOLD signal and RAC ΔBP derived from Spearman correlation analysis (see 2.3.4). Although coupling between speech-induced BOLD signal and RAC ΔBP was found only in the left APU, we also conducted functional connectivity analysis using the corresponding seed region in the right APU in order to test our prediction that speech-induced dopaminergic function has lesser modulatory effects on the right APU networks. Seed time series in each subject were extracted during speech production and silent fixation, multiplied by the task vector, regressed with the time series from the entire brain in each subject, and submitted to group analysis using a two-sample t-test at a corrected p ≤ 0.01 (minimum cluster size of 40 mm3 at a voxelwise threshold of 0.001).
To examine striatal structural circuits underlying functional networks of speech control, diffusion tensor modeling with probabilistic tractography from the same seeds in the bilateral APU as used for functional network analysis was performed using the FSL software package (FDT Diffusion Toolbox (Behrens et al., 2007)). After correction of diffusion-weighted images for eddy current and head motion artifacts, the voxelwise diffusion tensor was calculated using multivariate fitting and diagonalization. A probabilistic streamline and the connectivity distribution were constructed based on the distribution probabilities of each fiber direction sampled in each voxel. The APU structural connections were identified using unconstrained probabilistic tractography in each subject. The probabilistic tractography parameters were set at 5000 streamline samples, 2000 steps with 0.5 mm step length and 0.1-curvature threshold. The group maps of left/right APU structural connectivity were created by averaging resultant probabilistic tractography maps in all subjects normalized to the standard Talairach space and thresholded at a corrected p ≤ 0.01.
2.3.6 Assessment of hemispheric laterality
To examine the lateralization of striatal activation and functional/structural networks, whole-hemispheric ROIs were used to obtain the number of significantly activated and connected voxels, respectively. Statistical significance of lateralization was assessed across all subjects using paired t-test at p ≤ 0.01 to correct for multiple comparisons.
Additionally, a laterality index (LI) was used to examine the lateralization of functional activation and networks, defined as: LIactivity = (# active voxels in LH − # active voxels in RH)/(# active voxels in LH + # active voxels in RH) and LInetwork = (# connected voxels of LS in LH − # connected voxels of RS in RH)/(# connected voxels of LS in LH + # connected voxels of RS in RH), where LH – left hemisphere, RH – right hemisphere, LS – left seed, RS – right seed. A positive LI indicated a left-hemispheric lateralization, and a negative LI denoted right-hemispheric lateralization of activation or network (Seghier, 2008). Statistical significance of LI was determined using paired t-tests at p ≤ 0.01.
2.3.7 Methodological limitations
The combined use of PET, fMRI and DTI methodologies as well as a combination of univariate data analyses and network analyses were essential for obtaining unique information about different properties (i.e., neurotransmitter, functional, and structural) of the striatal system involved in the control of normal speech production within the same study. Specifically, such a multimodal approach allowed us not only to map the extent of striatal dopamine release and brain activation during speech production but also to integrate information about speech-induced dopaminergic transmission (derived from RAC PET) with neural function (derived from fMRI) and anatomical connectivity (derived from DTI tractography) for identification of potential mechanisms of dopamine action on the speech controlling system. However, our study had some limitations.
As an experimental task, we intentionally chose production of sentences with correct grammatical and lexical structure to reflect normal ‘everyday’ speech and language. We recognize that such a task carried both sensorimotor and a number of linguistic and cognitive components, for example, working memory due to multiple repetitions of the same sentences throughout the scanning session. On the other hand, our task did not have obvious reward and emotional components, frequently present in human speech. These are important aspects of human speech and language control, which should be addressed in the future studies. In the present study, the conduct of multiple PET scanning sessions separating each speech-forming factor was technically not feasible due to limitations in PET radiation exposure to subjects. Moreover, choosing to focus on one of the components or having a different baseline task would have required making a specific hypothesis about dopaminergic mechanisms during speaking prior to knowing the fundamentals of dopamine action on the speech production system. As our study identified where and how dopamine interacts with the speech production system, the future studies may be undertaken to dissect its contribution to the control of separate components of speech and language production.
3. RESULTS
3.1. Striatal dopamine release during speech production
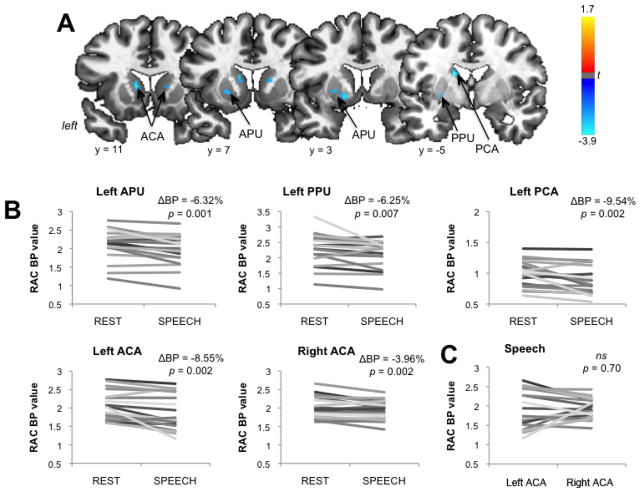
We found that endogenous dopamine release during sentence production led to predominantly left-sided RAC displacement (measured by a decrease in RAC BP between resting and speech conditions) in the ventromedial portion of the associative (AST) and sensorimotor (SMST) functional divisions of the dorsal striatum (Fig. 2A). While both striatal divisions receive dopaminergic input through the nigro-striatal pathway, functionally, the AST is known to play a role in cognition and includes the APU, dorsal anterior caudate nucleus (ACA), and dorsal posterior caudate nucleus (PCA), whereas the SMST is involved in movement coordination and is comprised of the dorsal posterior putamen (PPU) (Mawlawi et al., 2001). Within the AST, statistically significant decreases in RAC BP during speaking were found in the left APU (ΔBP = −6.32%, p = 0.001) and left PCA (ΔBP = −9.54%, p = 0.002), whereas the ACA showed a bilateral RAC BP reduction (left ΔBP = −8.55%, t19 = −3.30; right ΔBP = −3.96%, p = 0.002) (Fig. 2B, Table 1). A follow-up paired t-test found no lateralization effect in dopamine release within the ACA (t19 = −0.53, p = 0.70) (Fig. 2C). A speech-induced RAC BP decrease in the SMST was also found only in the left PPU (ΔBP = −6.25%, p = 0.007).
Figure 2.
Striatal dopamine release during speech production. A speech-induced decrease of RAC BP was found in the left APU, PPU, PCA and in the bilateral ACA. (A) Significant changes in RAC BP during speech production compared to resting are shown on a series of brain images in the coronal plane in the standard Talairach space. The color bar represents the t statistic. (B) The graphs display the RAC BP values and the corresponding RAC ΔBP values in each statistically significant striatal region during resting and speech production in 20 subjects. (C) The graph compares right and left RAC BP values of 20 subjects in the ACA during speech production. ns – non-significant. APU – anterior putamen; PPU – posterior putamen; PCA – posterior caudate nucleus; ACA – anterior caudate nucleus; RAC BP – binding potential of the [11C]raclopride radiotracer.
Table 1.
Striatal regions of significant [11C]raclopride displacement during speech production
| Striatum | RAC BP | Peak Coordinates | Peak t-level | |||
|---|---|---|---|---|---|---|
| Division | Region | Baseline | Speech | ΔRAC BP (%) | x y z | |
| Left AST | ACA | 2.06±0.38 | 1.89±0.47 | −8.55 | −11 11 8 | −3.30 |
| PCA | 0.99±0.20 | 0.89±0.23 | −9.54 | −15 −5 20 | −3.32 | |
| APU | 2.13±0.40 | 1.99±0.41 | −6.32 | −15 3 2 | −3.74 | |
| Right AST | ACA | 2.03±0.29 | 1.94±0.25 | −3.96 | 15 7 12 | −3.29 |
| Left SMST | PPU | 2.30±0.51 | 2.14±0.44 | −6.25 | −27 −7 0 | −2.73 |
Coordinates are given in the Talairach-Tournoux standard space. Statistical significance is set at p ≤ 0. 013, corrected for multiple comparisons. AST – associative striatum; SMST – sensorimotor striatum; ACA – anterior caudate; PCA – posterior caudate; APU – anterior putamen; PPU – posterior putamen; RAC – BP binding potential of the [11C]raclopride radiotracer.
No significant changes in RAC BP from baseline were found in the ventral (limbic) striatum, which receives dopaminergic input through the mesolimbic pathway. The lack of dopamine release in the ventral striatum was consistent with the fact that our speech task did not contain a reward, penalty, or emotional component. It may also point to minimal, if any, involvement of episodic memory associated with sentence production in our experimental setting (Cervenka et al., 2008).
3.2 Dopamine release is coupled with neural activity in the left APU
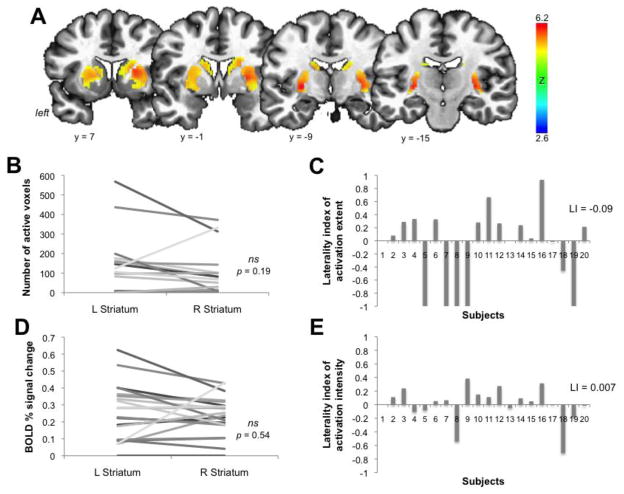
In the same subjects, the univariate analysis of fMRI data during production of the same sentences as during the PET study found widely spread bilateral activation in the entire striatum (Fig. 3A) without hemispheric lateralization of either its extent (t19 = 1.34, p = 0.19, mean laterality index (LI) = −0.09) or intensity (t19 = 0.62, p = 0.54; mean LI = 0.007) (Fig. 3B–E).
Figure 3.
(A) Striatal activation during speech production is shown on a series of coronal images in standard Talairach space. Color bar represents the Z value. Graphs display the number of active voxels (B) and BOLD percent signal change (D) in the left and right striatum in 20 subjects, showing no significant difference between hemispheres. Bar graphs demonstrate the corresponding laterality indices of activation extent (C) and activation intensity (E).
However, despite the whole-striatal activation during speaking, only the left APU (part of AST) showed a significant linear relationship between speech-induced RAC ΔBP and BOLD signal change during speaking (jackknife estimated rs = −0.59, p = 0.006) (Fig. 4).
Figure 4.
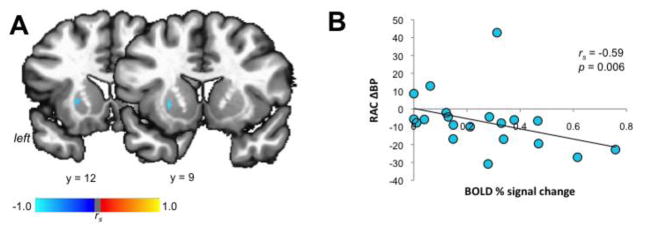
The left APU shows coupling between dopamine release and brain activation. Relationship between speech-induced RAC ΔBP and BOLD percent signal change during speaking is shown on a series of coronal images (A) and on a bar graph and correlation plot (B). The color bars depict Spearman’s correlation coefficients (rs). APU – anterior putamen; RAC BP – binding potential of the [11C]raclopride radiotracer.
3.3 Dopamine influences the organization of APU functional but not structural speech networks
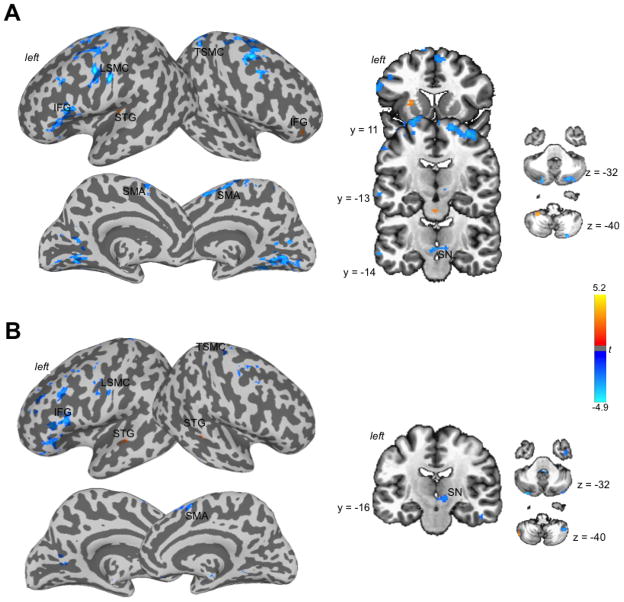
Functional and structural striatal networks were examined from the APU region, which showed significant relationship between the speech-related endogenous dopamine release and neural activity. During speech production, the left APU showed positive functional connections with the right inferior frontal gyrus (IFG), left superior temporal gyrus (STG), including Heschl’s gyrus, cerebellum (lobule VIII), pons, and the APU itself. Negative functional connections of the left APU were observed with the left laryngeal sensorimotor cortex (LSMC), right trunkal sensorimotor cortex (TSMC), bilateral premotor cortex, precuneus, supplementary motor area (SMA), midbrain including the substantia nigra (SN), and cerebellum (Crus 1–2) (Fig. 5A). The right APU showed positive functional connections only with the bilateral STG and left cerebellum (Crus 2). The negative connections were similar to those from the left APU (Fig. 5B).
Figure 5.
Functional networks of the left (A) and right (B) APU during speech production. Cortical connections using the APU as a seed are shown on the inflated brain surfaces in the standard Talairach space; subcortical and cerebellar connections are shown on a series of coronal and axial images. The color bar represents the t statistics of the network. APU – anterior putamen.
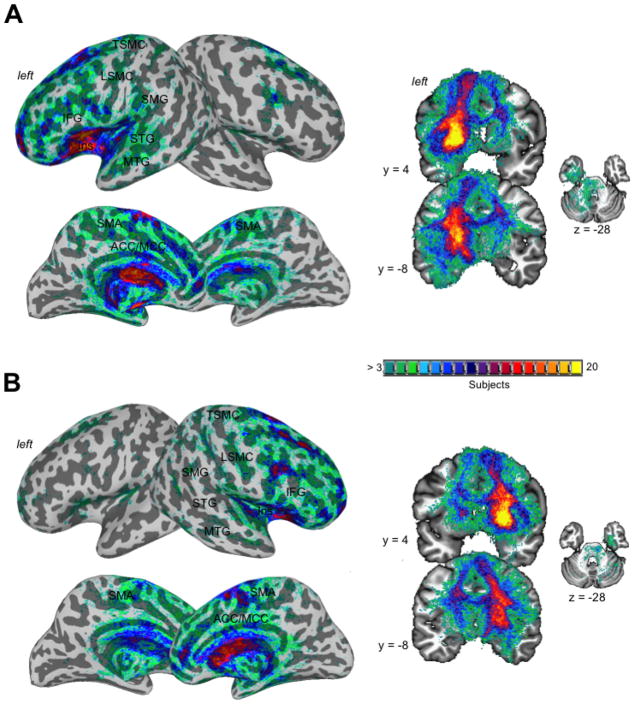
Structurally, both left and right APU networks had the heaviest connections (> 51–100% probability) with frontal cortical regions, including the IFG, dorsal and ventral premotor cortex, dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), insula, SMA, and anterior/middle cingulate cortex (ACC/MCC) (Fig. 6). Somewhat weaker striatal connections (> 15–50% probability) were found with the primary sensorimotor cortex, including the LSMC and TSMC, as well as with the STG, middle temporal gyrus (MTG), supramarginal gyrus (SMG), midbrain, and cerebellum.
Figure 6.
Probabilistic structural networks of the left (A) and right (B) APU. Cortical connections determined by probabilistic tractography with APU as a seed are shown on the inflated brain surfaces in the standard Talairach space; subcortical and cerebellar connections are shown on a series of coronal and axial images. The color bar represents the degree of projection overlap across 20 subjects. APU – anterior putamen.
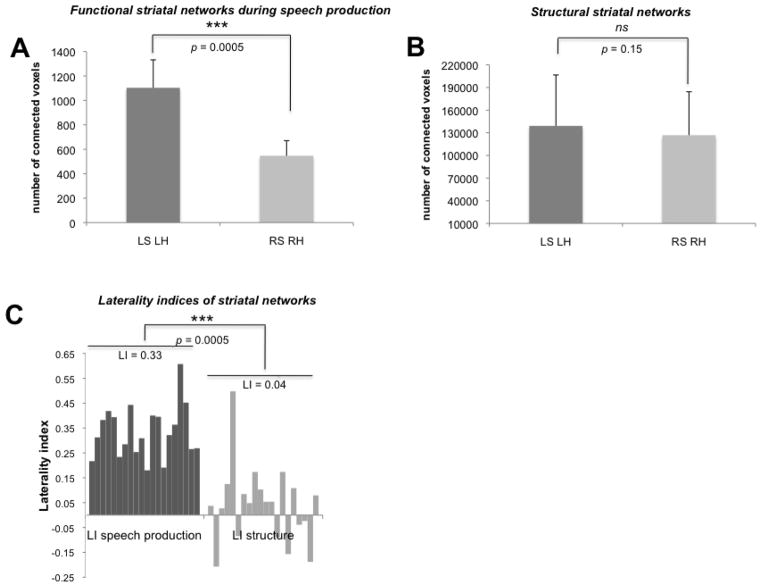
Similar to the left-striatal RAC displacement during speech production and despite the bilateral striatal neural activity, speech-related APU functional networks exhibited left-hemispheric lateralization (LI = 0.33±0.10; p = 0.0005) (Fig. 7A). In contrast, APU structural networks were bilaterally distributed without hemispheric lateralization (LI = 0.04±0.14, p = 0.15) (Fig. 7B). Left-hemispheric lateralization of speech production functional networks was significantly greater compared to structural networks (all p = 0.0005) (Fig. 7C).
Figure 7.
Bar graphs show lateralization effects of APU functional connectivity during speech production (A) and structural connectivity (B) as well as the corresponding laterality indices (C). Each bar in (C) corresponds to a single subject. (*) indicates the statistical significance of the comparison; ns non-significant comparison; LS – left seed, RS – right seed, LH – left hemisphere; RH – right hemisphere; LI – laterality index; APU – anterior putamen.
4. DISCUSSION
Despite the long recognized role of dopamine as a neuromodulator of striatal functions (Albin et al., 1989; Schultz, 2006; Wickens et al., 2003), information on dopaminergic regulation of human speech and language has remained limited. Using a multi-modal neuroimaging approach, we present here the first evidence of striatal dopaminergic transmission during normal speech production in healthy humans and define the extent of dopaminergic effects on the organization of striatal networks involved in speech control.
4.1 Dopamine release and lateralization of striatal speech networks
We demonstrated that sentence production was associated with significant release and binding of endogenous dopamine to the D2/D3 receptors in the left putamen and bilateral caudate nucleus within the AST and SMST divisions of the striatum. We found that these striatal regions were similar to those known to receive direct projections from the laryngeal motor cortex in non-human primates, that is the ventromedial portions of both AST and SMST (Jurgens, 1976; Simonyan and Jurgens, 2003). While the laryngeal motor cortical input into the SMST has been shown to overlap with the input from the facial motor cortex, the input into the AST appears to be segregated from other descending motor cortical projections, thus forming a ‘larynx’ striatal representation (Jurgens, 1976; Kunzle, 1975; Simonyan and Jurgens, 2003). Based on our findings of coupling between the speech-related dopamine release and neural activity in the same APU region, which receives descending laryngeal motor cortical projections, we may suggest that functional importance of APU dopaminergic transmission in speech control may, in part, be in its modulatory effects on descending laryngeal cortico-striatal terminal activity and speech cortical network organization. Earlier studies have shown that the D2 receptors are localized not only on the striatal medium spiny neurons (MSNs) but also on subpopulations of cortico-striatal terminals (Fisher et al., 1994; Hersch et al., 1995; Sesack et al., 1994; Wang and Pickel, 2002), excitability of which is also modulated by dopamine release (Bamford et al., 2004; Garris et al., 1994; Gonon, 1997; Nicola and Malenka, 1998). In line with this assumption, significant left-hemispheric lateralization of APU functional networks found in our study may possibly be due to modulatory influences of dopamine on neural activity in the left but not right APU. On the other hand, the more stable APU structural networks showed a bilateral hemispheric distribution, suggesting that they may represent an underlying framework for functional networks controlling different types of vocal behaviors, and are less receptive to dopaminergic influences.
Lateralization of dopaminergic function has been previously reported to be dependent on the direction of motor behavior and type of reinforcement task in rodents (Glick et al., 1981; Morice et al., 2005; Szostak et al., 1986; Zimmerberg et al., 1974). However, results of studies in songbirds, whose singing is considered to be analogous to human speaking, give no indication of striatal lateralization of dopamine release during vocal learning and song production (Hara et al., 2007; Huang and Hessler, 2008; Jarvis and Nottebohm, 1997; Sasaki et al., 2006; Yanagihara and Hessler, 2006), despite the species-specific hemispheric lateralization of avian forebrain vocal learning pathways (Nottebohm et al., 1976; Williams et al., 1992). In humans, recent studies have reported a range of task effects on the levels of nigro-striatal dopamine release. While no lateralization of striatal dopamine levels was found in postmortem human brain tissue (Rossor et al., 1980), more recent PET studies have established that the levels of striatal dopamine release may depend on the task performed. Specifically, the degree of right hand preference during self-paced freely chosen movements was found to be correlated with increased dopaminergic function in the left putamen (de la Fuente-Fernandez et al., 2000), whereas performance of a button press task was associated with bilateral striatal dopamine release compared to right-sided striatal lateralization of dopaminergic transmission in response to unpredictable reward (Martin-Soelch et al., 2011). The functional importance of left-striatal dopamine release during speech production appears to be in its modulatory influences contributing to hemispheric lateralization of speech controlling networks. Our finding of left-striatal dopaminergic transmission is in line with left-hemispheric lateralization of brain activation during speech production, which is known since the time of Broca, and may point to unique higher-order behavior-specific integration and adaptation of the striatal modulatory dopaminergic system for human speech and language control. The significance of left-striatal dopaminergic transmission is further supported by clinical evidence that suggests that abnormalities in lateralization of dopamine release, such as higher uptake of a dopamine precursor in the left but not right striatum in persons who stutter (Wu et al., 1997), and the presence of left putaminal lesions may contribute to the pathophysiology of various voice and speech disturbances, ranging from stuttering to aphasia and auditory agnosia (Ciabarra et al., 2000; D’Esposito and Alexander, 1995; Heuer et al., 1996; Lee et al., 1996; Metter et al., 1986; Taniwaki et al., 2000). Collectively, our findings provide evidence for neurochemical basis of a still elusive physiological concept of hemispheric dominance of human speech and language production.
4.2 Organization of striatal speech networks
An interesting feature of the APU functional speech-related networks was its predominantly negative connectivity. Although the mechanisms underlying negative correlations between brain regions within a network remain unclear (Bandettini, 2009), a possible explanation of our results may be suggested based on functional properties of striatal control. The cortico-striatal projections provide excitatory (i.e., glutamatergic) input to the striatum and can be differentiated into two types of projections, an inter-telencephalically projecting type (IT-type) and a pyramidal tract type (PT-type), which give rise to the direct and indirect basal ganglia output pathways, respectively (Kreitzer and Malenka, 2007; Lei et al., 2004). Neurons of the direct pathway, projecting from the striatum to the internal segment of the globus pallidus and the substantia nigra, express dopamine D1 receptors and produce robust D1-dependent long-term potentiation (LTP) allowing facilitation of movement. Neurons of the indirect pathway, projecting from the striatum to the external segment of the globus pallidus, express D2 receptors and exert long-term depression (LTD) resulting in supersession of unwanted movements that would otherwise interfere with ongoing movement (Fino et al., 2005; Kreitzer and Malenka, 2007, 2008; Mahon et al., 2004; Shen et al., 2008; Walsh, 1993). This balance between direct and indirect basal ganglia circuits is presumably maintained due to greater responsiveness of indirect pathway neurons, which reduce cortical activation (Ballion et al., 2008; Gertler et al., 2008; Kreitzer and Malenka, 2007; Mallet et al., 2006). In the current study, we mapped the striatal portion of the inhibitory indirect pathway involved in speech control because RAC binds to dopamine D2 receptors expressed within the indirect pathway but has no affinity to D1 receptors expressed within the direct pathway (Laruelle, 2000). Negative connectivity of speech-related functional APU networks may thus be explained by suppression of cortical networks due to activation of D2 receptors within the net inhibitory indirect basal ganglia pathway, which, in turn, may have led to inhibition of the activity of cortico-striatal terminals and subsequent suppression of speech-controlling cortical networks, evident as negative APU functional connectivity. This is consistent with a recent study showing that dopamine effects on D2 receptors lead to inhibition of the activity of cortico-striatal terminals (Bamford et al., 2004). Furthermore, our data reveal which brain regions within the speech production network may be suppressed due to the action of dopamine on indirect pathway neurons via their D2 receptors during speech production.
It is important to acknowledge that all regions showing both functional and structural connectivity with the APU have been known to be involved in playing critical roles in various stages of speech control, ranging from spectrotemporal analysis (dorsal STG) to participating as part of a combinatorial and lexical interface (MTG), phonological processing (middle-posterior STS) and articulatory execution (IFG, SMA, primary sensorimotor cortex, premotor cortex) (Hickok and Poeppel, 2007; Jurgens, 2002). These regions are also included in the DIVA (Directions into Velocities of Articulators) neural network model of speech control, in which feedforward and feedback control subsystems, comprising cortical, subcortical (including the striatum) and cerebellar regions, are integrated to generate speech motor commands (Golfinopoulos et al., 2010; Guenther et al., 2006). According to this model, the striatum establishes reciprocal connections with the SMA to define its activation timing (referred as to the Initiation Map in the DIVA model), which subsequently releases the motor commands (referred to as Articulator Velocity and Position Maps) associated with the selected speech motor programs (referred as to Speech Sound Map). Our current findings on the extent of APU functional and structural connectivity thus fit well with both data from human and animal research as well as neurocomputational simulations of speech motor control system. The wide array of APU connections with cortical regions found here may be related to the integral role of language in speech formation, which requires not only pure motor control of the laryngeal muscles for voice production but also a number of higher cognitive functions, such as construction of grammatically correct sentences, listening to one’s own speech, implicit processing of the meaning of the words, accurate phonetic processing, and verbal working memory.
We found that APU structural networks were more widely distributed compared to more segregated speech production-related networks. The differences in the connectivity extent were most notable in the temporo-parietal (STG, MTG, SMG), prefrontal (dlPFC, vlPFC) and cingulate (MCC/PCC) regions. It is plausible to suggest that striatal structural network likely represents a shared framework, which may be adapted to task production (i.e., speaking) by enhancing or reducing the strength of connectivity with selected brain regions and by changing the degree of hemispheric lateralization. Therefore, it is not surprising that this common structural network is likely to show connectivity with a larger set of brain regions compared to functional networks underlying performance of a specific task.
It is also possible that nigro-striatal dopaminergic input facilitates more than suppresses speech-related activity in some temporo-parietal, prefrontal and cingulate regions (e.g., STG, SMG, dlPFC, vlPFC, MCC/PCC). Indeed, one of the few brain regions showing positive connectivity with the APU during speech production was Heschl’s gyrus, which is the first cortical region to processes an auditory input. This connectivity may have been influenced by the presence of the subject’s own speech perception during sentence production. The Hesch’s region has been shown to decrease the functional value of its cortical and subcortical re-entrant inputs in the presence of syntactic and prosodic violations (David et al., 2011). We may assume that the activity of D1-family receptors within the net excitatory direct basal ganglia circuitry, as oppose to activity of D2-family receptors within the net inhibitory indirect basal ganglia circuitry, might be more important for modulation of striatal connectivity with the temporo-parietal, prefrontal and cingulate brain regions. This, however, remains to be explored in future studies with the use of D1-specific radioligands.
4.3 Speech-induced dopamine release in other dorsal striatal regions
While the relationship between speech-induced dopamine release and striatal neural activity was found in the left APU only, statistically significant dopamine release during speech production without correlations with neural activity was also observed in other regions of the AST (i.e., the bilateral ACA and left PCA) and SMST (i.e., left PPU). Speech-induced dopamine release in different regions of both AST and SMST may be important for functional integration between parallel and mainly segregated striatal circuits (Alexander et al., 1990; Draganski et al., 2008; Kelly and Strick, 2004) in order to orchestrate equally important speech motor control via the SMST with a number of speech-related higher cognitive functions, such as lexical and semantic processing, via the AST.
Coupling between neural activity and dopamine release in different striatal divisions may depend on the task production. In our experimental setting, correlation between speech-induced dopamine release and neural activity at the level of the goal-directed (AST) but not the habitual (SMST) control system suggests that, while sensorimotor controlling aspects remain important during normal speech production, dopaminergic influences on higher-level cognitive aspects weigh in more significantly for information processing during ongoing speech. On the other hand, the modulatory effects of dopamine on sensorimotor striatal control (i.e., exerted by PPU) might prevail in such situations as speech and language development and acquisition of a second language, during which sensorimotor learning of novel word articulation may require higher integration of the sensorimotor system (including SMST) for shaping the accurate production of a new word or sentence.
Brain activation studies have suggested that, within the AST, the caudate nucleus may be involved in monitoring of phonological accuracy and suppression of unintended responses, while the putamen has been associated with speech motor initiation, the speed of learned vocal task production, semantic processing, and verbal semantic and episodic memory (Davis and Gaskell, 2009; Koylu et al., 2006; Price, 2010; Riecker et al., 2005; Ystad et al., 2010). A recent neuroreceptor mapping study has further confirmed the role of dopaminergic function in monitoring phonological accuracy in the left caudate nucleus and speed of phonological processing in the left putamen (Tettamanti et al., 2005). Another study, investigating the relationship between the dopaminergic transmission and cognitive performance, has shown strong correlation between D2 receptor binding and spontaneous word generation in the AST and SMST, whereas both verbal and non-verbal episodic memory tasks were significantly associated with D2 receptor binding in the limbic striatum only (Cervenka et al., 2008). Based on these studies, we suggest that no relationship between the neural activity and dopamine release in the caudate nucleus may have been due to our experimental task, which did not contain any phonological violations and thus did not require the recruitment of the dopaminergic function in the caudate nucleus for error feedback monitoring. On the other hand, while our experimental task included repetition of the same ten sentences throughout the scanning session, the impact of verbal memory appeared to be minimal, if any, on our findings as we did not indentify any dopamine release in the limbic striatum, that is the region shown to be associated with both verbal and non-verbal memory (Cervenka et al., 2008).
5. CONCLUSION
In summary, we presented here a detailed investigation on dopaminergic regulation of the striatal networks involved in normal speech control in healthy humans. We identified predominantly left-striatal lateralization of speech-induced dopamine release within the ventromedial regions of AST and SMST and a segregated region within the left APU, where speech-induced dopamine release appeared to modulate the neural activity to influence the lateralization of functional but not structural speech networks. As such, our study provides the first evidence for the neurochemical underpinnings of hemispheric dominance of human speech and language control. These findings further lay a foundation for future studies directed toward the identification of the pathophysiological mechanisms underlying dopaminergic dysfunction in patients with neurological and psychiatric speech and language problems, such as hypophonia in Parkinson’s disease, stuttering, spasmodic dysphonia, vocal tics in Tourette’s syndrome, and auditory visual hallucinations in schizophrenia.
HIGHLIGHTS.
Striatal dopamine release is left-lateralized during speech production
Dopamine release is coupled with striatal activation in the left associative putamen
Dopamine release influences left lateralization of putaminal functional but not structural speech networks
Acknowledgments
We would like to thank the PET Department of the NIH Clinical Center for assistance with PET data acquisition, Pamela Kearney, M.D., for subject evaluation, and Richard Reynolds, M.S., for help with data processing. Supported by DC009629 grant to KS, the Intramural Programs of the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, and the NIH Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Aronson AE. An interdisciplinary approach. Thieme; Stuttgart: 1980. Clinical voice disorders. [Google Scholar]
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin C, Horvath J, Christe B, Landis T, Burkhard PR. Compulsive singing: another aspect of punding in Parkinson’s disease. Ann Neurol. 2007;62:525–528. doi: 10.1002/ana.21202. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–493. [PubMed] [Google Scholar]
- Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27:657–660. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, Herscovitch P. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- Cervenka S, Backman L, Cselenyi Z, Halldin C, Farde L. Associations between dopamine D2-receptor binding and cognitive performance indicate functional compartmentalization of the human striatum. Neuroimage. 2008;40:1287–1295. doi: 10.1016/j.neuroimage.2007.12.063. [DOI] [PubMed] [Google Scholar]
- Ciabarra AM, Elkind MS, Roberts JK, Marshall RS. Subcortical infarction resulting in acquired stuttering. J Neurol Neurosurg Psychiatry. 2000;69:546–549. doi: 10.1136/jnnp.69.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Vinney L, Wahoske EJ, Connor NP. A translational approach to vocalization deficits and neural recovery after behavioral treatment in Parkinson disease. Journal of communication disorders. 2010;43:319–326. doi: 10.1016/j.jcomdis.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Alexander MP. Subcortical aphasia: distinct profiles following left putaminal hemorrhage. Neurology. 1995;45:38–41. doi: 10.1212/wnl.45.1.38. [DOI] [PubMed] [Google Scholar]
- David O, Maess B, Eckstein K, Friederici AD. Dynamic causal modeling of subcortical connectivity of language. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2712–2717. doi: 10.1523/JNEUROSCI.3433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philos Trans R Soc Lond B Biol Sci. 2009;364:3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization. Behavioural brain research. 2000;112:63–68. doi: 10.1016/s0166-4328(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Tait RJ, Acosta-Cabronero J, Hong YT, Izquierdo-Garcia D, Smith R, Aigbirhio FI, Sahakian BJ, Muller U, Robbins TW, Fryer TD. Quantification of receptor-ligand binding potential in sub-striatal domains using probabilistic and template regions of interest. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.11.071. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Carson RE. Assessment of dynamic neurotransmitter changes with bolus or infusion delivery of neuroreceptor ligands. J Cereb Blood Flow Metab. 1998;18:1196–1210. doi: 10.1097/00004647-199811000-00006. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, Eckelman WC, Carson RE. Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet neurology. 2012;11:429–442. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Levine MS, Sibley DR, Ariano MA. D2 dopamine receptor protein location: Golgi impregnation-gold toned and ultrastructural analysis of the rat neostriatum. Journal of neuroscience research. 1994;38:551–564. doi: 10.1002/jnr.490380508. [DOI] [PubMed] [Google Scholar]
- Friedman JH. Involuntary humming in autopsy-proven Parkinson’s disease. Mov Disord. 1993;8:401. doi: 10.1002/mds.870080333. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garraux G, Peigneux P, Carson RE, Hallett M. Task-related interaction between basal ganglia and cortical dopamine release. J Neurosci. 2007;27:14434–14441. doi: 10.1523/JNEUROSCI.1595-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Weaver LM, Meibach RC. Amphetamine enhancement of reward asymmetry. Psychopharmacology. 1981;73:323–327. doi: 10.1007/BF00426459. [DOI] [PubMed] [Google Scholar]
- Golfinopoulos E, Tourville JA, Guenther FH. The integration of large-scale neural network modeling and functional brain imaging in speech motor control. Neuroimage. 2010;52:862–874. doi: 10.1016/j.neuroimage.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G. Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse. 1996;23:115–123. doi: 10.1002/(SICI)1098-2396(199606)23:2<115::AID-SYN7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Knable MB, Wolf SS, Jones DW, Gorey JG, Hyde TM, Weinberger DR. Tourette’s syndrome: [I-123]beta-CIT SPECT correlates of vocal tic severity. Neurology. 1998;51:1069–1074. doi: 10.1212/wnl.51.4.1069. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer RJ, Sataloff RT, Mandel S, Travers N. Neurogenic stuttering: further corroboration of site of lesion. Ear Nose Throat J. 1996;75:161–168. [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI. Age differences in intercorrelations between regional cerebral metabolic rates for glucose. Ann Neurol. 1986;19:60–67. doi: 10.1002/ana.410190111. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Swedo SE, Grady CL, Pietrini P, Schapiro MB, Rapoport JL, Rapoport SI. Cerebral metabolic pattern in obsessive-compulsive disorder: altered intercorrelations between regional rates of glucose utilization. Psychiatry Res. 1991;40:221–237. doi: 10.1016/0925-4927(91)90014-h. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS One. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens U. Projections from the cortical larynx area in the squirrel monkey. Exp Brain Res. 1976;25:401–411. doi: 10.1007/BF00241730. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neuroscience and biobehavioral reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Karlsson F, Blomstedt P, Olofsson K, Linder J, Nordh E, van Doorn J. Control of phonatory onset and offset in Parkinson patients following deep brain stimulation of the subthalamic nucleus and caudal zona incerta. Parkinsonism & related disorders. 2012;18:824–827. doi: 10.1016/j.parkreldis.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Ueno S. Compulsive singing associated with a dopamine agonist in Parkinson disease. Cogn Behav Neurol. 2010;23:140–141. doi: 10.1097/WNN.0b013e3181c12af2. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Koylu B, Trinka E, Ischebeck A, Visani P, Trieb T, Kremser C, Bartha L, Schocke M, Benke T. Neural correlates of verbal semantic memory in patients with temporal lobe epilepsy. Epilepsy research. 2006;72:178–191. doi: 10.1016/j.eplepsyres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lee SB, Kim WC. Spasmodic dysphonia associated with a left ventrolateral putaminal lesion. Neurology. 1996;47:827–828. doi: 10.1212/wnl.47.3.827. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Adler CH, Berke GS, Bielamowicz SA, Blitzer A, Bressman SB, Hallett M, Jinnah HA, Juergens U, Martin SB, Perlmutter JS, Sapienza C, Singleton A, Tanner CM, Woodson GE. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Deniau JM, Charpier S. Corticostriatal plasticity: life after the depression. Trends Neurosci. 2004;27:460–467. doi: 10.1016/j.tins.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, Carson RE, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–1715. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Jackson C, Kempler D, Riege WH, Hanson WR, Mazziotta JC, Phelps ME. Left hemisphere intracerebral hemorrhages studied by ((F-18)-fluorodeoxyglucose PET. Neurology. 1986;36:1155–1162. doi: 10.1212/wnl.36.9.1155. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morice E, Denis C, Macario A, Giros B, Nosten-Bertrand M. Constitutive hyperdopaminergia is functionally associated with reduced behavioral lateralization. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:575–581. doi: 10.1038/sj.npp.1300570. [DOI] [PubMed] [Google Scholar]
- Newton-John H. Acute upper airway obstruction due to supraglottic dystonia induced by a neuroleptic. BMJ. 1988;297:964–965. doi: 10.1136/bmj.297.6654.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. Journal of neurophysiology. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. The Journal of comparative neurology. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT (R)) in individuals with Parkinson’s disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders. 2001;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rossor M, Garrett N, Iversen L. No evidence for lateral asymmetry of neurotransmitters in post-mortem human brain. J Neurochem. 1980;35:743–745. doi: 10.1111/j.1471-4159.1980.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011 doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B, Jarvis ED. Dopaminergic regulation of human speech and bird song: a criticial review. Brain and Language. 2011 doi: 10.1016/j.bandl.2011.12.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nuclear medicine and biology. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Szostak C, Jakubovic A, Phillips AG, Fibiger HC. Bilateral augmentation of dopaminergic and serotonergic activity in the striatum and nucleus accumbens induced by conditioned circling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:2037–2044. doi: 10.1523/JNEUROSCI.06-07-02037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T, Tagawa K, Sato F, Iino K. Auditory agnosia restricted to environmental sounds following cortical deafness and generalized auditory agnosia. Clin Neurol Neurosurg. 2000;102:156–162. doi: 10.1016/s0303-8467(00)00090-1. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16:397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Urban N, Abi-Dargham A. How have developments in molecular imaging techniques furthered schizophrenia research? Imaging in medicine. 2009;1:135–153. doi: 10.2217/IIM.09.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T, Holl E, Petersen E, Roughton M, Hariz MI, Limousin P. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011;76:80–86. doi: 10.1212/WNL.0b013e318203e7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lancker Sidtis D, Rogers T, Godier V, Tagliati M, Sidtis JJ. Voice and fluency changes as a function of speech task and deep brain stimulation. Journal of speech, language, and hearing research : JSLHR. 2010;53:1167–1177. doi: 10.1044/1092-4388(2010/09-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des. 2010;16:2026–2032. doi: 10.2174/138161210791293097. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A. Linguistic complexity, speech production, and comprehension in Parkinson’s disease: behavioral and physiological indices. Journal of speech, language, and hearing research : JSLHR. 2011;54:787–802. doi: 10.1044/1092-4388(2010/09-0085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JP. Depression of excitatory synaptic input in rat striatal neurons. Brain Res. 1993;608:123–128. doi: 10.1016/0006-8993(93)90782-i. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. The Journal of comparative neurology. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Warren J, Thompson P. Drug-induced supraglottic dystonia and spasmodic dysphonia. Mov Disord. 1998;13:978–979. doi: 10.1002/mds.870130623. [DOI] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–530. [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. Right-side dominance for song control in the zebra finch. Journal of neurobiology. 1992;23:1006–1020. doi: 10.1002/neu.480230807. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Wu JC, Maguire G, Riley G, Lee A, Keator D, Tang C, Fallon J, Najafi A. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8:767–770. doi: 10.1097/00001756-199702100-00037. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhang Y, Zheng Z, Liu A, Wang X, Zhuang P, Li Y. Changes in speech characters of patients with Parkinson’s disease after bilateral subthalamic nucleus stimulation. Journal of voice : official journal of the Voice Foundation. 2011;25:751–758. doi: 10.1016/j.jvoice.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly--a resting state fMRI study. Neuroimage. 2010;52:379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Glick SD, Jerussi TP. Neurochemical correlate of a spatial preference in rats. Science. 1974;185:623–625. doi: 10.1126/science.185.4151.623. [DOI] [PubMed] [Google Scholar]