Abstract
White matter is an essential component of the central nervous system and is of major concern in neurodegenerative diseases such as multiple sclerosis (MS). Recent MRI studies have explored the unique anisotropic magnetic properties of white matter using susceptibility tensor imaging. However, these measurements are inhibited in practice by the large number of different head orientations needed to accurately reconstruct the susceptibility tensor. Adding reasonable constraints reduces the number of model parameters and can help condition the tensor reconstruction from a small number of orientations. The macroscopic magnetic susceptibility is decomposed as a sum of molecular magnetic polarizabilities, demonstrating that macroscopic order in molecular arrangement is essential to the existence of and symmetry in susceptibility anisotropy and cylindrical symmetry is a natural outcome of an ordered molecular arrangement. Noise propagation in the susceptibility tensor reconstruction is analyzed through its condition number, showing that the tensor reconstruction is highly susceptible to the distribution of acquired subject orientations and to the tensor symmetry properties, with a substantial over- or under-estimation of susceptibility anisotropy in fiber directions not favorably oriented with respect to the acquired orientations. It was found that a careful acquisition of three non-coplanar orientations and the use of cylindrical symmetry guided by diffusion tensor imaging allowed reasonable estimation of magnetic susceptibility anisotropy in certain major white matter tracts in the human brain.
Keywords: Susceptibility tensor imaging, magnetic susceptibility anisotropy, diffusion tensor imaging, white matter, cylindrical symmetric susceptibility tensor
Introduction
The white matter of the brain is organized in long fibers that trace throughout the central and peripheral nervous system (Koenig, 2009). Myelin sheaths of axons, which are composed of concentric phospholipid bilayers, are intrinsically tied to this fiber structure (Waxman et al., 1995; Koenig, 2009). The phase of the MRI signal (Lee et al., 2010; Denk et al., 2011) and T2* relaxation (Bender, 2010; Lee et al., 2011) of the white matter have been found to depend on the orientation of the fibers with respect to the main magnetic field. These effects can be attributed to the existence of an orientation-dependent magnetization or magnetic susceptibility anisotropy (MSA) of the macroscopic white matter tract. It is well known that macroscopically well-organized materials with anisotropic electron configurations, such as polymers (Kimura et al., 2001), minerals (Hrouda and Schulmann, 1990) and liquid crystals (Prosser et al., 1998a), can give rise to anisotropic magnetic susceptibility properties. Like these materials, the highly structured and organized nature of the myelin sheath of the white matter leads to its anisotropic magnetic behavior (Liu, 2010; Li et al., 2012a; Wisnieff et al., 2012a; Wisnieff et al., 2012b). This intrinsic anisotropic nature of phospholipid membranes has been previously observed in NMR in vitro within a model system (Prosser et al., 1998b). Few other structures in the brain present the same level of macroscopic organization as the white matter, without which the magnetic anisotropy may not be observable in MRI.
To assess the orientation-dependent magnetization of materials like white matter, susceptibility tensor imaging (STI) can be estimated from MRI data acquired at 12 or more subject orientations (Li et al., 2011a; Li et al., 2012a), which unfortunately are not acceptable in clinical practice. Furthermore, unlike studying the MSA of rocks using multiple acquisitions of the sample over a free range of orientations (Hext, 1963), the limited range of orientations with human subjects may lead to substantial noise propagation in the observed MSA. Constraining the system with reasonable prior information and assumptions can help condition the problem and reduce error propagation. Specifically, reducing the number of model parameters will reduce the number of required orientations to recover the susceptibility tensor. For example, a cylindrically symmetric susceptibility tensor (CSST) will reduce the number of parameters for the susceptibility tensor to two from three in the orthonormal tensor frame (Wharton and Bowtell, 2011). Exploiting symmetry in tensors has been explored in other applications as well including characterization of diffusivities in the spinal cord (Clark et al., 2000) and the orientation of minerals in rocks (Hrouda and Schulmann, 1990). The parallel and perpendicular axes of the white matter fiber tract derived from diffusion tensor imaging (DTI) can be used to define this orthonormal frame on a voxel-by-voxel basis (Fig. 1). As a result, the original 6-parameter STI problem is reduced to a 2-parameter CSST problem (Li et al., 2012b).
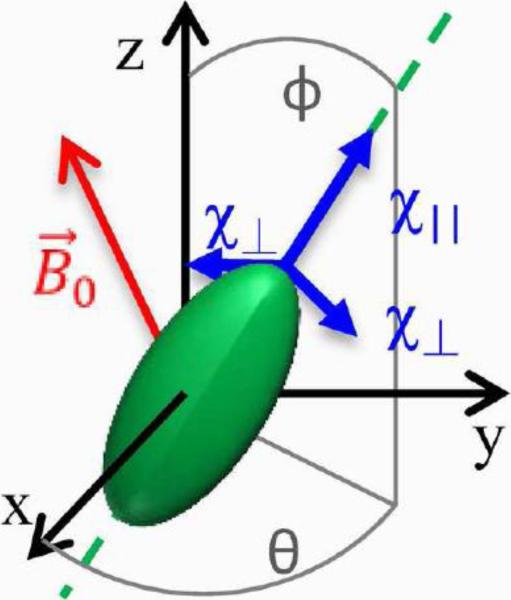
Figure 1.
The tensor frame is defined by the axes along χ∥ and χ⊥. The subject frame is defined by x, y and z axes. These two frames are related by a rotation around an axis along the cross product of z and χ∥ axes with a rotation angle ϕ equal to the angle between of z and χ∥ axes. For the purpose of describing the fiber orientation defined by the χ∥ axis, we also characterize the χ∥ axis using its polar angle ϕ and azimuthal angle θ as viewed in the subject frame xyz.
The purpose of this work is to systematically explore the symmetry of susceptibility anisotropy in the white matter of the human brain and investigate the performance of MSA estimation using the CSST and three subject orientations. This work employs theoretical calculation, numerical simulations, phantoms and human in vivo data. The macroscopic susceptibility anisotropy is connected with molecular magnetic polarization anisotropies through their arrangement in space, demonstrating that macroscopic order of molecule arrangement is essential to the existence of and the symmetry in susceptibility anisotropy observed in MRI. Error propagation in the CSST estimation is analyzed according to the symmetry characteristics of the tissue susceptibility tensor and the possible sets of subject orientations acquired in MRI, showing that DTI guided estimation of cylindrical MSA in major white matter tracts of the brain can be reasonable with as few as three non-coplanar orientations.
Theory
Origins of Magnetic Susceptibility Anisotropy Observed in MRI from the Macroscopic Arrangement of Molecules with Magnetic Polarizability Anisotropy
In general, macroscopic tissue magnetization, M, can be described as its susceptibility χ times the applied magnetic field, H, M = χH ≅ χB0 / μ0, B0 is the main magnetic field of the MR scanner and μ0 is the magnetic permeability of free space (Schenck, 1996). χ can be a general 3×3 matrix or tensor to account for the possible orientation dependent relationship between the magnetization and the applied field (Bertini et al., 2002). The magnetization is the sum of the magnetic moments of molecules in a unit volume, which is related to the applied magnetic field by the molecular polarizability tensor , where the summation index a is over all molecules in a unit volume. Therefore the susceptibility within a voxel is the sum of magnetic polarizabilities of all molecules,
| [1] |
where n is a factor accounting for the density of molecules within a voxel. The orientation of a molecule can be represented by its rotation matrix, Ra, and its magnetic polarizability tensor can be described in terms of its eigenvalues in a molecular specific frame where the tensor is diagonal:
| [2] |
Biological tissues may contain both isotropic and anisotropic sources of magnetic susceptibility (Lounila et al., 1994; Prosser et al., 1998b). One major source of susceptibility consists of paramagnetic ions due to unpaired electrons (Schenck, 1996) including iron deposition (Haacke et al., 2005) and MRI contrast agents (de Rochefort et al., 2008b). These paramagnetic ions are known to be isotropic (βa = βI = constant times identity matrix) with their magnetic moments independent of molecular orientation and have been widely examined using scalar susceptibility mapping techniques (de Rochefort et al., 2010; Li et al., 2011b; Bilgic et al., 2012; Liu et al., 2012b; Schweser et al., 2012a), and according to Eqs.1&2, they will not contribute to the macroscopic susceptibility anisotropy. Another major type of susceptibility source is diamagnetism from most biological molecules, which are mostly anisotropic in spatial structure. They can contribute to the macroscopic susceptibility anisotropy observable in MRI through the summation over all molecules in a macroscopic unit volume. When anisotropic molecules are oriented uniformly over all possible angles, i.e., there is no macroscopic order in the molecular alignment, the average over many molecules in a volume with molecular density n eliminates anisotropy, rendering the macroscopic susceptibility isotropic, χiso (Eq. A.2 in Appendix A),
| [3] |
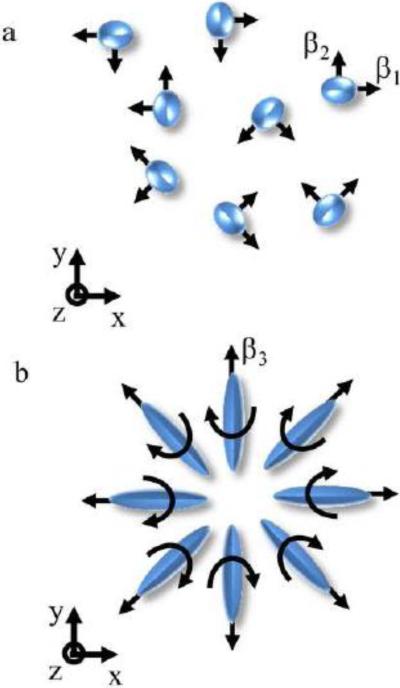
For molecular magnetic anisotropy to be observable at the resolution of MRI, macroscopic order of the molecular orientation is needed. An example is the order within white matter fiber tracts, where the lipid molecules in myelin are arranged in a radial pattern perpendicular to the fiber direction (Waxman et al., 1995), resulting in anisotropy in the macroscopic magnetic susceptibility (Li et al., 2012a). One possible model of myelin consists of constraining the β3 component to lie along the fiber axis, while the other axes are uniformly distributed in the plane orthogonal to the β3 (or fiber) axis, Fig. 2a. The resulting macroscopic anisotropic susceptibility, χCS1 is given by (Eq. A.4, in Appendix A),
| [4] |
Fixing one of the molecular axes to lie along the axis of the white matter fiber may not be entirely realistic as the molecules are free to diffuse in the membrane since they are not chemically bound (Bagatolli et al., 2010; Kusumi et al., 2012). More realistic configurations would allow the molecule to rotate about an axis that lies perpendicular to the fiber direction. The cylindrical symmetry of myelin may be represented by constraining the β3 component to lie in the transverse plane, while the other axes of the molecule are uniformly distributed in the plane orthogonal to the β3 axis, Fig. 2b. Resulting in a macroscopic anisotropic susceptibility, χCS3 given by (Eq. A.6, in Appendix A),
| [5] |
Eqs.4&5 indicate that when the molecules are ordered in space along a line or within a plane, the resulting macroscopic susceptibility tensor is always cylindrically symmetric for molecular magnetic polarizabilities with any anisotropy.
Figure 2.
a, shows the configuration of molecules examined for the first cylindrically symmetric case. b, shows the more realistic configuration of molecules that describes the organization of lipids that exists in myelin.
Data from a model of dysmyelination in shiverer mice brains showed near complete loss of anisotropy in magnetic susceptibility suggesting that myelin is largely responsible for the observed susceptibility anisotropy (Liu et al., 2011a).
Estimating Cylindrical Symmetric Susceptibility Tensor (CSST) from MRI
The MRI signal phase permits determination of the magnetic field generated by a tensor source according to Maxwell's equations in k-space (Liu, 2010):
| [6] |
where X is the second rank tensor of the magnetic susceptibility in the Fourier domain, Δ is the relative field change, also in the Fourier domain, normalized by the main field strength, is the orientation of the main field, and k is the Fourier space vector. In general susceptibility tensor imaging (STI), the inverse problem of finding the magnetic susceptibility tensor from the MRI field measurements is solved by imaging the subject at multiple directions, which can be achieved by reorienting the subject in the magnet. Let the subject frame be the orthogonal coordinate system coincident with the natural axes defined by the subject, such as the superior-inferior (SI), anterior-posterior (AP) and left-right (LR) axes of the head of a volunteer when positioned supine and head first in the scanner. Within the subject frame, the solution for Eq.6 can be formulated as:
| [7] |
where p is the index of the acquired orientation and wp is a weighting factor to minimize the propagation of noise (approximated as Gaussian) in the measured field (de Rochefort et al., 2008a). In general, the inverse problem of Eq.6 is poorly conditioned and upwards of 11 orientations has been reported to generate STI (Li et al., 2011a; Li et al., 2012a). The cylindrical symmetry described in Eq.4 or 5 with the cylindrical axis estimated from diffusion tensor imaging (DTI) can be used to reduce the number of unknowns in Eq.6, therefore improving the condition of inversion and reducing the number of orientations needed (Wharton and Bowtell, 2011; Li et al., 2012b). Important questions that remain to be addressed are to examine the improvement in the condition of the problem from assuming symmetry and to investigate the accuracy in the estimated CSST that can be achieved with a limited number of orientations.
The experimental part of this work endeavors to answer the latter of these questions. We begin by introducing the following notation for the tensor frame. Let the tensor frame for a given voxel be the orthonormal basis in which the susceptibility tensor at that voxel is strictly diagonal. For the CSST, the cylindrical symmetry can be imposed in this tensor frame by making two diagonal elements identical and the other diagonal element correspond to the myelin cylindrical axis as estimated from DTI data:
| [8] |
The susceptibility tensor in the subject frame, in image space, is then given by χ =RTχT R, where R is the rotation matrix that relates the subject frame to the tensor frame. For the CSST, the expression for the forward problem in Eq. 6 is updated as:
| [9] |
The principal diffusion direction derived from DTI is here assumed to coincide with the direction of the parallel component of the susceptibility tensor. The rotation matrix is defined by the Euler principal rotation vector formula according to the axis and angle of rotation between any two coordinate systems (Schaub and Junkins, 2003):
| [10] |
where and ϕ are the axis unit vector and angle of rotation. The angle of rotation, ϕ, is the angle between the principal axes in the tensor and subject frames; for example the angle between the z axis in the subject frame and the z' axis in the tensor frame.
Materials and Methods
Estimation of the Condition Number for the 3-CSST
A detailed discussion of the condition number and how it is calculated in this work is presented in Appendix B. To investigate the condition number of the CSST we examine the case where the rotation is an identity matrix simplifying the convolution in k-space. In this case the parallel component of the cylindrically symmetric tensor lies along the z axis over the entire field of view in both the subject and tensor frames; there is no assumed distribution of tensor coefficients only the orientation of the tensor is necessary to estimate the condition of the CSST system. Coefficients for the blocks of the system were generated according to Eq. B.6 and eigenvalues were calculated numerically using Matlab. The condition numbers of the 560 sets of 3 orientations used in the simulated phantom, Fig. 3, were examined. Since matrix components were explicitly constructed, to simplify eigenvalue calculations a smaller matrix size of 32×32×32 in k-space was used to reduce calculation time and memory requirements.
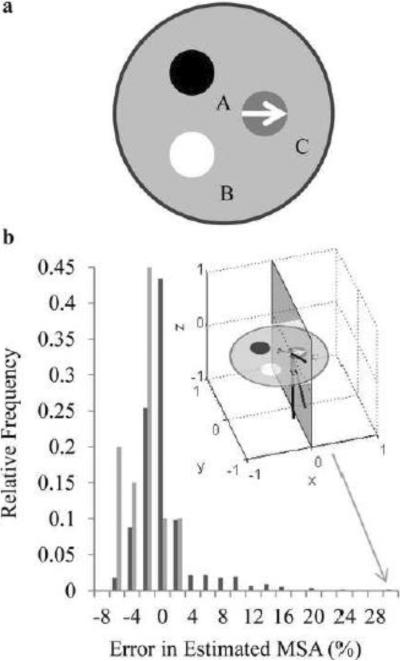
Figure 3.
a, shows a slice through the simulated phantom displaying the configuration of the isotropic sources, A and B, and anisotropic susceptibility source C with its orientation along the x axis (white arrow). b, Error in mean anisotropy detected in the simulated fiber over all combinations of possible 3-CSST reconstructions. Light gray bars indicate human feasible acquisitions and dark gray bars indicate all possible combinations of three orientations. The three black lines on the y-z plane in the inlaid figure indicate the three directions for the outlier 3-CSST MSA result of 29.6% error (gray arrow).
Numerical Simulation: Sensitivity of 3-CSST to Acquisition Orientations
A numerical simulation consisting of three spherically shaped regions of interest (ROIs): two contained isotropic susceptibility (regions A and B in Fig. 3a), while the third (region C in Fig. 3a) contained susceptibility anisotropy similar to that observed in human white matter in vivo (Li et al., 2012a). The values for χ⊥ and χ∥ were chosen as -0.03 and -0.01 parts per million (ppm), respectively. The simulated data consisted of 16 orientations evenly distributed over a unit sphere. A range of tensor reconstructions were investigated. First, CSST and STI reconstructions using all 16 orientations were performed. Second, a CSST reconstruction was performed for each set of 3 orientations chosen from the total set of 16 orientations (560 combinations) to investigate the variability in results from the limited number of orientations. Reconstructions are referred to as N-STI or N-CSST, with N indicating the number of orientations used in the reconstruction. A signal-to-noise ratio (SNR) of 100 was chosen to approximate the noise observed in human studies; initial simulations in preparation for this work with this numerical phantom showed that relatively high SNR was required for accurate estimation of the tensor components in the simulations. The field map was generated according to the forward model in Eq.6. It was assumed that the principal diffusion tensor eigenvector of the phantom was known (taken to be the simulated one) as diffusion data was not simulated.
The magnetic susceptibility anisotropy (MSA) for a susceptibility tensor with cylindrical symmetry was defined as the difference between parallel and perpendicular principal components of the tensor,
| [11] |
In the unconstrained 16-STI reconstruction, parallel and perpendicular components are not well defined; the second eigenvalue may or may not clearly resemble either the maximum or minimum eigenvalue, which is needed for cylindrical symmetry in general. In this work for comparison among STI and CSST reconstructions, the MSA from STI was defined as,
| [12] |
where the tensor eigenvalues λi are ordered from large to small. This difference was chosen since it is expected that the parallel component of the tensor is more paramagnetic as observed in previous studies of white matter (Li et al., 2011a; Li et al., 2012a). A histogram of the mean MSA of the fibers oriented along the x-axis (C in Fig. 3a) from all possible 3-CSST reconstructions was obtained to examine possible bias caused by the limited number of orientations. The orientations that were within ±50°, from the x-axis of the simulation were considered to be within the range of human feasible orientations.
Numerical Simulation: Realistic Fiber Orientations and MSA Estimation
To investigate the error in the susceptibility tensor reconstructed from a limited number of orientations, a numerical simulation was performed using the brain anatomy of a volunteer in this study. An automatic segmentation algorithm, FAST (Zhang et al., 2001), was used to segment a T2 weighted image obtained in this volunteer into white matter, grey matter and cerebrospinal fluid (CSF). Specific grey matter regions were identified by hand from this initial segmentation. The white matter (WM) was set to have a homogeneous susceptibility tensor (in the voxel specific tensor frame) that was cylindrically symmetric, χ∥ = −0.01ppm and χ⊥ = − 0.03 ppm. The grey matter and CSF regions were set to have isotropic magnetic susceptibility described by scalars as follows: CSF, 0 ppm; globus pallidus (GP), 0.19 ppm; putamen (PU), 0.09 ppm; thalamus (T), 0.07 ppm; red nucleus (RN), 0.07 ppm; substantia nigra (SN), 0.09 ppm; dentate nucleus (DN), 0.09 ppm; caudate nucleus (CN), 0.09 ppm; and cortex (C) 0.05 ppm, similar to values observed in vivo (Liu et al., 2011b). The orientation of the tensor for each voxel was defined by the principal diffusion eigenvector, obtained from a DTI acquisition in the same subject and registered to the anatomical image. The susceptibility tensor in the subject frame, defined by the anatomical image, was calculated using the relation χ =RTχT R, and the field inhomogeneity due to this susceptibility distribution was simulated using the forward problem Eq.6. Noisy phase data for this simulation was generated in the same fashion as the above numerical simulation.
The sensitivity of the anisotropy estimation to various acquisition orientations was investigated in this simulated data set. Three types of tensor reconstructions were generated as follows: 1) using the same three orientations as acquired on the Subject 1, 2) using the 12 orientations acquired on the Subject 1 and 3) using twelve orientations evenly distributed over a sphere; these acquisition schemes are referred to as human 3, human 12 and uniform 12 respectively. The anisotropies generated from these reconstructions were evaluated against the known anisotropy in the simulation. An error map was generated by plotting the average error for every encountered fiber direction. To depict the orientations represented in this numerical brain, a 3D histogram was displayed as a 2D intensity map.
Experimental Anisotropic Phantom
The anisotropic phantom consisted of a bundle of parallel carbon fibers (12K carbon fiber tow, Aerospace Composites), a known source of cylindrically symmetric susceptibility anisotropy (Kimura et al., 2001) approximately 3cm in length and 3mm in diameter immersed in a 1% agarose gel background in a cylindrical container (10cm diameter, 6cm height). The direction of the fibers used for the CSST reconstruction was obtained by determining the axis of the bundle from the magnitude image. Two water balloons were added as isotropic references containing 2.5 and 5mM of Magnevist, which corresponded to susceptibilities of 0.8ppm and 1.6ppm, respectively. Multi-echo gradient data was acquired in twelve distinct orientations uniformly distributed over the unit sphere.
For this phantom, three types of tensor reconstructions were performed on the acquired data: the CSST and STI reconstructions using all twelve orientations and the 3-CSST for each of the 220 combinations of three orientations out of the available twelve. The relationship between the molar susceptibility of gadolinium is well known; since there is no inherent anisotropy within the gadolinium solutions, their magnetic susceptibility tensors should be defined by a single scalar susceptibility. Accordingly, anisotropy within the eigenvalues of the gadolinium phantom was used as a measure of error in the estimated susceptibility tensor. The MSA from each concentration of gadolinium across the reconstructions was compared for consistency of the estimated anisotropy. A histogram of the MSA of the 220 3-CSST reconstructions was constructed to examine bias in the anisotropy from these reconstructions. The mean magnetic susceptibility, or MMS, defined as
| [13] |
was calculated for each of the balloons. The prepared concentration of Gadolinium was converted to ppm using the molar susceptibility of 326 L/mol (de Rochefort et al., 2008a). A regression analysis against the prepared gadolinium susceptibility was performed to examine errors in the reconstructions of isotropic materials.
Human Subject Study
Human studies were performed with approval from our institutional review board. In four healthy volunteers (3 male, 1 female, age 28±2.9 years), the 3-CSST was calculated from 3 acquisitions with isotropic 1.5×1.5×1.5mm3 resolution in the supine position, including neutral, left- and right-leaning orientations. One volunteer, Subject 1, consented and was able to perform an additional 9 orientations using a combination of neutral, left-, right-, forward- and backward-leaning orientations in supine and prone positions. On this volunteer, all twelve orientations were used to reconstruct the tensor with and without symmetry constraints (12-CSST and 12-STI), and all possible 3-CSST reconstructions were performed for each set of 3 orientations within the available set of twelve (220 combinations). On all susceptibility tensor reconstructions, the MSA and MMS were measured in predefined ROIs in four major white matter tracts, including the splenium, body and genu of the corpus callosum (SCC, BCC and GCC, respectively), and optic radiations (OR). Summary statistics were calculated across all volunteers and reported as mean ± standard deviation unless otherwise stated. Histograms were generated over all 3-CSST reconstructions in Subject 1 in the white matter ROIs. For human subjects, the analysis of the results focused on major white matter tracts, where the principal fiber direction was assumed to be uniform with few crossing fibers. The principal eigenvectors for the estimated DTI and STI reconstructions in Subject 1 correspond to the principal eigenvalue, signed maximum value, in the eigenvalue decomposition. The absolute value of the dot product of these vectors, |V1STI·V1DTI|, is used here to indicate the degree of alignment between them, with 1 indicating aligned and 0 orthogonal vectors. Alignment in ROIs identified in the brain, SCC, BCC, GCC, OR and centrum semiovale (CS) were compared with a one-way ANOVA.
Data Acquisition
All experiments were performed on a 3T clinical MR scanner (General Electric Excite HD; GE Healthcare, Waukesha, WI, USA). An 8-channel head coil was used for the phantom study. A sample holder was constructed from a Styrofoam ball to reproducibly reorient the sample evenly over a sphere. A total of 12 acquisitions were obtained at an isotropic resolution of 1mm3. These orientations were acquired with a multi echo gradient echo (MEGRE) sequence with a total of 8 echoes and echo time (TE) spacing of 3.4ms and repetition time (TR) of 71.6ms, flip angle of 15°, bandwidth of 62.5kHz, acquisition matrix of 130×130×116, field of view (FOV) of 13cm, and a slice thickness of 1mm. In the phantom, DTI information was not reliable due to the lack of observed restricted diffusion signal in both the isotropic and anisotropic sources.
The MEGRE sequence was also used for the volunteer studies, but with the larger transmit/receive head coil to allow for a greater degree of rotation. Imaging parameters were as follows: 11 echoes with TE spacing of 2.64ms, TR of 46.94ms and 1.5×1.5×1.5mm3 resolution. The 9 additional orientations were acquired on Subject 1 to allow a 12-STI reconstruction. A 2D echo planar imaging (EPI) dual spin echo DTI sequence was used to acquire diffusion tensor data on each of the volunteers with a resolution of 2×2×2.4mm3, with parameters: 33 directions, b value of 1000s/mm2, 22cm FOV, acquisition matrix of 110×110, 2.4mm slice thickness, 17sec TR, 85.3ms TE and bandwidth per pixel of 1953.12Hz.
Data Processing
Phase information was calculated from the complex MRI signal. Phase data were first temporally unwrapped across echoes. Next, the resulting multi-echo phase data were linearly fit to estimate the field inhomogeneity relative to the B0 field (de Rochefort et al., 2008a; Kressler et al., 2010). Subsequent spatial unwrapping of this field map was performed with a region growing algorithm (Cusack and Papadakis, 2002). The background field was removed using the projection onto dipole fields method (Liu et al., 2012a). All tensor calculations were performed in MATLAB using a conjugate gradient solver. Spatial warping of the diffusion data was corrected and registered to the gradient echo data using FUGUE and FLIRT (Crum et al., 2004). The registration between the orientations and the subject frame was used to determine the vector in each of the orientations. Estimated MMS was calculated from the reconstructed susceptibility components. Since the MSA is insensitive to the choice of reference, no such reference was determined in phantom and human data.
Results
Estimation of the Condition Number for the 3-CSST
The 560 sets of 3 orientations in the simulated phantom showed the following condition min/median/max numbers: 9.4×103/ 3.2×105/ 3.1×1011, after excluding one infinite condition number for the set of 3 orientations that were aligned close to a single plane. Twenty of these 560 sets contained human feasible orientations, having the min/median/max condition numbers of 2.0×104/ 7.1×105/ 1.8×108. Preliminary experiments with larger matrix sizes did not significantly affect the calculated condition number of this system.
Numerical Phantom Simulation
Both 16-CSST (MSA:20±1.2 parts per billion, ppb) and 16-STI (MSA:19±1.6ppb) gave reconstructed MSA in close agreement with the truth; giving −0.5% and −5.5% error, respectively. 96% of all simulated 3-CSST reconstructions estimated the MSA of the fiber within 10% of the true MSA; the 3-CSST of human orientations was in this 10% error group with an average of −2.4% error. There was a tail in the distribution biased towards higher MSA. An outlier at the end of the tail had sampled directions (represented in the inlaid plot of Fig. 3b) that were largely perpendicular to the χ∥ direction (along the x axis).
Numerical Brain Simulation
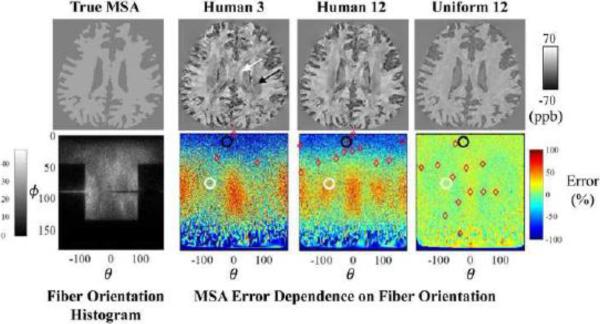
The true MSA map and reconstructed MSA maps are shown in the top row of Fig.4. The distribution of fiber orientations in the simulation and the observed error in the reconstructed MSA with respect to fiber orientation are shown in the bottom row of Fig.4.
Figure 4.
Estimation of white matter MSA using realistic fiber orientations with various CSST reconstructed MSA (top row) and associated errors (bottom row). The lower left corner shows a histogram of the fiber orientations estimated from experimental human data (the black region corresponds to few samples). Red diamonds in the error maps represent the directions in each of the corresponding MSA maps in the top row. The white and black circles in the error maps correspond to the regions indicated by the white and black arrows in the MSA maps respectively.
The MSA as measured by the 3-CSST had erroneous hyper- and hypo-intense bands (over- and under-estimation) in the regions of the body of corpus callosum and the centrum semiovale (BCC and CS, respectively; white and black arrows/circles in the top/bottom of Fig. 4). The intensity of these error bands was reduced when the reconstruction was based on the set of 12 human feasible orientations and was significantly diminished with a uniformly distributed set of 12 orientations. The MSA error varied substantially with fiber orientations.
Experimental Anisotropic Phantom
The image quality of the 12-CSST and 12-STI reconstructions were similar, and both were superior to that of the 3-CSST (Fig.5a), consistent with observations in the simulated phantom; data not shown. The MSA estimated by the 12-CSST (740 ppb) was not significantly different from that estimated by the 12-STI (770 ppb, p=0.56). The mean MSA of the 3-CSST reconstructions was 650 ppb, 12% lower than the 12-CSST/12-STI estimates. In the 3-CSST with orientations restricted to a human feasible range, the MSA was further skewed towards the lower end of the distribution (Fig.5b). All estimated MSAs for the carbon fiber were within the range of values reported in the literature (Kimura et al., 2000).
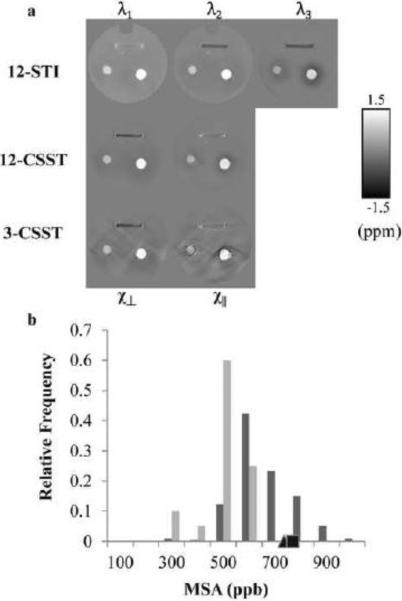
Figure 5.
a, Eigenvalues of the STI and parallel and perpendicular components of the CSST reconstructions of the phantom consisting of both isotropic and anisotropic materials. b, Histograms of MSA detected in the carbon fiber over all 3-CSST reconstructions of the phantom (dark gray bars) and the sets of 3-CSSTs that lie within a human feasible range (light gray bars). The black triangle and square indicate the location of the mean anisotropy detected in the 12-CSST and 12-STI reconstructions respectively.
In the gadolinium balloons of this phantom, regression analysis showed similar fits of mean susceptibility in the gadolinium balloons with a slope of 0.86 (R2 = 0.98) for 12-CSST, 0.86 (R2 = 0.91) for one 3-CSST and 0.89 (R2 = 0.97) for 12-STI; no statistical difference, P>0.05 between regressions.
Human Subject Study
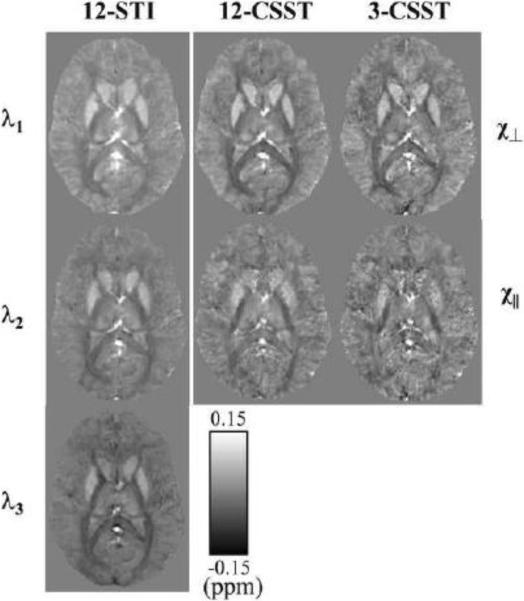
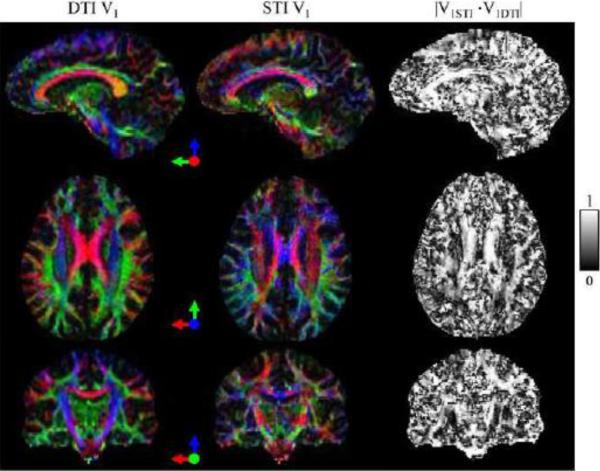
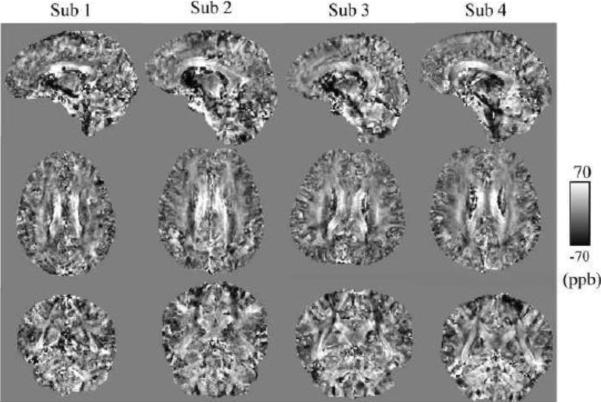
For Subject 1 with all 12 orientations, 12-STI, 12-CSST and 3-CSST were successfully reconstructed. The traces of these reconstructions (in Fig. 6) demonstrated striking similarity with increased noise with fewer orientations. The MSA maps of 12-CSST and 3-CSST were similar, but this visual similarity was limited to the major white matter tracts when compared to the MSA map of 12-STI (Fig. 7). A comparison of the STI versus DTI principal eigenvector maps in Subject 1 showed high correspondence in some anatomical regions like the corpus callosum and optic radiations, but regions that were oriented along the SI direction showed poor alignment. It was found that the absolute value of the dot product resembled the pattern in the estimated anisotropy maps, particularly in the axial plane, Fig. 7 versus the middle row of Fig. 8. The SCC (|V1STI ·V1DTI|: 0.44±0.30), BCC (0.69±0.29), GCC (0.68±0.28) and OR (0.56±0.27) were all regions that laid in planes mostly perpendicular to the SI direction and all showed significantly stronger alignment compared to the CS (0.38±0.25, P <0.05 using a multiple comparison test), which was mostly oriented along the SI direction as determined by DTI. The mean susceptibility maps of all three reconstructions showed many similarities with the scalar reconstruction of the neutral orientation of the brain (Fig. 9). The 3-CSST MSA maps were largely consistent across volunteers for the axial, sagittal and coronal planes (Fig. 10).
Figure 6.
Diagonal components of the 12-CSST and 3-CSST reconstructions and the eigenvalues of the 12-STI reconstructions from Subject 1.
Figure 7.
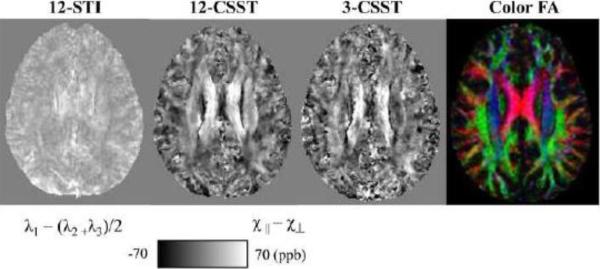
Comparison of MSA maps generated within Subject 1 compared to the color FA generated from the diffusion data.
Figure 8.
First and second columns: maps of the principal eigenvector direction as derived from DTI and STI (both weighted by the FA from DTI) in sagittal (top), axial (middle) and coronal (bottom) section. Directions are color coded according to the arrows. Third column: the absolute value of the dot product between the vectors.
Figure 9.
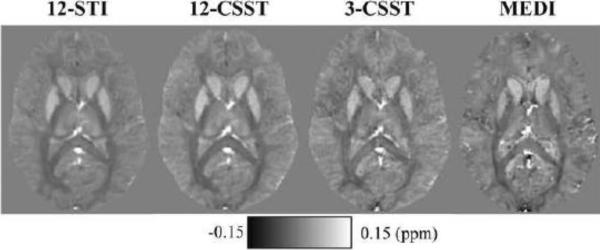
Mean magnetic susceptibility, from each of the susceptibility tensor reconstructions compared to a scalar susceptibility reconstruction of the neutral orientation.
Figure 10.
MSA maps generated from the 3-CSST of all of the volunteers, view in sagittal (top), axial (middle) and coronal (bottom) section.
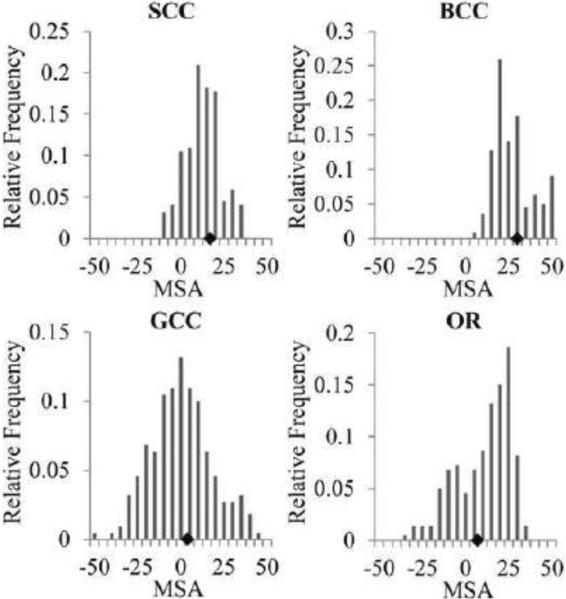
The 3-CSST mean MSA values measured in 4 major white matter tracts were listed in Table 1 for all subjects. The MSA for reconstructions in Subject 1 are listed in Table 2. There was reasonable consistency among the measured MSA values of the BCC across all subjects and among all reconstructions in Subject 1. There was also reasonable consistency among all subjects for 3-CSST MSA values of the OR. Compared to BCC, there were larger variations for MSA values of GCC and SCC. The MSA values for all 220 possible 3-CSST reconstructions were plotted for the 4 identified white matter tracts (Fig. 11), showed less spread in BCC and SCC than GCC and OR.
Table 1.
Mean AMS detected across 3-CSST reconstructions of all volunteers, in ppb
| SCC | BCC | GCC | OR | |
|---|---|---|---|---|
| Subject 1 | 5 ± 21 | 20 ± 38 | −24 ± 28 | 30 ± 24 |
| Subject 2 | 14 ± 12 | 31 ± 24 | −31 ± 14 | 30 ± 22 |
| Subject 3 | 8 ± 16 | 22 ± 23 | −35 ± 13 | 31 ± 22 |
| Subject 4 | −7 ± 18 | 37 ± 24 | −1 ± 13 | 27 ± 23 |
Table 2.
Mean AMS detected across reconstructions of 12 orientations in Subject 1, in ppb
| SCC | BCC | GCC | OR | |
|---|---|---|---|---|
| 12-STI | 34 ± 13 | 37 ± 13 | 43 ± 14 | 37 ± 13 |
| 12-CSST | 17 ± 14 | 30 ± 38 | 4 ± 20 | 7 ± 18 |
| 3-CSST | 5 ± 21 | 20 ± 38 | −24 ± 28 | 30 ± 24 |
Figure 11.
Histograms of the mean MSA (ppb) in white matter regions over all 220 combinations of 3-CSST from acquired orientations in Subject 1; the black diamonds mark the mean MSA estimated from the 12-CSST.
Discussion
Our analysis of decomposing macroscopic tissue magnetic susceptibility into a sum of microscopic molecular magnetic polarizabilities demonstrates that susceptibility anisotropy observed in MRI requires both intrinsic anisotropy in molecular polarizibility and macroscopic order in molecular arrangement. Order achieved by restricting a molecules' axis to lie in a plane or a line lead to cylindrical symmetry in the susceptibility tensor. Myelin lipids are a major source of susceptibility anisotropy of the major white matter tracts in the human brain. These structures have a macroscopic arrangement observable at the resolution of MRI, allowing the cylindrical symmetry model to be used in susceptibility tensor imaging (STI).
Neurological diseases such as multiple sclerosis (Stys et al., 2012) or rare hereditary neuropathies can affect the formation of myelin and lipid metabolism, which can greatly affect the presence and appearance of myelin (Sander et al., 2000; Cai et al., 2001; Perlman and Mar, 2012). The abnormal formation of myelin would undoubtedly affect the symmetry of the myelin sheath. These changes are very inconsistent on a microscopic scale and all such syndromes appear lead to eventual demyelination (Sander et al., 2000; Cai et al., 2001; Perlman and Mar, 2012). As a consequence to the reduced macroscopic order in lipids, we may expect reduced anisotropy in macroscopic susceptibility in diseased myelin. It has been observed within Shiverer mice that demyelinated white matter showed significant loss in MSA (Li et al., 2012a). Further evidence may be obtained from other mouse models of demyelination or dysmyelination and from patients with demyelinating diseases. Conversely remyelinated axons appear to regain some of the function of normal neurons and some of the cylindrical symmetry in the organization of lipid layers. (Keough and Yong, 2012). This remyelination process could be monitored by measuring the increase in MSA.
The cylindrical symmetry of the susceptibility tensor (CSST) can be estimated from diffusion tensor imaging (DTI) and can then be used to obtain an estimation of MSA of the white matter tracts of the brain from a reduced number of subject orientations. Our results in simulation, phantom and human studies show the reconstructed susceptibility tensor is very sensitive to the distribution of acquired subject orientations used in the reconstruction, which was not explored previously. With judiciously chosen 3 non-planar orientations in the human study, it was found that, the 3-CSST reconstruction had similar tensor anisotropy in main white matter regions compared to the 12-CSST and 12-STI reconstructions directly in this study, the in vivo anisotropy maps in earlier work with more orientations and/or higher field strength (Li et al., 2012a; Li et al., 2012b), and the anisotropy of white matter measured in vitro (Lee et al., 2010).
The reduction in the number of sampling orientations is highly desired in practice for STI. Acquiring all 12 orientations in a single volunteer required a total of 4 hours of scan time. In addition to the long scan time to acquire a single orientation (~10 min in this study without parallel imaging), repositioning and stably holding a human head at various orientations required particular care and time in preparing for each of the scans. Completing all 12 orientations demanded substantial cooperation from the subject and the subject had to take breaks between scan sessions. For this reason, we were only able to achieve 12 orientations in one subject. In contrast, the three orientations studies were easily acquired in every subject within 1 hour, which may be further shortened using parallel imaging. The three orientations (neutral, left and right leaning) performed by all volunteers in this study were tolerable. Additional orientations increase subject discomfort particularly when tilted forward or backward, which leads to decreased image quality due to motion and poorer field measurements. This reduction in time to within 1 hour and increase in subject tolerance are critical for both exploring clinical possibilities and research investigations requiring larger numbers of subjects.
Characterization of tensor anisotropy in STI is not as easily defined as it is in DTI. The fractional anisotropy (FA) commonly used in DTI for the characterization of the anisotropy of a tensor no longer has a meaningful interpretation with FA=0 as isotropic and FA=1 as a line for the susceptibility tensor; because components can be negative resulting in values for FA greater than 1. We also found FA to be too sensitive to the noise in STI, which has much poorer SNR than DTI. One may achieve an anisotropy measurement robust against noise and sensitive to high susceptibility anisotropy by adding a regularizing constant to the tensor components (Liu et al., 2012a). However, this may introduce a bias that varies with the regularization constant. With cylindrical symmetry each voxel is constrained to two principal susceptibility coefficients parallel and perpendicular to the fiber axis. Their difference, or MSA, is 0 for isotropic materials. Deviations away from 0 indicate anisotropy. This measure has been previously used to define susceptibility tensor anisotropy (Kimura et al., 2000; Li et al., 2012b), and can be extended for a general susceptibility tensor using its eigenvalues in STI.
Fundamentally, STI is an extension of the inverse problem of quantitatively determining the scalar susceptibility map from MR field measurements (QSM), which has an intrinsic ill-posedness arising from the zero cone surfaces of the dipole kernel in the Fourier domain. STI requires additional susceptibility parameters, further exacerbating the ill-conditioned nature of the STI inversion problem. The CSST uses tensor frame orientation information to reduce the number of unknowns to two parameters per voxel. This reduces but does not eliminate the ill-conditioning of determining the two tensor susceptibility parameters, perhaps due to the zeroes of coefficients (resembling dipole kernel coefficient in QSM) of the 2×2 linear system of equations (Eq.B.6). This ill-posedness can be characterized in a detailed analysis of the condition number for the CSST, similar to that for the COSMOS method of QSM (Liu et al., 2009; Wharton and Bowtell, 2010). Examination of 3-CSST over a range of orientations showed poor condition numbers ranging from 104 to 1011. The choice of 3 human orientations limits how far the condition number can be brought down. The worst error propagation was observed when the 3 orientations were roughly planar, leading to erroneous estimation of MSA. Accordingly, coplanar 3 orientations have to be avoided in implementing 3-CSST. Further investigation to improve estimation of the susceptibility tensor may include identifying an optimal configuration of orientations that produces the best conditioned system, and formulating a Bayesian approach that allows use of prior information similar to QSM (de Rochefort et al., 2008a; de Rochefort et al., 2010; Wharton and Bowtell, 2010; Bilgic et al., 2012; Liu et al., 2012b; Liu et al., 2012c; Schweser et al., 2012b).
It was found that the simulated 3-CSST reconstruction of orientations uniformly spread over a sphere provided fairly accurate estimation of the true susceptibility anisotropy, most within 10%. However, the 3-CSST is sensitive to the sampling angles (Fig. 3). In addition to the sampling angle dependence shared with QSM, there is an additional susceptibility tensor angle dependence unique to tensor imaging. When the 3 orientations are nearly coplanar, tensor components perpendicular to the plane do not contribute significantly to the observed field shifts in any orientation (Eq. 6), which poorly conditions the inverse problem contributing to large noise propagation in these components. Specifically, for the limited human range of orientations, the 3-CSST reconstruction may appear to be skewed, and the MSA detected may be erroneously high (Fig. 3).
Further simulations of realistic fiber directions and estimation of MSA show that within a particular set of three acquisitions, the MSA error maps (Fig. 4) show regions of consistent over- and under-estimation. The MSA error maps reflect the noise propagation into the reconstructed tensor components; the variance in the components was on the order of 0.01ppm in white matter and yielded possible errors of up to ± 50% for any particular fiber, Fig. 4. Less error is observed when more orientations are incorporated into the cylindrically symmetric reconstruction (such as the 12 orientations acquired in Subject 1), and when those orientations are more evenly distributed (such as the 12 uniformly distributed orientations). From these observations, high SNR and evenly distributed orientations were shown to be necessary for consistent estimation of susceptibility anisotropy with a realistic distribution of fiber orientations like those observed in the human brain even with the symmetry constraint.
A comparison of the MSA maps for the simulation and the volunteers, Fig. 4 vs. Fig. 10, showed similar regions of hyper- and hypo-intensity. In the simulation, this led to both under- and over-estimation of the anisotropy due to the distribution of acquisition directions unfavorable to those fibers. In the human data, there are many factors that are not accounted for in the simulation, such as the density of fibers and crossing fibers that exist in various regions in the human brain, which would also affect the MSA of the white matter. The brain simulation demonstrates that the distribution of orientations is a contributing factor to errors in the MSA in the human brain, particularly in the BCC and CS, as shown in Fig. 4.
There is a high degree of alignment between principal eigenvectors of diffusion tensors (DT) and susceptibility tensors (ST) in the corpus callosum and optic radiations, but there is also poor alignment in the centrum semiovale (Fig. 8). The pattern of the observed errors in the estimated MSA in the brain simulation is consistent with that of misalignment of the DT and ST eigenvectors of major white matter tracts in the human study. MSA underestimation for fibers parallel to the SI direction in the simulated brain (ϕ=0 in Fig. 4) corresponds to poor alignment between DTI and STI eigenvectors in the human data (Fig. 8). Differences in alignment could be due to errors in the STI reconstruction from limited human orientations or intrinsic differences between the ST and DT principal eigenvectors. The limited range of human orientations causes errors in the STI reconstruction, which may contribute to misalignment between the ST and DT. In the periphery of the brain, there are crossing fibers that may make it difficult to estimate DT eigenvectors and their alignment with ST eigenvectors. Our experimental data show that, for most major white matter tracts, the DTI derived prior information could be a reasonable estimate of the principal direction of the susceptibility tensor.
It is not straightforward to compare estimates of anisotropy between STI and CSST reconstructions. MSA from the STI reconstruction is always positive due to the ordering of the eigenvalues and there is no natural way to define parallel and perpendicular components explicitly in this reconstruction. This leads to decreasing variability of the MSA estimates, which are now by definition positive. The error in susceptibility tensor reconstruction is determined in large part by the noise amplification of the poorly conditioned inverse problem. This suggests that the constrained reconstruction also has the added benefit of denoising in situations where the known tensor symmetry can be used to improve the condition of the inversion.
Susceptibility tensor imaging of the human brain is challenging due to the limited range of orientations available to sample the orientation dependence of the magnetization. The lack of ground truth adds to the challenge. The center of k-space for the susceptibility tensor map is not determined, making a reference for the absolute susceptibility of any particular region difficult to define in a human subject. The focus of this study, MSA, is based on a difference of components, which should not be sensitive to this issue. The genu of the corpus callosum is particularly difficult to reconstruct in both the scalar and tensor susceptibility cases due to reduced SNR near the air tissue interfaces of the frontal sinuses. Imperfect background field removal further complicates reconstructing susceptibility tensor in some regions of the brain. Consequently, the reliability in estimating MSA is low in some regions of the brain. This may explain the greater variability observed in the genu of the corpus callosum than in other regions of the brain Table 2 and Fig. 11. While it is relatively easy for healthy volunteers to perform three orientations in the MR scanner, patients may have difficulties performing three head orientations, and the acquisition time of three orientations may be too long to be part of a clinical protocol. Given the dependence of MSA on the choice of the orientations for the 3-CSST (Fig. 11) the ability of a volunteer to follow instructions to perform the orientations may also affect the estimated anisotropy map.
STI shares many of the problems of DTI in estimating tensor components and anisotropy on a voxel level. For example, even with an ideal distribution of samples over a sphere, voxels containing crossing fibers pose a problem when reconstructing the tensor. A single MSA value as determined by the average tensor in the voxel may not reflect the different components present in that voxel. In the diffusion tensor case, this phenomenon effectively decreases the detected diffusion anisotropy erroneously (Basser and Jones, 2002). In these voxels, the principal diffusion direction may not be the parallel axis of the susceptibility tensor. Additionally, for the CSST reconstructions, DTI has a lower resolution when compared to the gradient echo data and thus the resolution with which DTI can determine the direction for thinner white matter tracts in the brain is reduced. This in turn lowers the confidence with which the assumption of symmetry can be applied in these voxels. This phenomenon may account for some of the differences in the susceptibility observed at the edges of anatomical structures or in the center of the corpus callosum at the gap between the hemispheres. However, increasing the resolution of the EPI based DTI acquisition also increases the noise in the images, which then reduces the reliability of the diffusion directions. As a result, the CSST constraint may only be appropriate for specific regions of the brain. Further investigations are needed to explore similarities and differences between diffusion and susceptibility tensor frames. For qualitative mapping of susceptibility anisotropy of white matter tracts, our volunteer data suggest that the instructions for this 3-CSST may be robust enough in practice.
Conclusion
In this work we have explored theoretical and experimental aspects of estimating MSA in white matter tracts from human feasible data. We have shown cylindrical symmetry in susceptibility anisotropy from configurations of molecules with orientations similar to the non-rigid structure of the myelin sheath. Cylindrical symmetry can be used to improve the condition of estimating the susceptibility tensor, CSST, from MRI acquired with few subject orientations. Our analysis of noise propagation shows the error in the estimated MSA of the CSST is sensitive to the relative angle between the fiber direction and the acquired orientations of the subject, with substantial error observed when the subject orientations are coplanar. We find that DTI guided estimation of cylindrical MSA in major white matter tracts of the brain can be reasonable with as few as three non-coplanar orientations particularly in the corpus callosum and optic radiations.
Highlights
Theoretical analysis of bulk susceptibility anisotropy from the magnetic polarizability of molecules within a voxel
Cylindrically symmetric susceptibility tensor estimation guided by DTI from human feasible three orientations (3CSST)
Consistent estimation of magnetic susceptibility anisotropy in vivo at 3T using 3CSST in major white matter tracts
Analysis of susceptibility anisotropy error sensitivity to fiber directions and sampling orientation distribution
Acknowledgements
This work was supported in part by NIH grants R01EB013443, R01NS072370, R43NS076092, and T35EB006732.
Appendix A The Susceptibility Tensor of a Voxel with Molecules of Various Organizations
In this section we will apply Schur's second lemma from group theory to express the expectation value of the magnetic polarizability tensor. To begin, we define irreducible and reducible representations; in this paper we are interested in matrix representations of the rotation groups. If a representation can be written in a block diagonal form, such that its action can be defined on its individual subspaces, it is said to be reducible otherwise the representation is irreducible (Hammermesh, 1962). For example a rotation about the z-axis can be expressed in 2 or 3 dimensions, the 2D representation (2×2 matrices) is irreducible, whereas the 3D representation (3×3 matrices, which are block diagonal with the 2D matrix as one block and 1 as the other block) is reducible. Schur's second lemma states: if the matrices R are irreducible representations of a group and if a matrix A commutes with R, RA = AR, for all R in the group then A is scalar, A = constant·I (Hammermesh, 1962).
As stated in Eq. 1 the susceptibility of a voxel is the sum of magnetic polarizabilities within a unit volume. Considering a specific organization of molecules, we introduce the expected mean value of the magnetic polarizability tensor as:
| [A.1] |
where Ra is a 3-dimenional rotation described by Eq. 10, and the integration is over the possible angles. The explicit matrix form of the rotation Ra is the representation of the 3D rotation group. In the form described by equation 10, all unconstrained 3D rotations form an irreducible representation. The mean polarizability 〈β〉 commutes with all 3D rotations Ra. By Schur's second lemma, 〈β〉 is a scalar in 3D space, and its value can be determined from its trace, which is invariant under rotations. As such the resulting susceptibility tensor is:
| [A.2] |
where n is a factor to account for the density the molecules within the voxel. Therefore, it follows directly from Schur's lemma that freely oriented molecules will result in an isotropic susceptibility tensor(χ =χI = constant times identity matrix). This result can also be shown numerically.
Now we consider fixing one of the components of the magnetic polarizability along the z-axis of the voxel. This only allows the molecular axes corresponding to β1 and β2 to rotate about the β3 axis freely; β3 remains along the z axis of the voxel as in Fig 2a.The group under consideration is the 2D rotation group about the z-axis. The matrix representation RZ(α), for the rotation about the z axis by an angle, α, is reducible in 3 dimensions, while the block in the x-y plane is irreducible. Correspondingly, we introduce a 3D mean polarizabilty tensor as:
| [A.3] |
and we can apply Schur's lemma similarly to the 2D block. From this we can obtain one expression for a cylindrically symmetric susceptibility tensor:
| [A.4] |
This is one possible configuration that could describe the anisotropy of white matter that restricts the β3 axis of the tensor to lie along the fiber direction, but allows the other components to orient freely in transverse plane, Fig. 2a. This organization shows susceptibility that is cylindrically symmetric with molecules that have any arbitrary anisotropy. This configuration may not be entirely realistic for white matter as it restricts one component to lie along the fiber direction, while it is known that the phospholipids of the myelin sheath can undergo diffusion within the membrane as the molecules are not chemically bound (Bagatolli et al., 2010; Kusumi et al., 2012).
We can obtain more realistic cylindrical symmetry in the organization of molecules by imposing that the β3 axis of the tensor must lie in the x-y plane, but the β1 and β2 axes are oriented evenly in the plane perpendicular to the β3 axis. This allows the molecules to have the freedom to rotate about the β3 axis in the transverse plane to account for their freedom of movement, Fig. 2b. To do this we decompose the 3-dimensional rotation matrix into its Euler angles using the zxz-convention such that (Roberts and Winch, 1984) : Ra = RZ(ϕa)RX(π/ 2)RZ(ψa), where RZ and RX are rotations about the z axis and x axis respectively at angles ϕa, π/2 and ψa. Here ϕa and ψa take all possible angles. Correspondingly, we introduce the mean polarizability tensor as:
| (A.5) |
Similar to Eq. A.3, we can apply Schur's lemma to the two 2D rotations, RZ (ϕa) and RZ (ψa). The resulting susceptibility tensor is:
| (A.6) |
This organization also shows cylindrical symmetry in the susceptibility tensor with arbitrary anisotropy in the molecules within the voxel. Both equations A.4 and A.6 produce a cylindrically symmetric tensor, similar to the white matter tensor model presented by Li et al. (Li et al., 2012a) without assuming that the lipid molecules themselves are cylindrically symmetric.
Appendix B Condition Number for Estimating the Susceptibility Tensor With and Without Constraints
In examining linear systems of the form, Ax = b, it is useful to have an indicator of the stability of the solution of a system from perturbations in the input data. Using numerical methods to find a solution for a linear system Ax =b, where A is Hermitian, it can be shown that under perturbations on a non-trivial b, δb, the error, δX, in the estimate of the solution, is bound by(Demmel, 1997):
| (B.1) |
where κ(A) is the condition of a linear system A and . In this case if the condition number is finite the error in the solution is bound by the condition number; if the condition number is infinite the error is unbounded and as such this system would be ill-posed. For a system of the form Ax = b, with A Hermitian, the condition number of A is an indicator of the error propagation that can be expected from small perturbations. The condition number with respect to the 2 norm is defined as the ratio of the maximum to minimum eigenvalues of the system, (Demmel, 1997). In estimating the STI and CSST, the original system matrix A is not Hermitian and the condition number of AHA is computed instead, whereH indicates the Hermitian transpose. In matrix form the STI and CSST systems can be written as block diagonal matrices for which it is possible to calculate the eigenvalues similar to COSMOS, (Liu et al., 2009).
For STI, Eq. 6 in matrix form is written as , where Δp,is the measured difference field for each orientation p and Dp,ij is a block diagonal matrix whose blocks are px6 matrices for every point in k-space, whose coefficients are given by the STI system described by Liu, (Liu, 2010), where the pth row has the form:
| (B.2) |
for every point, k, in k-space, where ki is the ith component of this vector, is the applied field vector for orientation p, bp,i is the ith component of this vector, each combination ij takes values ij = [ 11, 12, 13, 22, 23, 33]. After adopting the compact notation for these coefficients, Dp,jj (K) = [Dp,12 (K) ⋅ Dp,33 (K)], the system [DHD]ij,lm can be seen to be block diagonal with 6x6 blocks having coefficients:
| (B.3) |
where ij and lm take values: [11, 12, 13, 22, 23, 33]. The eigenvalues of the overall system are determined by the eigenvalues of these 6×6 blocks and the condition of the system is the ratio of the maximum to minimum eigenvalue. In order for this block to have full rank the number of orientations must be greater than or equal to 6.
With symmetry constraints Eq. 9 can be written in the form: , where the constraint in image space for the CSST introduces the complication where it is difficult to decouple the susceptibility coefficients from the forward problem for the CSST without forming a convolution in k-space. For the purposes of analysis, the condition number of the case where the rotation matrix is the identity matrix is examined such that the tensor is the same in subject and tensor frame. With this assumption the convolution in k-space reduces to convolution with a delta function. For this case the CSST in matrix form is written as Δp = DM XT, where M is a block diagonal matrix mapping function that allows us to write the parallel and perpendicular components in the tensor frame to the six components in the subject frame, whose blocks are:
| (B.4) |
The system DM can be shown to be block diagonal with px2 blocks of the form:
| (B.5) |
Similar to the unconstrained case the system MHDHDM, is a block diagonal system with 2×2 blocks of the form:
| (B.6) |
The coefficients of these square block matrices can be easily calculated to evaluate the eigenvalues and condition of this system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG. An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog Lipid Res. 2010;49:378–389. doi: 10.1016/j.plipres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Bender B, Klose U. The in vivo influence of white matter fiber orientation towards B0 on T2* in the human brain. NMR in Biomedicine. 2010;23:1071–1076. doi: 10.1002/nbm.1534. [DOI] [PubMed] [Google Scholar]
- Bertini I, Luchinat C, Parigi G. Magnetic susceptibility in paramagnetic NMR. Progress in Nuclear Magnetic Resonance Spectroscopy. 2002;40:249–273. [Google Scholar]
- Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Cash K, Swift J, Sutton-Smith P, Robinson M, Thompson PD, Blumbergs PC. Focal myelin swellings and tomacula in anti-MAG IgM paraproteinaemic neuropathy: novel teased nerve fiber studies. J Peripher Nerv Syst. 2001;6:95–101. doi: 10.1046/j.1529-8027.2001.01013.x. [DOI] [PubMed] [Google Scholar]
- Clark CA, Werring DJ, Miller DH. Diffusion imaging of the spinal cord in vivo: estimation of the principal diffusivities and application to multiple sclerosis. Magn Reson Med. 2000;43:133–138. doi: 10.1002/(sici)1522-2594(200001)43:1<133::aid-mrm16>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Crum WR, Rueckert D, Jenkinson M, Kennedy D, Smith SM. A framework for detailed objective comparison of non-rigid registration algorithms in neuroimaging. Medical Image Computing and Computer-Assisted Intervention - Miccai 2004. 2004;(Pt 1):679–686. Proceedings 3216. [Google Scholar]
- Cusack R, Papadakis N. New robust 3-D phase unwrapping algorithms: application to magnetic field mapping and undistorting echoplanar images. NeuroImage. 2002;16:754–764. doi: 10.1006/nimg.2002.1092. [DOI] [PubMed] [Google Scholar]
- de Rochefort L, Brown R, Prince M, Wang Y. Quantitative MR Susceptibility Mapping using piece-wise contast regularized inversion of the magnetic field. Magnetic Resonance in Medicine. 2008a;60:1003–1009. doi: 10.1002/mrm.21710. [DOI] [PubMed] [Google Scholar]
- de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, Wu J, Wang Y. Quantitative Susceptibility Map Reconstruction from MR Phase Data Using Bayesian Regularization: Validation and Applications to Brain Imaging Magnetic Resonance in Medicine. 2010;63:194–206. doi: 10.1002/mrm.22187. [DOI] [PubMed] [Google Scholar]
- de Rochefort L, Nguyen T, Brown R, Spincemaille P, Choi G, Weinsaft J, Prince MR, Wang Y. In vivo quantification of contrast agent concentration using the induced magnetic field for time resolved arterial input function measurement with MRI Medical Physics. 2008b;35 doi: 10.1118/1.3002309. [DOI] [PubMed] [Google Scholar]
- Demmel JW. Applied Numerical Linear Algebra. SIAM; Philadelphia: 1997. [Google Scholar]
- Denk C, Hernandez Torres E, MacKay A, Rauscher A. The influence of white matter fibre orientation on MR signal phase and decay. NMR Biomed. 2011;24:246–252. doi: 10.1002/nbm.1581. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hammermesh M. Group Theory and Its Application to Physical Problems. Dover Publications, Inc.; New York: 1962. [Google Scholar]
- Hext G. The Estimation of Second-Order Tensors, with Related Tests and designs. Biometrika. 1963;50 [Google Scholar]
- Hrouda F, Schulmann K. Conversion of the Magnetic-Susceptibility Tensor into the Orientation Tensor in Some Rocks. Physics of the Earth and Planetary Interiors. 1990;63:71–77. [Google Scholar]
- Keough MB, Yong VW. Remyelination Therapy for Multiple Sclerosis. Neurotherapeutics. 2012 doi: 10.1007/s13311-012-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Yamato M, Aoki H, Yamamoto I, Ishikawa F, Yamaguchi M, Tobita M. Determination of anisotropic diamagnetic susceptibility of polymeric fibers suspended in liquid. Japanese Journal of Applied Physics Part 1-Regular Papers Short Notes & Review Papers. 2001;40:2237–2240. [Google Scholar]
- Kimura T, Yamato M, Koshimizu W, Koike M, Kawai T. Magnetic orientation of polymer fibers in suspension. Langmuir. 2000;16:858–861. [Google Scholar]
- Koenig E. Cell Biology of the Axon. Springer; 2009. [Google Scholar]
- Kressler B, de Rochefort L, Liu T, Spincemaille P, Jiang Q, Wang Y. Nonlinear regularization for per voxel estimation of magnetic susceptibility distributions from MRI field maps. IEEE Transactions on Medical Imaging. 2010;29 doi: 10.1109/TMI.2009.2023787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KG. Dynamic Organizing Principles of the Plasma Membrane that Regulate Signal Transduction: Commemorating the Fortieth Anniversary of Singer and Nicolson's Fluid-Mosaic Model. Annu Rev Cell Dev Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- Lee J, Shmueli K, Fukunaga M, Van Gelderen P, Merkle H, Silva A, Duyn JH. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. PNAS. 2010;107:5130–5135. doi: 10.1073/pnas.0910222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, van Gelderen P, Kuo LW, Merkle H, Silva AC, Duyn JH. T2*-based fiber orientation mapping. NeuroImage. 2011;57:225–234. doi: 10.1016/j.neuroimage.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Avram AV, Liu C. Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. NeuroImage. 2012a;59:2088–2097. doi: 10.1016/j.neuroimage.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu B, Liu C. In Vivo Evidence of Susceptibility Anisotropy and Susceptibility Tensor Imaging of Human Brain. Proc. Intl. Soc. Mag. Reson. Med. 2011a;19:121. [Google Scholar]
- Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage. 2011b;55:1645–1656. doi: 10.1016/j.neuroimage.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Vikram DS, Lim IA, Jones CK, Farrell JA, van Zijl PC. Mapping magnetic susceptibility anisotropies of white matter in vivo in the human brain at 7T. NeuroImage. 2012b;62:314–330. doi: 10.1016/j.neuroimage.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Susceptibility tensor imaging. Magn Reson Med. 2010;63:1471–1477. doi: 10.1002/mrm.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li W, Johnson GA, Wu B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. NeuroImage. 2011a;56:930–938. doi: 10.1016/j.neuroimage.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li W, Wu B, Jiang Y, Johnson GA. 3D fiber tractography with susceptibility tensor imaging. NeuroImage. 2012a;59:1290–1298. doi: 10.1016/j.neuroimage.2011.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, Tsiouris AJ, Wisnieff C, Spincemaille P, Prince MR, Wang Y. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. NeuroImage. 2012b;59:2560–2568. doi: 10.1016/j.neuroimage.2011.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu J, de Rochefort L, Spincemaille P, Khalidov I, Ledoux JR, Wang Y. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: Comparison with COSMOS in human brain imaging. Magn Reson Med. 2011b doi: 10.1002/mrm.22816. [DOI] [PubMed] [Google Scholar]
- Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y. Calculation of Susceptibility Through Multiple Orientation Sampling (COSMOS): A Method for Conditioning the Inverse Problem From Measured Field Map to Susceptibility Source Image in MRI. Magnetic Resonance in Medicine. 2009;61:196–204. doi: 10.1002/mrm.21828. [DOI] [PubMed] [Google Scholar]
- Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2012c doi: 10.1002/mrm.24272. [DOI] [PubMed] [Google Scholar]
- Lounila J, Ala-Korpela M, Jokisaari J, Savolainen MJ, Kesaniemi YA. Effects of orientational order and particle size on the NMR line positions of lipoproteins. Physical Review Letters. 1994;72:4049–4052. doi: 10.1103/PhysRevLett.72.4049. [DOI] [PubMed] [Google Scholar]
- Perlman SJ, Mar S. Leukodystrophies. Advances in Tnf Family Research. 2012;724:154–171. doi: 10.1007/978-1-4614-0653-2_13. [DOI] [PubMed] [Google Scholar]
- Prosser RS, Hwang JS, Vold RR. Magnetically aligned phospholipid bilayers with positive ordering: a new model membrane system. Biophys J. 1998a;74:2405–2418. doi: 10.1016/S0006-3495(98)77949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RS, Volkov VB, Shiyanovskaya IV. Solid-state NMR studies of magnetically aligned phospholipid membranes: taming lanthanides for membrane protein studies. Biochem Cell Biol. 1998b;76:443–451. doi: 10.1139/bcb-76-2-3-443. [DOI] [PubMed] [Google Scholar]
- Roberts PH, Winch DE. On Random Rotations. Advances in Applied Probability. 1984;16:638–655. [Google Scholar]
- Sander S, Ouvrier RA, McLeod JG, Nicholson GA, Pollard JD. Clinical syndromes associated with tomacula or myelin swellings in sural nerve biopsies. Journal of Neurology Neurosurgery and Psychiatry. 2000;68:483–488. doi: 10.1136/jnnp.68.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub H, Junkins JL. Analytical Mechanics of Space Systems. American Institute if Areonautics and Astronautics; 2003. [Google Scholar]
- Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Medical Physics. 1996;23:815–850. doi: 10.1118/1.597854. [DOI] [PubMed] [Google Scholar]
- Schweser F, Deistung A, Sommer K, Reichenbach JR. Toward online reconstruction of quantitative susceptibility maps: Superfast dipole inversion. Magn Reson Med. 2012a doi: 10.1002/mrm.24405. [DOI] [PubMed] [Google Scholar]
- Schweser F, Sommer K, Deistung A, Reichenbach JR. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. NeuroImage. 2012b;62:2083–2100. doi: 10.1016/j.neuroimage.2012.05.067. [DOI] [PubMed] [Google Scholar]
- Stys PK, Zamponi GW, van Minnen J, Geurts JJG. Will the real multiple sclerosis please stand up? Nature Reviews Neuroscience. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- Waxman S, Kocsis J, Stys P. The Axon: Structure Function and Pathophysiology. Oxford University Press; 1995. [Google Scholar]
- Wharton S, Bowtell R. Whole-Brain Susceptibility Mapping at High Field: A Comparison of Multiple- and Single-Orientation Methods. NeuroImage. 2010;53:515–525. doi: 10.1016/j.neuroimage.2010.06.070. [DOI] [PubMed] [Google Scholar]
- Wharton S, Bowtell R. A Simplified Approach for Anisotropic Susceptibility Map Calculation. Proc. Intl. Soc. Mag. Reson. Med. 2011;19:4515. [Google Scholar]
- Wisnieff C, Liu T, Spincemaille P, Wang Y. Proc. Intl. Soc. Mag. Reson. Med. Melbourne: 2012a. Feasible 3-Orientation Acquisition for Detecting Susceptibility Anisotropy in the Human Brain Using Prior Structural Information; p. 3444. [Google Scholar]
- Wisnieff C, Liu T, Wang Y. Proc. Intl. Soc. Mag. Reson. Med. Melbourne: 2012b. Anatomic Prior and Cylindrical Symmetry Constraints for Reconstructing Susceptibility Tensor; p. 3445. [Google Scholar]
- Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]