Abstract
Background
This study compared Cox-Maze IV (CMIV) outcomes for the treatment of atrial fibrillation (AF) in patients with lone AF vs those with AF and mitral valve (MV) disease.
Methods
Since 2002, 200 patients have undergone a CMIV procedure for lone AF (n = 101) or concomitantly with MV operations (n = 99). Preoperative, perioperative, and late outcomes between these groups were compared. Data were collected prospectively and reported at 3, 6, and 12 months.
Results
Lone AF patients had AF of longer duration; patients with AF and MV disease were older, with larger left atria and worse New York Heart Association classification (p < 0.05). Operative mortality (1% vs 4%, p > 0.05, respectively) was similar between both groups. Perioperative atrial tachyarrhythmias were more prevalent in patients with concomitant MV operations (57% vs 41%, p = 0.03); however, freedom from AF and antiar-rhythmics was similar for both groups at 12 months (76% and 77%). The only predictor for atrial tachyarrhythmia recurrence or arrhythmic drug dependence was failure to isolate the posterior left atrium (p < 0.01).
Conclusions
Patients with AF and MV disease have distinct comorbidities compared with patients with lone AF. However, the CMIV is safe and effective in both groups and should be considered for patients with AF undergoing MV operations. Patients with MV disease had more atrial tachyarrhythmias at 3 months, but freedom from AF and antiarrhythmics was similar to patients with lone AF at 1 year. The posterior left atrium should be isolated in every patient, because this was the only predictor for failure of the CMIV for either group.
The Cox-Maze (CM) procedure, created by Dr James Cox, was initially designed to treat atrial fibrillation (AF) in patients with mitral valve (MV) disease. However, because initial iterations of the CM procedure were complex, technically difficult, and time consuming, the procedure was preferentially performed as a stand-alone procedure on patients with lone AF [1, 2]. As the procedure evolved, and with increasing experience, the CM procedure was more broadly applied to patients with AF and concomitant structural heart disease. The final version introduced by Dr Cox was the Cox-Maze III (CMIII) procedure, which became the gold standard for the surgical treatment of AF. The overall freedom from symptomatic AF in patients undergoing the CMIII procedure is reported as exceeding 90% in several series with more than 5 years of follow-up [1].
To further simplify the CMIII procedure, the Cox-Maze IV (CMIV) was developed in 2002. This procedure uses bipolar radiofrequency energy to create linear lines of ablation that replace most of the atrial incisions required for the CM lesion set [3]. The CMIV is technically easier, requires shorter operative times, and has demonstrated similar efficacy to the CMIII [4]. Our experience with the CMIV is now more than 300 cases, and it is readily offered to patients with lone AF, as well as to patients with AF and concomitant structural heart disease.
Atrial fibrillation is a common coincident process in patients undergoing MV operations, with a prevalence of upwards of 30% in this population. Additionally, more than half of all AF correction operations are performed in patients undergoing MV repairs, demonstrating the significant role of AF procedures in this group [5]. Given these data, it is clear that characterizing the efficacy and outcomes data of the CMIV among patients undergoing mitral operations is critical. The purpose of this study is to evaluate the efficacy of the CMIV in patients with lone AF compared with patients with AF and concomitant MV disease.
Material and Methods
This study was approved by the Washington University School of Medicine Institutional Review Board. Informed consent and permission for release of information were obtained from each participant.
Patients
From January 2002 through May 2010, 200 patients underwent a CMIV procedure for AF as a stand-alone procedure (n = 101) or concomitantly with a MV operation (n = 99). The procedure has been previously described [3]. For all patients, pulmonary vein isolation was confirmed by exit block from each of the pulmonary veins, unless left atrial (LA) appendage thrombus precluded cardioversion or patients were unable to be successfully cardioverted. The lesion set performed was similar for all patients during the study period, with the exception of a “superior connecting lesion” that was added in 2005, connecting the right and left superior pulmonary veins. This additional lesion effectively electrically isolated the entire posterior LA [6] as part of the “box-lesion set.”
Patients were discharged on therapy with class I or III antiarrhythmic drugs and warfarin, unless contraindicated; antiarrhythmic agents were discontinued 2 months postoperatively if patients were in normal sinus rhythm. β-Blockers or calcium channel blockers were not considered as antiarrhythmic drugs.
At 3 months, 24-hour Holter monitoring and echocardiograms were obtained, and anticoagulation was stopped if patients had no evidence of atrial tachyarrhythmias (ATA) or atrial stasis by echocardiogram. Electrocardiograms or prolonged monitoring were obtained at 3, 6, and 12 months. As indicated in the new guidelines introduced in 2006, 24-hour Holter monitoring was routinely obtained in all patients after that time [7].
Late recurrence was defined as any episode of AF, atrial flutter, or ATA that lasted more than 30 seconds. Any patient requiring an interventional electrophysiologic procedure was deemed a permanent failure. Patients were only considered a success if they stopped taking antiarrhythmic drugs and were free of ATA.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or as median with interquartile range (IQR: 25% to 75%). Categoric variables are expressed as frequencies and percentages with outcomes compared using the χ2 or the Fisher exact test. Continuous outcomes were compared using the t test for means of normally distributed continuous variables and the Wilcoxon ranksum nonparametric test for skewed distributions.
Eleven preoperative and perioperative variables were evaluated by univariate analysis to identify predictors of late ATA recurrence and dependence on antiarrhythmic medications. These included age, sex, AF type, AF duration, New York Heart Association (NYHA) classification, left ventricular ejection fraction (LVEF), failed catheter ablation, LA diameter, box vs nonbox lesion set, early ATA (within 30 days), and postoperative pacemaker implantation within 90 days. Significant covariates on univariate analysis (p ≤ 0.10) or covariates deemed clinically relevant based on experience were entered into a multivariate binary logistic regression analysis. Kaplan-Meier analysis was used to estimate freedom from recurrence (need for antiarrhythmic drugs or the presence of ATA). All data analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL).
Results
A total of 101 patients (51%) underwent a stand-alone CMIV procedure and 99 (49%) underwent the CMIV concomitantly with MV operation. The MV procedures included MV repair in 61 patients and MV replacement in 38. A tricuspid valve procedure was additionally performed in 15 patients undergoing MV operations.
Follow-up for this series was 100% complete, and 24-hour monitoring was performed for 95% of all patients since 2006 [7]. Patients undergoing a stand-alone CMIV were more likely to be men with a longer duration of preoperative AF and were also more likely to have had a previous failed catheter ablation for AF (Table 1). Conversely, patients undergoing mitral procedures and concomitant CMIV were older, had a worse functional NYHA class, and larger LA size. There were no differences in AF type, LVEF, or preoperative pacemaker incidence between the groups (Table 1).
Table 1.
Demographics
| Variablesa | CMIV (n =101) |
CMIV + MV (n = 99) |
p Value |
|---|---|---|---|
| Preoperative | |||
| Age, years | 56 ± 10 (29–74) | 65 ± 12 (34–83) | <0.001 |
| Male sex | 77 (76) | 38 (38) | <0.001 |
| AF duration, years | 7.6 ± 6.8 | 4.8 ± 6.0 | 0.003 |
| Paroxysmal AF | 31 (31) | 40 (41) | 0.139 |
| Left atrial diameter | 4.7 ± 1.1 | 5.7 ± 1.2 | <0.001 |
| NYHA class III or IV | 22 (22) | 80 (81) | <0.001 |
| LVEF | 0.50 ± 0.13 | 0.50 ± 0.12 | 0.832 |
| Failed catheter ablation | 41 (41) | 9 (9) | <0.001 |
| Pacemaker | 10 (10) | 14 (15) | 0.387 |
Continuous variables are presented as mean ± standard deviation (range) and categoric variables as number (%).
AF = atrial fibrillation; CMIV = Cox-Maze IV; LVEF = left ventricular ejection fraction; MV = mitral valve; NYHA = New York Heart Association.
The operative mortality was 2.5% for the entire series and 1% in the stand-alone CMIV cohort. Aortic cross-clamp and cardiopulmonary bypass times were longer for patients undergoing a concomitant MV procedure. The percentage of posterior LA isolations, or “box lesions,” performed was similar between the two groups (78% vs 82%). There was a trend for the procedure to be performed through a right thoracotomy approach in the stand-alone group (Table 2).
Table 2.
Perioperative Details
| Variablesa | CMIV (n = 101) |
CMIV + MV (n = 99) |
p Value |
|---|---|---|---|
| Operative | |||
| Aortic cross-clamp time, min | 40 ± 13 | 92 ± 26 | <0.001 |
| Cardiopulmonary bypass, min | 135 ± 29 | 166 ± 34 | <0.001 |
| Right thoracotomy approach | 19 (19) | 10 (10) | 0.08 |
| Box lesion set | 79 (78) | 81 (82) | 0.6 |
| Postoperative | |||
| Operative deaths (<30 days) | 1 (1) | 4 (4) | 0.21 |
| Stroke | 1 (1) | 1 (1) | 1.0 |
| Reoperation for bleeding | 0 (0) | 5 (5) | 0.03 |
| Need for IABP | 1 (1) | 5 (4) | 0.17 |
| Renal failure requiring dialysis | 0 (0) | 5 (5) | 0.03 |
| Pneumonia | 6 (6) | 11 (11) | 0.21 |
| Mediastinitis | 1 (1) | 0 | 1.0 |
| Length of stay, days | 7 (6–10) | 10 (7–13) | 0.02 |
| Early atrial tachyarrhythmias | 41 (41) | 57 (57) | 0.03 |
| Permanent PM (<90 days) | 7 (7) | 10 (11) | 0.45 |
Continuous data are presented as mean ± standard deviation or median (range), and categoric data are presented as number (%).
CMIV = Cox-Maze IV; IABP = intraaortic balloon pump; MV = mitral valve; PM = pacemaker.
Several postoperative complications were higher in patients undergoing a concomitant MV procedure, including the rates of reoperation for bleeding and renal failure (Table 2). The incidence of stroke, pneumonia, mediastinitis, or need for an intraaortic balloon pump was similar between the groups (Table 2). Early postoperative ATAs were documented in 98 patients (49%) and occurred more frequently in patients with MV disease (57% vs 41%). The need for permanent pacemaker placement within 90 days of operation was 9% (n = 17) and no different between the two groups. Hospital length of stay was higher for the concomitant group (Table 2).
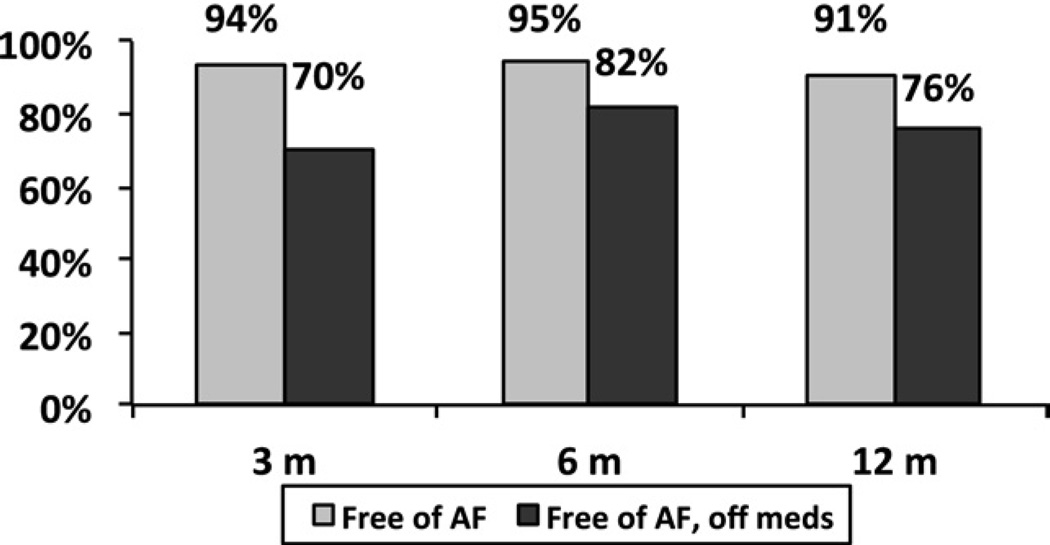
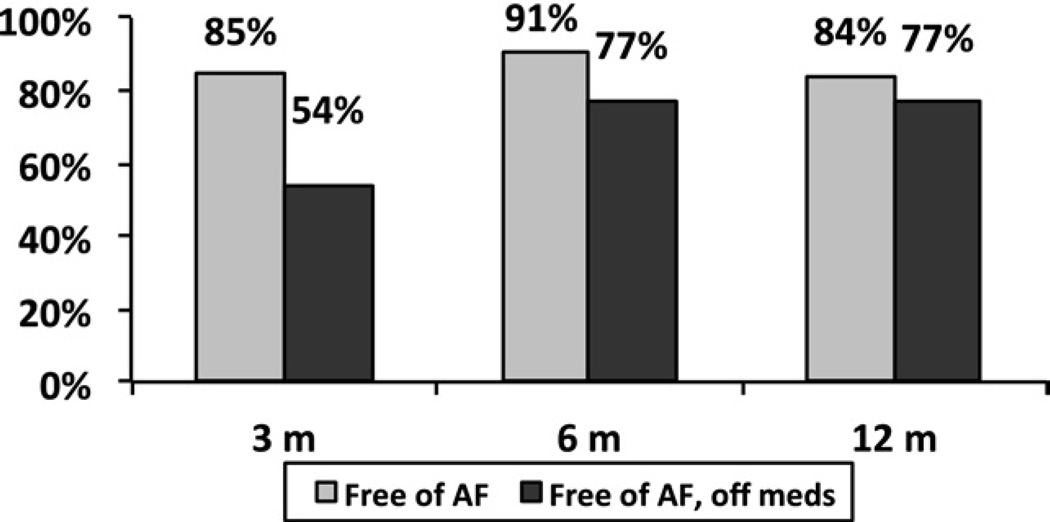
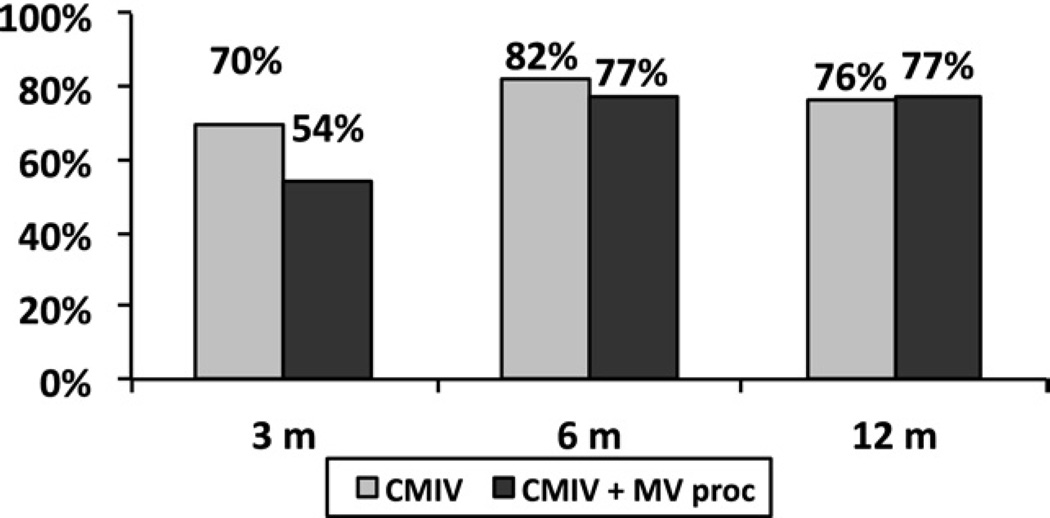
At 1 year, freedom from ATAs was 94%, 95%, 91% at 3, 6 and 12 months, respectively, for a stand alone CMIV (Fig 1). In the same group, freedom from both ATAs and antiarrhythmic drugs was 70%, 82%, and 76% at the same time points. For patients with MV disease and concomitant AF, freedom from ATAs was 85%, 91%, 84% at 3, 6 and 12 months, respectively (Fig 2), and freedom from ATAs and antiarrhythmic drugs was 54%, 77%, and 77% at the same time points. At 12 months, all failures in both the lone AF group (n = 6) and the concomitant group (n = 10) were recurrent AF, with no episodes of atrial flutter observed. In a direct comparison of each group, more early ATAs occurred in the MV group (Table 2) at 3 months, but freedom from AF and antiarrhythmic medications was similar in both groups at 1 year (Fig 3).
Fig. 1.
One-year follow-up shows freedom from atrial fibrillation (AF, gray bars) and freedom from AF and antiarrhythmic medications (black bars) for patients undergoing a stand-alone Cox-Maze IV procedure.
Fig. 2.
One-year follow-up shows freedom from atrial fibrillation (AF, gray bars) and freedom from AF and antiarrhythmic medications (black bars) for patients undergoing a mitral valve procedure and a concomitant Cox-Maze IV procedure.
Fig. 3.
Freedom from atrial fibrillation and antiarrhythmic medications is shown at the 1-year follow-up for patients undergoing a Cox-Maze IV procedure (CMIV, gray bars) or a combined CMIV and mitral valve (MV, black bars) procedure.
Univariate analysis of the entire series identified early ATA (p < 0.08), LA size (p < 0.004), and failure to perform a box lesion set (p < 0.001) as predictors of failure (AF recurrence or antiarrhythmic drug dependence). Arrhythmia failure was specifically not affected by the type of procedure (lone vs concomitant CMIV) or the year the procedure was performed (p <0.05). Each cohort was then further examined by multivariate analysis to determine if any variables predictive of AF recurrence or anti-arrhythmic drug dependence could be identified. According to the multivariate analysis, the only predictor of ATA recurrence or dependence on antiarrhythmic medications for either group at 1 year was failure to perform the box lesion set and isolate the posterior LA (Table 3).
Table 3.
Multivariate Analysis for 12-Month Freedom From Drugs and Atrial Fibrillation
| Variables | OR (95% CI) | p Value |
|---|---|---|
| Lone CMIV | ||
| Nonbox lesion set | 4.884 (1.371–17.401) | 0.014 |
| NYHA class III or IV | 2.028 (0.355–11.605) | 0.427 |
| LVEF | 0.959 (0.909–1.012) | 0.124 |
| Early pacemaker | 4.144 (0.528–32.533) | 0.176 |
| Early ATA | 2.395 (0.591–9.705) | 0.221 |
| Left atrial diameter | 1.728 (0.963–3.101) | 0.067 |
| Concomitant MV and CMIV | ||
| Nonbox lesion set | 6.699 (1.772–25.328) | 0.004 |
| Left atrial diameter | 1.541 (0.874–2.716) | 0.135 |
| Age | 1.049 (0.988–1.114) | 0.117 |
ATA = atrial tachyarrhythmia; CI = confidence interval; CMIV = Cox-Maze IV; LVEF = left ventricular ejection fraction; MV = mitral valve; NYHA = New York Heart Association; OR = odds ratio.
Comment
This study evaluated the CMIV when used as a concomitant procedure with mitral operations and represents a large comparison of two specific and discrete populations of AF patients: those with lone AF vs those with AF and concomitant MV disease [8]. Previous series have investigated the efficacy of CM procedures as concomitant operations, but have primarily focused on the CMIII procedure [1, 2, 9]. Moreover, previous analyses of Maze series and concomitant operations have been hampered by the assimilation of multiple pathologies into a single “concomitant” cohort. The inferences that can be drawn from a heterogeneous cohort that includes patients undergoing concomitant operations for coronary artery disease, aortic valve disease, and mitral valve disease are necessarily limited [10]. As a result, this study offers a unique opportunity to identify differences between patients with lone AF and those with AF requiring only MV operations. Lastly, the follow-up reported here is 100% complete, and our results are reported in accordance with current guidelines in order to provide a more accurate representation of recurrence, and a clearer elucidation of risk factors for procedural failure.
The efficacy of the CMIV for both the lone and concomitant groups was similar with respect to freedom from AF and antiarrhythmic agents at the 6-month and 12-month intervals. Although other centers have reported worse AF outcomes for patients undergoing a CMIII procedure in the setting of MV disease, this is not consistent with our data. The results from this series of CMIV patients also agree with previous reports from our institution that demonstrate equivalent success of the CMIII as a lone or concomitant procedure [9, 10]. The rates of freedom from AF and freedom from AF and antiarrhythmic medications was 87% and 75% for the entire series, respectively. This finding compares favorably with our larger CMIII series, supporting our hypothesis that the two procedures do have similar, excellent outcomes for the treatment of AF [4]. The differences in outcomes between CMIII and CMIV iterations are most likely the result of the different methods and techniques used for follow-up with each of these series.
Not unexpectedly, the two patient groups have distinct demographics depending on their underlying disease. The patients with concomitant MV disease were older, had worse functional status, were more likely to be women, and had a larger LA size. This demographic profile of AF and MV disease patients is consistent with reports from other centers [8, 9, 11], and demonstrates the worse overall health status of this population when compared with lone AF patients. Many of these comorbidities, LA size in particular, are risk factors for Maze failures from other surgical series, which makes the excellent results from this investigation for the MV cohort particularly noteworthy [10, 12, 13].
Although the etiology of AF in the concomitant group remains poorly understood, it is reasonable to assume that structural heart disease does play a role in the development of the arrhythmia for these patients [14, 15]. Animal data suggest that the mechanisms of AF are different between lone AF and valvular heart disease patients and that these two groups simply manifest the arrhythmia as a common end point. That the CMIV is equally effective for each group, irrespective of the etiology of AF, supports the notion that the Maze is effectively a salvage procedure that prevents the atria from fibrillating in a wide variety of patients, independent of the nature of their arrhythmia [16].
The only risk factor associated with CMIV failure after multivariate analysis in either group was a failure to electrically isolate the posterior LA. Our initial version of the CMIV procedure used only one inferior connecting lesion between the pulmonary veins, leaving most of the posterior LA in continuity with the rest of the LA. Before 2005, the superior connecting lesion was only performed on the largest of atria, but as a result of good outcomes in these patients, the superior connecting lesion became routinely added for all patients undergoing a CMIV. This procedural modification makes the lesion sets of the CMIV and CMIII more similar and has significantly increased our arrhythmia drug-free success rates [6, 17]. The importance of posterior LA isolation in surgical patients also agrees with data from the electrophysiology laboratory, in which wide-area circumferential ablation involving most of the posterior LA was more effective than targeting only the pulmonary veins [18, 19]. Our present recommendation is that the entire posterior LA be electrically isolated in all patients undergoing surgical ablation for AF.
The patients who underwent a concomitant CMIV experienced more ATAs than the lone AF group, which is likely the result of the larger atrial sizes and longer cardiopulmonary bypass times in the MV cohort [20, 21]. Although this investigation did not test these hypotheses, recent work from our basic science laboratory has shown that ATAs do occur more frequently with increasing atrial surface area and the presence of myocardial inflammation [22, 23]. Despite these findings, the relevance of ATAs to clinical success remains unclear. As seen in this study, the presence of ATAs failed to affect CMIV efficacy at 6 or 12 months (Fig 3), a finding consistent with earlier CMIII series.
More perioperative complications occurred among patients undergoing a concomitant CMIV procedure, but the risk appears relatively low. The overall mortality rate was similar between our two groups, despite the MV group being older and having more heart failure symptoms. Furthermore, the rates of complications in the concomitant group were no different than what would be expected for AF patients undergoing isolated MV repair or replacement procedures with respect to renal failure, reoperation, or stroke [24]. That more perioperative morbidity was not seen in the concomitant group is most likely because ablative technology was incorporated for the CMIV. Minimizing the need for atrial incisions has shortened myocardial ischemic and cardiopulmonary bypass times for the CMIV and allowed us to perform AF operations more safely in patients with more comorbidities than previously seen in earlier CM series [4]. The increased risks found with a concomitant CMIV procedure are offset not only by a realistic expectation for the elimination of AF, a leading cause of early and late morbidity and death in patients undergoing mitral operations, but also by an improved quality of life after successful AF interventions [25, 26].
This study has several limitations: Although this patient cohort has been well-monitored with no patient lost to follow-up, the actual failure rate may be underestimated. This is due to the fact that the follow-up in this series remains short, and that some patients did not have 24-hour Holter monitoring [27].
Although the addition of the superior connecting lesion, and consequential posterior LA isolation, was not random and thus may represent a “learning curve” for the procedure, multivariate analysis failed to identify year of operation as a predictor of CMIV failure.
Lastly, the precise mechanism of procedural failure was not defined in this series. Thus, the question remains unanswered whether the recurrence of ATAs was due to technical difficulty in performing a complete CMIV lesion set, or was simply inherent to atrial pathology. Some of the lines of ablation may not have been transmural, as only the efficacy of pulmonary vein isolation was tested by confirmation of exit block during pacing from the pulmonary veins. In the future, our hope is to perform noninvasive electrocardiographic imaging to better define the mechanisms of failure and allow us to develop strategies to improve surgical success rates.
In conclusion, patients with AF and MV disease have unique demographics compared with the lone AF population. The risks and benefits of a concomitant AF intervention must obviously be weighed for each patient; however, the CMIV appears safe and equally effective for the treatment of AF in either group at 1 year, and should thus be considered for patients with AF undergoing MV operations. Additionally, this report emphasizes the importance of isolating the entire posterior LA every time the CMIV procedure is performed. Although the etiology and progression of the underlying disease responsible for AF in these two groups may require different long-term approaches for the treatment of AF, only with improved mapping techniques [28] and continued rigorous follow-up will we be able to delineate the mechanisms and differences in AF therapy for these two distinct groups.
Acknowledgments
This study was funded in part by National Institutes of Health Grants R01 HL032257–21 and F32 HL082129–02.
Abbreviations and Acronyms
- AF
atrial fibrillation
- ATA
atrial tachyarrhythmia
- CI
confidence interval
- CMIII
Cox-Maze III
- CMIV
Cox-Maze IV
- IABP
intraaortic balloon pump
- LA
left atrial
- LVEF
left ventricular ejection fraction
- MV
mitral valve
- NYHA
New York Heart Association
- OR
odds ratio
- PM
pacemaker
DISCUSSION
DR EUGENE GROSSI (New York, NY): Is there any hard evidence on whether having a silent posterior left atrium has any impact on left atrial transport, or of the need for long-term anticoagulation?
DR MANIAR: We’ve looked at that previously using MR technology, and demonstrated that isolating the posterior left atrium really didn’t impact atrial transport function. In fact, the posterior left atrium is relatively tethered because of its association to other posterior mediastinal structures and as a result cannot contract very well, minimizing any contribution it might have to atrial pump function or meaningful contraction.
DR YOSUKE ISHII (Tokyo, Japan): Congratulations, it was a beautiful presentation. The patient with atrial fibrillation and mitral valve disease has a higher incidence of postoperative atrial fibrillation after surgery than the patient with lone atrial fibrillation. So, I think one of the reasons of that might be atrial scar or atrial fibrosis. Did you check the left atrial appendage histologically?
DR MANIAR: Thank you for your question. We didn’t look at histology in this series. I am aware of a few papers that have looked at atrial histology of the left atrial appendage and it appears there are some differences in atrial architecture between people with lone AF, AF and mitral disease, and patients with normal sinus rhythm.
DR ISHII: I have another question for you. Could you tell me the postoperative management for the postoperative atrial fibrillation. Should we use antiarrhythmic drugs after surgery or anticoagulant therapy? What do you think about that?
DR MANIAR: Good question. We have standardized the treatment for our patients. All of our patients go home on antiarrhythmic therapy and warfarin unless there is a contraindication. The antiarrhythmics are continued for the first 2 months. At the end of the second month, if we’ve had no evidence of atrial fibrillation or ATAs, the drugs are discontinued, but the patients are still maintained on warfarin. At 3 months, after the first Holter exam, if we see no evidence of ATAs or AF we then feel comfortable in stopping the warfarin as well. So our goal is that by the end of 3 months, our patients are off both antiarrhythmics and warfarin.
DR JAMES EDGERTON (Dallas, TX): I want to acknowledge the fine work with this paper and the contributions of your group over the last 20 years in the field of Afib, and my great friendship with Dr Cox and Dr Damiano. You’ve published previously in another paper that isolating the posterior wall results in better outcomes. However, in your previous paper you actually only had fewer episodes of Afib, atrial tachycardia, and atrial flutter during the first 3 months. Most people would consider this a blanking period. But months 4, 12, and presumably the rest of the patient’s life, there was no difference in boxing out the posterior wall and not boxing out the posterior wall. Accordingly, I’ve called into question your implication that “boxing out the posterior wall results in less Afib” because it only results in less Afib during what most of us consider a blanking period. And after the lesions are healed in, there is no difference in the ADULT CARDIAC two. Am I correct in interpreting that this paper is the same as the previous paper and we’re not showing any difference in long-term effect of Afib?
DR MANIAR: Thanks for the question, Dr Edgerton. Obviously, your contributions in this area are well recognized. There seems to be a bit of a misinterpretation here and I’ll try to clarify. This slide demonstrates that AF patients with concomitant mitral disease have more early ATAs; however, at 12 months the efficacy of the Cox-Maze IV is similar between both groups. And your second question is really asking what role does isolation of the posterior left atrium “the box lesion” have at 6- or 12-month efficacy of the procedure. The data in this manuscript confirms what we have previously shown, which is failure to isolate the entire posterior left atrium “box lesion set” is the greatest predictor for failure with the Cox-Maze IV procedure at 12 months. I am sorry I don’t have the 6-month data here to show you. So our take home message is that we do see early ATAs in the mitral valve patients and in and of themselves, these arrhythmias don’t necessarily mean much in terms of late predictors of recurrence. We demonstrated the same finding with our Cox Maze III series. And most importantly, if you fail to isolate the posterior left atrium you are setting yourself up for a significantly higher failure rate with AF recurrence.
DR EDGERTON: Nice clarification. Thank you. Stress that in your manuscript, please.
DR MANIAR: We will. Thank you.
Footnotes
Presented at the Forty-seventh Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 31–Feb 2, 2011.
Ms Bailey discloses that she has a financial relationship with AtriCure Inc; Dr Schuessler with AtriCure, Inc, Medtronic, Inc, and Estech; Dr Damiano with AtriCure Inc, Medtronic Inc, and ATS Medical; and Dr Maniar with nContact Surgical and Estech.
References
- 1.Cox JL, Ad N, Palazzo T, et al. The Maze-III procedure combined with valve surgery. Semin Thorac Cardiovasc Surg. 2000;12:53–55. doi: 10.1016/s1043-0679(00)70017-7. [DOI] [PubMed] [Google Scholar]
- 2.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 3.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 4.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85:909–914. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 6.Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–877. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ad N, Cox JL. Combined mitral valve surgery and the Maze III procedure. Semin Thorac Cardiovasc Surg. 2002;14:206–209. doi: 10.1053/stcs.2002.34395. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D., 3rd The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25–29. doi: 10.1016/s1043-0679(00)70013-x. [DOI] [PubMed] [Google Scholar]
- 10.Stulak JM, Sundt TM, 3rd, Dearani JA, Daly RC, Orsulak TA, Schaff HV. Ten-year experience with the Cox-Maze procedure for atrial fibrillation: how do we define success? Ann Thorac Surg. 2007;83:1319–1324. doi: 10.1016/j.athoracsur.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Chua YL, Schaff HV, Orszulak TA, Morris JJ. Outcome of mitral valve repair in patients with preoperative atrial fibrillation. Should the maze procedure be combined with mitral valvuloplasty? J Thorac Cardiovasc Surg. 1994;107:408–415. [PubMed] [Google Scholar]
- 12.Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: predictors of late recurrence. J Thorac Cardiovasc Surg. 2005;129:104–111. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Gillinov AM, Sirak J, Blackstone EH, et al. The Cox maze procedure in mitral valve disease: predictors of recurrent atrial fibrillation. J Thorac Cardiovasc Surg. 2005;130:1653–1660. doi: 10.1016/j.jtcvs.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Qian Y, Meng J, Tang H, et al. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010;12:371–377. doi: 10.1093/europace/eup438. [DOI] [PubMed] [Google Scholar]
- 15.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 16.Damiano RJ, Jr, Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg. 2011;141:113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weimar T, Bailey MS, Watanabe Y, et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011;31:47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–2360. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 19.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 20.Cox JL, Boineau JP, Schuessler RB, Kater KM, Lappas DG. Five-year experience with the maze procedure for atrial fibrillation. Ann Thorac Surg. 1993;56:814–824. doi: 10.1016/0003-4975(93)90338-i. [DOI] [PubMed] [Google Scholar]
- 21.Ishii Y, Gleva MJ, Gamache MC, et al. Atrial tachyarrhythmias after the maze procedure: incidence and prognosis. Circulation. 2004;110(11 Suppl 1):II164–II168. doi: 10.1161/01.CIR.0000138400.44799.65. [DOI] [PubMed] [Google Scholar]
- 22.Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005;112(9 Suppl):I7–I13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 23.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 24.The Society of Thoracic Surgeons. Online STS Risk Calculator. 2008 http://209.220.160.181/STSWebRiskCalc261/de.aspx.
- 25.Jovin A, Oprea DA, Jovin IS, Hashim SW, Clancy JF. Atrial fibrillation and mitral valve repair. Pacing Clin Electrophysiol. 2008;31:1057–1063. doi: 10.1111/j.1540-8159.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Melby SJ, Zierer A, Lubahn JG. Normal quality of life after the Cox Maze procedure for atrial fibrillation. Innovations (Phila) 2008;3:142–146. doi: 10.1097/IMI.0b013e31819165d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgerton JR, McClelland JH, Duke D, et al. Minimally invasive surgical ablation of atrial fibrillation: six-month results. J Thorac Cardiovasc Surg. 2009;138:109–114. doi: 10.1016/j.jtcvs.2008.09.080. [DOI] [PubMed] [Google Scholar]
- 28.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]