Abstract
The role of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)- and SNARE-associated proteins have not yet been assessed in regulation of cardiac glucose uptake, nor in the regulation of long-chain fatty acid (LCFA) uptake in any tissue. Munc18c is a SNARE-associated protein that regulates GLUT4 translocation in skeletal muscle and adipose tissue. Using cardiomyocytes from Munc18c−/+ mice (with 56% reduction of Munc18c protein expression), we investigated whether this syntaxin4-associated protein is involved in regulation of cardiac substrate uptake. Basal, insulin- and oligomycin (a 5′ AMP-activated protein kinase-activating agent)-stimulated glucose and LCFA uptake were not altered significantly in Munc18c−/+ cardiomyocytes compared to wild-type cells. We conclude, therefore, that Munc18c is not rate-limiting for cardiac substrate uptake, neither under basal conditions nor when maximally stimulated metabolically.
Keywords: Munc18c, Long-chain fatty acid uptake, Glucose uptake, Cardiomyocytes
Introduction
In the heart, long-chain fatty acids (LCFA) and glucose are the predominant substrates. Glucose is mainly taken up into cardiomyocytes via glucose transporter-4 (GLUT4), and LCFA largely via fatty acid translocase (CD36) [1]. Both transporters recycle between intracellular stores and the sarcolemma, and can be induced to translocate to the sarcolemma by several physiological stimuli, such as an increase in circulating concentrations of insulin [2], and an increase in contractile activity [3]. Hence, both the translocation of GLUT4 and CD36 appear to be similarly regulated. This similarity also applies to the signaling components activated by each of these stimuli. Insulin recruits both GLUT4 and CD36 via activation of the phosphatidylinositol-3-kinase—Akt/protein kinase B (PKB) axis, and contraction recruits these transporters via activation of the LKB1—5′ AMP-activated protein kinase (AMPK) axis [2, 3].
The translocation of GLUT4 and CD36 to the sarcolemma in response to physiological stimuli occurs by vesicular trafficking [2, 3], but for the heart there is little information on the routes involved. However, much more information is available for GLUT4 translocation in adipocytes [4], and to a lesser extent in skeletal muscle [5], upon insulin stimulation. This work revealed that GLUT4 translocation is a vesicle-mediated exocytotic process, obeying principles of the soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE) hypothesis. Accordingly, in vesicular trafficking events a unique vesicle SNARE (vSNARE) specifically recognizes and interacts with a cognate target SNARE (tSNARE) localized at the target membrane. This specific vSNARE–tSNARE recognition ensures that transport vesicles do not fuse randomly with subcellular membrane compartments, but deliver their cargo at the appropriate intracellular address. Cardiomyocytes contain multiple types of SNARE proteins [6], which include the vSNARE vesicle-associated membrane protein-2 (VAMP2), the tSNAREs soluble NSF-attachment protein-23 (SNAP23), and syntaxin4, which each were found to be involved in insulin-induced GLUT4 translocation in adipocytes and skeletal muscle [7].
Besides SNARE proteins, a number of accessory proteins have been shown to be important in formation of the SNARE complex [7]. One of these accessory proteins is Munc18c, which belongs to a family of Sec1p-like/Munc18 proteins. Munc18c forms a complex with syntaxin4 [8], and is known to be involved in several trafficking processes including GLUT4 translocation, as has been shown in cell lines [9] and in skeletal muscle from heterozygous Munc18c knockout mice [10].
Driven by the lack of information about the role of SNAREs and accessory proteins in GLUT4 translocation in the heart and in CD36 translocation in any tissue, we sought to investigate (1) whether in the heart Munc18c fulfills a similar role in insulin-stimulated GLUT4 translocation, as has been observed in adipose tissue and in skeletal muscle, and (2) whether this role can be extended to AMPK-stimulated GLUT4 translocation and/or to (3) insulin- and contraction-induced CD36 translocation. Because homozygous Munc18c mice are not viable [10, 11], we used heterozygous Munc18c mice to study the potential effects of partial ablation of this protein in the stimulation of glucose and LCFA uptake into cardiomyocytes by insulin and by oligomycin, an AMPK-activating contraction-mimetic agent.
Materials and methods
Materials
[1-14C]palmitic acid and 2-deoxy-d-[1-3H]glucose were obtained from GE Healthcare (Piscataway, NJ, USA). BSA (fraction V, essentially fatty acid free), phloretin, oligomycin, insulin, and DMSO were obtained from Sigma (St. Louis, MO). Liberase blendzyme 1 was purchased from Roche Diagnostics (Indianapolis, IN). Sulfo-NHS-LC-biotin and immobilized streptavidin were from Perbio Science (Etten-Leur, the Netherlands). The rabbit anti-Munc18c antibody was generated as previously described [9]. The antibody directed against phosphorylated ACC was obtained from Upstate (Dundee, UK) and anti-GAPDH, anti-phospho-PKB, and anti-phospho-AMPK from Cell Signalling (Danvers, MA). Antibodies directed against CD36 and GLUT4 were obtained from Chemicon International Inc. (Temecula, USA).
Animals
The generation of Munc18c−/+ mice on the C57Bl/J6 background was as previously described [10]. The Experimental Animal Committee of Maastricht University gave approval for all experiments involving animals.
Isolation and pre-incubation of mouse cardiomyocytes
Adult mouse cardiomyocytes (male mice 2–3 months of age) were isolated using a Langendorff perfusion system described previously [12]. Suspensions of cardiomyocytes (2.0 ml; 5–10 mg wet mass/ml; viability 60–80%) were pre-incubated in capped 20-ml incubation vials either with 0.35% dimethyl sulfoxide (DMSO) (Ctrl), 1 µmol/l oligomycin, or 100 nmol/l insulin for 20 min at 37°C under continuous shaking. Neither oligomycin nor insulin was found to affect the percentage of cells that (1) were sod-shaped and (2) excluded trypan blue, as parameters of cellular integrity.
Previously we have shown that oligomycin, a mitochondrial F1F0-ATPase inhibitor, stimulates LCFA uptake in a non-additive manner to contractions, indicating that oligomycin activates the same signaling cascade as contractions [3].
Sarcolemmal CD36 and GLUT4 content of cardiomyocytes
Cardiomyocytes were plated in laminin-coated culture plates. After 1 h recovery, cells were incubated with 0.35% DMSO (ctrl) or 1 µmol/l oligomycin (20 min at 37°C). As previously described [13], cells were biotinylated with the cell-impermeable reagent sulfo-NHS-LC-biotin whereafter the cells were washed and lysed. Lysates were incubated overnight with streptavidin beads, followed by their centrifugation and washing. Biotinylated proteins were eluted in sample buffer, whereafter CD36 and GLUT4 expression was detected.
Phosphorylation of AMPK, ACC, PKB, and total expression of Munc18c, GADPH, CD36, and GLUT4 in cardiomyocytes
Pellets of stimulated cell suspensions were dissolved in sample buffer, as previously described [12], and subjected to SDS-polyacrylamide gel electrophoresis, followed by Western blotting for the detection of Munc18c [9], GAPDH, phospho-AMPKα (Thr172), phospho-ACC (Ser79), phospho-PKB (Ser473), and of total GLUT4 and CD36 expression levels by applying the antibodies according to the manufacturer’s instructions.
Deoxyglucose and palmitate uptake into cardiomyocytes
To study the rate of deoxyglucose and palmitate uptake, 0.5 ml of a mixture of [1-3H]deoxyglucose and [1-14C]palmitate/BSA complex was added to pre-incubated cell suspensions at the start of the incubations with a final concentration of 100 µmol/l deoxyglucose and 100 µmol/l palmitate with a corresponding palmitate/bovine serum albumin (BSA) ratio of 0.3. Cellular uptake of 3H-deoxyglucose (3-min incubation) and 14C-palmitate (3-min incubation) was determined upon washing the cells, and measuring the radioactivity of the cell pellets [14].
Data presentation and statistics
All data are presented as means ± SEM for the indicated number of cardiomyocyte preparations. Statistical difference between means was analyzed by the paired Student’s t test. P values ≤ 0.05 were considered significant.
Results
Sarcolemmal content of CD36 and GLUT4 after oligomycin and insulin treatment
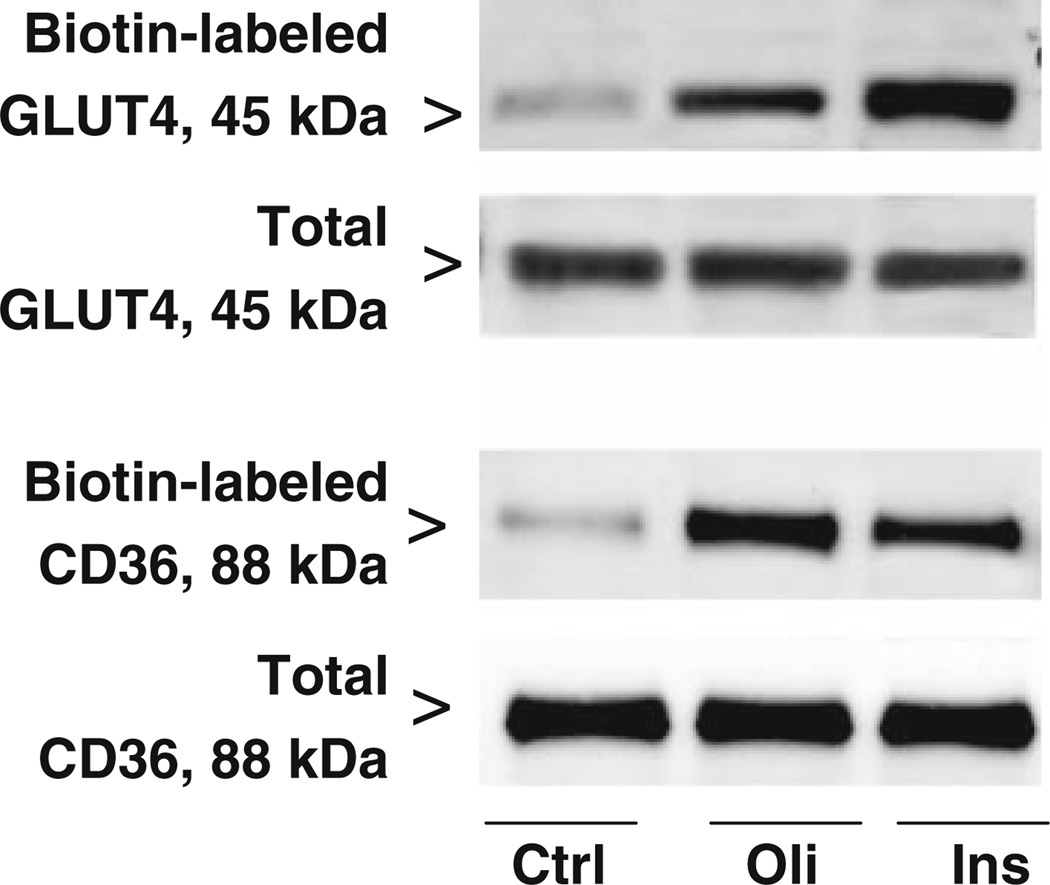
Oligomycin treatment of wild-type mouse cardiomyocytes increased the sarcolemmal contents of GLUT4 and CD36 by 2.5-fold (±0.5; n = 4, P < 0.05) and 3.9-fold (±0.6; n = 4, P < 0.05), respectively, whereas the total cellular content of both transporters did not change (Fig. 1). Similarly, after insulin stimulation the sarcolemmal contents of GLUT4 (3.8-fold ± 0.7; n= 4, P < 0.05) and CD36 (3.0-fold ± 0.4; n = 4, P < 0.05) were increased (Fig. 1).
Fig. 1.
Effects of oligomycin and insulin on GLUT4 and CD36 expression at the sarcolemma. Cardiomyocytes (n = 4) from WT mice were incubated for 20 min in the absence (Ctrl) or presence of 1 µmol/l oligomycin (Oli) or 100 nmol/l insulin (Ins) whereafter biotin-labeled (cell surface) GLUT4 and CD36, and total cellular GLUT4 and CD36 were determined. A representative Western Blot is displayed
Expression of Munc18c, GLUT4, and CD36 in WT and Munc18c−/+ cardiomyocytes
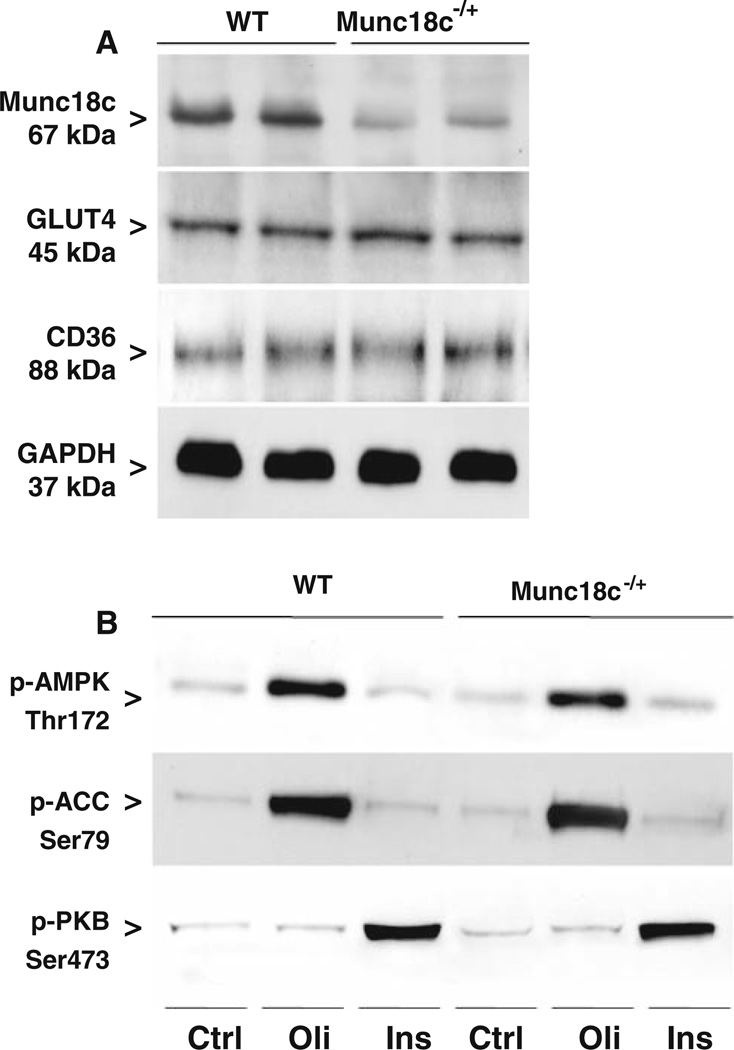
Quantification of Western blots showed Munc18c to be reduced in Munc18c−/+ cardiomyocytes by 56% (P values ≤ 0.05; Fig. 2a), in agreement with the previously observed 50% reduction in heart homogenates of these mice [10]. Expression of GLUT4, CD36, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; loading control) was similar in WT and Munc18c−/+ cardiomyocytes (Fig. 2a). This indicates that the cardiac transport capacity for glucose and LCFA is not altered due to heterozygous knockout of Munc18c.
Fig. 2.
Munc18c, GLUT4, and CD36 expression in cardiomyocytes from WT and Munc18c−/+ mice and the effects of oligomycin and insulin treatment on phosphorylation of AMPK, ACC, and PKB. WT and Munc18c−/+ cardiomyocytes were used to determine Munc18c, and GAPDH (loading control) expression (panel a), and after incubation for 20 min in the absence (Ctrl) or presence of 1 µmol/l oligomycin (Oli) or 100 nmol/l insulin (Ins) phospho-AMPK (Thr172), phospho-ACC (Ser79), phospho-PKB (Ser473) (panel b) by Western blotting. A representative Western blot is presented out of five experiments with different cardiomyocyte preparations
Effects of oligomycin and insulin on signal transduction pathways in WT and Munc18c−/+ cardiomyocytes
Activation of AMPK was assessed by determining phosphorylation of the specific threonine residue (Thr172) at the α-subunit of this heterotrimeric enzyme, and by the phosphorylation of its main substrate acetyl-CoA carboxylase (ACC; Ser79). AMPK-Thr172 phosphorylation in quiescent cardiomyocytes (referred to as Ctrl) was similar in WT and Munc18c−/+ cardiomyocytes. Oligomycin-treatment of cardiomyocytes increased the degree of phosphorylation of both AMPK and ACC by 5.9-fold (±0.5; n = 4, P < 0.05) and 7.5-fold (±0.5; n = 4, P< 0.05), respectively, in WT cardiomyocytes and by 5.3-fold (±0.4; n = 4, P < 0.05) and 7.9-fold (±0.6; n = 4, P < 0.05), respectively, in Munc18c−/+ cardiomyocytes (Fig. 2b). Stimulation of cardiomyocytes with insulin did not alter the phosphorylation states of AMPK or ACC, neither in WT cardiomyocytes nor in Munc18c−/+ cardiomyocytes (Fig. 2b).
Activation of the insulin signaling pathway was determined by phosphorylation of PKB (Ser473). In quiescent cardiomyocytes, phosphorylation of PKB was similar in WT and Munc18c−/+ cardiomyocytes. Treatment of WT cardiomyocytes with insulin (4.3-fold ± 0.5; n = 5, P < 0.05) but not oligomycin increased the phosphorylation of PKB (Fig. 2b). This increase after insulin treatment was similar in Munc18c−/+ cardiomyocytes (4.1-fold ± 0.4; n = 5, P < 0.05). Hence, activation of signal transduction pathways normally leading to increased myocardial substrate uptake are not disrupted in Munc18c−/+ cardiomyocytes.
Oligomycin- and insulin-stimulated deoxyglucose and palmitate uptake into WT and Munc18c−/+ cardiomyocytes
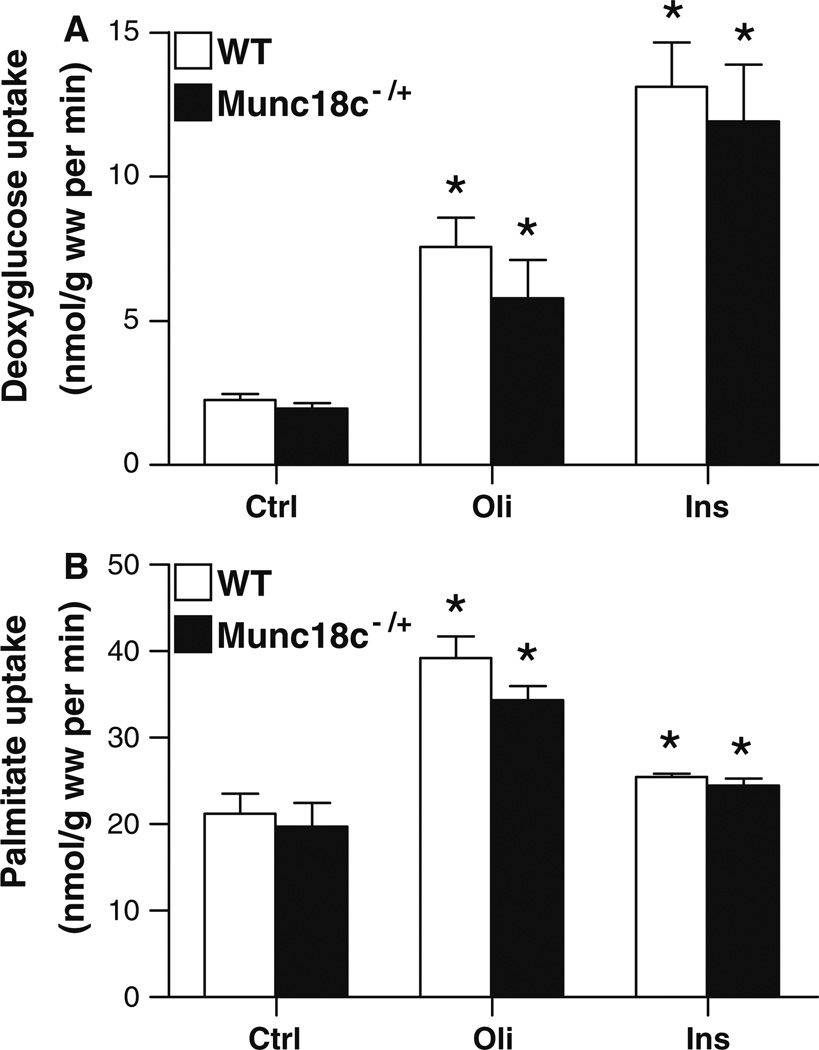
Deoxyglucose uptake into quiescent cardiomyocytes was similar between WT and Munc18c−/+ cardiomyocytes (Fig. 3a). Oligomycin treatment increased deoxyglucose by 3.4-fold in WT and by 2.9-fold in Munc18c−/+ cardiomyocytes. Insulin enhanced the deoxyglucose uptake by 5.8-fold and 6.1-fold into WT and Munc18c−/+ cardiomyocytes, respectively. These enhancing actions of both stimuli on glucose uptake are of the same order of magnitude as their enhancing actions on surface GLUT4 appearance (compare Fig. 1a with Fig. 3a).
Fig. 3.
Effects of oligomycin and insulin stimulation on deoxyglucose and palmitate uptake in cardiomyocytes from WT and Munc18c−/+ mice. WT (□) and Munc18c−/+ cardiomyocytes (■) were incubated for 20 min in the absence (Ctrl) or presence of 1 µmol/l oligomycin (Oli) or 100 nmol/l insulin (Ins) whereafter uptake rates of 3H-deoxyglucose (panel a) and 14C-palmitate (panel b) and were measured. Data are means ± SEM for 10 experiments carried out with different cardiomyocyte preparations. *Significantly different from Ctrl (P < 0.05)
Palmitate uptake into quiescent cardiomyocytes was not significantly different between WT and Munc18c−/+ cardiomyocytes (Fig. 3b). Treatment of WT and Munc18c−/+ cardiomyocytes with oligomycin increased palmitate uptake by 1.9-fold and 1.7-fold, respectively. In addition, there was no significant difference in insulin-induced palmitate uptake into WT (1.2-fold increase) and Munc18c−/+ cardiomyocytes (1.2-fold increase). The fact that insulin-stimulated LCFA uptake is dwarfed by insulin-stimulated glucose uptake could include the following reasons. (1) Whereas insulin-stimulated LCFA uptake (just like oligomycin-stimulated glucose uptake and oligomycin-stimulated LCFA uptake) relies on only one compartment for transporter recruitment (i.e., recycling endosomes) [15], insulin-stimulated glucose uptake uses, besides the recycling endosomes, also an extra compartment for transport recruitment, being a specialized pre-endosomal compartment, uniquely dedicated to storage of GLUT4 [16]. (2) Whereas glucose uptake is solely dependent on glucose transporters, there is always a passive diffusion component in uptake of LCFA, due to their lipophilic nature. The contribution of passive diffusion to basal LCFA uptake amounts to ~60% in mouse cardiomyocytes [12] which is markedly greater than in rat cardiomyocytes, being 20–30% [14]. This passive diffusion masks the relative increases in protein (CD36)-mediated LCFA uptake, i.e., upon insulin stimulation. Taken together, WT and Munc18c−/+ cardiomyocytes, in the absence or presence of a stimulus, showed no significant differences in deoxyglucose and palmitate uptake rates.
Discussion
The SNARE-associated protein Munc18c is on the steadily growing list of proteins found to be involved in insulin-stimulated GLUT4 translocation, as established in adipocytes and skeletal muscle [17]. Whether Munc18c fulfils a similar role in insulin-induced GLUT4 translocation in the heart is not known. It is also of interest to study whether Munc18c is involved in CD36 translocation, because differences in the regulation of the translocation of CD36 relative to GLUT4 would disclose targets for modulation of the substrate preference of the heart [1]. Using cardiomyocytes from heterozygous Munc18c knockout mice, we made the following observations: (1) basal, oligomycin- or insulin-induced glucose uptake rates were not altered by partial Munc18c deletion, nor were (2) basal, oligomycin-or insulin-induced LCFA uptake rates. Together, these findings suggest that Munc18c is not rate-limiting for basal and stimulus-induced cardiac substrate uptake.
The lack of effect upon insulin signaling (PKB-Ser473 phosphorylation) in cardiomyocytes as seen in this study in heterozygous Munc18c knockout mice is consistent with the lack of effect in skeletal muscle, supporting the concept that Munc18c plays a more direct role in modulating SNARE protein machinery at the plasma membrane in exocytosis events. With respect to the regulation of glucose uptake through GLUT4 translocation, the heterozygous Munc18c mice display significantly impaired skeletal muscle insulin-stimulated GLUT4 translocation [10]. Obviously, the unimpaired glucose uptake rate into insulin-treated cardiomyocytes with 56% reduced Munc18c content, as observed in this study, contrasts markedly with the effects in skeletal muscle. There are two explanations for this discrepancy: (1) Munc18c is obligatorily involved in insulin-stimulated GLUT4 translocation in muscle, but not in heart, or (2) Munc18c is redundantly present in heart, but not in skeletal muscle, so that a partial reduction in its content is not rate-limiting for glucose flux under maximal conditions. We speculate that the latter option is more likely. Notably, in heart and muscle glucose uptake is similarly regulated by signaling cascades inducing GLUT4 translocation from endosomal and pre-endosomal stores to the sarcolemma [18, 19]. Moreover, besides Munc18c, components of the SNARE complex involved in insulin-induced fusion of GLUT4-containing transport vesicles with the sarcolemma, as found in muscle [20], including VAMP2, syntaxin4, and SNAP23, are also present in heart [6]. When taken into consideration that this redundancy hypothesis cannot be explained by the degree of Munc18c expression in heart versus muscle, being of similar magnitude [6, 20], a higher activation state of Munc18c in heart versus muscle may be compatible with a functional redundancy in the heart. This might be related to the permanent contractile state of the heart resulting in the permanent elevation of second messengers which could be involved in Munc18c activation. The recent finding that tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis [21] might elude to the notion that in the heart tyrosine phosphorylation of Munc18c is chronically upregulated. Alternatively, the ratio of Munc18c expression to expression of the other components of the SNARE complex could be different in heart versus muscle. In this instance, either one of the proteins VAMP2, syntaxin4, or SNAP23 could display a lower expression level in heart compared to muscle, so that these proteins, and thus not Munc18c, are rate-limiting for SNARE complex formation in heart.
Concerning regulation of LCFA uptake via CD36 translocation, no studies have yet been undertaken to examine the involvement of SNARE- or SNARE-associated proteins in this process. The inability of partial Munc18c deletion to alter LCFA uptake into quiescent and stimulated cardiomyocytes leads us to speculate that (1) Munc18c is not involved in the regulation of LCFA uptake, or (2) Munc18c is not rate-limiting for LCFA uptake. As above, we propose that the latter possibility is more likely, since the regulation of glucose uptake and LCFA uptake is very similar with respect to localization of intracellular compartments and involvement of signaling cascades. Taken together, Munc18c is not rate-limiting for uptake of both glucose and LCFA into cardiomyocytes, neither under basal nor under maximal metabolic demands.
Acknowledgments
This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw grant nr. 40-00812-98-03075), the European Community (Integrated Project LSHM-CT-2004-005272, Exgenesis), the National Institutes of Health (DK67912 to D.C.T.), and the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Heart and Stroke Foundation of Ontario, the Canada Research Chair program. A. Bonen is the Canada Research Chair in Metabolism and Health. J. F. C. Glatz is Netherlands Heart Foundation Professor of Cardiac Metabolism.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- AMPK
5′ AMP-activated protein kinase
- CD36
Fatty acid translocase
- GAPDH
Glyceraldehydes-3-phosphate dehydrogenase
- GLUT4
Glucose transporter-4
- LCFA
Long-chain fatty acids
- PKB
Protein kinase-B
- SNAP23
Soluble NSF-attachment protein-23
- t/vSNARE
Target/vesicular soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- VAMP2
Vesicle-associated membrane protein-2
Contributor Information
Daphna D. J. Habets, Department of Molecular Genetics, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, P.O. Box 616, 6200 MD Maastricht, The Netherlands, d.habets@gen.unimaas.nl
Debbie C. Thurmond, Department of Biochemistry and Molecular Biology, Center for Diabetes Research, Indiana University School of Medicine, Indianapolis, IN, USA
Will A. Coumans, Department of Molecular Genetics, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, P.O. Box 616, 6200 MD Maastricht, The Netherlands
Arend Bonen, Department of Human Health and Nutritional Sciences, Guelph University, Guelph, ON, Canada.
Jan F. C. Glatz, Department of Molecular Genetics, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, P.O. Box 616, 6200 MD Maastricht, The Netherlands
Joost J. F. P. Luiken, Department of Molecular Genetics, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, P.O. Box 616, 6200 MD Maastricht, The Netherlands
References
- 1.Glatz JF, Bonen A, Ouwens DM, et al. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther. 2006;20:471–476. doi: 10.1007/s10557-006-0582-8. [DOI] [PubMed] [Google Scholar]
- 2.Luiken JJ, Koonen DP, Willems J, et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 3.Luiken JJ, Coort SL, Willems J, et al. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 4.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal. 2007;19:2209–2217. doi: 10.1016/j.cellsig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Rudich A, Klip A. Push/pull mechanisms of GLUT4 traffic in muscle cells. Acta Physiol Scand. 2003;178:297–308. doi: 10.1046/j.1365-201X.2003.01163.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters CG, Miller DF, Giovannucci DR. Identification, localization and interaction of SNARE proteins in atrial cardiac myocytes. J Mol Cell Cardiol. 2006;40:361–374. doi: 10.1016/j.yjmcc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- 8.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 9.Thurmond DC, Ceresa BP, Okada S, et al. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J Biol Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- 10.Oh E, Spurlin BA, Pessin JE, et al. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes. 2005;54:638–647. doi: 10.2337/diabetes.54.3.638. [DOI] [PubMed] [Google Scholar]
- 11.Kanda H, Tamori Y, Shinoda H, et al. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habets DD, Coumans WA, Voshol PJ, et al. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355:204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 13.van Oort MM, van Doorn JM, Bonen A, et al. Insulin-induced translocation of CD36 to the plasma membrane is reversible and shows similarity to that of GLUT4. Biochim Biophys Acta. 2008;1781:61–71. doi: 10.1016/j.bbalip.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Luiken JJ, van Nieuwenhoven FA, America G, et al. Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: involvement of sarcolemmal proteins. J Lipid Res. 1997;38:745–758. [PubMed] [Google Scholar]
- 15.Luiken JJ, Coort SL, Koonen DP, et al. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448:1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- 16.Tomas E, Sevilla L, Palacin M, et al. The insulin-sensitive GLUT4 storage compartment is a postendocytic and heterogeneous population recruited by acute exercise. Biochem Biophys Res Commun. 2001;284:490–495. doi: 10.1006/bbrc.2001.4983. [DOI] [PubMed] [Google Scholar]
- 17.Thurmond DC, Pessin JE. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review) Mol Membr Biol. 2001;18:237– 245. doi: 10.1080/09687680110082400. [DOI] [PubMed] [Google Scholar]
- 18.Zorzano A, Sevilla L, Camps M, et al. Regulation of glucose transport, and glucose transporters expression and trafficking in the heart: studies in cardiac myocytes. Am J Cardiol. 1997;80:65A–76A. doi: 10.1016/s0002-9149(97)00459-1. [DOI] [PubMed] [Google Scholar]
- 19.Martin S, Slot JW, James DE. GLUT4 trafficking in insulin-sensitive cells. A morphological review. Cell Biochem Biophys. 1999;30:89–113. doi: 10.1007/BF02737886. [DOI] [PubMed] [Google Scholar]
- 20.St-Denis JF, Cushman SW. Role of SNARE’s in the GLUT4 translocation response to insulin in adipose cells and muscle. J Basic Clin Physiol Pharmacol. 1998;9:153–165. doi: 10.1515/jbcpp.1998.9.2-4.153. [DOI] [PubMed] [Google Scholar]
- 21.Oh E, Thurmond DC. The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J Biol Chem. 2006;281:17624–17634. doi: 10.1074/jbc.M601581200. [DOI] [PMC free article] [PubMed] [Google Scholar]