Abstract
Purpose
The observation periods and thresholds on serum creatinine and urine output defined in the Acute Kidney Injury Network (AKIN) criteria were not empirically derived. By continuously varying creatinine/urine output thresholds as well as observation period, we sought to investigate the empirical relationships among creatinine, oliguria, in-hospital mortality, and receipt of renal replacement therapy (RRT).
Methods
Using a high-resolution database (MIMIC-II), we extracted data from 17,227 critically ill patients with an in-hospital mortality rate of 10.9%. 14,526 patients had urine output measurements. Various combinations of creatinine/urine output thresholds and observation periods were investigated by building multivariate logistic regression models for in-hospital mortality and RRT predictions. For creatinine, both absolute and percentage increases were analyzed. To visualize the dependence of adjusted mortality and RRT rate on creatinine, urine output, and observation period, we generated contour plots.
Results
Mortality risk was high when absolute creatinine increase was high regardless of observation period, when percentage creatinine increase was high and observation period was long, and when oliguria was sustained for a long period of time. Similar contour patterns emerged for RRT. The variability in predictive accuracy was small across different combinations of thresholds and observation periods.
Conclusions
The contour plots presented in this article complement the AKIN definition. A multi-center study should confirm the universal validity of the results presented in this article.
Keywords: AKI, renal failure, creatinine, urine output, kidney injury, AKIN criteria
Introduction
Between 5% and 7% [1] of all hospitalized patients develop acute kidney injury (AKI) during their hospital stay, increasing to between 26% and 49% in the critically ill [2-5]. However, AKI has not been consistently defined, with over 30 different definitions appearing in the literature [6]. In 2004, the Acute Dialysis Quality Initiative (ADQI) published the first widely accepted definition of AKI, the RIFLE criteria [7, 8]. Subsequently, a number of studies, largely in vascular and cardiothoracic patients, demonstrated that even small absolute increases in serum creatinine can indicate AKI and are correlated with increased mortality [9, 10]. In 2005, the Acute Kidney Injury Network (AKIN) proposed a revision to the RIFLE criteria on creatinine. The urine output criteria had originally been established by RIFLE and were confirmed by AKIN. The AKIN criteria were first published by Mehta et al. in 2007 [7]. For a rigorous comparison between RIFLE and AKIN, please see the study conducted by Joannidis et al. [11]. In 2012, Kidney Disease: Improving Global Outcomes (KDIGO) published the most recent clinical guideline for AKI [12], which summarizes the AKIN and RIFLE criteria.
The AKIN criteria are based on both serum creatinine and urine output (Table E1 in the Electronic Supplemental Material), because creatinine and urine output may not always agree with each other. Recently, Prowle and colleagues demonstrated that transient oliguria was a common event in the intensive care unit (ICU) and that such incidents were not necessarily associated with development of AKI by creatinine criteria [13]. The AKIN threshold of 0.3mg/dl for absolute increase in creatinine was taken from a study by Chertow et al. [1] which used a large cohort of hospitalized patients. In that study, Chertow and colleagues demonstrated a 4-fold adjusted increase in mortality risk even with this minimal increase in serum creatinine. This finding was confirmed in two subsequent large retrospective cohort studies [14, 15]. Hourly urine output measurements are part of both the RIFLE and AKIN classifications, primarily because changes in urine output often precede the corresponding rise in serum creatinine [16]. The minimum hourly urine output rate currently in use to define oliguria (less than 0.5 ml/kg/h) was based on clinical experience and animal models, not on clinical investigation. Moreover, the minimum duration of oliguria currently being used by the AKIN criteria (6 hours) was proposed by clinicians at the Amsterdam consensus conference and was not experimentally determined. Hence, despite that the creatinine threshold of 0.3 mg/dl was based on a formal investigation and that severity of illness scoring systems (e.g., SAPS, APACHE, etc.) provided a general framework, the AKIN criteria were largely derived by consensus.
Using an intensive care database with high resolution measurements of creatinine and urine output (namely, MIMIC-II [17]), we have previously analyzed the relationship between the three AKIN stages and mortality [18]. The high-resolution feature of MIMIC-II facilitates a complete empirical AKI study. Therefore, in the present study, we sought to empirically examine the relationships among serum creatinine, urine output, mortality, and renal replacement therapy, in a large cohort of critically ill patients. The relationships were investigated by continuously varying observation period and thresholds on creatinine increase and urine output. The primary objective of the present study was to provide useful clinical information that complements the AKIN criteria, which implement only a few thresholds and observation periods.
Methods
Data Source and Patient Cohort
The Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC-II) database (version 2.5) [17] is a National Institutes of Health funded, publicly available database of high-resolution, de-identified clinical data developed at Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC). Data were collected from more than 25,000 ICU patients at BIDMC, an urban, tertiary care university hospital located in Boston, Massachusetts, USA. In particular, MIMIC-II contains high resolution urine output measurements and serum creatinine lab test results from a diverse sample of critically ill patients, making it ideally suited for the present study.
We included data from the first ICU stay of all adult (15 years or older) patients who were admitted to BIDMC’s ICUs between 2001 and 2007. We excluded patients who had a total length of stay less than 24 hours, less than two serum creatinine measurements, or end stage renal disease (ESRD) on admission (identified based on ICD9, admission creatinine > 4 mg/dl, and text search; please see [18] for details). In addition, we excluded patients weighing more than 130 kg due to suspected data entry errors.
The MIMIC-II project was approved by the Institutional Review Boards of MIT and BIDMC, and granted a waiver of informed consent [17]. Oracle (Redwood Shores, CA, USA) SQL Developer version 3.1.07 was used for data extraction.
Study Variables and Outcomes
The primary predictor variable was a binary AKI indicator based on a given choice of a measurement threshold and an observation period. This binary predictor was given a value of 1 if the patient had at least one time window that met the threshold and observation period under investigation. The measurement thresholds were varied from 0.1 to 1.0 mg/dl with a 0.1 increment, from 125 to 400 % with a 25%-point increment, and from 0.1 to 1.0 ml/kg/h with a 0.1 increment for absolute creatinine increase, percentage creatinine increase, and urine output, respectively. The observation periods were varied from 1 to 7 days with a 1 day increment for both absolute and percentage creatinine increases, and from 2 to 48 hours with a 1 hour increment for urine output. In MIMIC-II, serum creatinine and urine output are usually recorded daily and hourly, respectively. For increases in creatinine, the maximum increase between two creatinine measurements separated by less than a given observation period was considered. For urine output, the sum of all urine output measurements within an observation period was divided by the duration of the observation period and the patient’s weight.
ICU nurses at BIDMC sometimes recorded urine output measurements for a period longer than one hour. To ensure adequate quality on urine output data, valid windows were required to have a measurement available for at least 50% of the number of hours in the window and have a maximum gap of two hours between subsequent measurements. We excluded windows which did not meet the above criteria.
The primary outcome variable was in-hospital mortality. As a secondary outcome, we also analyzed the receipt of renal replacement therapy (RRT) during the hospital admission (for acute and other reasons, but not due to ESRD), which was identified using Current Procedural Terminology (CPT) codes. Whereas mortality represents general severity of illness, RRT may be a more relevant end point from an AKI management standpoint.
The following confounding variables were included: age, gender, administration of vasoactive medications, mechanical ventilation, primary diagnosis by Diagnosis Related Group (DRG), service type (medical or surgical), Sequential Organ Failure Assessment (SOFA) [19] scores from the first ICU day, and Elixhauser comorbidities [20].
Statistical Analyses
A separate multivariate logistic regression model was built for each combination of creatinine/urine output threshold and observation period. A total of three analyses were conducted: absolute creatinine increase, percentage creatinine increase, and urine output. Since each analysis varied two parameters (threshold for creatinine increase or urine output, and observation period), formation of all possible threshold combinations resulted in the generation of multiple regression models: 70, 84, and 470 models for the absolute creatinine increase, percentage creatinine increase, and urine output analyses, respectively.
Two values were obtained from each regression model for analysis: adjusted mortality or RRT rate, and predictive accuracy. For each combination of threshold and observation period, the adjusted mortality or RRT rate was computed to be the median of the output values of the corresponding logistic regression model among the patients who were deemed to have AKI according to the chosen threshold and observation period. The adjusted mortality and RRT rates took into account the confounders, hence making it possible to compare different sets of thresholds. The mortality or RRT predictive power was quantified by computing the area under the receiver operating characteristic curve (AUC) of the corresponding regression model using 10-fold cross-validation [21].
We generated contour plots to visualize the dependence of adjusted mortality/RRT rate and AUC on creatinine, urine output, and observation period. In each plot, the independent variables were creatinine or urine output threshold and observation period, whereas the dependent variable was either adjusted mortality or RRT rate or AUC. The contour plots employed two-dimensional cubic spline interpolation on the fine-grids generated by the regression models. Furthermore, in order to examine the AKIN criteria, the cross-sections of the adjusted mortality or RRT contour plots at the AKIN observation periods (2 days for creatinine and 6, 12, and 24 hours for urine output) were plotted separately.
All analyses were conducted using MATLAB (Natick, MA, USA) version R2010b.
Results
The MIMIC-II database (version 2.5) contains the records of 26,576 patients of whom 19,742 were aged 15 years or older at the time of admission and included in the study. 1,305 patients (6.6%) who had ESRD prior to their ICU admission were excluded. An additional 1,210 patients (6.1%) were excluded due to insufficient creatinine measurements, length of stay less than 24 hours, or missing data for the generation of the logistic regression models. The final analytic cohort used for the creatinine analyses (absolute and percentage) consisted of 17,227 patients. Of these, 14,526 had sufficient data for the urine output analysis. For a detailed description of the cohort, please see the Electronic Supplemental Material.
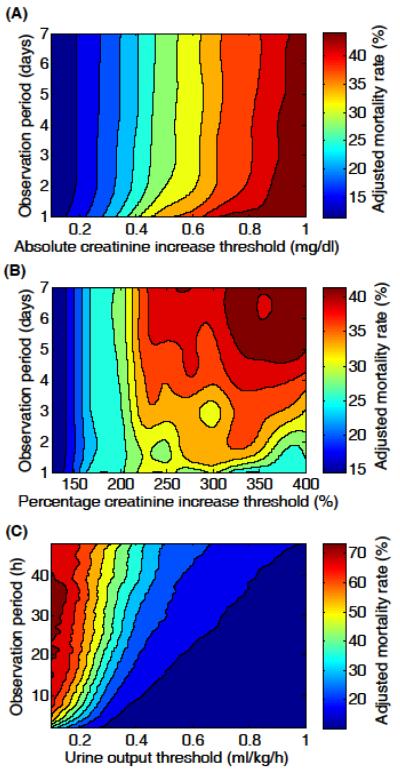
Figure 1(A) is the contour plot showing adjusted in-hospital mortality as a function of absolute creatinine increase and observation period. Regardless of observation period, mortality risk increased with greater absolute creatinine increases but especially rapidly when the observation period was less than two days. However, for observation periods longer than two days, mortality risk was independent of observation period.
Figure 1.
Adjusted in-hospital mortality rate contour plots for (A) absolute increase in serum creatinine (N=17,227), (B) percentage increase in serum creatinine (N=17,227), and (C) urine output (N=14,526). Adjusted mortalities were evaluated at a fine grid of observation periods and thresholds (steps of 1 day, 1 hour, 0.1 mg/dl, 25% points, and 0.1ml/kg/h), and the contour plots were produced using cubic spline interpolation.
Figure 1(B) illustrates adjusted in-hospital mortality as a function of percentage creatinine increase and observation period. When creatinine increases were below 200%, in-hospital mortality was independent of observation period. For creatinine increases greater than 200%, mortality risk increased with longer observation periods and larger percentage creatinine increases. The decrease in mortality when observation period was short and percentage increase was large (i.e., the bottom right corner of the contour plot) is likely an artifact due to a very small number patients meeting the AKI criteria (<100).
Figure 1(C) describes adjusted in-hospital mortality as a function of urine output and observation period. When urine output was less than 0.5 ml/kg/h, mortality rate increased rapidly as urine output decreased. However, when urine output was greater than 0.5 ml/kg/h, there was a minimal reduction in mortality rate as urine output increased. Furthermore, for urine outputs less than 0.3 ml/kg/h and observation periods shorter than 5 hours, mortality risk was especially sensitive to the degree of oliguria and longer observation time. Mortality risk became independent of the duration of oliguria for observation periods longer than approximately 24 hours (i.e., the upper half of the contour plot).
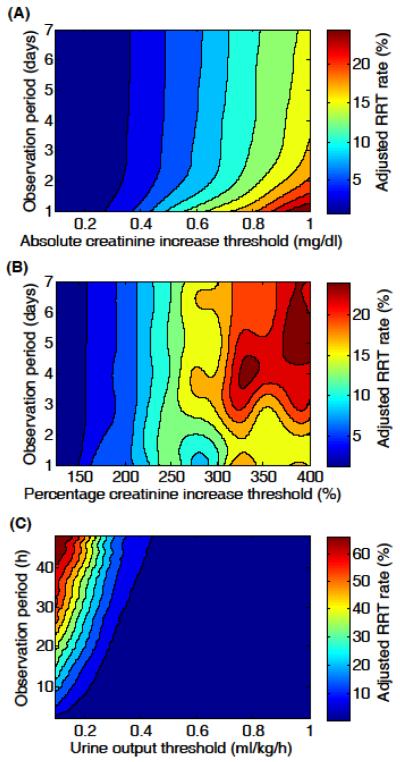
The three contour plots in Figure 2 depict adjusted RRT rate as a function of creatinine increase or urine output, and observation period. The patterns in Figure 2 are similar to those in Figure 1, suggesting that in-hospital mortality and receipt of RRT were closely related.
Figure 2.
Adjusted renal replacement therapy rate contour plots for (A) absolute increase in serum creatinine (N=17,227), (B) percentage increase in serum creatinine (N=17,227), and (C) urine output (N=14,526). Adjusted mortalities were evaluated at a fine grid of observation periods and thresholds (steps of 1 day, 1 hour, 0.1 mg/dl, 25% points, and 0.1ml/kg/h), and the contour plots were produced using cubic spline interpolation.
Please see the Electronic Supplemental Material for the cross-sections of Figure 1 and 2 at the AKIN criteria, as well as for the AUC contour plots for in-hospital mortality and RRT predictions.
Discussion
Using a large cohort of unselected critically ill patients, we aimed to investigate the empirical relationships among serum creatinine, urine output, observation period, in-hospital mortality, and RRT. The primary contribution of the present study is the contour plots shown in Figure 1 and 2. Whereas the criteria for the three AKIN stages (Table E1) are simply points on the contour plots (except some of the AKI 3 criteria such as need for RRT), Figure 1 and 2 provide physicians with more complete pictures of AKI with respect to in-hospital mortality risk and anticipated need for RRT. In general, the severity of AKI was high when 1) absolute creatinine increase was high regardless of observation period, 2) percentage creatinine increase was high and observation period was long, and 3) oliguria was sustained for a long period of time. Similar contour patterns emerged for both in-hospital mortality and RRT rates. In addition, Figure E1 and E2 present further insight into the AKIN criteria by focusing on the observation periods specified by AKIN.
One can argue that clinicians can utilize Figure 1 and 2 as nomograms for their own patients. Improved risk prediction may lead to improved AKI management in terms of medication adjustment and early initiation of appropriate preventative procedures. However, one major concern in this regard is the fact that Figure 1 and 2 were generated from a single-center database, which may limit the universal validity of the contour plots. Since it is unlikely that high-resolution urine output and creatinine measurements would be available in any multi-center database, a logical step is to generate knowledge from an appropriate database such as MIMIC-II and validate the results in a multi-center data set. Hence, we strongly advocate a multi-center study to follow the present study.
Furthermore, the present study simplified the AKI investigation by analyzing only one of the three variables (absolute and percentage creatinine increases, and urine output) at a time. It would be of great interest and importance to investigate the synergistic predictive performance arising from a combination of two or more variables. Such a joint analysis is worthy of future research.
The AUCs of all regression models were above the generally accepted value of 0.7 (see the Electronic Supplemental Material). Although the variability in AUC was small in Figure E3 and E4, the combinations of threshold and observation period associated with the highest AUCs can be useful in maximizing predictive accuracy. It is worthwhile to note that urine output slightly outperformed creatinine in mortality prediction (Figure E3), which corroborates our findings in a previous study [18]. From a clinical standpoint, urine output features several advantages over creatinine such as an earlier indication of deterioration in renal function and no need for blood draws. On the other hand, in RRT prediction, absolute increase in creatinine resulted in the highest median AUC (Figure E4).
This study has a number of limitations. First, the database contains data from a period of 7 years (2001-2007), during which there were changes in management of the critically ill (e.g. early goal directed therapy) and therefore possibly in patients outcome. Because the MIMIC II database is completely de-identified, we were unable to segment patients temporally. Second, we excluded patients with insufficient measurement data. The missing data were most likely data entry errors and not a result of deliberate clinical judgment. We treated them analytically as randomly distributed within the population. Third, we used AUC as a measure of predictive performance. While AUC is a good measure of discrimination, it assumes equal weights for sensitivity and specificity. In different clinical contexts, sensitivity may be more important than specificity and vice-versa. Fourth, because MIMIC-II contains urine output data only from ICU stays, we were unable to capture any AKI episodes that may have occurred prior to ICU admission. Finally, although our study included data from more than 17,000 patients and had strong statistical power, it was still a retrospective analysis with its characteristic limitations.
Conclusions
Using a massive high-resolution ICU database, we generated empirical relationships among serum creatinine increase, urine output, observation period, in-hospital mortality, and need for RRT. The results of the present study complement and extend upon the existing AKIN criteria. These results better reflect the complexity of the relationships between urine output, creatanine elevation and renal failure than do the existing criteria. A multi-center study is needed to confirm the external validity and clinical usefulness of the results presented in this article.
Supplementary Material
Acknowledgements
This research was supported in part by grant R01 EB001659 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH). Dr. Joon Lee also holds a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Support: This work was supported in part by NIH Grant No. R01-EB001659.
Footnotes
Disclosures: None of the authors have any financial interests or potential conflicts to disclose
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Barrantes F, Tian J, Vazquez R, Amoateng-Adjepong Y, Manthous CA. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 3.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostermann M, Chang R, Riyadh ICU Program Users Group Correlation between the AKI classification and outcome. Crit Care. 2008;12:R144. doi: 10.1186/cc7123. doi: 10.1186/cc7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–514. doi: 10.1097/00075198-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, Krumholz HM. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 10.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 11.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, Metnitz PG. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 13.Prowle JR, Liu YL, Licari E, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011;15:R172. doi: 10.1186/cc10318. doi: 10.1186/cc10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 15.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–37. doi: 10.1007/s00134-003-2078-3. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 17.Saeed M, Villarroel M, Reisner AT, et al. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Crit Care Med. 2011;39:952–960. doi: 10.1097/CCM.0b013e31820a92c6. doi: 10.1097/CCM.0b013e31820a92c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011;39:2659–2664. doi: 10.1097/CCM.0b013e3182281f1b. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Picard RR, Cook RD. Cross-Validation of Regression Models. J Am Stat Assoc. 1984;79:575–583. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.