Abstract
Purpose
The purpose of this study was to explore the effect of heparin on bone morphogenetic protein 6 (BMP6) osteogenic activity.
Methods
Western blot analysis was used to confirm the binding of BMP6 to heparin and to observe its effect on BMP6 signaling in C2C12-BRE-Luc myoblasts. Real-time RT-PCR was performed for the expression analysis of alkaline phosphatase (ALP) and osteocalcin (OC) in C2C12 myoblasts treated with BMP6 and heparin for 72 hours. Rat ectopic bone formation assay was performed to explore the effect of heparin on BMP6 osteogenic activity. Two weeks following implantation the implants were analysed morphologically and histologically. A mouse osteoporotic model was used to test the ability of BMP6 to improve the bone quality in vivo in the presence of heparin, followed by DEXA and μCT analyses. Blood coagulation was tested in rats previously treated with BMP6.
Results
BMP6 specifically bound to heparin and induced Smad1/5/8 phosphorylation which was inhibited by heparin. After 48 and 72 hours of treatment, heparin inhibited BMP6-induced ALP and OC expression in C2C12 cells. Heparin dose dependently inhibited BMP6-induced new bone and cartilage formation in the rat ectopic bone formation assay, while in osteoporotic mice heparin inhibited the BMP6 potential to improve the bone quality as evidenced by decreased bone mineral density and trabecular bone parameters. Interestingly, BMP6 prevented the effect of heparin on the blood coagulation parameters.
Conclusion
The interaction of BMP6 with heparin might contribute to the heparin-induced osteoporosis and blood coagulation.
Introduction
Osteoporosis is a rare, but potentially serious complication of long-term heparin therapy [1–5]. Although symptomatic fractures occur in less than 5 % of patients receiving heparin [6], around one third of them have a reduction in bone density [7]. Long-term administration of standard heparin is not prescribed frequently, but is indicated for the prevention of venous thromboembolism, treatment of pulmonary embolism and venous thrombosis, patients who undergo vascular surgery and coronary angioplasty, and selected patients with disrupted coagulation, such as protein C deficiency [8].
Heparin is a highly sulphated glycosaminoglycan and the most negatively charged naturally occurring molecule. Structurally, it is similar to heparan sulphate, which is, in the form of heparan sulphate (HS) proteoglycans (PGs), found at the cell surface and in the extracellular matrix (ECM). Both heparin and HS consist of a core protein and highly sulphated glycosaminoglycan (GAG) chains, although with different cellular localisation they share structural similarities [9]. GAGs are composed of disaccharide units of D-glucuronic acid-N-acetyl-D-glucosamine (GlcA-GlcNAc), modified by N-deacetylation/N-sulphation of GlcNAc, epimerisation of GlcA to α-D-iduronic acid (IdoA), 2-O-sulphation of GlcA and IdoA and 6-O-sulphation of N-sulfoglucosamine [10]. Compared to heparan sulphate, heparin N-acetyl groups correspond to less than 5 % of all glucosamine units, but the degree of sulphation is higher than in heparan sulphates [9]. Despite differences, heparin derivatives are frequently used in exploring the HS role in binding and interaction with various molecules to initiate cell signalling [9].
The preferred routes of heparin administration are continuous iv infusion and sc injections [8]. In plasma, it binds to a number of different plasma proteins, which reduces its anticoagulant activity to about one third. Using 125I-labelled heparin, it has been demonstrated that it accumulates and remains in bone long after stopping the treatment [11]. It induces bone resorption by increasing the number and activity of osteoclasts (OC), probably by inhibiting the expression of osteoprotegerin (OPG) [12]. In parallel, heparin reduces bone formation by decreasing the number and activity of osteoblasts (OB) [12]. However, the precise mechanism of heparin’s effect on bone remodelling remains undetermined.
It has been reported that heparin and HS influence the activity of bone morphogenetic proteins (BMPs) [10, 13–15], which are members of the transforming growth factor-β (TGF-β) superfamily of proteins that regulate a variety of developmental processes including proliferation, differentiation, pattern formation and apoptosis [13, 14, 16–19]. In addition, several BMPs have confirmed osteogenic activity in different animal models, with BMP2 and -7 already in clinical use [20, 21]. BMPs were originally purified using heparin affinity chromatography [22], and subsequently cell surface and ECM heparan sulphate proteoglycans were shown to be critical for the biological activity of BMPs and their antagonists [10, 13, 23]. BMP2 and BMP4 have a heparin-binding domain at their N-terminus and strongly bind to heparin and HS via clustered basic residues in that domain [14]. Their binding to HSPGs could restrict the amount of BMPs available for signalling or limit their diffusion [14, 24]. Heparin/HS chains bind to BMP4 and repress BMP4-mediated expression patterns in Xenopus embryos [24]. Furthermore, combined deficiency of BMP4 and glypican-3, a cell surface proteoglycan, results in an abnormal skeletal development [25]. Syndecan-3, another member of cell surface HSPGs, interferes with the interaction of BMP2 and its receptor, thus inhibiting its activity during normal limb cartilage differentiation [14]. In addition, HSPGs could act as co-receptors and facilitate the interaction between BMPs and their receptors [26]. For example, Dally, a Drosophila homolog of the glypican family of cell surface HSPGs, acts as a co-receptor for Decapentaplegic (Dpp), a homolog of vertebrate BMPs, and regulates the sensitivity of cells to Dpp signalling [27]. Dally-like (Dlp), another member of the glypican family of cell surface HSPGs, interacts with Hadgehog (Hh) and acts as an Hh co-receptor, promoting Hh signalling strength in the Drosophila wing disc [28]. Compared to BMP2 and -4, BMP5 to -8 have longer N-terminal sequences ahead of the first conserved cysteine and the allocation of basic residues within these sequences is quite different with the absence of clustered basic residues [29]. In spite of these differences BMP7 also binds to HS and heparin, which inhibits its activity in vitro [10, 30].
BMP6 has a major role in promoting OB differentiation and bone formation [31]. Haematopoietic stem cell (HSC)-derived BMP6 is responsible for enhanced OB differentiation and bone formation from bone marrow-derived stem cells (BMSCs) [32]. Also, exploring the role of BMP6 in the adult skeleton, it was found that it circulates in the plasma of healthy individuals [33], and when systemically applied to osteoporotic rats it restores the bone inductive capacity, microarchitecture and quality of the skeleton [34]. BMP6 expression is also strong in promoting the chondrocyte hypertrophy [35] and one study suggests its physiological role in maintaining growth plate function [36].
In our study, we investigated the role of heparin in BMP6 signalling using C2C12-BRE-Luc mouse premyoblast cell line stably transfected with a reporter plasmid consisting of a BMP response element (BRE) from the Id-1 promoter fused to a luciferase reporter gene [37]. Since BMP6 was shown to induce differentiation of C2C12 cells towards an OB phenotype [38], and is known to upregulate the Id-1 gene expression [39–41], this cell line is a good experimental model for investigating the BMP6-mediated signalling in vitro. In a mouse model of osteoporosis, we explored the effect of the exogenous heparin on the BMP6-mediated osteogenic activity. We show that BMP6 specifically binds to heparin which inhibits the BMP6-mediated bone formation. In addition, we show that the removal or disruption of the cell-surface HS inhibits BMP6 signalling. Interestingly, BMP6 prevented the heparin’s effect on blood coagulation. Since BMP6 circulates in humans, its interaction with exogenous heparin might contribute to the heparin-induced osteoporosis and blood coagulation.
Materials and methods
Materials
C2C12-BRE-Luc BMP reporter cells, stably transfected with a reporter plasmid consisting of BMP response element (BRE) from the Id-1 promoter fused to a luciferase reporter gene, were previously described [37]. DMEM/F-12, foetal bovine serum (FBS), antibiotic/antimicotic, phosphate buffer saline (PBS), NuPage 10 % Bis-Tris gels, sample buffer, and nitrocellulose membranes were from Invitrogen Life Technologies (Carlsbad, CA). Chondroitin sulphate, de-O-sulphated heparin, de-N-sulfated heparin, sodium chlorate, BCIP/NBT tablets, phosphatase inhibitor cocktail 2 and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO). Heparin for i.v. use (5000 IU/ml) was from Belupo (Croatia). Heparinase III (EC 4.2.2.8.) was from Iduron Ltd. (Manchester, UK). Recombinant human BMP6 and monoclonal anti-BMP6 antibody (7H2) were from Genera Research (Croatia). Anti-phospho-Smad1/5/8 antibody was from Cell Signaling Technology (Beverly, MA). Anti-β-actin antibody was from Millipore (Billerica, MA). Anti-Smad 1/5/8 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Protease and phosphatase inhibitor cocktails were from Roche (Basel, Switzerland). Luciferase Assay reagent and Reporter Lysis Buffer were from Promega (Madison, WI). Prestained SDS-PAGE marker and heparin-Sepharose beads were from BIO-RAD Laboratories Inc. (Hercules, CA). Sources of other materials are shown in the text.
Cell culture
C2C12-BRE-Luc cells were cultured in Dulbecco’s Modified Eagle’s Medium/F-12 (DMEM/F-12) containing 10 % FBS and 1 % antibiotic/antimicotic at 37 °C in a humidified atmosphere of 5 % CO2 in air. For sodium chlorate treatment, cells were cultured in DMEM/F-12 supplemented with 5 % dialysed foetal bovine serum and 50 mM sodium chlorate for four days. For heparinase III treatment, cells were incubated with 25 mIU/ml for three hours in DMEM/F-12 at 37 °C. All cell culture studies were performed with a cell passage less than 25.
BMP6 binding to heparin-Sepharose beads
A total of 100 ng BMP6 was incubated with 50 μl heparin-Sepharose beads in 100 μl binding buffer (10 mM Na2HPO4, pH 7) with or without 2 mg intact heparin for 20 minutes at room temperature (RT). After binding, the beads were washed twice with binding buffer. The bound protein was eluted with sample buffer and subjected to SDS-PAGE. BMP6 was detected by immunoblot using anti-BMP6 antibody. BMP6 (100 ng) was used as a positive control.
BCA protein assay
Protein concentration in lysates was determined by using BCA Protein Assay Kit (Thermo scientific) and the samples were normalised before immunoblot analysis.
Detection of Smad1/5/8 phosphorylation
C2C12-BRE-Luc cells were seeded in 10-cm Petri-dishes, 3 × 106 cells per dish and cultured overnight in DMEM/F-12. The cells were then incubated with 50 ng/ml BMP6 and various experimental reagents for 30 minutes at 37 °C. For BMP6 + heparin treatment, reagents were preincubated for 20 minutes at RT prior to the treatment. After incubation, the cells were washed twice with ice cold PBS, scraped and centrifuged at 4 °C for ten minutes at 1100 rpm. Pellets were resuspended in lysis buffer (50 mM HEPES, 150 mM NaCl, 1 % TritonX-100, phosphatase and protease inhibitors), and lysis was done for 30 minutes on ice using an orbital shaker. The lysates were microcentrifuged for ten minutes at 4 °C and BCA was performed to normalise the samples.
Western immunoblotting
45 μg of samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with 3 % BSA and 0.05 % Tween in TBS for 30 minutes at room temperature and then incubated overnight with primary antibodies at 4 °C. Immunolabelling was detected using alkaline phosphatase-conjugated secondary antibodies and BCIP/NBT substrate (Sigma) according to the manufacturer’s instructions. ImageJ software (NIH) was used for quantification of protein band intensities.
Id-1 reporter gene assay
C2C12-BRE-Luc cells were plated at a concentration of 3 × 104 cells per well in 48-well plates containing DMEM/F-12 plus 10 % FBS and allowed to attach overnight. Cells were washed with PBS and re-fed with 300 μl of DMEM/F-12 with 0.1 % FBS for 7 h. BMP6 and other reagents or their combinations were added to cells at appropriate concentrations for 15 hours, and then cells were washed with PBS and lysed using 60 μl of the reporter lysis buffer. To measure the luciferase activity, 20 μl of cell lysate was added to 100 μl Luciferase Assay Reagent and luminescence was quantified using a Victor Wallack luminometer. The protein content of each lysate was analysed using BCA Protein Assay Kit according to the manufacturer’s instructions. Luciferase units were normalised to the protein content of each well. All experiments were repeated at least three times with three independent wells per condition.
RNA isolation and real time PCR
Total RNA was isolated from C2C12 cells cultivated in 10 cm Petri-dishes and treated with BMP6 (50 ng/ml) with or without heparin (10 μg/ml) for different time points using TRIzol (Invitrogen) according to manufacturer’s instructions. The amount of RNA was determined by spectrophotometry. The cDNA was generated by reverse-transcription of 1 μg adjusted RNA using Super Script III First-Strand Synthesis System (Invitrogen) as indicated by the manufacturer’s instructions. Gene expression of interest was measured by using a LightCycler FastStart DNA Master SYBR Green kit in a LightCycler instrument (Roche Diagnostics), as described [42, 43]. Briefly, 1 μl of template was mixed with 9 μl of LightCycler FastStart DNA Master SYBR Green mix to which MgCl2 and gene-specific forward and reverse PCR primers had been added to a final concentration of 2 μM for MgCl2 and 0.5 μM for each primer. Results are represented as a fold change of the control expression level. Gapdh transcripts were used as a normaliser. The list of primers used is shown in Table 1.
Table 1.
Sequences of primers used for gene expression analysis
| Target gene | Name | Forward 5′–3′ | Reverse 3′–5′ |
|---|---|---|---|
| ALP | Alkaline phosphatase | GATCATTCCCACGTTTTCAC | TGCGGGCTTGTGGGACCTGC |
| OC | Osteocalcin | CAAGTCCCACACAGCAGCTT | AAAGCCGAGCTGCCAGAGTT |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Ectopic bone formation and morphometric analysis in rats
Bone matrix was prepared from six-month-old Sprague–Dawley rats. After sacrifice, diaphyses of femurs and tibiae were removed and then powdered, sieved, and demineralised as previously described [44]. BMP6 (10 μg) with heparin (2, 20 or 200 IU) (international units) was added to demineralised bone matrix (DBM) to form pellets, which were then implanted subcutaneously in the pectoral region of normal rats. Pellets with acetate buffer only were used as a negative control and BMP6 (10 μg) alone as a positive control. Two pellets per group were analysed, altogether ten rats. Two weeks following implantation of DBM, pellets were removed and embedded in paraffin, cut into 7-μm-thick sections, stained with toluidine blue, and examined for the presence of new cartilage and bone to reveal effects of the heparin on BMP6 osteogenic activity. Histomorphometric analysis was quantified using a computer-aided image analysis system (SForm Image Analyzer software, version 1.0). Data is shown as percentage of the area of interest in the implant tissue. All experiments involving animals were done in proper accordance and approved by the Institutional Animal Research Committee.
Ovariectomised mice
Four-month-old CD-1 female mice were subjected to ovariectomy (OVX). Animals were anaesthetised with an intraperitoneal injection of ketamine at doses of 100 mg/kg body weight. Six animals per experiment were subjected to sham surgery during which the ovaries were exteriorised but replaced intact. Bilateral ovariectomies were performed in the remaining mice from the dorsal approach and left untreated for a period of three weeks following surgery to await the development of osteopaenia. BMP6 at dose of 10 μg/kg was injected through the tail vein three times a week for seven weeks. Animals were divided into groups of six as follows: (1) sham, (2) OVX, (3) OVX (BMP6 10 μg/kg intravenously, three times/week), (4) OVX (heparin 0.5 IU/g, three times/week), (5) OVX (BMP6 10 μg/kg + heparin 0.5 IU/g intravenously, three times/week), and (6) OVX (BMP6 10 μg/kg + heparin 1 IU/g intravenously, three times/week); altogether there were 36 animals.
Bone mineral density (BMD)
Prior to ovariectomy and three and ten weeks after, the animals were anaesthetised and hind limbs were scanned for bone density by dual-energy X-ray absorptiometry (DEXA) (Hologic QDR-4000, Hologic, Waltham, MA) equipped with Regional High Resolution Scan software [45]. At the end of the experiment, animals were anaesthetised and killed by cervical dislocation. The hind limbs were removed and fixed in 4 % paraformaldehyde for further analysis by μCT. The right femur and tibia were also used for determination of BMD by dual-energy X-ray absorptiometry. The scan images were analysed and bone mineral density of whole femurs and tibiae were determined.
μCT analysis
The effect of heparin and BMP6 was tested on femur and tibia in OVX mice by μCT (μCT SkyScan 1076, Belgium). The distal femur and proximal tibia were scanned in 9-μm resolution with an aluminum filter of 0.5 mm in the dorsoventral direction. Three-dimensional reconstruction of bone was performed using the NRecon software (SkyScan, Belgium), and the volume of the trabecular bone (BV, U^3) was calculated. Trabecular parameters, including trabecular number (Tb. N, 1/U), trabecular thickness (Tb. Th, U), trabecular separation (Tb. Sp, U), and cortical parameters, including tissue volume (TV, U^3), bone volume (BV, U^3), and cortical thickness (Co. Th, U) were measured using CTAn software (Skyscan, Belgium) by using the method described previously [46].
BMP6 and heparin effect on rat blood coagulation
In the ex vivo experiment 1 ml of rat blood was collected in the citrate sterile tube using heparin-coated capillary tubes (n = 5) (Hirschmann Laborgeräte, Germany). One μg of BMP6 was added immediately after blood collection (n = 5), while control samples were treated with a vehicle (acetate buffer pH 4.0). In vivo rats were injected with 10 μg/kg of BMP6 (n = 5) or a vehicle (acetate buffer pH 4.0; n = 5). Ten minutes later blood was collected from the orbital plexus using heparin-coated capillary tubes. Coagulation parameters (PT, APTT, TT, fibrinogen and coagulation factor XI) were measured using standard laboratory procedures for human blood analysis. Altogether 25 animals were used in this experiment.
Statistics
Experimental values obtained by PCR analysis and DEXA are given as means ± SEM. Data obtained by μCT are presented as means ± STD. Data analyses were performed using Statistica 10 (StatSoft, USA) software. One way analysis of variance (ANOVA) with a Tuckey post hoc test was performed to determine the significance between the experimental groups. The results were considered significant if P value was lower than 0.05 (P < 0.05).
Results
BMP6 binds to heparin
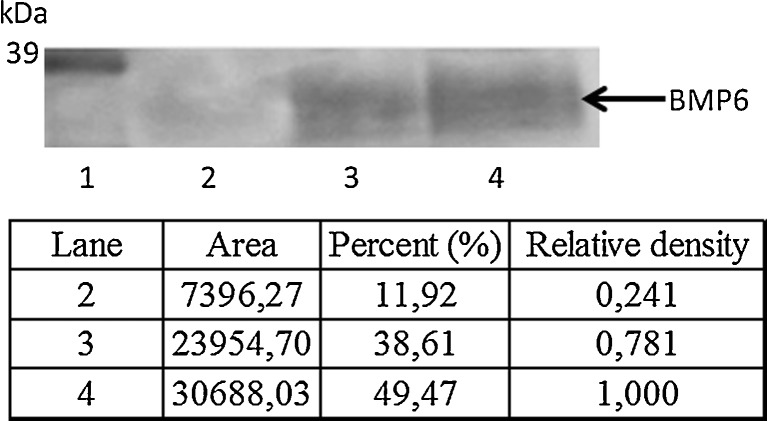
To demonstrate that BMP6 binds to heparin, we incubated BMP6 with heparin-Sepharose beads and detected the bound BMP6 by Western blotting (Fig. 1). Coincubation with exogenous-free heparin reduced the amount of the heparin-Sepharose bead bound fraction of BMP6, indicating that both immobilised and free heparin in solution bound BMP6.
Fig. 1.
BMP6 binds to heparin-Sepharose. 100 ng BMP6 was incubated with heparin-Sepharose beads in the presence or absence of heparin (2 mg) for 20 min in room temperature. BMP6 bound to beads was eluted and subjected to SDS-PAGE and detected by immunoblot analysis with an anti-BMP6 antibody. Lane 1 MWM (molecular weight marker); lane 2 eluted BMP6 (100 ng) incubated with heparin beads and heparin; lane 3 eluted BMP6 incubated with heparin beads; lane 4 BMP6. Arrow indicates mature BMP6 (35 kDa). Intensity of the bends was quantified using ImageJ software and expressed as relative density compared to BMP6 standard
Exogenous heparin inhibits BMP6 activity in vitro
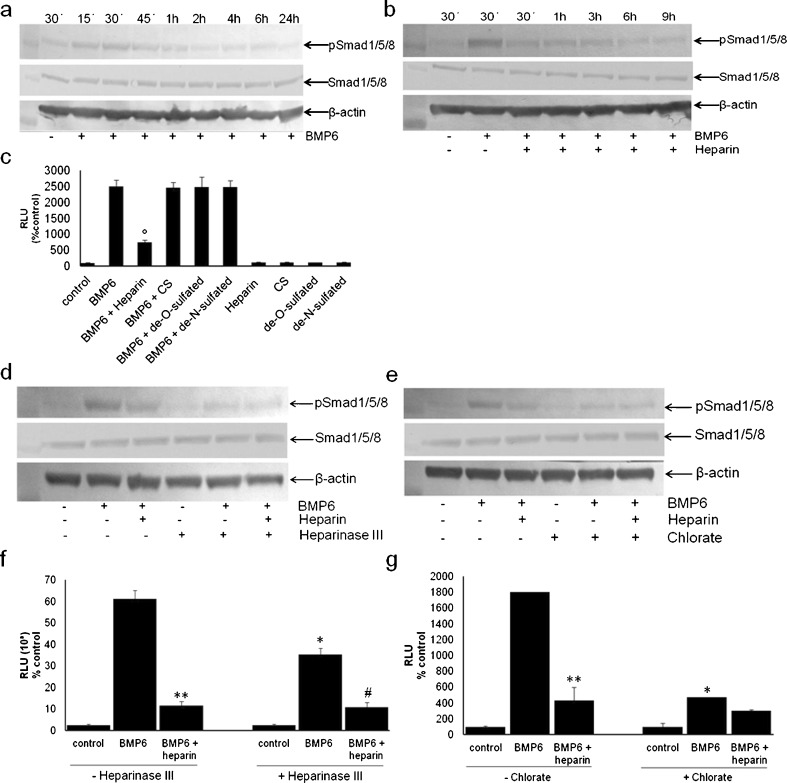
Phospho-Smad1/5/8 were activated in BMP6-stimulated C2C12-BRE-Luc cells (Fig. 2a). To determine whether heparin interacts directly with BMP6 and influences its signalling, C2C12-BRE-Luc cells were treated at different time points with a combination of BMP6 (50 ng/ml) and heparin (10 μg/ml) (Fig. 2b). BMP6-induced Smad1/5/8 phosphorylation was reduced by exogenous heparin treatment. The same was observed in the Luciferase reporter assay, where heparin significantly reduced the BMP6-induced luciferase signal, while galactosaminoglycan chondroitin sulphate, another member of proteoglycan sugar chains, had no influence (Fig. 2c). These results suggested that BMP6 specifically bound to heparin/HS GAG chains and we assumed that free heparin competed with cell surface HSPGs for BMP6 binding. Because heparin and HS are modified by N- and O- sulfation, and these sulphated structures are responsible for the interaction with HS-binding proteins, we treated the cells with de-O- and de-N-sulphated heparins and BMP6 to test whether BMP6 requires specific sulfate groups for binding to heparin/HS. Neither de-O- nor de-N-sulphated heparin interfered with BMP6-induced luciferase signal (Fig. 2c). These results suggested that both O- and N-sulphation were essential for the BMP6 binding to HS chains.
Fig. 2.
Exogenous heparin inhibits BMP6-mediated signaling. a BMP6 induces Smad1/5/8 phosphorylation. C2C12-BRE-Luc cells were stimulated with BMP6 (50 ng/ml) for indicated time points. b Cells were stimulated with BMP6 for 30 min or BMP6 and heparin (10 μg/ml) for various periods of time (30 min–9 h). c C2C12-BRE-Luc cells were stimulated with BMP6 alone or in combination with heparin, chondroitin sulphate and de-O- and de-N-sulphated heparin for 17 h. BMP6-mediated signalling is inhibited by heparinase III and chlorate treatment. d, e Heparinase III pretreatment interferes with BMP6-mediated signalling. C2C12-BRE-Luc cells were incubated in the presence or absence of heparinase III (25 mIU/ml) for three hours and then stimulated with BMP6 (50 ng/ml) for 30 min (d) or 10 ng/ml for 17 h (e). f, g Chlorate treatment inhibits BMP6-mediated signalling. C2C12-BRE-Luc were incubated with or without sodium chlorate (50 mM) for four days and then stimulated with BMP6 (50 ng/m) for 30 min (f) or 10 ng/ml for 17 h (g). a, b Cells were lysed and 45-μg aliquots were subjected to immunoblot analysis with an anti-phospho-Smad1/5/8 antibody as described in Materials and methods. β-Actin levels are shown as a loading control. Total Smad 1/5/8 levels showed no difference between samples. c, f, g The cells were lysed and the luciferase activity measured using Victor Wallack luminometer as described in Materials and methods. °P < 0.05, comparison between BMP6 with heparin and BMP6 alone; *P < 0.05, comparison between BMP6 with and without heparinase III/chlorate; ** P < 0.05, comparison between BMP6 without and with heparin; #P < 0.05, comparison between BMP6 without and with heparin, all with heparinase III
Removal of HS from cell surface inhibits BMP6 activity
Heparan sulphate (HS) is ubiquitously expressed on cell surfaces and we determined whether HS expressed in C2C12 cells was involved in BMP6-mediated signalling. C2C12-BRE-Luc cells were pretreated with heparinase III, HS lyase, to remove HS chains from the cell surface prior to stimulation with BMP6. As shown in Fig. 2d (lane 5), BMP6-induced Smad1/5/8 phosphorylation was inhibited in heparinase III pretreated cells, suggesting that cell surface HS was important for BMP6 signalling. Similarly, the same treatment in the luciferase assay decreased the BMP6-induced activity by two-fold (Fig. 2e).
Because sulphated structures of HS are responsible for its function and interaction with HS-binding proteins, these sulphate groups might be important for BMP6 signalling in C2C12-BRE-Luc cells. To explore if HS sulphation was involved in BMP6 signalling, C2C12-BRE-Luc cells were pretreated with sodium chlorate, an inhibitor of proteoglycan sulphation, and then BMP6-mediated Smad1/5/8 phosphorylation and luciferase induction were examined. The BMP6-induced Smad phosphorylation was inhibited (Fig. 2f, lane 5), as well as the luciferase induction, which was decreased five-fold as compared to chlorate non-treated cells (Fig. 2g).
Cell-surface-anchored HS is important for BMP6 signalling
Proteoglycans (PGs) can be classified on the basis of their localisation and type of the core protein. To determine the role of plasma membrane HS chains anchored through their core proteins in BMP6 signalling, we applied heparin exogenously and explored whether it substituted for the endogenous HS in heparitinase- and chlorate-treated C2C12-BRE-Luc cells. As shown in Fig. 2, exogenous heparin did not restore BMP6-mediated luciferase induction (Fig. 2e and g) nor Smad1/5/8 phosphorylation (Fig. 2d, lane 6 and 2f, lane 6) in either heparinase III- or chlorate-pretreated cells. Moreover, exogenous heparin inhibited BMP6-induced Smad phosphorylation in normal C2C12-BRE-Luc cells (Fig. 2b, lanes 3–7). These results indicate that exogenous heparin could not rescue BMP6 signalling in HS-disrupted cells and that HS should be anchored on the plasma membrane for normal BMP6 signalling. Also, chondroitin-sulphate did not inhibit BMP6-mediated luciferase signal (Fig. 2c), suggesting that heparin-induced inhibition was a specific event not caused by the negative charges of the sulphate groups. Compared to de-O- and de-N-sulphated heparins, intact heparin showed the highest level of inhibition (Fig. 2c), suggesting that N- and O-sulfate groups of exogenous heparin were required for BMP6 signalling.
Heparin inhibits BMP6-mediated C2C12 differentiation
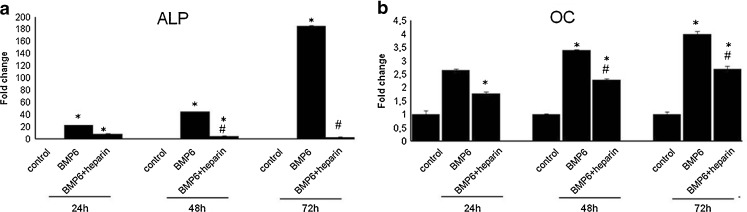
BMP6 is known to induce C2C12 myoblast differentiation towards the osteoblastic phenotype. However, at 24 hours following treatment (Graph 1a) heparin inhibited BMP6-induced alkaline phosphatase (ALP) expression, which was more pronounced after 48 and 72 hours. The same was observed for the osteocalcin (OC) gene expression (Graph 1b).
Graph 1.
Heparin inhibits BMP6-induced osteoblast differentiation. C2C12 cells were incubated with BMP6 (50 ng/ml) in the absence or presence of soluble heparin (10 μg/ml) for 24 h, 48 h and 72 h. Expression of osteoblast markers alkaline phosphatase (ALP) (a) and osteocalcin (OC) (b) was measured by real-time RT-PCR. Data presented are fold change in gene expression from three experiments ± standard deviation (SD). *P < 0.05 vs control, #P < 0.05 vs BMP6
Heparin inhibits ectopic bone formation induced by BMP6 in vivo
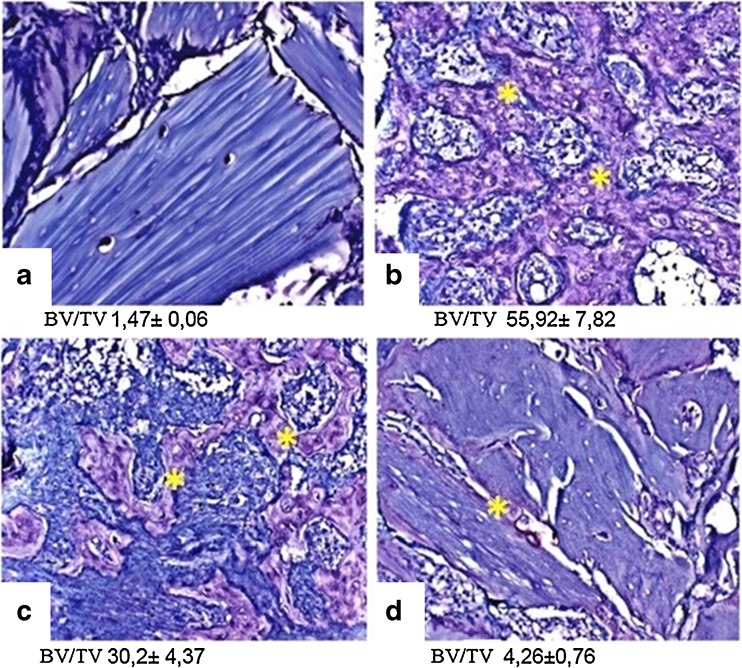
BMP6 induces bone when implanted ectopically [47–49]. Heparin implanted with BMP6 on DBM, which was previously extracted with urea, dose dependently inhibited BMP6-induced ectopic bone with 200 IU heparin being the most effective (Fig. 3).
Fig. 3.
Heparin inhibits the ectopic bone formation induced by BMP6 in vivo. BMP6 (10 μg) and 2, 20 or 200 IU (international units) of heparin were implanted subcutaneously to induce ectopic bone formation in rats. After two weeks implants were removed and examined histologically with toluidin blue staining. Morphometric analysis data is presented as BV/TV % of area of interest (newly formed bone) in the whole implant tissue. a Control (acetate buffer only). b BMP6 (10 μg). c BMP6 (10 μg) + heparin (20 IU). d BMP6 (10 μg) + heparin (200 IU)
Heparin inhibits osteogenic activity of systemically administered BMP6 in OVX mice
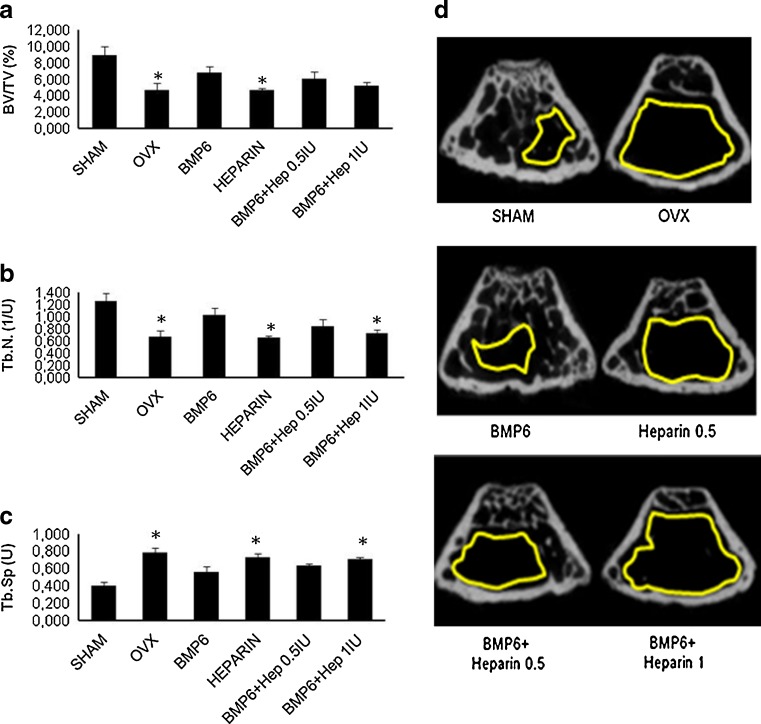
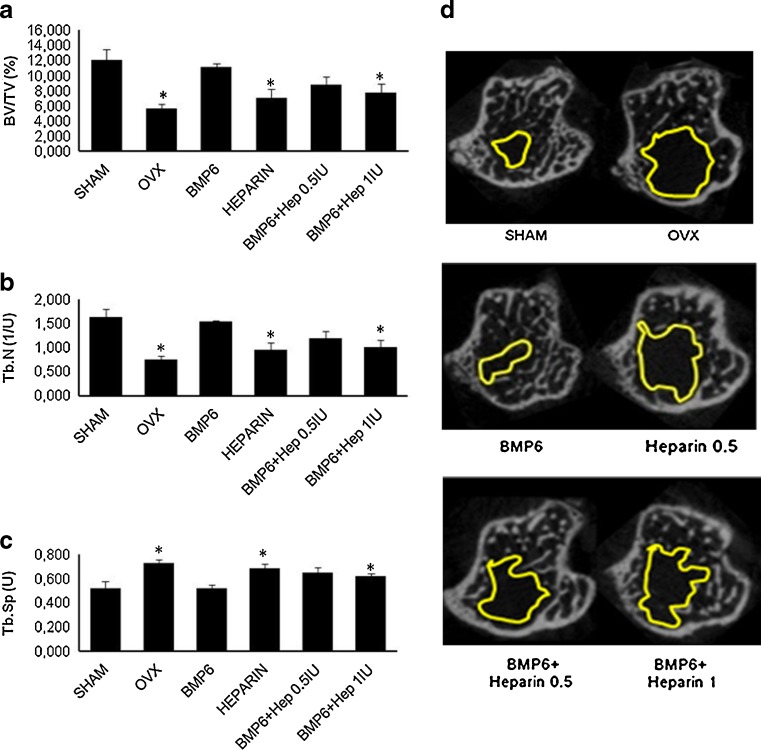
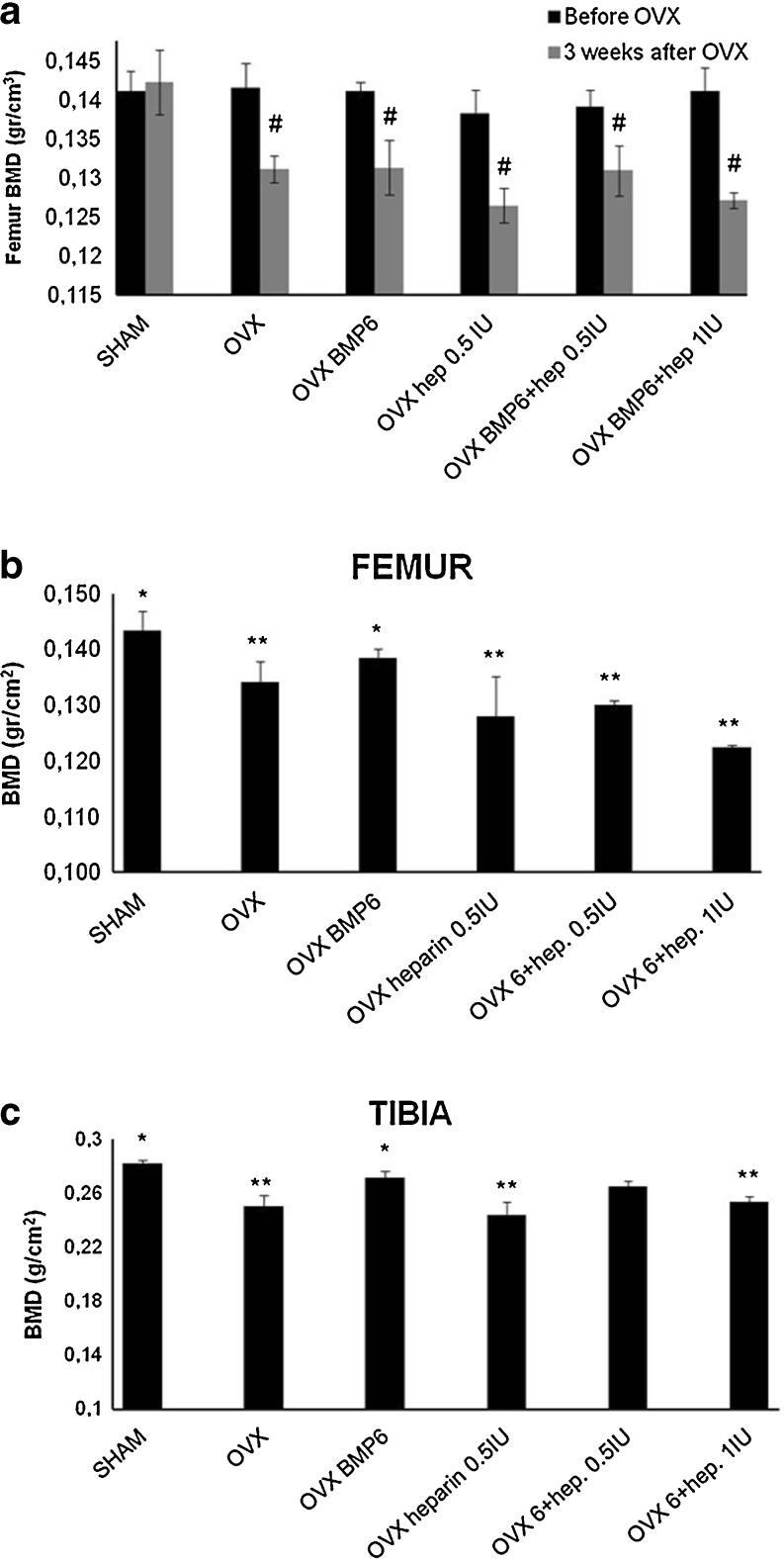
We have previously shown that BMP6, given systemically, can restore bone in rats with osteoporosis [34]. When OVX mice were treated intravenously with BMP6 and two heparin doses (0.5 and 1 IU/g/day) for seven weeks, μCT analyses showed that heparin reduced the BMP6 osteogenic activity. In the femur, the higher dose reduced the BV/TV by 24 % (Fig. 4a), the trabecular number by 29 % (Fig. 4b) and increased the trabecular separation by 25 % (Fig. 4c), as compared to BMP6 therapy alone. The lower dose showed a similar trend (Fig. 4). In tibiae, mice treated with heparin had the bone volume reduced by 35 % (data not shown) and BV/TV by 30 % (Fig. 5a). The trabecular number was reduced by 35 % (Fig. 5b), while the trabecular separation was increased by 17 % (Fig. 5c). Cortical bone parameters (bone volume, bone surface and cortical thickness) were not different (data not shown). The result was also supported by BMD values measured by DEXA, where both heparin-treated animals showed lower BMD as compared to BMP6 treated mice. After seven weeks BMD of femurs and tibiae in mice treated with BMP6 and heparin decreased dose dependently (Graph 2b and c).
Fig. 4.
μCT analysis of the distal femur of four-month-old OVX mice treated with BMP6 (10 μg/kg), heparin (0.5 IU/g), BMP6 (10 μg/kg) + heparin (0.5 IU/g) and BMP6 (10 μg/kg) + heparin (1 IU/g). 1 IU/g heparin with BMP6 significantly decreased the trabecular number (Tb.N.) (b) and increased the trabecular separation (Tb.Sp.) (c). Bone volume over tissue volume (BV/TV) values were also decreased (a). μCT images of femurs from all groups (d). Space encircled by the yellow line indicates the bone marrow cavity without trabeculi. *P < 0.05 vs BMP6
Fig. 5.
μCT analysis of the proximal tibiae of four-month-old OVX mice treated with BMP6 (10 μg/kg), heparin (0.5 IU/g), BMP6 (10 μg/kg) + heparin (0.5 IU/g) and BMP6 (10 μg/kg) + heparin (1 IU/g). One IU/g heparin with BMP6 significantly decreased the bone volume/tissue volume (BV/TV) (a) and the trabecular number (Tb.N.) (b) and increased the trabecular separation (Tb.Sp.) (c) compared to BMP6-treated mice. μCT images of tibiae from all groups of mice (d). Space encircled by the yellow line indicates the bone marrow cavity without trabeculi. *P < 0.05 vs BMP6
Graph 2.
DEXA analysis of tibiae and femurs of four-month-old mice prior and three weeks after OVX (a), followed by seven weeks of therapy (b, c). Mice were treated with BMP6 (10 μg/kg), heparin (0.5 IU/g), BMP6 (10 μg/kg) + heparin (0.5 IU/g) and BMP6 (10 μg/kg) + heparin (1 IU/g). One IU/g heparin with BMP6 significantly decreased BMD values in both femur (b) and tibiae (c) compared to BMP6 treated mice. Decreased BMD values were also seen in mice treated with a lower dose of heparin (0.5 IU/g) and BMP6 (b). #P < 0.05 vs before OVX, *P < 0.05 vs OVX, **P < 0.05 vs OVX BMP6
BMP6 improves coagulation parameters
To further explore the specificity of BMP6 and heparin interaction, we used the blood coagulation pathway as an experimental model. When rat blood was collected in heparinised tubes the resulting activated partial thromboplastin time (APTT) and thrombin time (TT) were prolonged, while the coagulation factor XI value was undetectable (Table 2). Following addition of 1 μg of BMP6 to 1 ml of rat blood, the coagulation factor XI and the coagulation time were normalised (Table 2). To confirm these results in vivo, we administered BMP6 (10 μg/kg) intravenously and ten minutes later collected the blood using similar heparin-coated capillary tubes. The prothrombin time (PTT), activated partial thromboplastin time, thrombin time, fibrinogen (FBG) and coagulation factor XI were measurable and reached normal values (Table 2). These results confirmed that BMP6 interacts with the heparin activity in vivo. Surprisingly, in these experiments BMP6 had a similar effect as the enzyme hepzyme (Table 2), a heparinase which specifically cleaves and inactivates heparin.
Table 2.
Ex vivo and in vivo correction of coagulation parameters after BMP6 treatment
| Group | n | PT (%) | APTT (s) | TT (s) | FBG (mg/dl) | F XI |
|---|---|---|---|---|---|---|
| Ex vivo | ||||||
| Control | 5 | 84.6 | NM | NM | 85 | NM |
| BMP6 1 μg | 5 | 86.3 | 22.23 | 69.95 | 80 | 0.25 |
| Hepzyme | 5 | 80.0 | 21.60 | 78.30 | 80 | 0.21 |
| In vivo | ||||||
| Control | 5 | 87.7 | 89.33 | 93.80 | 70 | <0.025 |
| BMP6 10 μg/kg | 5 | 93.3 | 21.26 | 70.60 | 90 | 0.26 |
NM not measurable, APTT activated partial thromboplastin time, PT Prothrombin time, TT thrombin time, F XI coagulation factor XI, FBG fibrinogen
Values are presented as a mean (n = 5 mice per group)
Discussion
Long-term heparin treatment induces bone loss and increases the risk of fractures in patients [1–6] and in experimental animals [7, 11]. Here we explored the effect of exogenous heparin on BMP6 and found that BMP6 binds to heparin which, in contrast to endogenous heparan sulphate proteoglycans, blocks BMP6 signal transduction in C2C12 myoblasts and inhibits its osteogenic activity in vivo after local and systemic application.
Heparin belongs to highly sulphated glycosaminoglycans and is often used in exploring structurally similar heparan sulphate (HS) roles in binding and interaction with various molecules in cell signalling. It was revealed that BMPs are heparin/HS-binding molecules and their binding to specific HS GAG chains is necessary for their biological activity. GAGs have been found to have opposite effects on BMP activity in various in vivo and in vitro systems, enhancing it in some, while inhibiting their activity in others [10, 13, 30]. These effects may depend on multiple factors, such as different BMPs examined [10, 13, 30, 50], the source, concentration and sulphation structure of specific GAGs, the availability or absence of cell surface GAGs, as well as BMP receptors and the assay used. Similarly, variable effects on the BMP efficacy were also observed in osteogenic assays [15, 30, 50]. In some studies it has been shown that heparin and HS enhance BMP2 activity in C2C12 cells [30, 50], while in others heparin had an opposite effect on the BMP signalling [15], inhibiting BMP2 [15] and BMP7 [10] binding to receptors and induction of Smad signalling. In contrast to BMP2 and BMP4, BMP6-induced alkaline phosphatase activity in C2C12 cells was inhibited by heparin [50]. BMP7 signalling was inhibited by heparin in rat osteosarcoma cells [10].
Here we showed that both N- and O-sulphate structures are needed for BMP6 binding and signalling in C2C12 cells. According to similarity in structure of mature domains of BMP6 and BMP7, we assume that BMP6 like BMP7 binds to negatively-charged HS chains through basic lysine and arginine residues [10]. Alteration of the endogenous HS structure with chlorate treatment or its removal using heparinase III inhibits BMP6-mediated signalling in C2C12-BRE-Luc cells, indicating that endogenous HS plays an important role in the BMP6 activity. Similarly, BMP7 activity was inhibited by removal/desulphation of cell surface GAGs [10]. We showed that exogenous heparin competitively inhibited BMP6 binding to endogenous HS, which disrupted the BMP6 signalling. Thus, for the proper BMP6 signalling, HS should be anchored on the plasma membrane. It is possible that HS binds BMP6, concentrating it on the plasma membrane, allowing the ligand-receptor interaction. On the other hand, it is interesting that exogenous heparin also binds to BMP6, probably preventing it from interacting with the receptor complex.
It is known that BMPs support differentiation of C2C12 cells towards an osteoblastic phenotype [51, 52]. BMP6 in concentration of 50 ng/ml induced the expression of ALP by 180-fold and OC by four-fold after 72 h. The addition of heparin (10 μg/ml) attenuated the expression of both osteoblastic markers, which is also consistent with previous reports demonstrating that exogenous heparin can inhibit BMP2 and BMP7 binding to their receptors [10, 15].
We also showed that exogenous heparin dose-dependently inhibited BMP6-mediated ectopic bone formation in rats which is in contrast to previous results showing that heparin potentiates BMP2 osteogenic activity [50, 53]. These differences are consistent with in vitro data, showing that heparin influences the activity of various BMPs differently [15, 30, 50]. Using μCT analysis of femur and tibiae in a mice osteoporotic model, we demonstrated that exogenous heparin reduced the BMP6 osteogenic activity and its potential to improve the bone quality after seven weeks of therapy. These results are consistent with previous studies showing that heparin induces bone loss leading to development of osteoporosis in rats [7].
The observation that BMP6 inhibited the heparin anticoagulant activity, probably by preventing its binding to coagulation factors, suggests that endogenous circulating BMP6 level might also influence the coagulation process in patients.
We demonstrated that the addition of exogenous heparin to OVX mice significantly reduces the BMP6 osteogenic activity. These results support the clinical observations that heparin, when used as a thromboprophylactic agent after orthopaedic, delays fracture healing. Based on these results, we propose that exogenous heparin binds to BMP6 which influences the activity of mature osteoblasts as well as the differentiation of early osteoblasts and may be responsible for reduced bone formation leading to osteoporosis, a well-known side effect of the long-term heparin therapy. Previous studies have shown that in pregnant women, almost one third of patients receiving heparin have a significant decrease of BMD, and that fractures occur in 2.2–3.6 % of all patients [6]. In non-pregnant women, the numbers are even higher. Our in vivo data support these observations.
BMP6 plays an important role in iron metabolism by directly modulating the expression of hepcidin, a major regulator of iron homeostasis [54]. Endogenous BMP6 increases the hepcidin expression in the liver and reduces serum iron in mice [54–56]. In addition, loss of BMP6 leads to reduced hepatic hepcidin expression and the iron overload in Bmp6 -/- mice which supports involvement of the BMP6 signalling pathway in regulation of hepcidin [54, 57]. A recent publication showed that patients treated with low doses of heparin for prevention of a deep vein thrombosis showed a major decrease in serum hepcidin levels and increase in the serum iron [58]. This suppressed BMP6-mediated hepcidin expression may be due to a similar heparin mediated inhibition of BMP6 signalling as we describe here.
We propose that endogenous plasma membrane heparan sulphate stimulated BMP6 signalling, while exogenous heparin inhibited the BMP6 osteogenic activity.
Acknowledgments
Acknowledgments
We thank Durdica Car for providing animal care in mice and rat studies. The authors declare that they have no conflict of interest.
Abbreviations
- BMP
Bone morphogenetic protein
- ALP
Alkaline phosphatase
- OC
Osteocalcin
- IU
International unit
- HS
Heparan sulphate
- HSPG
Heparan sulphate proteoglycans
- OVX
Ovariectomy
- μCT
Microcomputerised tomography
- BMD
Bone mineral density
- DEXA
Dual X-ray absorptiometry
- DBM
Demineralised bone matrix
Footnotes
This study was supported by a grant (number 108-1080327-0320) from the Ministry of Science and Technology of the Republic of Croatia.
Contributor Information
Jelena Brkljacic, Email: jelena.brkljacic@mef.hr.
Martina Pauk, Email: martina.pauk@mef.hr.
Igor Erjavec, Email: igor.erjavec@mef.hr.
Antonio Cipcic, Email: antonio.cipcic@gmail.com.
Lovorka Grgurevic, Email: lovorka.grgurevic@mef.hr.
Renata Zadro, Email: rzadro@mef.hr.
Gareth J. Inman, Email: g.j.inman@dundee.ac.uk
Slobodan Vukicevic, Phone: +385-1-4566812, FAX: +385-4566822, Email: vukicev@mef.hr.
References
- 1.Avioli LV. Heparin-induced osteopenia: an appraisal. Adv Exp Med Biol. 1975;52:375–387. doi: 10.1007/978-1-4684-0946-8_33. [DOI] [PubMed] [Google Scholar]
- 2.Miller WE, DeWolfe VG. Osteoporosis resulting from heparin therapy. Report of a case. Cleve Clin Q. 1966;33:31–34. doi: 10.3949/ccjm.33.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Murphy MS, John PR, Mayer AD, Buckels JA, Kelly DA. Heparin therapy and bone fractures. Lancet. 1992;340:1098. doi: 10.1016/0140-6736(92)93118-7. [DOI] [PubMed] [Google Scholar]
- 4.Rupp WM, McCarthy HB, Rohde TD, Blackshear PJ, Goldenberg FJ, Buchwald H. Risk of osteoporosis in patients treated with long-term intravenous heparin therapy. Curr Surg. 1982;39:419–422. [PubMed] [Google Scholar]
- 5.Street JT, McGrath M, O’Regan K, Wakai A, McGuinness A, Redmond HP. Thromboprophylaxis using a low molecular weight heparin delays fracture repair. Clin Orthop Relat Res. 2000;381:278–289. doi: 10.1097/00003086-200012000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Douketis JD, Ginsberg JS, Burrows RF, Duku EK, Webber CE, Brill-Edwards P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb Haemost. 1996;75:254–257. [PubMed] [Google Scholar]
- 7.Muir JM, Andrew M, Hirsh J, Weitz JI, Young E, Deschamps P, Shaughnessy SG. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood. 1996;88:1314–1320. [PubMed] [Google Scholar]
- 8.Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. doi: 10.1378/chest.119.1_suppl.64S. [DOI] [PubMed] [Google Scholar]
- 9.Dreyfuss JL, Regatieri CV, Jarrouge TR, Cavalheiro RP, Sampaio LO, Nader HB. Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An Acad Bras Cienc. 2009;81:409–429. doi: 10.1590/S0001-37652009000300007. [DOI] [PubMed] [Google Scholar]
- 10.Irie A, Habuchi H, Kimata K, Sanai Y. Heparan sulfate is required for bone morphogenetic protein-7 signaling. Biochem Biophys Res Commun. 2003;308:858–865. doi: 10.1016/S0006-291X(03)01500-6. [DOI] [PubMed] [Google Scholar]
- 11.Shaughnessy SG, Hirsh J, Bhandari M, Muir JM, Young E, Weitz JI. A histomorphometric evaluation of heparin-induced bone loss after discontinuation of heparin treatment in rats. Blood. 1999;93:1231–1236. [PubMed] [Google Scholar]
- 12.Rajgopal R, Bear M, Butcher MK, Shaughnessy SG. The effects of heparin and low molecular weight heparins on bone. Thromb Res. 2008;122:293–298. doi: 10.1016/j.thromres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Khan SA, Nelson MS, Pan C, Gaffney PM, Gupta P. Endogenous heparan sulfate and heparin modulate bone morphogenetic protein-4 signaling and activity. Am J Physiol Cell Physiol. 2008;294:C1387–C1397. doi: 10.1152/ajpcell.00346.2007. [DOI] [PubMed] [Google Scholar]
- 14.Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006;25:27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Jiao X, Billings PC, O’Connell MP, Kaplan FS, Shore EM, Glaser DL. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J Biol Chem. 2007;282:1080–1086. doi: 10.1074/jbc.M513414200. [DOI] [PubMed] [Google Scholar]
- 16.Vukicevic S, Stavljenic A, Pecina M. Discovery and clinical applications of bone morphogenetic proteins. Eur J Clin Chem Clin Biochem. 1995;33:661–671. doi: 10.1515/cclm.1995.33.10.661. [DOI] [PubMed] [Google Scholar]
- 17.Helder MN, Ozkaynak E, Sampath KT, Luyten FP, Latin V, Oppermann H, Vukicevic S. Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J Histochem Cytochem. 1995;43:1035–1044. doi: 10.1177/43.10.7560881. [DOI] [PubMed] [Google Scholar]
- 18.Vukicevic S, Kopp JB, Luyten FP, Sampath TK. Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7) Proc Natl Acad Sci USA. 1996;93:9021–9026. doi: 10.1073/pnas.93.17.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vukicevic S, Luyten FP, Reddi AH. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci USA. 1989;86:8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pecina M, Giltaij LR, Vukicevic S. Orthopaedic applications of osteogenic protein-1 (BMP-7) Int Orthop. 2001;25:203–208. doi: 10.1007/s002640100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35:1271–1280. doi: 10.1007/s00264-011-1301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampath TK, Coughlin JE, Whetstone RM, Banach D, Corbett C, Ridge RJ, Ozkaynak E, Oppermann H, Rueger DC. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J Biol Chem. 1990;265:13198–13205. [PubMed] [Google Scholar]
- 23.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol. 2001;231:31–46. doi: 10.1006/dbio.2000.0127. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205–209. doi: 10.1016/S0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- 25.Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S. glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development. Dev Biol. 2000;225:179–187. doi: 10.1006/dbio.2000.9831. [DOI] [PubMed] [Google Scholar]
- 26.Kuo WJ, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21:4028–4041. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 2003;130:1515–1522. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- 28.Yan D, Wu Y, Yang Y, Belenkaya TY, Tang X, Lin X. The cell-surface proteins Dally-like and Ihog differentially regulate Hedgehog signaling strength and range during development. Development. 2010;137:2033–2044. doi: 10.1242/dev.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans. 2006;34:458–460. doi: 10.1042/BST0340409. [DOI] [PubMed] [Google Scholar]
- 30.Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–43235. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- 31.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441–448. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grgurevic L, Macek B, Healy DR, Brault AL, Erjavec I, Cipcic A, Grgurevic I, Rogic D, Galesic K, Brkljacic J, Stern-Padovan R, Paralkar VM, Vukicevic S. Circulating bone morphogenetic protein 1-3 isoform increases renal fibrosis. J Am Soc Nephrol. 2011;22:681–692. doi: 10.1681/ASN.2010070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simic P, Buljan-Culej J, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 35.Carey DE, Liu X. Expression of bone morphogenetic protein-6 messenger RNA in bovine growth plate chondrocytes of different size. J Bone Miner Res. 1995;10:401–405. doi: 10.1002/jbmr.5650100310. [DOI] [PubMed] [Google Scholar]
- 36.Perry MJ, McDougall KE, Hou SC, Tobias JH. Impaired growth plate function in bmp-6 null mice. Bone. 2008;42:216–225. doi: 10.1016/j.bone.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 37.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 39.Sivertsen EA, Huse K, Hystad ME, Kersten C, Smeland EB, Myklebust JH. Inhibitory effects and target genes of bone morphogenetic protein 6 in Jurkat TAg cells. Eur J Immunol. 2007;37:2937–2948. doi: 10.1002/eji.200636759. [DOI] [PubMed] [Google Scholar]
- 40.Darby S, Cross SS, Brown NJ, Hamdy FC, Robson CN. BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J Pathol. 2008;214:394–404. doi: 10.1002/path.2292. [DOI] [PubMed] [Google Scholar]
- 41.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigmond J, Kroep JR, Loves W, Codacci-Pisanelli G, Peters GJ. Quantitative real time PCR of deoxycytidine kinase mRNA by Light Cycler PCR in relation to enzyme activity and gemcitabine sensitivity. Cancer Lett. 2004;213:173–179. doi: 10.1016/j.canlet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Sampath TK, Nathanson MA, Reddi AH. In vitro transformation of mesenchymal cells derived from embryonic muscle into cartilage in response to extracellular matrix components of bone. Proc Natl Acad Sci USA. 1984;81:3419–3423. doi: 10.1073/pnas.81.11.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karahan S, Kincaid SA, Lauten SD, Wright JC. In vivo whole body and appendicular bone mineral density in rats: a dual energy X-ray absorptiometry study. Comp Med. 2002;52:143–151. [PubMed] [Google Scholar]
- 46.Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 47.McCullough KA, Waits CA, Garimella R, Tague SE, Sipe JB, Anderson HC. Immunohistochemical localization of bone morphogenetic proteins (BMPs) 2, 4, 6, and 7 during induced heterotopic bone formation. J Orthop Res. 2007;25:465–472. doi: 10.1002/jor.20340. [DOI] [PubMed] [Google Scholar]
- 48.Gitelman SE, Kobrin MS, Ye JQ, Lopez AR, Lee A, Derynck R. Recombinant Vgr-1/BMP-6-expressing tumors induce fibrosis and endochondral bone formation in vivo. J Cell Biol. 1994;126:1595–1609. doi: 10.1083/jcb.126.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 50.Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, Chung UI, Koike T, Takaoka K, Kamijo R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006;281:23246–23253. doi: 10.1074/jbc.M511039200. [DOI] [PubMed] [Google Scholar]
- 51.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grasser WA, Orlic I, Borovecki F, Riccardi KA, Simic P, Vukicevic S, Paralkar VM. BMP-6 exerts its osteoinductive effect through activation of IGF-I and EGF pathways. Int Orthop. 2007;31:759–765. doi: 10.1007/s00264-007-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, Stein GS, Nurcombe V, van Wijnen AJ, Cool SM. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50:954–964. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corradini E, Schmidt PJ, Meynard D, Garuti C, Montosi G, Chen S, Vukicevic S, Pietrangelo A, Lin HY, Babitt JL. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139:1721–1729. doi: 10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meynard D, Vaja V, Sun CC, Corradini E, Chen S, López-Otín C, Grgurevic L, Hong CC, Stirnberg M, Gütschow M, Vukicevic S, Babitt JL, Lin HY. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118:747–756. doi: 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 58.Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, Nai A, Arosio P. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117:997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]