Abstract
Purpose
Tranexamic acid (TXA) reduces blood loss in patients undergoing total knee arthroplasty (TKA). However, few studies have reported the optimum timing and dosage for administration of TXA. The purpose of this study was to evaluate the effect of repeat-dose TXA on blood loss during TKA and the necessity of autologous blood donation or postoperative autotransfusion.
Methods
We enrolled 78 patients with primary osteoarthritis undergoing cemented TKAs. Consecutive patients were divided into three groups, as follows: control group (n = 31), single-TXA group (n = 21) in whom TXA (1,000 mg) was intravenously administered 10 min before deflation of the tourniquet, and twice-TXA group (n = 26) in whom TXA (1,000 mg) was intravenously administered 10 min before deflation of the tourniquet and 3 h after the operation. We measured the volume of drained blood after the operation. Haemoglobin (Hb) levels were measured at days 1, 4 and 7 postoperation. Venous thromboembolic events (VTE) were screened using compression ultrasonography at enrollment and 1 and 7 days after operation.
Results
The mean volume of drained blood after the operation was lower in the twice-TXA group than in the single-TXA (p < 0.001) and control (p < 0.0001) groups. No significant differences were observed in the incidence of VTE between these groups.
Conclusion
Administration of TXA twice reduced postoperative blood loss after TKA, and TXA was not associated with the risk of deep-vein thrombosis (DVT) or pulmonary embolism (PE). Further, administration of TXA twice may eliminate the need for blood transfusion during TKA.
Introduction
Total knee arthroplasty (TKA) is usually performed using a tourniquet. Several studies have shown that postoperative blood loss from drainage ranges from 500 to 1,000 ml, and a hidden loss of >700 ml also occurs [1]. Allogeneic blood transfusion carries important risks of an immunological reaction and transmission of disease, particularly infective diseases. Furthermore, blood transfusion involves additional cost [2, 3]. A surgeon may consider reducing the postoperative risks of allogeneic blood transfusion in patients by preoperative autologous blood donation [4], postoperative blood salvage [5, 6], anaesthetic techniques [7] and drain clamping [8, 9].
Tranexamic acid (TXA) inhibits fibrinolysis by blocking the lysine-binding sites of plasminogen, which degrades fibrin. There are two ways to apply TXA: intravenous injection, and intra-articular injection. Recently, the effect of intra-articular TXA and drain clamping was reported [10, 11]. Although this is a simple and more efficient method to deliver TXA, TXA is applied at the only chance available, i.e. the end of the operation. On the other hand, several studies have shown that administration of 10–15 mg/kg of intravenous TXA before tourniquet release and after the operation decreases the blood loss associated with TKA [12, 13]. Administration of intravenous TXA was not associated with increased risk of deep vein thrombosis (DVT) in those studies [14]. However, few studies have reported the optimum timing and dosage for TXA administration. Thus, we performed a cohort study, not a randomised study, to evaluate the effect of repeat-dose TXA on blood loss during TKA and the necessity of autologous blood donation or postoperative autotransfusion.
Patients and methods
Between August 2009 and April 2011, we enrolled 78 patients with primary osteoarthritis who were undergoing cemented TKAs. In all patients, TKA was performed by one surgeon (ST). Exclusion criteria were preoperative hepatic or renal dysfunction, serious cardiac or respiratory disease, congenital or acquired coagulopathy and history of thromboembolic disease. Consecutive patients were divided into three groups: the control group (n = 31); the intraoperative TXA group (single-TXA group, n = 21), who received intravenous administration of TXA (1,000 mg; Transamin 1 g/10 ml; Daiichi-Sankyo Co Ltd, Japan) 10 min before tourniquet deflation; and intra- and postoperative-TXA group (twice-TXA group, n = 26), which received intravenous administration of TXA (1,000 mg) 10 min before tourniquet deflation and 3 h after the operation. In the control group, we prepared for both autologous donation and postoperative autotransfusion (CBCII; Stryker, USA). We prepared postoperative auto-transfusion in the single-TXA group and no transfusion in the twice-TXA group.
A tourniquet was placed around the thigh and inflated to a pressure of 280 mmHg after exsanguination. An anteromedial skin incision was made, and the mini-incision mid-vastus approach was used in all patients. Patellar replacement was performed in all patients, and all components were fixed using cement. The medullary cavity was plugged using an autologous bone.
At the end of the procedure, the tourniquet was deflated, and major bleeding was controlled by diathermy before closure. An intra-articular drain was used. On the first postoperative day, the drain was removed and physiotherapy was initiated. Haemoglobin (Hb) levels were measured at days1, 4 and 7 postoperation. Blood loss after surgery was estimated using two different methods. The first was the standard clinical method in which blood loss was measured as the volume recovered in drains. Apparent blood loss was determined the sum of intraoperative blood loss and postoperative volume of drained blood. We measured the increase in blood volume (BV) during the following four periods: from the time of arrival in the recovery room to 3 h after the operation, from 3 h to 6 h after the operation, from 6 h to 9 h after the operation and from 9 h to 12 h after the operation. The second method was based on Hb balance [15]. We assumed that BV in litres on postoperative day 4 was the same as that before surgery. BV was estimated according to the method of Nadler and colleagues [16]. The loss of Hb was then estimated according to the following formula:
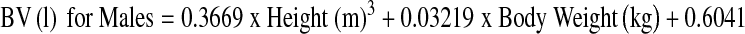 |
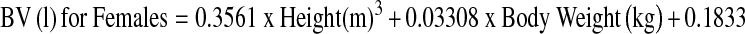 |
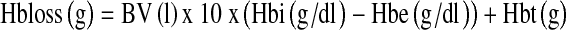 |
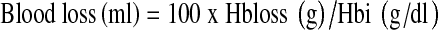 |
where Hbloss (g) was the amount of Hb lost, Hbi (g/dl) was the Hb concentration before surgery, Hbe (g/dl) was the Hb concentration on postoperative day 4 and Hbt (g) was the total amount of allogeneic and autologous Hb transfused
Standard thromboembolic prophylaxis was performed after surgery. Antiembolic stockings and a foot pump were used for all patients, and 2,000 IU enoxaparin sodium (Kaken Pharmaceutical Co Ltd, Tokyo, Japan) was administered starting 36 h after surgery and continuing every 24 h for 10 days [17]. Venous thromboembolic (VTE) events included symptomatic VTE or asymptomatic DVT screened routinely by compression ultrasonography at enrollment, on postoperative day1 and on postoperative day 7. When the result of compression ultrasonography was positive, enhanced computed tomography (CT) was performed to confirm the diagnosis as proximal DVT or pulmonary embolism (PE).
In all groups, we used the principles of transfusion based on the guidelines for postoperative surgical patients suggested by the American Association of Blood Banks (AABB) [18]. Transfusion was considered at a haemoglobin concentration of ≤8 g/dl or for symptoms of acute anemia. In the end, the need for transfusion was decided upon by the orthopaedic surgeon (ST) on the basis of the symptoms of acute anemia.
For statistical analysis, quantitative variables were expressed as mean and standard deviation (SD) and qualitative variables by absolute and relative frequencies. The quantitative variables were compared using analysis of variance (ANOVA) and Bonferroni multiple comparison. Pearson’s chi-square test was used to calculate adverse events (AEs). Stat View Ver.5.0 was used as the statistical software. This observational study was approved by the local ethical committee, and all patients signed informed consent.
Results
The three groups were comparable in terms of age, gender, height, bodyweight, body mass index (BMI), operating time, duration of tourniquet inflation and preoperative Hb (Table 1).
Table 1.
Patient characteristics
| Control | Single-TXA | Twice-TXA | ||
|---|---|---|---|---|
| (n = 31) | (n = 21) | (n = 26) | ||
| Age (years) | 73.7 ± 7.3 | 74.7 ± 5.3 | 75.0 ± 5.0 | |
| Height (cm) | 151.9 ± 5.9 | 151.8 ± 7.6 | 150.4 ± 8.3 | |
| Body weight (kg) | 64.9 ± 9.9 | 60.6 ± 11.5 | 59.0 ± 9.0 | |
| BMI (kg/m2) | 28.1 ± 3.8 | 26.2 ± 4.2 | 26.0 ± 3.1 | |
| Operating time (min) | 145.7 ± 18.8 | 143.6 ± 15.1 | 140.7 ± 17.5 | |
| Tourniquet time (min) | 113.8 ± 16.4 | 107.9 ± 10.1 | 103.6 ± 15.2 | |
| Preoperative Hb (g/dl) | 12.6 ± 1.1 | 12.4 ± 1.5 | 12.8 ± 1.6 | |
There was no significant difference between groups
TXA tranexamic acid, BMI body mass index, Hb haemoglobin
Blood loss measured using the clinical method
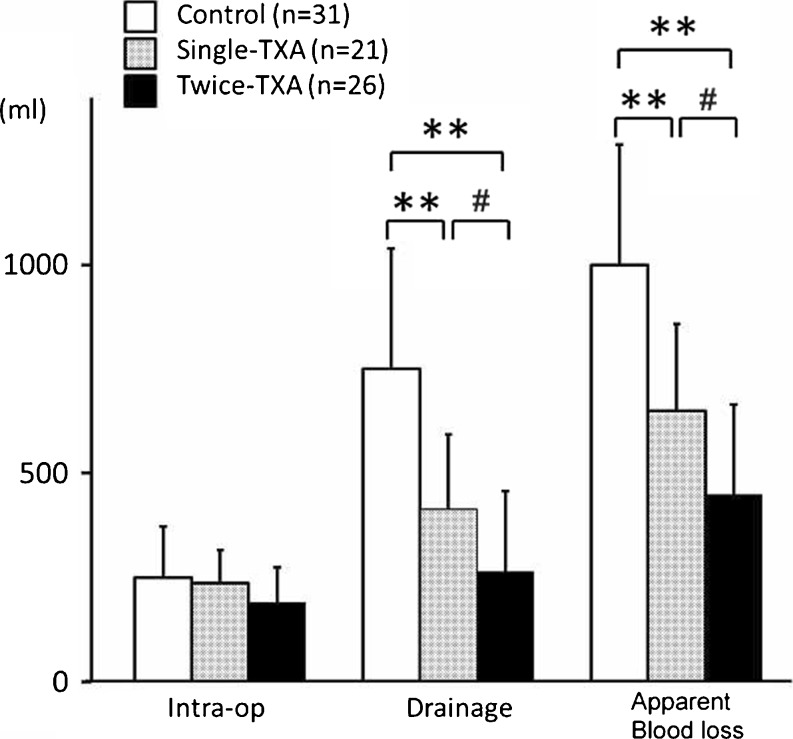
All groups were similar, with no significant differences in mean intraoperative blood loss (Fig. 1).
Fig. 1.
Blood loss measured by the clinical method. Data are presented as mean. Asterisks indicate a significant difference (**p < 0.0001, # p < 0.05)
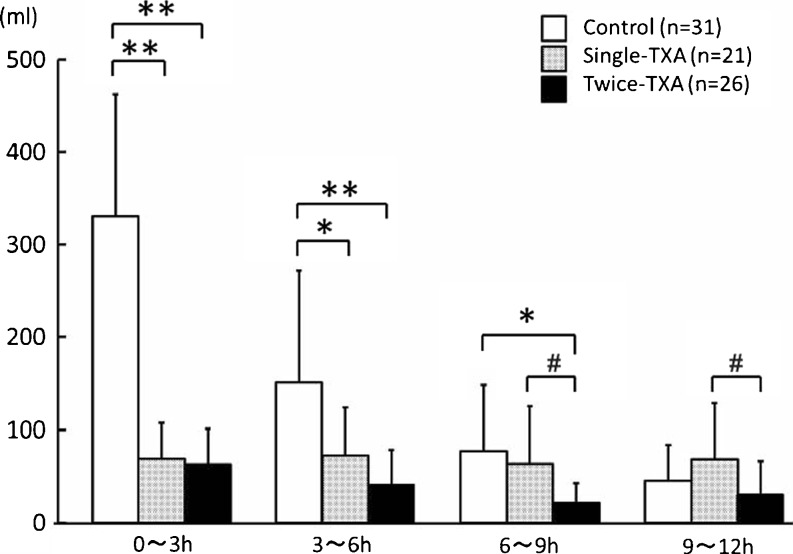
Mean postoperative volume of drained blood was lower in the twice-TXA group (263.7 ml) than in the single-TXA (413.3 ml; p = 0.030) and control (749.7 ml; p < 0.0001) groups. Mean apparent blood loss was lower in the twice-TXA group (454.5 ml) than in the control (999.4 ml; p < 0.0001) and single (649.8 ml; p = 0.0067) groups. The mean volume of drained blood from the time of arrival in the recovery room to 3 h after operation (po3 h) and from po3 h to po6 h was lower in the twice-TXA (p < 0.0001, p < 0.0001) and the single-TXA (p < 0.0001, p = 0.0008) groups than in the control group. However, no significant difference was observed between the single-TXA and control groups from po6 h to po9 h (p = 0.39); tmean BV was lower in the twice-TXA group than in other two groups (vs. control group, p = 0.0003; vs. single-TXA group, p = 0.011) (Fig. 2).
Fig. 2.
Mean drained blood volume during each 3-h postoperatively. Data are presented as mean. Asterisks indicate a significant difference (**p < 0.0001, *p < 0.001, # p < 0.05)
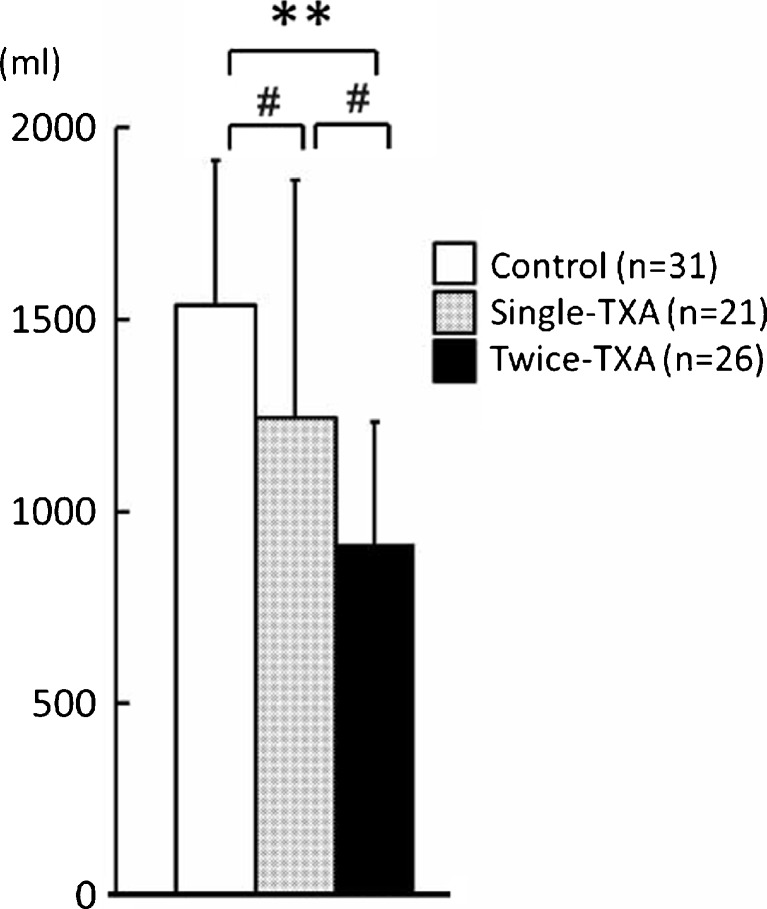
Estimated blood loss
Mean estimated total blood loss on postoperative day 4 was lower in the twice-TXA group (915.3 ml) than in the single-TXA group (1246.6 ml) and control group (1538.3 ml) (vs. control group, p < 0.0001; vs. single-TXA group, p = 0.012) (Fig. 3).
Fig. 3.
Estimated blood loss on the fourth day after surgery. Data are presented as mean. Asterisks indicate a significant difference (**p < 0.0001, # p < 0.05)
Hb balance and transfusion requirement
Hb level on postoperative day 1 was similar in the three groups. On postoperative days 4 and day 7, it was greater in the control group with autologous donation than in the single-TXA group (p < 0.05). Mean volume of postoperative autotransfusion was lower in the single-TXA group (34.0 ml) than in the control group (396.8 ml) (p < 0.0001). However, our results showed that allogeneic blood transfusion was not required in the TXA and control groups (Table 2).
Table 2.
Haemoglobin (Hb) balance and transfusion requirement
| Control | Single-TXA | Twice-TXA | ||
|---|---|---|---|---|
| (n = 31) | (n = 21) | (n = 26) | ||
| Postoperative Hb day 1 (g/dl) | 10.6 ± 1.2 | 10.2 ± 1.1 | 10.7 ± 1.6 | |
| Postoperative day 4 (g/dl) | 9.9 ± 1.2 | 8.7 ± 1.3a | 9.4 ± 1.3 | |
| Postoperative day 7 (g/dl) | 9.9 ± 1.2 | 9.2 ± 1.3a | 9.6 ± 1.4 | |
| Autologous donation (ml) | 371.6 ± 100 | – | – | |
| Postoperative autotransfusion (ml) | 396.8 ± 221 | 34.0 ± 66.6b | – | |
| Allogeneic transfusion (ml) | 0 | 0 | 0 | |
TXA tranexamic acid
aMean postoperative Hb was greater in the control group with autologous donation than in the single-TXA group (p < 0.05)
bMean volume of postoperative autotransfusion was lower in the single-TXA group than in the control group (p < 0.0001)
Adverse events
The incidence of DVT on postoperative day 1 was 12.9 % (4/31) in the control group, 9.5 % (2/21) in the single-TXA group and 11.1 % (3/26) in the twice-TXA group; and on postoperative day 7, it was 22.6 % (7/31) in the control group, 19.0 % (4/21) in the single-TXA group and 19.2 % (5/26) in the twice-TXA group. No significant differences were observed between these groups on postoperative days 1 and 7. The incidence of PE was 3 % (1/31) in the control group on postoperative day 1, and PE was not observed in the TXA group (Table 3).
Table 3.
Adverse events
| Control (n = 31) | Single-TXA (n = 21) | Twice-TXA (n = 26) | |||
|---|---|---|---|---|---|
| DVT [n (%)] | Day 1 | 4/31 (12.9) | 2/21 (9.5) | 3/26 (11.1) | |
| Day 7 | 7/31 (22.6) | 4/21 (19.0) | 5/26 (19.2) | ||
| PE [n (%)] | Day 1 | 1/31 (3.2) | 0/21 (0) | 0/26 (0) | |
| Day 7 | 1/31 (3.2) | 0/21 (0) | 0/26 (0) | ||
There was no significant difference between groups
Discussion
Several studies have shown that preoperative autologous blood donation, perioperative transfusion, anaesthetic techniques and normovolemic haemodilution are useful methods for avoiding allogeneic blood transfusion. Preoperative autologous blood donation followed by autotransfusion is an expensive procedure with logistic problems in many hospitals. Furthermore, about 45 % of predonated units may be discarded for of different reasons [19]. Although the use of air tourniquet decreases intraoperative blood loss in TKA, postoperative blood loss occurs because of increased fibrinolysis in response to exsanguination [20]. Because hyperfibrinolysis is considered to be the major cause of postoperative bleeding after TKA, antifibrinolytic drugs, including aprotinin, aminocaproic acid (EACA) and TXA have been proposed.
TXA has gained popularity in reducing perioperative blood loss, particularly after the publication of a trial in high-risk cardiac surgery [21]. TXA is cheaper and safer than aprotinin, much more potent than EACA and has overall good penetration into the major joints [22]. In our study, administration of TXA reduced postoperative blood loss and eliminated the need of autologous blood donation and autotransfusion. In the control group, 88 % of all drained BV was discharged until 9 h after the operation. Blood levels of TXA are reduced by half from 2 to 3 h after intravenous administration. Therefore, administration of TXA 3 h after the operation reduced blood loss from 6 h to 9 h after the operation. There was no significant difference between the three groups in mean intraoperative blood loss. We believe the reason was due to the small number of patients and the delay of TXA administration in the operation.
The use of antifibrinolytics has increased anxiety about increased thrombotic tendency. However, only case reports for cerebral thrombosis, arterial thrombosis, acute renal failure and coronary graft occlusion exist for TXA administration [23]. Furthermore, several dose-ranging studies have recommended TXA dose of up to 100 mg/kg in patients undergoing cardiac surgery [24]. Murkin et al. reported that a high dose of TXA ranging from 61 to 259 mg/kg had no adverse events [25]. In the case of our patients, we found no differences between the control and the two TXA groups regarding VTE rate, including asymptomatic DVT and PE.
Our study has several limitations. The major limitation was the small sample size. Further, he study was not randomized. Because we excluded patients with a history of thromboembolic disease, we did not examine the risk of TXA administration for patients with VTE. The requirement for transfusion was decided upon on the basis of the symptoms of acute anemia determined by the orthopaedic surgeon (ST).
Administration of TXA twice reduced postoperative blood loss after TKA, and TXA was not associated with the risk of DVT and PE. Further, administration of TXA twice may eliminate the need for blood transfusion during TKA.
Acknowledgments
Conflict of interest
The authors received no financial support and have no competing interest with regard to this manuscript.
Contributor Information
Takao Iwai, Phone: +81-728-402641, FAX: +81-728-4022662.
Shigeyoshi Tsuji, Phone: +81-72-8402641, FAX: +81-72-8402266, Email: shige-tt@nike.eonet.ne.jp.
Masayuki Hamada, Phone: +81-728-402641, FAX: +81-728-4022662.
References
- 1.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86–4:561–565. [PubMed] [Google Scholar]
- 2.Nozoe T, Miyazaki M, Saeki H, Ohga T, Sugimachi K. Significance of allogenic blood transfusion on decreased survival in patients with esophageal carcinoma. Cancer. 2001;92–7:1913–1918. doi: 10.1002/1097-0142(20011001)92:7<1913::AID-CNCR1709>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Spahn DR, Casutt M. Eliminating blood transfusions. New aspects and perspectives. Anesthesiology. 2000;93:242–255. doi: 10.1097/00000542-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 4.Dalen T, Brostrom LA, Engstrom KG. Autotransfusion after total knee arthroplasty. Effects on blood cells, plasma chemistry, and whole blood rheology. J Arthroplast. 1997;12–5:517–525. doi: 10.1016/S0883-5403(97)90174-1. [DOI] [PubMed] [Google Scholar]
- 5.Adalberth G, Bystrom S, Kolstad K, Mallmin H, Milbrink J. Postoperative drainage of knee arthroplasty is not necessary: a randomized study of 90 patients. Acta Orthop Scand. 1998;69–5:475–478. doi: 10.3109/17453679808997781. [DOI] [PubMed] [Google Scholar]
- 6.Sinha A, Sinha M, Burgert S. Reinfusion of drained blood as an alternative to homologous blood transfusion after total knee replacement. Int Orthop. 2001;25–4:257–259. doi: 10.1007/s002640100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juelsgaard P, Larsen UT, Sorensen JV, Madsen F, Soballe K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med. 2001;26–2:105–110. doi: 10.1053/rapm.2001.21094. [DOI] [PubMed] [Google Scholar]
- 8.Prasad N, Padmanabhan V, Mullaji A. Comparison between two methods of drain clamping after total knee arthroplasty. Arch Orthop Trauma Surg. 2005;125–6:381–384. doi: 10.1007/s00402-005-0813-7. [DOI] [PubMed] [Google Scholar]
- 9.Raleigh E, Hing CB, Hanusiewicz AS, Fletcher SA, Price R. Drain clamping in knee arthroplasty, a randomized controlled trial. ANZ J Surg. 2007;77–5:333–335. doi: 10.1111/j.1445-2197.2007.04053.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acidreduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35:1639–1645. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutsuzaki H, Ikeda K (2012) Intra-articular injection of tranexamic acid via a drain plus drain-clamping to reduce blood loss in cementless totalknee arthroplasty. 7(1):32 [DOI] [PMC free article] [PubMed]
- 12.Kakar PN, Gupta N, Govil P, Shah V. Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian J Anaesth. 2009;53(6):667–671. [PMC free article] [PubMed] [Google Scholar]
- 13.Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206–1211. doi: 10.1034/j.1399-6576.2002.461007.x. [DOI] [PubMed] [Google Scholar]
- 14.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg. 2011;93(12):1577–1585. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 15.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 16.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 17.Fuji T, et al. Prevention of postoperative venous thromboembolism in Japanese patients undergoing total hip or knee arthroplasty: two randomized, double-blind, placebo-controlled studies with three dosage regimens of enoxaparin. J Orthop Sci. 2008;13(5):442–451. doi: 10.1007/s00776-008-1264-0. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 19.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg [Am] 1999;81–1:2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Petaja J, Myllynen P, Myllyla G, Vahtera E. Fibrinolysis after application of a pneumatic tourniquet. Acta Chir Scand. 1987;153(11–12):647–651. [PubMed] [Google Scholar]
- 21.Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysin analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 22.Ellis MH, Fredman B, Zohar E, Ifrach N, Jedeikin R. The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. J Clin Anesth. 2001;13–7:509–513. doi: 10.1016/S0952-8180(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 23.Dunn CJ, Goa KL. Tranexamic Acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 24.Karski JM, Dowd NP, Joiner R, Carroll J, Peniston C, Bailey K, Glynn MF, Teasdale SJ, Cheng DC. The effect of three different doses of tranexamic acid on blood loss after cardiac surgery with mild systemic hypothermia. J Cardiothorac Vasc Anesth. 1998;12:642.6. doi: 10.1016/S1053-0770(98)90235-X. [DOI] [PubMed] [Google Scholar]
- 25.Murkin JM, Falllter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;1–110(2):350–353. doi: 10.1213/ANE.0b013e3181c92b23. [DOI] [PubMed] [Google Scholar]