Abstract
Background
The human personality trait of sensation seeking (SS) indicates an attraction to novel sensations and experiences, and is associated with greater likelihood of drug abuse. In rodents, locomotor activity in a novel environment (Loco) has been found to predict drug self-administration (SA), and has been hypothesized to be a translational model of human SS. Previously, we reported (Gancarz et al., 2011 [12]) that high responder (HR) animals responded more than low responder (LR) animals to produce a response contingent light onset. The primary goal of this paper was a detailed analysis of the association between Loco and light contingent responding in a large sample of rats (n = 93).
Methods
Male rats were pre-exposed to dark operant test chambers for ten 30 min sessions and baseline levels of responding (snout poking) were determined. The pre-exposure phase was followed by 6 sessions during which active responding produced a visual sensory reinforcer (VSR; 5 s light onset) according to a variable interval 1 min schedule of reinforcement. After completion of the VSR phase, Loco was tested.
Results
The activating effects (total responding) of light were associated with Loco, but the response guiding effects (proportion of active responding) of the light were not. In addition, HR rats habituated more slowly in both the VSR and Loco tests than LR rats.
Conclusions
These data indicate that VSR measures aspects of the rodent’s response to novel sensations and experiences that are not detected by Loco. These data provide some evidence for the use of light reinforcement as an animal model of SS.
Keywords: Operant conditioning, Drug abuse, Rat, Self-administration, Visual stimuli, Individual differences
1. Introduction
Individual differences in the initial locomotor response to an novel locomotor chamber (Loco) is predictive of self-administration (SA) of cocaine [1,2], amphetamine [3,4], ethanol [5], morphine [6] and nicotine [7]. In these experiments, rats with high levels of locomotor activity in a novel environment are identified as high responders (HRs) and SA more drug than rats with low levels of locomotor activity, identified as low responders (LRs). This model has been suggested to be an animal model of sensation seeking (SS) [8,9]. The activity measures obtained using these procedures are referred to in the literature as the animal’s “response to novelty”.
Animals identified as HR have been reported to differ from LR on a number of behavioral tasks other than acquisition of drug SA. HR rats have greater preference for the novel arms of a Y-maze compared to LR rats [8] and spend more time in novel arms of a radial arm maze [9]. In a dark-light emergence test, where animals are familiarized to a dark compartment and a guillotine door is removed exposing a novel light compartment, HR rats visited the novel illuminated compartment more rapidly and more frequently in the initial exposure period than LR rats [8]. These differences between HR and LR rats in maze exploration and dark emergence tests only occurred when the compartment was novel. Taken together, these results suggest HR rats have a greater sensitivity to novel environmental stimuli, but that differences between HR and LR rats disappear when the environment becomes familiar. This is of interest, because similar observations have been reported in humans. For example, human subjects identified as high SS have been reported to have a greater response to novel environmental stimuli but habituated to the levels of low SS with repeated presentations of the stimulus [10]. Differential performance on a variety of different tasks suggests broad sensitivity to novel stimuli and supports the hypothesis that Loco measures an important underlying trait which may be analogous to SS in humans [11].
We have previously reported that locomotor response to novelty predicts both responding for a visual sensory reinforcer (VSR) and acquisition of methamphetamine (METH) SA [12]. The primary goal of this study was to perform a detailed analysis of the association between Loco and light contingent responding in a large sample of rats. In addition to comparing overall levels of activation, we also compared the rates of within-session habitation. The results of the present study are of particular importance when considering the use of HR and LR classification as an animal model of SS.
We hypothesize that both Loco and operant responding to produce light onset involve sensory reinforcement, as Loco and VSR have been shown to have similar characteristics. Previous reviews of sensory reinforcement have classified both exploration of a novel environment and operant responding to produce light onset as investigatory behaviors [11,13,14]. In agreement with previous studies, we have found that the highest levels of activation occur at the beginning of exposure to novel stimulation, followed by both between- and within-session decreases in responding with repeated testing for both Loco and VSR [12,15]. In addition, both Loco and VSR responding are increased by non-contingent systemic injections of psychomotor stimulants [12,15–23]. These commonalities suggest locomotor activation elicited by a novel test chamber and responding to produce a novel sensory stimulus may be mediated by common underlying behavioral and neurophysiological processes.
2. Methods
2.1. Subjects
Ninety-three Male Holtzman Sprague Dawley rats were used in this experiment. Subjects weighed approximately 250 g at the start of the experiment and were housed in pairs in plastic cages (42.5 cm × 22.5 cm × 19.25 cm). Lights were on in the colony room from 6:00 pm to 8:00 am. All behavioral testing occurred 7 days/week during the dark phase of the light/dark cycle. Food (Harlan Teklad Laboratory Diet #8604, Harlan Inc., Indianapolis, IN) was continuously available. Access to water was restricted to 20 min following daily testing. Animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals and the experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at of the University at Buffalo, The State University of New York.
2.2. Apparatus
2.2.1. VSR apparatus
Twenty-four locally constructed experimental chambers were used. The chambers had stainless-steel grid floors, aluminum front and back walls, Plexiglas sides, and a Plexiglas top. The test chambers were 20 cm × 22 cm × 23 cm (inside dimensions). The right side wall served as the test panel. The test panel had two snout poke holes (4 cm diameter) located on either side of a centrally located smaller snout-poke hole (2.5 cm diameter). The larger snout poke holes were centered 2.5 cm above the floor and 5 cm from the closest side wall of the chamber. Stimulus lights were mounted above the two large snout poke holes and the smaller center snout-poke hole. The center stimulus light was positioned directly above the center snout-poke hole, 7 cm from the floor and 11.5 cm from the side walls. The stimulus lights for the larger snout poke holes were positioned 5.5 cm above the floor of the chamber and 2 cm from the closest side wall. Snout pokes and head entries into the snout poke holes were monitored with infrared detectors located 0.5 cm behind the front panel. A second stimulus light was located in the middle of the back wall of the test chamber. The front center light was equipped with a 28-V light bulb (SPC Technology, Model # 1819), white lens cap (Dialight, Model # 081-0135-303). The back house-light is equipped with a 28-V light bulb (SPC Technology, Model # 1864), covered by a clear lens cap (Dialight, Model # 081-0135-303). Snout pokes and head entries were monitored with infrared detectors. The entire apparatus was computer controlled through a MED Associates interface with MED-PC (version 4). The temporal resolution of the system was 0.01 s.
2.2.2. Locomotor chamber apparatus
Locomotor activity was recorded by an infrared motion-sensor system (Hamilton-Kinder) fitted outside a standard plastic cage tub (42.5 cm × 22.5 cm × 19.25 cm). The tubs used for locomotor testing did not have shavings in them and clean tubs were used for each test session. Each locomotor chamber was located in a sound and light attenuating enclosure. Each enclosure was equipped with a wall mounted fan that provided masking noise and was illuminated by an 8 W light bulb (light output 450 lm). This arrangement provided a novel environment for locomotor testing. Two levels of infrared motion sensors were set at 5.5 (for recording horizontal movement) and 15.5 cm above the cage floor (for recording vertical movements). The sensors at the lower level consist of eight pairs along the long axis and five pairs along the short axis each spaced 5.5 cm apart and were used to determine the position of the animal. The sensors at the upper level were spaced 5.5 cm apart along the short axis and recorded vertical rearing movements. The activity-monitoring system monitors each of the beams at a frequency of 0.01 s to determine whether the beams were interrupted. The interruption of any beam not interrupted during the previous sample was interpreted as an activity score.
2.3. Procedure
2.3.1. Overall timeline
As is outlined in Table 1, operant responding for light onset was tested first and locomotor response to novelty was tested second.
Table 1.
Overall timeline of behavioral testing.
| Phase | Days |
|---|---|
| Light reinforcement testing | |
| Pre-exposure to operant chamber | 1–10 |
| Response contingent light onset | 11–17 |
| Locomotor response to novelty | 18 |
2.3.2. Pre-exposure phase
Animals were placed in dark operant chambers for a 10-day pre-exposure period. Daily sessions were 30 min in duration, during which snout pokes to either side were recorded, but resulted in no programmed consequences. This phase was conducted in order to (i) familiarize/pre-expose the rat to the operant chamber, (ii) measure operant level of responding, and (iii) determine the preferences of individual rats for the left and right snout poke holes. The information about preference was used to counterbalance the side assigned to be active during the VSR phase.
2.3.3. VSR phase
Animals were placed in operant chambers for six 30 min test sessions. The test chambers were dark during testing except when a response contingent visual stimulus (VS) was presented. The VS consisted of the onset of two stimulus lights. One of the lights was located on the front control panel above and midway between the two snout poke holes, and the other light was located in the middle of the back wall of the test chamber. Onset of the VS combination produced a total of 43 lx light as measured from the side snout poke hole of the test chamber. Snout pokes into the active alternative resulted in illumination of the stimulus light for 5 s according to a variable interval (VI) 1 min schedule of reinforcement. Each snout poke response was operationally defined as interruption of the infrared beam; additional responses were not recorded until the infrared beam was restored and re-interrupted. Snout pokes to the inactive alternative had no programmed consequences. The active alternative was determined by counterbalancing the operant level of snout pokes during the pre-exposure phase, so that for half of the rats, the active snout poke hole was the preferred hole during the pre-exposure phase.
The VI 1 min schedule was produced by selecting without replacement from a list of 20 intervals with a mean of 1 min generated using a Fleshler–Hoffman progression [24]. The first response after the interval elapsed resulted in presentation of the visual stimulus and the selection of the next VI value from the list. Animals were tested 7 days/week for sixteen sessions (10 pre-exposure sessions and 6 response contingent light sessions) during the VSR phase of the experiment.
2.3.4. Locomotor activity in a novel environment
Loco testing occurred the day following the last day of VSR testing. Subjects were placed into activity monitors for 60 min. Total number of horizontal beam breaks, total number of vertical beam breaks, and basic movements, (defined as the total number of horizontal and vertical beam breaks) during the 60 min test period were used as the dependent measures. Loco tests vary in duration from 5 to 10 min [25], 30 min [26–28], 1 h [2] to 2 h [29,30] in the literature. We have evaluated both 1 and 2 h Loco durations, and the associations with Loco and VSR were very similar across both durations (unpublished observations), and therefore the 60 min duration was used for the analysis.
2.4. Data analysis
2.4.1. Dependent variables
The primary measure of Loco was basic movements. The primary dependent measures for VSR were total responding (active + inactive responding) and the relative frequency of active responding. The total responses measure indicates the increasing and/or possible decreasing effects of the light on responding (or activating effects of light onset). The relative frequency of active responses (defined as: active responses/total responses) is a measure of preference (or “guiding effects of light onset”) for the response alternative that produced the visual stimulus. The relative frequency indicates the proportion of snout pokes into the side that produced response contingent light onset. We have previously discussed the rationale for the use of relative frequency of active responses as a dependent measure for VSR [12,15] (see Section 4).
2.4.2. Data analysis
The data were analyzed using two different approaches: In the first data analysis approach, we determined correlation coefficients between: (i) Loco and VSR using Pearson’s correlations tests. The two-tail alpha level used to identify significant association was p < 0.05.
The second analysis used an ‘extreme groups’ approach in which animals were divided into groups that differed with respect to Loco using one-third splits. In this analysis, animals that scored in the highest third on Loco were determined as HR (n = 30) and animals that scored in the lowest third on Loco were determined as LR (n = 30). Animals in the middle third were not used for this ‘extreme groups’ analysis. This division was made to produce distinct populations of animals based on Loco. We [12], and others [1,31,32], have previously used an extreme group approach to study Loco as a predictor of drug SA. A two-factor mixed analysis of variance (ANOVA) with one factor being the grouping variable (HR or LR) and the other factor being days of acquisition were used for repeated measure comparisons in acquisition of VSR. Post hoc t-tests with Bonferroni corrections were conducted to elaborate upon significant F-tests. For all statistical tests an alpha criterion of p = 0.0083 (calculated as alpha level/number of time points = (0.05)/6) was used.
2.4.3. Within-session performance of HR and LR groups
In order to examine habituation in the Loco and VSR tasks, the within-session pattern of responding was analyzed. For Loco, data from the 1 h test session was divided into six 10 min epochs (i.e., 0–10 min, 10–20 min, etc.). For VSR, the data from the 30 min session was similarly divided in to six 5 min epochs. Each epoch was the number of locomotor counts (or snout pokes) that occurred in the specified epoch. These data were analyzed using a using a mixed two-factor repeated measures ANOVA with group (HR or LR) as the independent variable and epoch as the repeated measure.
The higher initial levels of Loco and VSR responding in the HR rats made it difficult to compare the rates of habituation between the HR and LR. In order to control for differences in the absolute rate of responding, a second analysis of the within-session data was done on the proportion of total responding that occurred in each epoch. In this analysis, the number of activity counts (or responses) that occurred in each epoch was divided by the total activity counts (or responses) that occurred in the test session. Since the proportions must sum to 1.0, this analysis describes how the animals distributed their responding across the test session independently of the absolute rates of responding allowing a direct comparison of the rates of habituation between HR and LR. These data were analyzed using a using a mixed two-factor repeated measures ANOVA with group (HR or LR) as the independent variable and epoch as the repeated measure. Post hoc t-tests with Bonferroni corrections were conducted to elaborate upon significant F-tests. For all statistical tests, an alpha criterion of p = 0.0083 (calculated as alpha level/number of time points = (0.05)/6) was used.
3. Results
3.1. VSR performance in all animals
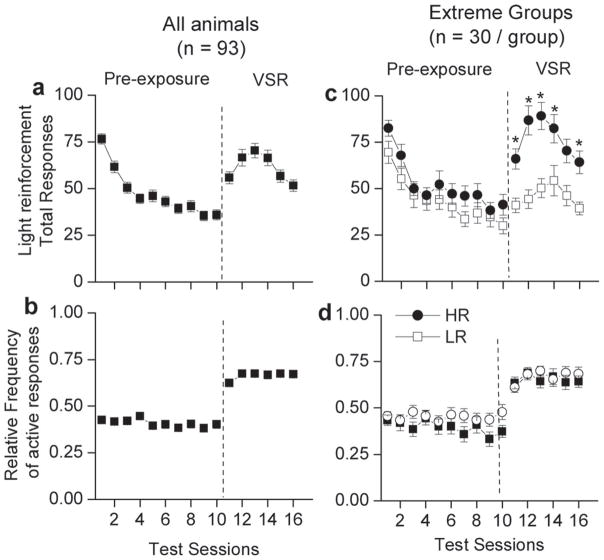
Fig. 1a shows total responding during the pre-exposure and VSR phases. Total responses were significantly greater on Day 1 of pre-exposure compared to total responses on Day 10 of pre-exposure testing [t(92) = 12.701, p < 0.01]. This comparison indicates that the operant level of responding decreased across days during the pre-exposure phase. A second within-subject t-test indicates that there was a significant increase in responding between the average of Days 9 and 10 of habitation and the average of Days 1–6 of VSR testing [t(92) = −9.936, p < 0.05] indicating that introduction of the response contingent light stimulus had activating effects on snout poke responding (Fig. 1a).
Fig. 1.
Performance across all days of VSR testing. In each plot, performance during the pre-exposure phase is shown to the left of the dashed line, and performance in the VSR phase is shown to the right of the dashed line. Data are averages (±SEM) across all 16 test sessions. The left column (a and b) shows performance of all animals (n = 93) tested on VSR. The right column (c and d) shows performance in animals identified as high (HR) and low (LR) locomotor responders during VSR testing. The closed circles are animals identified as HR responders (n = 30), the open squares are animals identified as LR responders (n = 30). (a) This plot shows that there was a decline in responding during pre-exposure and an increase in responding during the VSR phase. VSR responding increases from session 11 to 14 of testing with peak performance occurring on the third test session of VSR (test session 14). (b) This plot shows relative frequency of active responding across pre-exposure and VSR phases. The response contingent light increased the relative frequency of active responding. (c) This plot illustrates total responses across pre-exposure and VSR phases for HR and LR rats. There is no difference in total responses between HR and LR rats during pre-exposure. HR rats have significantly greater total responses across all days of VSR testing. (d) This plot illustrates relative frequency to the active alternative across pre-exposure and VSR phases for HR and LR rats. There were no differences in relative frequency to the active alternative in HR and LR rats for pre-exposure or light reinforced phases. (*) indicates a significant difference between HR and LR rats at p < 0.0083.
A one-way ANOVA on total responses revealed a significant change in total responses across days of VSR testing [F(5, 460) = 10.561, p < 0.05]. Follow-up paired samples t-tests revealed a significant difference between Days 1 and 3 [t(92) = −4.608, p < 0.05] and between Days 3 and 6 of VSR testing [t(92) = 6.364, p < 0.05]. This pattern of results reveals that total responses increased from Days 1 to 3 of VSR and then decreased from Days 3 to 6. The peak in total responding occurred on Day 3 of testing.
Fig. 1b shows the relative frequency of active responding during the pre-exposure and VSR phases. A within-subject t-test comparing the average of Days 9 and 10 of pre-exposure with the average of Days 1–6 of VSR revealed a significant increase in relative frequency of active responding during VSR testing [t(92) = −11.051, p < 0.05]. These data indicate that the rats responded more to the active alternative that produced the visual stimulus onset. A one-way ANOVA for relative frequency of active responding revealed a significant change in relative frequency of active responding across the 6 sessions of VSR testing [F(5, 460) = 3.001, p < 0.05]. Follow-up paired t-tests revealed a significant difference between Days 1 and 3 of VSR testing [t(92) = −3.493, p < 0.05]. No significant difference was detected between Days 3 and 6 of VSR testing.
Fig. 1a shows that introduction of response contingent light onset caused an increase in total responding, while Fig. 1b shows that the relative frequency of active responding increased from below 50% during pre-exposure (the non-preferred side was made active) to approximately 70% during the VSR phase. Taken together, the increase in total responding and the increase in the proportion of active responses indicate that the active responding was increased more than inactive responding indicating that the visual stimulus was a reinforcer. If the active alternative was not differentially increased, and both active and inactive responding were effected to the same degree, then the relative frequency of active responding would have remained unchanged while the total responding was increased.
Furthermore, changes in the pattern of the relative frequency of active responding differed from the pattern of changes observed for total responding. The relative frequency of active responding increased across Days 1–3 of testing and then remained high. In contrast, total responding increased across Days 1–3 of testing and then decreased indicating a separation between total responding and the relative frequency of active responding as measures of VSR.
3.2. Association between locomotor response to novelty and VSR
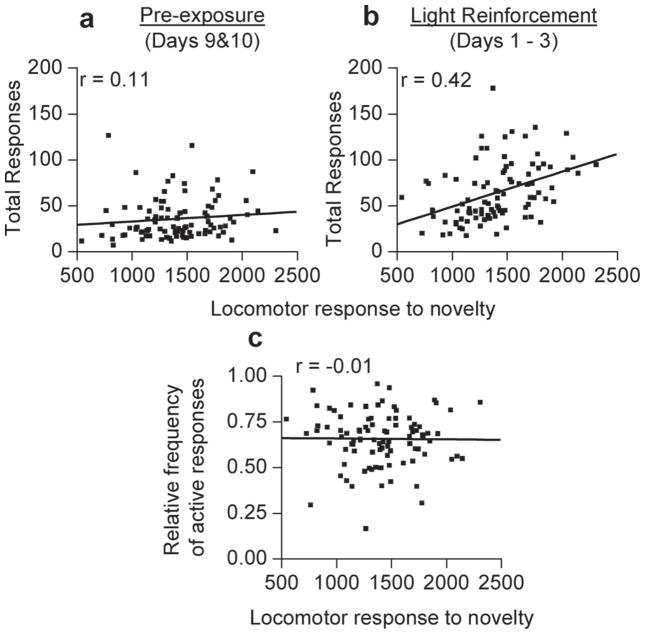
Loco was compared to the average of Days 1–3 of VSR testing because Days 1–3 encompassed the ‘acquisition’ period with increases in responding peaking at Day 3 of testing (see Fig. 1a). No correlation was observed between Loco (Number of Beam Breaks: Mean (M) = 1399.24 ± 36.27, SD = 347.93) and number of responses emitted during Days 9 and 10 of pre-exposure (M = 35.66 ± 2.35485, SD = 22.58; r = 0.11, p > 0.05, Fig. 2a). In contrast, Loco predicted the total responses emitted (M = 64.26 ± 3.345, SD = 32.09; r = 0.42, p < 0.01, Fig. 2b) during the VSR phase. There was no correlation between Loco and relative frequency of active responding (M = 0.66 ± 0.01, SD = 0.146; r = −0.01, p > 0.05, Fig. 2c).
Fig. 2.
Scatterplots showing associations between Loco and total responses of pre-exposure and VSR testing in all animals (n = 93). (a and b) Locomotor scores are represented on the X-axis, and total responding during pre-exposure or VSR is represented on the Y-axis. (a) A scatterplot of locomotor movements in 1 h exposure to novel environment and total responses made during last 2 days of pre-exposure testing (i.e., dark chamber with no programmed consequences for snout pokes). There is no association between pre-exposure and Loco. (b) A scatterplot of locomotor movements in 1 h exposure to novel environment and total responses made during the first 3 days of VSR testing. There is a significant positive association between Loco and performance on VSR testing. (c) A scatterplot of locomotor movements in 1 h exposure to novel environment and relative frequency of active responses made during the first 3 days of VSR testing. There is no association between pre-exposure and Loco.
3.3. Extreme locomotor groups performance on VSR
3.3.1. Pre-exposure
There was a significant main effect of test session [F(9, 522) = 20.926, p < 0.05] on total responses during pre-exposure. There was no significant effect of group or of the interaction of group and time. Total responding during the pre-exposure phase, when there were no programmed consequences to either hole, was not different between animals identified as HR and LR rats (Fig. 1c). An a priori t-test was conducted on total responses during Day 1 of pre-exposure (when the experimental chamber was novel) and there is a non-significant trend (p = 0.07) for HR rats to have greater total responses than LR rats. There were no significant effects of time or group during pre-exposure for relative frequency of responding to the alternative that would be designated as active during the VSR phase. HR and LR rats were also not different in allocation of responses to the snout poke hole that would be designated as the active alternative during the VSR phase (Fig. 1d). Taken together, these results show that HR and LR groups were not different in basal levels of responding in the operant chamber during pre-exposure testing. The absence of a difference in snout poke responses between HR and LR rats on the first day of the pre-exposure phase indicates that exploring the snout poke hole is only one aspect of the experimental chamber during the first day of pre-exposure, when the entire experimental chamber is novel. HR rats may be directing their behavior towards different aspects of the experimental chamber that do not result in differences in total recorded snout poke responses.
3.3.2. VSR
There was a significant main effect of test session [F(5, 290) = 7.100, p < 0.05] on total responses during the VSR phase. There was also a significant main effect of Loco group on total responses [F(1, 58) = 20.858, p < 0.05]. The interaction between test session and Loco group was not significant. Follow-up t-tests revealed that HR rats had significantly greater total responses than LR rats on Days 1 [t(58) = −3.823, p < 0.0083], 2 [t(58) = −4.639, p < 0.0083], 3 [t(58) = −4.462, p < 0.0083], 5 [t(58) = −2.957, p < 0.05], and 6 [t(58) = −3.604, p < 0.05]. On Day 4 of testing, there was a non-significant trend that HR rats had significantly greater total responses than LR rats [t(58) = −2.559, p = 0.013]. This result indicates HR rats were more activated than LR rats by the response contingent light (Fig. 1c).
There was a trend for a main effect of test sessions on relative frequency of responding to the active alternative [F(5, 290) = 2.038, p = 0.073]. There were no other significant effects of relative frequency. These results show that there were no differences between HR and LR groups on preference for the visual stimulus reinforcer (Fig. 1d).
3.4. Extreme locomotor groups within-session performance on Loco and VSR
3.4.1. Within-session Loco performance
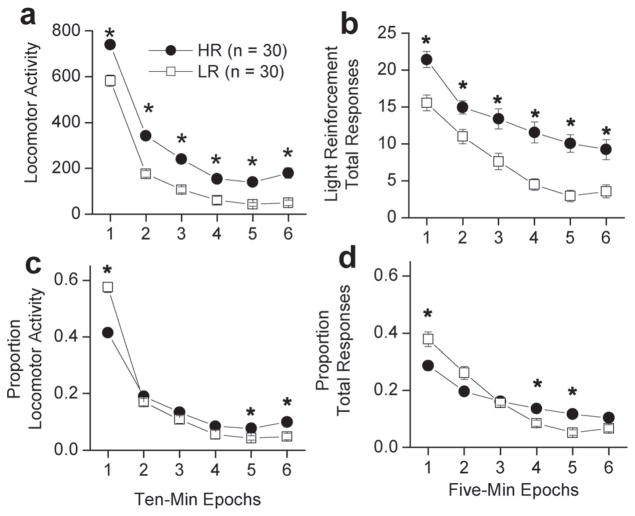
An analysis of the within-session pattern of locomotor activity was performed for the HR and LR groups (Fig. 3a). There was a significant main effect of epoch [F(5, 290) = 327.79, p < 0.05] and Loco group [F(1, 58) = 280.70, p < 0.05]. No interactions between epoch and Loco group were observed. Follow-up t-tests revealed HR rats had greater locomotor activity at epochs 1 [t(58) = −5.45, p < 0.0083], 2 [t(58) = −8.15, p < 0.0083], 3 [t(58) = −6.51, p < 0.0083], 4 [t(58) = −3.70, p < 0.0083], 5 [t(58) = −5.28, p < 0.0083], and 6 [t(58) = −5.25, p < 0.0083]. These data indicate HR rats had greater locomotor activity compared to LR rats across the entire test session.
Fig. 3.
Within-session performance during Loco and VSR testing in HR and LR rats. The closed circles are animals identified as HR rats (n = 30), the open squares are animals identified as LR rats (n = 30). (a) Data are within-session locomotor activity in 10 min epochs. HR rats have significantly greater locomotor activity at each 10 min epoch. (b) Data are within-session total responding in 6 min epochs. Data are mean (±SEM) total responses from Days 1 to 3 of VSR testing. HR rats have significantly more responses at each 6 min epoch. (c) Data are the mean (±SEM) of the proportion of locomotor activity in each 10 min epoch. (d) Data are the mean (±SEM) of the proportion of total responses in each five min epoch. (*) indicates a significant difference in between HR and LR rats at p < 0.0083.
In order to compare the within-session decline in locomotor activity for HR and LR groups, the within-session declines in responding shown in Fig. 3a were plotted as a proportion of total locomotor activity that occurred in each of the six 10 min epochs (Fig. 3c). There was an interaction between epoch and Loco group [F(5, 290) = 20.16, p < 0.05]. HR responder rats had a smaller proportion of activity counts at epoch 1 [t(58) = 8.135, p < 0.0083], but greater proportion of activity counts at epochs 5 [t(58) = −2.88, p < 0.0083] and 6 [t(58) = −3.18, p < 0.0083]. These data indicate the HR rats distributed their activity more evenly across the session, and habituated more slowly to the novel locomotor chamber than LR counterparts.
3.4.2. Within-session VSR performance
An analysis of the within-session pattern of total VSR responses (Days 1–3) parallel to the one performed for locomotor activity for the HR and LR groups (Fig. 3b) was carried out. There was a significant main effect of epoch [F(5, 290) = 63.56, p < 0.05] and Loco group [F(1, 58) = 29.74, p < 0.05]. The interaction between epoch and Loco group was not significant. Follow-up t-tests revealed HR had greater total responding for light onset at epochs 1 [t(58) = 3.91, p < 0.0083], 2 [t(58) = 3.00, p < 0.0083], 3 [t(58) = 3.33, p < 0.0083], 4 [t(58) = 4.47, p < 0.0083], 5 [t(58) = 5.10, p < 0.0083], and 6 [t(58) = 3.61, p < 0.0083]. These data indicate HR rats had greater total light responses compared to LR rats across the entire test session.
In order to compare the within-session decline in VSR responding for HR and LR groups, the within-session declines in responding were calculated as a proportion of total responding as was described above for locomotor activity, except that the data were plotted as 5 min rather than 10 min epochs. There was an interaction between epoch and Loco group [F(5, 290) = 8.06, p < 0.05]. HR rats had a smaller proportion of total responses at epochs 1 [t(58) = −3.11, p < 0.0083] and a trend at epoch 2 [t(58) = −2.64, p = 0.012], but a greater proportion of total responses at epochs 4 [t(58) = 3.16, p < 0.0083], 5 [t(58) = 4.40, p < 0.0083] and a trend at epoch 6 [t(58) = 2.34, p = 0.023]. The results were similar to those obtained for locomotor activity. The results indicate the HR rats distributed responding more evenly across the session and habituated more slowly to the response contingent light than LR rats (Fig. 3d).
4. Discussion
The results indicate that there were consistent individual differences in reactivity to sources of novel stimulation. Rats identified as HRs in the locomotor test demonstrated greater reactivity in both the locomotor and light reinforcement tests compared to rats identified as LRs (Figs. 1c and 2b). HR rats also demonstrated slower habituation of locomotor activity and operant responding for light onset than LR rats (Fig. 3c and d). Presumably, decreases in activity in the Loco procedure and operant responding for light onset in the VSR reflect decreases in novelty as novel stimuli become familiar. These results support the hypothesis that locomotor activity in a novel environment and operant responding for light onset share some common behavioral and neural processes.
The results of the current study replicate and extend the results of a previous study with a smaller number of subjects [12]. As is shown in Table 2, there were a number of methodological differences between the previously reported Gancarz et al. study and the current experiment: (i) number rats tested, (ii) strain of rat tested, (iii) testing time of day, (iv) order of testing, (v) number of pre-exposure sessions, and (iv) water restriction. Replication of the major findings of the previous study despite many methodological differences supports the robustness of the relationship between Loco and VSR.
Table 2.
Comparison of methodological differences of current study with previous Gancarz et al. [12,15] study.
An important feature of the current manuscript is the use of total responses and relative frequency of active responding as the dependent measures of VSR performance. The use of these two measures allows for a differentiation to be made between overall changes in responding induced by the novel stimulus and preference for the novel stimulus. For example, an animal may increase from a baseline of 100 active and 10 inactive responses to 200 active and 20 inactive responses. This represents a substantial increase in the absolute rate of active responding. However, the relative frequency of active responding (active/total) is .91 in both cases, indicating that there was no selective increase in active responding (preference). Rather, both active and inactive responding was increased in proportionally equivalent manner. This example illustrates the importance of using total responses and relative frequency of active responses as dependent measures in order to differentiate between the response activating (total responding) and response guiding (relative frequency of active responding) effects.
4.1. Association between locomotor response to novelty and visual stimulus reinforcement
The difference between animals in the HR and LR groups cannot be explained by differences in general basal activity. In this large sample, there were no significant correlations between Loco and total responses during the last two sessions of the 10-day session pre-exposure period of the VSR test. The association found between Loco and VSR reflects individual differences in each animal’s reaction to a source of novel stimulation since the association between snout poking and Loco emerged only following introduction of the novel response contingent light.
Both locomotor response in a novel environment and VSR are investigatory behaviors in rats. One of the initial studies of operant responding for a visual reinforcer was an effort by Girdner [33], to arrange a situation in which an organism could make a precisely measured operant response that would produce novel environmental change [see, 34 p. 111]. Girdner considered the response measured in this situation as being analogous to locomotor and orienting investigatory responses which expose organisms to novel environmental change. Tapp [13] has pointed out that the activity in novel environments is directed at specific aspects of the environment which evoked similar stereotyped response patterns across rats. An advantage of the VSR procedure for measuring investigatory behavior over Loco is that the source of novel stimulation (light onset) is precisely identified. In accord with Tapp, the view point of Girdner described above, and others [14,35–39], we have suggested [15] that stimulus-directed behaviors which create the general activity measured in a novel locomotor chamber may, in part, reflect responses reinforced by novel sensory stimulation.
Although locomotor activity in a novel environment and operant responding for light onset may share some common behavioral and neural processes, the association between them is only partial, suggesting that there are other behavioral and neural processes involved which the two tests do not share in common. For example, an unexpected result of this study was that while total VSR responding was correlated with Loco, preference for the active alternative (as indicated by relative frequency of active responding) was not correlated with Loco. This result confirmed the results of a previous study in which we also did not find a significant relationship between relative frequency and Loco [12]. These results indicate that the activating effects of the light (total responses), but not response guiding effects of the light (relative frequency of active responding), are related to locomotor reactivity in a novel environment.
The present results indicating a significant positive association between VSR and Loco are different from results previously reported by Bardo and co-workers, who found no significant positive association between locomotor response to novelty and approach to novel places and objects [26–28]. Bardo and coworkers characterize locomotor response in a novel environment as “inescapable novelty” emphasizing that locomotor activity measured in a novel enclosure may reflect attempts to escape which are associated with stress related HPA-axis activation [40,41]. According to Bardo and co-workers, “free-choice” measures of novelty seeking such as approach to novel objects or places (in comparison to familiar objects and places) does not include an escape component and is not associated with stress related HPA-axis activation [41,42]. Thus, according to these investigators, the absence of a positive association between “free-choice” measures of novelty seeking and Loco may be explained by the fact that there is no escape component in “free-choice” novelty tests.
One way to reconcile the results indicating that VSR shares some common behavioral and neural processes with Loco and that “free-choice” novelty tests do not, is to hypothesize that novelty has both activating and response guiding effects. Increased locomotor activity in a novel environment primarily reflects the response activating effects of novelty, whereas the free-choice novelty tests measure mainly response guiding effects. This interpretation is consistent with the results of the present study indicating a positive association between Loco and the absolute rate of responding for novel light onset (activating effects) but no association of Loco with the relative frequency of active responding (guiding effects). This hypothesis predicts that the relative frequency of active responding in the VSR procedure should be positively associated with the “free-choice” novelty tests used by Bardo and co-workers.
Further support for the concept of separate activating and guiding effects of novel stimuli is provided by the effects of systemic injections of amphetamine on the Loco, VSR and “free-choice” novelty tests. Amphetamine increases locomotor activity [43]. We have reported that amphetamine increases the absolute rate of VSR responding (activating effect) without altering the relative frequency of active responding (response guiding effect) [15]. Bardo et al. have reported that amphetamine does not alter preference for novelty in “free-choice” novelty tests [44]. One possible implication of these results is that the activating effects of novelty are increased by amphetamine and that the response guiding effects of novel stimuli are not. To summarize, VSR has several potential advantages over the Loco novelty test as a measure of sensation-seeking. First, in the VSR procedure, the source of novel stimulation is precisely identified and its availability can be easily manipulated. In contrast, the precise sources of novelty in the Loco procedure are unspecified and cannot be independently manipulated. Second, the VSR procedure produces two unrelated measures of novelty seeking: (i) absolute rate of responding indicating the response activating effects and (ii) the relative frequency of active responding indicating the response guiding effects. In contrast, the Loco procedure provides only a single locomotor activity measure which may be more sensitive to response activating effects of novelty than the response guiding effects of novelty. An important unanswered question is the association between the absolute and relative frequency of active responding obtained using the VSR procedure with approach to novelty in “free-choice” novelty procedures. We hypothesize that the relative frequency of responding in the VSR procedure will be positively associated with “free-choice” measures of novelty. It is possible that the Loco procedure is most sensitive to the response activating effects of novelty and that the “free-choice” novelty procedures are most sensitive to the response guiding effects of novelty while, the VSR procedure is sensitive to both the response activating and response guiding effects of novelty.
4.2. Operant and Pavlovian processes and novelty
An alternative way to view the differences between the VSR, “free-choice” and Loco novelty tests is in terms of the distinction between operant and Pavlovian processes. Although both “free-choice” and VSR tests involve approach to novelty, VSR is an operant processes in which a response is emitted to produce novel light onset. “Free-choice” tests, however, appear to rely more on Pavlovian conditioning, because the stimulus/location is presented non-contingently resulting in elicitation of an approach response. That being said, it is possible to speculate that approach responses in the “free-choice” novelty procedures are operant responses which are reinforced by interactions with the novelty. The Loco procedure can also perhaps be best described as a Pavlovian procedure, although the stimulus (novel locomotor chamber) and elicited response (movements) are defined in a relatively non-specific fashion in comparison to the VSR and “free-choice” novelty tests. As was described for “free-choice” novelty procedure, a portion of the responses causing movement in the Loco procedure could be operant.
Only the VSR procedure allows a clear distinction to be made between operant and Pavlovian conditioning. The response guiding effects indicated by the relative frequency of active responding clearly fit the definition of operant responding. In contrast, the activating effects indicated by non-specific increases in total responding may be the result of both operant and Pavlovian processes. Operant and Pavlovian processes may play distinct roles in the production and reaction to novel stimuli. This distinction may be important in describing sensation seeking phenotypes.
4.3. Sensory reinforcement and SA
The finding that locomotor activity in a novel environment is a predictor of operant responding for light onset has implications for Loco as a predictor of drug SA. Although it has been frequently demonstrated that light onset and offset can be primary reinforcers [for reviews see, 13,14,34,45], the potential primary reinforcing effects light are often not taken into account in SA procedures where the onset (or offset) of a light is paired with drug delivery. In these studies, changes in illumination are often used to indicate a time-out period when drug is unavailable and/or to act as a conditioned reinforcer in studies of cue-induced reinstatement. In studies using light as a cue, it is possible that prediction of SA by Loco is related to the ability of Loco to predict VSR [for example, see, 12]. A further complication is that there is evidence that systemic administration of methamphetamine [15], D-amphetamine [16–20], and nicotine [21,22] increase responding for visual reinforcers.
A number of studies have indicated that the primary reinforcing effects of light stimuli play a role in rodent SA studies. Deroche-Gamonet et al. [46] demonstrated that the presence of a VS enhanced acquisition of cocaine SA. Furthermore, a series of experiments [47–55] have shown that the rate of nicotine SA is greater when nicotine infusions are accompanied by light onset. One conclusion drawn from this work is that nicotine enhances responding for VSRs. SA studies offer important information regarding sensitivity to the reinforcing properties of drugs of abuse. However, inclusion of potentially reinforcing sensory stimuli without proper controls may weaken the conclusions that can be drawn from these experiments.
4.4. SS and habituation
Zuckerman [56–58] has theorized that high SS should be initially more responsive to novel stimuli but that this responsiveness should habituate more rapidly. In the present study, HR rats were more reactive to novel stimulation and sustained this reactivity for a longer period of time within the test session than LR rats, indicating that they habituated more slowly. Slower habilitation was found for both locomotor response to novelty and light contingent responding (Fig. 3c and d).
Evidence indicating faster habituation in high human SS is mixed. Neary and Zuckerman [10] showed that high SS exhibit an enhanced skin conductance response to a novel visual stimulus presentation compared to low SS. As was the case for the present study, no basal differences of arousal existed prior to stimulus presentation. Differences in skin conductance were no longer detectable with repeated presentation of the visual stimulus, presumably because it had become familiar. Presentations of additional novel stimuli resulted in the same behavioral and physiological pattern as the initial novel stimulus. However, these authors observed no differences in the rate of habituation between high and low SS.
There is also some evidence that high SS habituate more rapidly. Eye blink responses elicited by acoustic startle stimuli have been reported to habituate more rapidly in participants that scored high on the SS [59]. Furthermore, there is evidence that high SS engaging in extreme sports show no overall differences in P3a amplitude from low SS as measured by event-related potentials in a three-stimulus oddball task. Yet, high SS exhibit a greater decline in P3a amplitude across trials, presumably as the task becomes more familiar compared to low SS counterparts [60]. There is also evidence that high SS exhibit qualitatively different event-related brain potential responses after seeing a novel visual stimulus, and there is some indication that habituation to the repeated visual stimulus may be localized to the ventral prefrontal cortex [61]. In summary, there are indications of differences in habituation between high and low SS, but the nature of these differences is unclear.
A contributing factor that may account for different results between studies is a lack of experimental control in human studies. The experiments in rats described in this paper were conducted in highly controlled environments designed to eliminate competing stimulation. In the case of the VSR experiment, the animals were pre-exposed to the test environment for 10 days ensuring that the animals were very familiar with contextual stimuli during the test phase except for the novel light onset. In the absence of interference from competing contextual stimuli, the relative novelty of light onset may have been sustained for a longer period.
4.5. Conclusions: VSR as an animal model of SS
VSR has construct validity as a measure of SS. Both VSR and the thrill and adventure seeking subscales of SSS-V involve novelty. In present experiment, VSR was tested in an environment that was made familiar through pre-exposure, so that when the response contingent light was finally available it was both surprising (unexpected) and novel. Light reinforcement also has face validity. In the VSR paradigm, rats approach the novel stimulus by performing an operant response. Human SS also actively approach novel stimuli. In both rats and humans, voluntary interaction with the novel stimulus appears to be motivated by degree of interest in investigating or exploring novel events. Another similarity between VSR in rats and SS in humans is that both are characterized by individual variation which is consistent across different contexts. HR rats are different from LR rats in Loco [31], VSR [12], maze exploration [8,9] and drug SA [1–7]. Similarly, high human SS are different from low SS on gambling [62], extreme sports [60] and drug abuse [58]. The value of VSR as a non-human animal model of SS lies in its translational utility. Using behavioral tasks to measure psychological constructs such as SS in both human and non-human animals allows the development of behaviorally based operational definitions of SS. Development of an operational definition in non-human animals will help advance research into the underlying neural and behavioral determinants of SS.
Acknowledgments
This work was conducted in partial fulfillment of the requirements of a doctoral degree at the State University of New York at Buffalo for Amy M. Gancarz. We would like to thank Linda Beyley for her assistance in conducting the experiments and Mark Kogutowski for his technical expertise in computer programming for the current experiments. We wish to thank Drs. Michael Bozarth, Micheal Dent, and Larry Hawk for their critical comments and editorial assistance on earlier versions of the manuscript. This work was partly supported by DA10588 to Jerry B. Richards and NIAAA training grant T32-AA007583-11.
Abbreviations
- VSR
visual sensory reinforcer
- Loco
locomotor response to novelty
- SS
sensation seeking
- HR
high responder
- LR
low responder
- SA
self-administration
- VI
variable interval
- METH
methamphetamine
Footnotes
Financial disclosures
The authors on this manuscript reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–32. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–84. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- 4.Cain ME, Dotson WF, Bardo MT. Individual differences in the effect of novel environmental stimuli prior to amphetamine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2006;14:389–401. doi: 10.1037/1064-1297.14.3.389. [DOI] [PubMed] [Google Scholar]
- 5.Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–8. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–37. [PubMed] [Google Scholar]
- 7.Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–80. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- 8.Dellu F, Mayo W, Piazza PV, Le Moal M, Simon H. Individual differences in behavioral responses to novelty in rats. Possible relationship with the sensation-seeking trait in man. Pers Indiv Differ. 1993;4:411–8. [Google Scholar]
- 9.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats—biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 10.Neary RS, Zuckerman M. Sensation seeking, trait, and state anxiety, and the electrodermal orienting response. Psychophysiology. 1976;13:205–11. doi: 10.1111/j.1469-8986.1976.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–54. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Gancarz AM, San George MA, Ashrafioun L, Richards JB. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav Processes. 2011;86:295–304. doi: 10.1016/j.beproc.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapp JT. Activity, reactivity, and the behavior-directing properties of stimuli. In: Tapp JT, editor. Reinforcement and behavior. New York: Academic Press; 1969. pp. 148–78. [Google Scholar]
- 14.Eisenberger R. Explanation of rewards that do not reduce tissue needs. Psychol Bull. 1972;77:319–39. doi: 10.1037/h0032483. [DOI] [PubMed] [Google Scholar]
- 15.Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr, Richards JB. Exploratory studies in sensory reinforcement: effects of methamphetamine. Exp Clin Psychopharmacol. 2012;20(1):16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glow PH, Russell A. Effects of dexamphetamine, amylobarbitone sodium and their mixture on sensory contingent bar pressing behaviour in the rat. Psychopharmacologia. 1973;31(3):239–51. doi: 10.1007/BF00422514. [DOI] [PubMed] [Google Scholar]
- 17.Glow PH, Russell A. Drug enhanced sensory contingent bar pressing: comparing the effect of contingent and noncontingent sensory change. Psychopharmacologia. 1973;32(3):285–92. doi: 10.1007/BF00422151. [DOI] [PubMed] [Google Scholar]
- 18.Glow PH, Russell A. Sensory-contingent barpressing for familiar and novel change under a dexamphetamine–amylobarbitone mixture. Anim Learn Behav. 1974 Feb;2(1):27–30. [Google Scholar]
- 19.Gomer FE, Jakubczak LF. Dose-dependent selective facilitation of response-contingent light-onset behavior by D-amphetamine. Psychopharmacologia. 1974;34(3):199–208. doi: 10.1007/BF00421961. [DOI] [PubMed] [Google Scholar]
- 20.Winterbauer NE, Balleine BW. The influence of amphetamine on sensory and conditioned reinforcement: evidence for the re-selection hypothesis of dopamine function. Front Integr Neurosci. 2007;1:9. doi: 10.3389/neuro.07.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, et al. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–9. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behav Processes. 2009;82:95–9. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–30. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–61. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- 26.Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–75. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- 27.Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self-administration in rats classified as high and low responders. Psychopharmacology (Berl) 2004;176:129–38. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- 28.Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–75. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–9. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- 30.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–85. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 32.Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–45. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- 33.Girdner JB. An experimental analysis of the behavioral effects of a perceptual consequence unrelated to organic drive states. Duke University; 1953. [Google Scholar]
- 34.Kish GB. Studies of sensory reinforcement. In: Honig W, editor. Operant behavior: areas of research and application. New York: Appletone-Century-Crofts; 1966. pp. 100–59. [Google Scholar]
- 35.Glow PH. Response contingent schedule-control: control over the environment motivates bar pressing. Aust J Psychol. 1985 Dec;37(3):233–55. [Google Scholar]
- 36.Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions. Nat Rev Neurosci. 2006;7:967–75. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 37.Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals. Brain Res Rev. 2008;58:322–39. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Berlyne DE. Exploratory behavior: III. Investigatory responses. In: Berlyne DE, editor. Conflict, arousal, and curiosity. New York, NY, USA: McGraw-Hill Book Company; 1960. pp. 136–62. [Google Scholar]
- 39.Renner MJ. Neglected aspects of exploratory and investigatory behavior. Psychobiology. 1990;18:16–22. [Google Scholar]
- 40.Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9:790–8. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 42.Misslin R, Herzog F, Koch B, Ropartz P. Effects of isolation, handling and novelty on the pituitary–adrenal response in the mouse. Psychoneuroendocrinology. 1982;7:217–21. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 43.Cole SO. Brain mechanisms of amphetamine-induced anorexia, locomotion, and stereotypy: a review. Neurosci Biobehav Rev. 1978;2:89–100. [Google Scholar]
- 44.Bardo MT, Neisewander JL, Pierce RC. Novelty-induced place preference behavior in rats: effects of opiate and dopaminergic drugs. Pharmacol Biochem Behav. 1989;32:683–9. doi: 10.1016/0091-3057(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 45.Berlyne DE. The reward-value of indifferent stimulation. In: Tapp JT, editor. Reinforcement and behavior. New York: Academic Press; 1969. pp. 179–214. [Google Scholar]
- 46.Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–70. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- 47.Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–7. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- 48.Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–30. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 50.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190:353–62. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 53.Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- 54.Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- 55.Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- 56.Zuckerman M. Psychobiology of personality. 2. Cambridge University Press; 2005. [Google Scholar]
- 57.Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- 58.Zuckerman M. Sensation seeking and risky behavior. Washington, DC: American Psychological Association; 2008. [Google Scholar]
- 59.LaRowe SD, Patrick CJ, Curtin JJ, Kline JP. Personality correlates of startle habituation. Biol Psychol. 2006;72:257–64. doi: 10.1016/j.biopsycho.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Fjell AM, Aker M, Bang KH, Bardal J, Frogner H, Gangas OS, et al. Habituation of P3a and P3b brain potentials in men engaged in extreme sports. Biol Psychol. 2007;75:87–94. doi: 10.1016/j.biopsycho.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Jiang Y, Lianekhammy J, Lawson A, Guo C, Lynam D, Joseph JE, et al. Brain responses to repeated visual experience among low and high sensation seekers: role of boredom susceptibility. Psychiatry Res. 2009;173:100–6. doi: 10.1016/j.pscychresns.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fortune EE, Goodie AS. The relationship between pathological gambling and sensation seeking: the role of subscale scores. J Gambl Stud. 2010;26:331–46. doi: 10.1007/s10899-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]