Abstract
Purpose
The anterior cruciate ligament (ACL) is known to have a poor healing ability, especially in comparison with the medial collateral ligament (MCL) which can heal relatively well. Interleukin-1beta (IL-1β) is considered to be an important chemical mediator in the acute inflammatory phase of ligament injury. The role of IL-1β-induced expressions of lysyl oxidases (LOXs) and matrix metalloproteinases (MMPs), which respectively facilitate extracellular matrix (ECM) repair and degradation, is poorly understood. In this study, we aim to determine the intrinsic differences between ACL and MCL by characterising the differential expressions of LOXs and MMPs in response to IL-1β in the injury process.
Methods
Semi-quantitative polymerase chain reaction (PCR), quantitative real-time PCR, Western blot, and zymography were performed.
Results
We detected high expressions of IL-1β-induced LOXs in normal ACL and MCL. Then, we found IL-1β induced injured MCL to express more LOXs than injured ACL (up to 2.85-fold in LOX, 2.58-fold in LOXL-1, 1.89-fold in LOXL-2, 2.46-fold in LOXL-3 and 2.18-fold in LOXL-4). Meanwhile, we found IL-1β induced injured ACL to express more MMPs than injured MCL (up to 1.72-fold in MMP-1, 1.95-fold in MMP-2, 2.05-fold in MMP-3 and 2.3-fold in MMP-12). The further protein results coincided with gene expressions above.
Conclusions
Lower expressions of LOXs and higher expressions of MMPs might help to explain the poor healing ability of ACL.
Introduction
The anterior cruciate ligament (ACL) and medial collateral ligament (MCL) are two commonly injured areas of knee joints [1, 2]. In general, the MCL has the capacity to self-heal and restore joint function of a completely ruptured mid-substance within one month, and in most cases, without the need for surgery [3, 4]. On the other hand, the ACL does not heal satisfactorily, even if surgical repair is attempted [5], and ACL injuries often lead to knee instability, pain and even progressive degeneration of other joint tissues [6, 7].
Ligament healing is a complicated process which contains old extracellular matrix (ECM) collagen degradation and new ECM collagen synthesis and deposition [5, 8]. Failure to heal could be influenced by a lower potential for structural protein synthesis leading to a lower amount of scar tissue formation or rapid degradation of ligament substance following injury, or both [9]. Interleukin-1beta (IL-1β) is a strong pro-inflammatory cytokine that appears in the knee joint immediately after ligament injury and remains there through the following days [10–12]. IL-1β works both directly and indirectly on ligament injury by binding to its receptors and initiating a cellular response [10, 13]. Kondo and Ohshima’s research using a skin wound model in mice suggested a close relationship between IL-1β, the migration of fibroblasts and the formation of new granulation tissue [14]. However, the healing potential of intra-articular soft tissues is generally lower than an ordinary wound. To better understand the different healing abilities of ACL and MCL, we focused on the lysyl oxidases (LOX) and matrix metalloproteinases (MMP) response to IL-1β.
The LOX family has the capability of oxidising peptidyl lysine to peptidyl aldehyde residues within collagen, thus initiating formation of the covalent cross-linkages that precipitate these extracellular proteins, which facilitates the formation and repair of ECM [15, 17, 18, 45]. The LOX family contains five members characterised by conserved C-terminal copper binding and functional catalytic domains. They include LOX [18], LOX-like proteins 1 (LOXL-1) [19], 2 (LOXL-2) [20], 3 (LOXL-3) [21] and 4 (LOXL-4) [22], whose residue lengths are 417, 574, 638, 753 and 756 kDa, respectively. Compared with the significant homology within the C-terminal domains that appear among the human LOX family, there is little homology in the N-terminal regions of these proteins with the LOX propeptide domain [18]. As for the relationship between LOXs and IL-1β, previous data were mainly based on adult skin fibroblasts [23] and human lung fibroblasts [24].
MMPs play an important role in ECM degradation of ligaments [9, 25]. They could cleave one or more of the components of the ECM of ligaments. MMP-1 has the capability to cleave interstitial collagens I, II and III at a specific site relative to the N terminus [26]. MMP-2 containing three repeats of a type II fibronectin domain in the catalytic domain which bind to gelatin, collagens and laminin [27] can degrade gelatin as well as type I and III collagens [28]. MMP-3 has substrates including collagen type III, IV, V, VII, IX, X and XI, and also has activity against gelatin type I and elastin [29]; furthermore, it has a pivotal role in activating other MMPs [30]. The newest member of the stromelysin subgroup, MMP-12, is clearly the most active MMP against elastin [31], but it is also able to cleave, e.g. fibronectin, type IV collagen, and laminin-1 [32]; additionally, it has been shown to up-regulate the inflammatory response by stimulating the release of tumour necrosis factor alpha (TNF-α) from pro-TNF-α fusion protein [33].
Here, we investigate the intrinsic differences between the poor self-healing adult ACL and the relatively good self-healing adult MCL by characterising the differential expressions of LOXs and MMPs in response to IL-1β in the injury process. We mimicked injured ACL and MCL in vitro by an equibiaxial stretching chamber [43, 44] and sought to elucidate the differential variations of cross-linked enzyme LOXs and matrix-degraded protease MMPs in an injured ACL/MCL comparison model.
Materials and methods
Cell culture
The human materials used for this study were obtained according to ethical principles and the protocol was reviewed and approved by our Institutional Review Board. Human ACL and MCL fibroblasts were harvested from six donor tissues with an age from 23 to 56 as described previously [41]. In short, the donor ligament tissues were collected from patients who underwent total knee replacement surgery at the First Affiliated Hospital of Chongqing Medical University, Chongqing, China. The samples were immediately washed with 1 × phosphate-buffered saline (PBS) and cut into small pieces of dimension 2 × 2 × 2 mm3 (peri-portion of ACL tissue was discarded to avoid peri-fibroblast contamination). The small pieces of ligament were suspended in 10 % foetal bovine serum (FBS) media (low-glucose DMEM, 0.1 mM nonessential amino acids, 4 mM L-glutamine and antibiotics) and incubated at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. After the fibroblasts migrated out from small tissues and attached to the bottom, the tissues were transferred to another flask and the remaining cells were allowed to grow to confluence. The cells were then frozen into arrest growth with liquid nitrogen. Cells were then cultured in 10 % FBS-DMEM at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Experiments were carried out with cells from only passage 3 to passage 5. Cells from six different donor patients were used separately in the experiments. Experiments were repeated at least three times.
In vitro injury
The area inside each stretch chamber was coated with 5 pg/ml poly-D-lysine (MW 220,000: Sigma, St. Louis, MO, USA) at 37 °C for 40 minutes and then washed twice with PBS to remove unattached poly-D-lysine. The substrate molecule, collagen I (Sigma), was coated at concentrations of 5 pg/ml for 30 minutes at 37 °C. Excess collagen was washed away with two rinses of PBS and the chambers were then incubated with 0.5 % bovine serum albumin in PBS to prevent cell adhesion to areas of poly-D-lysine left uncovered by the collagen. Cells were trypsinised and seeded onto the collagen I-coated silicone membrane within an equibiaxial stretch chamber [43, 44] at the concentration of 107 cells per chamber (90–95 % confluence). Cells were allowed 24 hours to seed and equilibrate. Then, the culture media were removed and replaced by 2 % FBS media for 16 h for starvation. Right before stretching, the culture media were replaced with 1 % FBS fresh media. ACL and MCL cells were subjected to injurious (12 %) and control (0 %) stretch conditions. Then, 600 μl culture media samples were collected at 12, 24, 48 and 72 hours for protein expressions; 1,000 μl cell lysate samples (according to the RNA Isolation Kit, Qiagen, Hilden, Germany) were collected at zero, two, six, 12 and 24 hours for gene expressions.
IL-1β treatment
Cells were seeded onto the collagen I-coated silicone membrane within an equibiaxial stretch chamber. Cells were allowed 24 hours to seed and equilibrate. Then, the culture media were removed and replaced by 2 % FBS media for 16 hours for starvation. Right before being treated with IL-1β (PeproTech, Rocky Hill, NJ, USA) at different concentrations (1, 5 and 20 ng/ml), the culture media were replaced with fresh 1 % FBS media and cells were subjected to control (0 %) and injurious (12 %) stretch. Samples of 1,000 μl cell lysates were collected two hours after treatments with 1, 5 and 20 ng/ml IL-1β for semi-quantitative polymerase chain reaction (PCR) and quantitative real-time PCR assays; at 5 ng/ml, samples were collected at zero (control), two, six, 12 and 24 h after IL-1β treatments. At the protein level, samples of 500 μl cell lysates and 600 μl culture media were collected at 72 hours for Western blot of LOXs; 600 μl culture media were collected at 12, 24, 48 and 72 hours for zymography of MMP-2.
Semi-quantitative PCR
RNA samples from cells at two, six, 12 and 24 hours after IL-1β treatments in normal and injured ACL/MCL fibroblasts were isolated using the RNeasy Plus Mini Kit (Qiagen) with a genomic DNA eliminator, according to the manufacturer’s instructions. Isolated RNA was dissolved in RNAse-free water and quantified by measuring the absorbance at 260 nm with a spectrophotometer. The RNA samples were then treated with DNAse I (Mbi, Glen Burnie, MD, USA), and cDNA was prepared for each sample, using 0.5 mg of total RNA and the cDNA synthesis kit (Mbi) in a final volume of 20 μl.
To evaluate the expression levels of LOXs and MMPs in both ACL and MCL normalised to the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (or beta-actin gene, we finally displayed GAPDH as the control gene), semi-quantitative PCR was performed with a PCR kit (Mbi) using a thermal cycler (Bio-Rad, Hercules, CA, USA) according to described techniques. PCR primer pairs of these genes were designed using Primer5.0. The selected sets of primers are shown in Table 1. The BLAST was used to search for all primer sequences to ensure gene specificity. Semi-quantitative PCR reactions were performed in a 25 μl volume containing 1 μl cDNA sample following the manufacturer’s recommendations (here we used the OD method to detect the isolated mRNA concentration to make sure the total mRNA was suited for reactions). PCR consisted of an initial 30 second denaturising at 94 °C, 30 second for annealing at 55–65 °C and 72 °C, 30 second for elongation and 25–28 amplification cycles (to make sure the reaction is finished at exponential amplification zone). Products were resolved by 2 % agarose gel electrophoresis in Tris-borate/ethylenediaminetetraacetic acid (EDTA) buffer and visualised by staining with ethidium bromide.
Table 1.
Lysyl oxidase family (LOXs) and matrix metalloproteinases (MMPs) primers designed for semi-quantitative PCR and quantitative real-time PCR
| mRNA | Primer pairs |
|---|---|
| GAPDH | Forward GCACCGTCAAGGCTGAGAAC |
| Reverse TGGTGAAGACGCCAGTGGA | |
| LOX | Forward GCATACAGGGCAGATGTCAGA |
| Reverse TTGGCATCAAGCAGGTCATAG | |
| LOXL-1 | Forward TGCCACCAGCATTACCACAG |
| Reverse GAGGTTGCCGAAGTCACAGG | |
| LOXL-2 | Forward CTGCAAGTTCAATGCCGAGT |
| Reverse TCTCCACCAGCACCTCCACTC | |
| LOXL-3 | Forward CAACAGGAGGTTTGAACGCTAC |
| Reverse GCTGACATGGGTTTCTTGGTAA | |
| LOXL-4 | Forward TTCACCCACTACGACCTCCTCA |
| Reverse CAGCAGCCTACAGTCACTCCCT | |
| MMP-1 | Forward: GGCTGAAAGTGACTGGGAAACC |
| Reverse: TGCTCTTGGCAAATCTGGCGTG | |
| MMP-2 | Forward: GTGACGGAAAGATGTGGTG |
| Reverse: GGTGTAGGTGTAAATGGGTG | |
| MMP-3 | Forward: GACAAAGGATACAACAGGGAC |
| Reverse: TGAGTGAGTGATAGAGTGGG | |
| MMP-12 | Forward: AGGCACCAACTTGTTCCTCAC |
| Reverse: GAATGCCACGTATGTCATCAGC |
Quantitative real-time PCR
Quantitative real-time PCR was performed with Quanti-Tect SYBR Green PCR Kit (Qiagen) using iCycler (Bio-Rad) according to described techniques. PCR reactions were performed at 0.5 μM for each primer in a 25 μl volume containing 1 μl cDNA sample. The reaction was initiated by activating the polymerase with a 5-min pre-incubation at 95 °C. Amplification was achieved with 45 cycles of 15 seconds denaturation at 94 °C, 20 s annealing at 65 °C and ten seconds extension at 72 °C. The programme was concluded by a melting curve analysis. All experiments were performed in triplicate. The copy numbers of each gene were determined with cycle threshold (△CT) methods. The means of the copy numbers of GAPDH were used as internal controls to normalise the data. Standards for establishing standard curves of all primers were prepared from total normal cDNA, amplified by semi-quantitative PCR, and cloned using TOPO II TA Cloning Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s recommendations.
Western blot
Protein samples were prepared by mixing one part of sample with one part of Bio-Rad Laemmli Sample Buffer and then boiled at 100 °C for five minutes. Proteins were separated in 10 % sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (according to the molecular weight of LOX) and transferred to a polyvinylidene difluoride (PVDF) membrane at 200 mA for one hour at room temperature (RT). The blot was blocked with 5 % nonfat dry milk suspended in 1 × TBST for two hours at room temperature. The resulting blot was incubated with 1:1,000 goat LOX polyclonal antibody from Santa Cruz Biotechnology [LOX (E-19): sc-32410] for one hour at room temperature, followed by incubation with 1:5,000 rabbit anti-goat IgG-HRP from Santa Cruz Biotechnology for two hours at room temperature. Between the first and second incubation, the blot was washed three times with 1 × TBST for five minutes each time. Signals from blots were obtained using Santa Cruz Western Blotting Luminol Reagent Kit (sc-2048). Proteins were visualised via chemiluminescence with hydrogen peroxide using Kodak X-AR and luminol as substrate.
Zymography
MMP-2 activity was assayed from the collected culture media samples using 0.05 % gelatin zymography. Briefly, 10 ml of each sample was mixed with an equal amount of Laemmli Sample Buffer (62.5 mM Tris-HCl, pH 6.8, 25 % glycerol, 2 % SDS, 0.01 % bromophenol blue, no β-mercaptoethanol) and separated on a 10 % SDS-PAGE gel that was copolymerised with 0.05 % gelatin. To regain enzyme activity by removing the SDS, gels were washed three times for 1.5 hours in 2.5 % Triton X-100 at RT after electrophoresis. Washed gels were then bathed in proteolysis buffer (50 mM CaCl2, 0.5 M NaCl, 50 mM Tris, pH 7.8) and incubated at 37 °C for 15 hours. Following incubation, gels were rinsed in a 2.5 % Triton X-100 solution and stained at room temperature with Coomassie blue (45 % methanol, 44.75 % H2O, 10 % acetic acid, 0.25 % Coomassie blue R-250) for one hour on a rotator. Gels were destained (40 % methanol, 7.5 % acetic acid, 52.5 % H2O) until white bands appeared clearly from the Coomassie blue background. Bands were scanned using a densitometer (Bio-Rad), and quantification was performed using Quantity One 4.6.3 software (Bio-Rad).
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) to determine whether differences existed among groups. Post hoc analysis used Fisher’s protected least significant differences (PLSD). In each analysis, the critical significance level was set to be p < 0.05.
Results
Cell viability
No cytotoxic effects of exogenous inflammatory factor IL-1β were observed on the ACL and MCL cells at the different doses used in this study by trypan blue staining. Additionally, in our lab, cell activities of ACL and MCL were determined using the MTT assay, which is based on the mitochondrial conversion of the tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide. Increasing doses of IL-1β did not significantly alter cell viability in our lab (IL-1β up to 50 ng/ml).
IL-1β promoted gene expressions of the LOX family in normal ACL and MCL fibroblasts
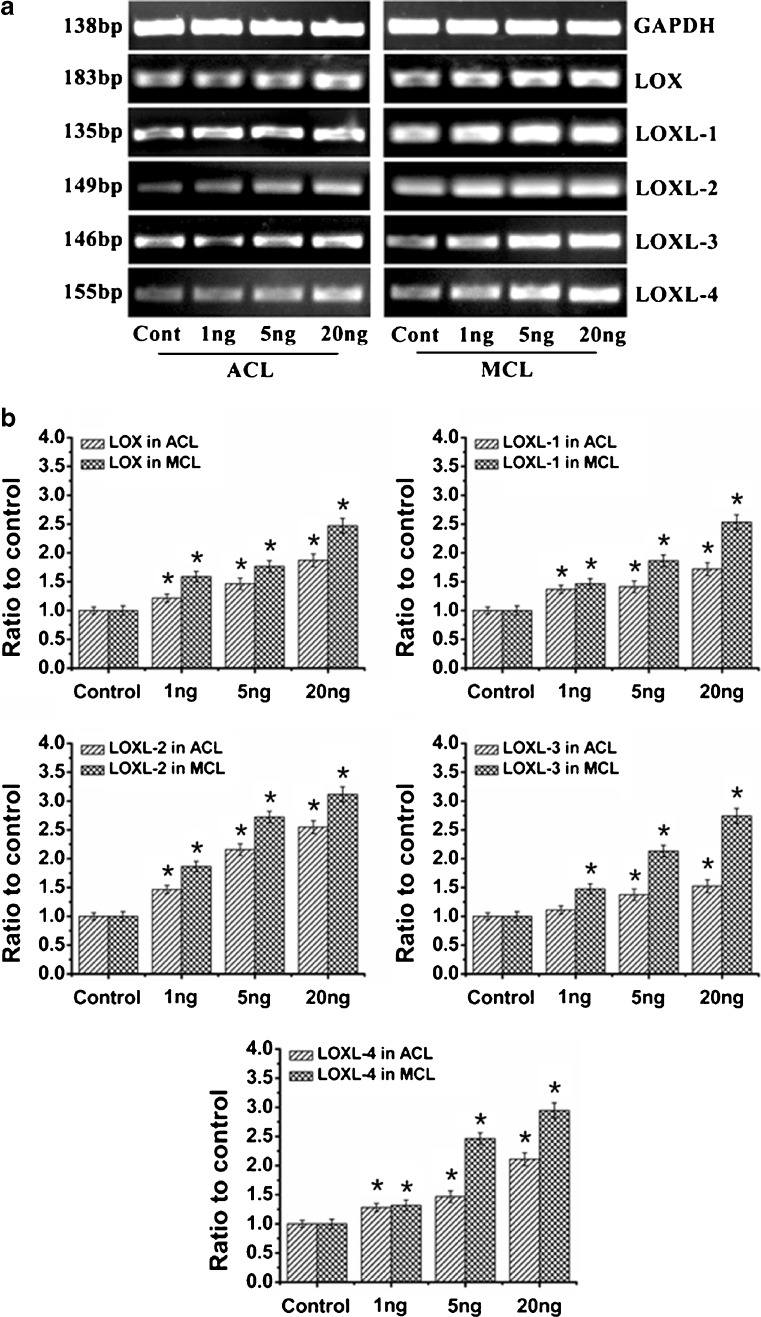
To determine the effects of IL-1β on expressions of the LOX family, ACL and MCL fibroblasts were treated with 0 (control), 1, 5 and 20 ng/ml IL-1β for the indicated time courses. The gene expressions of LOXs were detected by semi-quantitative PCR (Fig. 1a) and quantitative real-time PCR (Fig. 1b). We observed that IL-1β induced gene expressions of LOXs in a dose-dependent manner in ACL and MCL fibroblasts. Moreover, expressions of IL-1β-induced LOXs in MCL were higher than those in ACL fibroblasts. Specifically, at 20 ng/ml, LOX was 1.87 ± 0.15- and 2.47 ± 0.17-fold in ACL and MCL compared to non-treated controls, respectively; LOXL-1 1.72 ± 0.11- and 2.53 ± 0.13-fold; LOXL-2 2.55 ± 0.14- and 3.12 ± 0.13-fold; LOXL-3 1.52 ± 0.10- and 2.74 ± 0.16-fold; LOXL-4 2.11 ± 0.15- and 2.95 ± 0.13-fold. From Fig. 1, we could see the results of semi-quantitative PCR coincided with those of quantitative real-time PCR.
Fig. 1.
IL-1β induced dose-dependent increases of LOXs genes in both ACL and MCL fibroblasts. a Semi-quantitative PCR showed IL-1β induced higher gene expressions of LOXs in MCL than those in ACL after 2-h IL-1β treatments. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The gels shown were representative of four different experiments (n = 4); ACL and MCL fibroblasts in the comparison model of the experiments came from the same donors. Cont control, 1 ng, 5 ng and 20 ng 1, 5 and 20 ng/ml IL-1β, respectively. b Quantitative real-time PCR confirmed the different increases of LOXs in both ACL and MCL after 2-h IL-1β treatments. GAPDH was used as the reference gene. The △Ct method was used for measuring the fold changes. 1 ng, 5 ng and 20 ng concentrations of 1, 5 and 20 ng/ml IL-1β. The data presented were the mean of five different experiments (n = 5); scale bars SD. *p < 0.05 vs non-treated control
IL-1β induced higher expressions of the LOX family in injured MCL than those in injured ACL fibroblasts
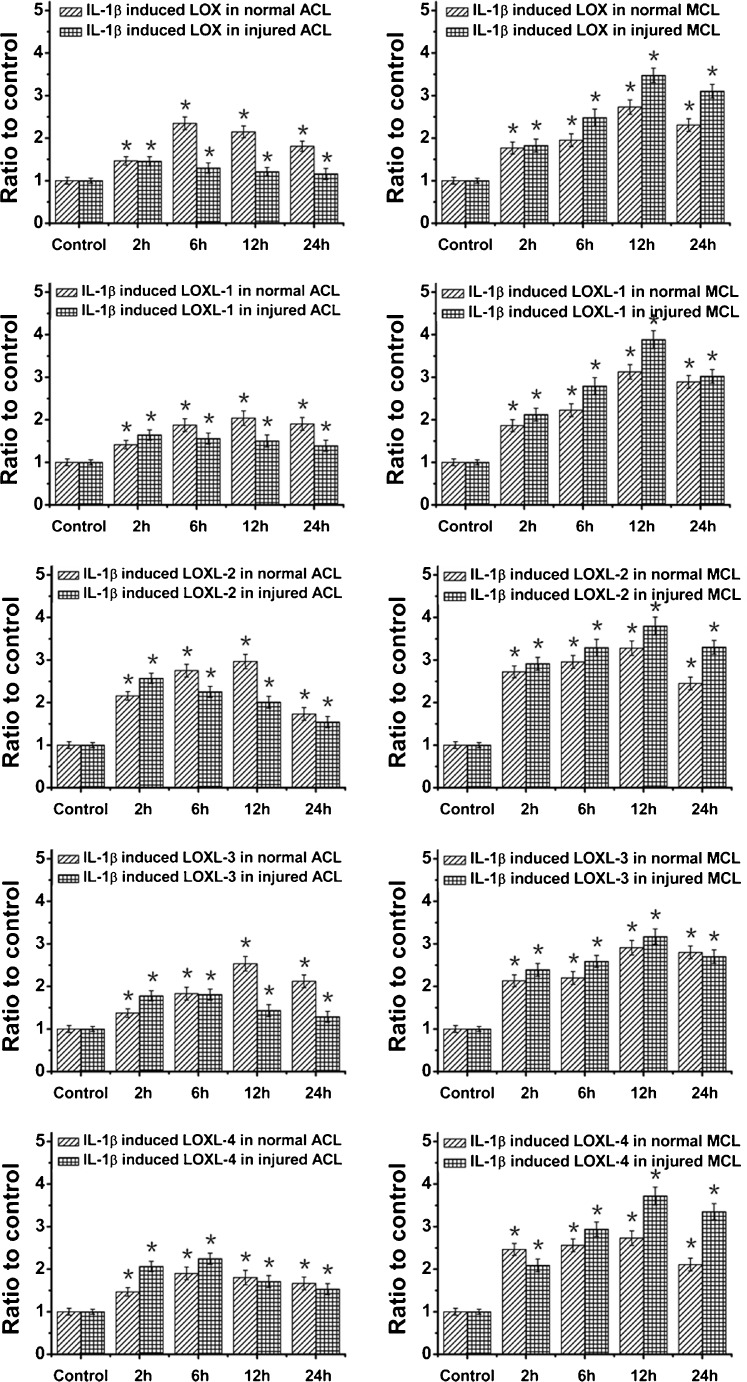
We used an equibiaxial stretch chamber to injure ACL and MCL cells, and then we treated these cells with 5 ng/ml IL-1β. As the time points at which a gene responds to stimulus vary, we determined the mRNA levels of LOX family genes at 0 (control), 2, 6, 12 and 24 hours after initiation of the 12 % injury stretch and 5 ng/ml IL-1β. We found that almost mRNA level peaks of LOXs were at 12 hours, except for LOX and LOXL-4 in ACL (at six hours). Specifically, IL-1β induced injured MCL to express more LOXs than normal MCL fibroblasts, while in ACL, although IL-1β up-regulated LOXs expressions in both normal and injured cells, the IL-1β induced injured ACL to express less LOXs compared with normal ACL as time points varied (Fig. 2).
Fig. 2.
An amount of 5 ng/ml IL-1β induced different high expressions of LOXs in normal and injured ACL/MCL fibroblasts by quantitative real-time PCR. After Fig. 1 experiments with different concentrations of IL-1β, we chose 5 ng/ml IL-1β to show the LOX family gene variations at the different time points following treatments. We collected samples at 0 (control), 2, 6, 12 and 24 h after 5 ng/ml IL-1β treatments in normal and injured ACL/MCL fibroblasts. GAPDH was used as the reference gene. The △Ct method was used for measuring the fold changes. The data presented were the mean of four different experiments (n = 4). *Significant difference with respect to control (p < 0.05)
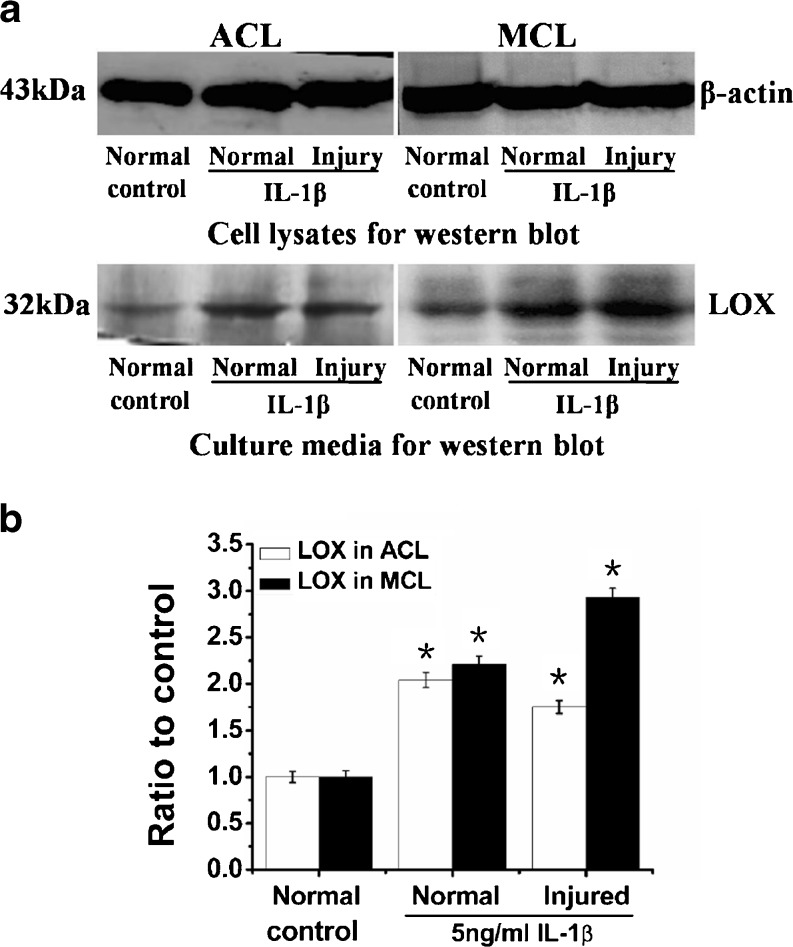
As we focused on the role of cross-linkage of LOX in the ECM, we further collected culture media to analyse LOX expressions at the protein level (cell lysates for β-actin as control). The culture media were collected at 72 hours after 5 ng/ml IL-1β treatments. We found 5 ng/ml IL-1β promoted higher expressions of LOX in both normal ACL and MCL fibroblasts compared with non-treated controls (up to 204 % in ACL and 225 % in MCL). In the injured state, IL-1β induced LOX expression up to 175 % in ACL and 293 % in MCL (Fig. 3).
Fig. 3.
IL-1β promoted protein expressions of LOX in normal and injured ACL/MCL fibroblasts. a Western blot showed LOX expressions in normal and injured ACL/MCL fibroblasts after being treated with 5 ng/ml IL-1β. The blot gels shown are representative of four different experiments (n = 4). The culture media samples were collected at 72 h following treatments for LOX expressions and the cell lysates samples for β-actin. b Quantitative analysis of the Western blot with Bio-Rad image software (Quantity One 4.6.3 software). The data were the mean of four different experiments (n = 4); SD. *Significant difference with respect to control (p < 0.05)
IL-1β induced higher levels of MMPs in injured ACL than those in injured MCL fibroblasts
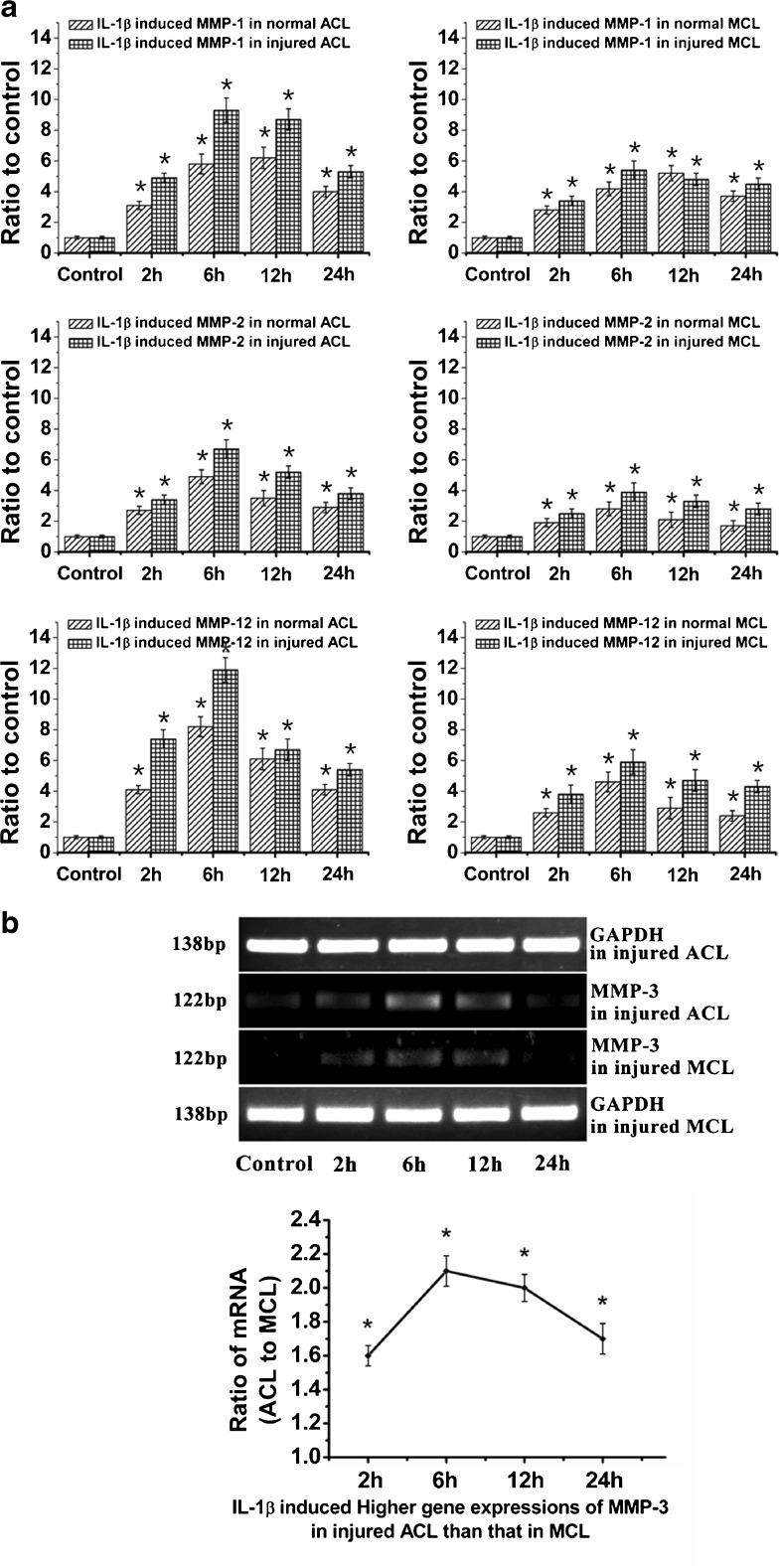
MMPs were known for their roles in ECM degradation after injury. Our lab previously reported the increases of MMPs in ACL and MCL fibroblasts after 12 % mechanical stretch injury [5, 8]. We further determined inflammatory factor IL-1β-induced MMPs in both ACL and MCL fibroblasts. We collected samples at 0 (control), 2, 6, 12 and 24 hours after initiation of 5 ng/ml IL-1β treatments in normal and injured ACL/MCL. We found MMP-1, -2 and -12 were highly expressed in injured ACL and MCL; furthermore, the expression peaks of MMP-1, -2 and -12 were all higher in injured ACL than those in MCL (MMP-1 9.30 ± 0.80-fold in ACL and 5.40 ± 0.60-fold in MCL compared to control; MMP-2 6.72 ± 0.70- and 3.91 ± 0.65-fold; MMP-12 11.91 ± 0.9- and 5.80 ± 0.81-fold) (Fig. 4a). Semi-quantitative PCR detected that MMP-3 was higher in injured ACL than that in MCL (Fig. 4b).
Fig. 4.
IL-1β induced higher gene expressions of MMPs in injured ACL than those in MCL fibroblasts. a Quantitative real-time PCR showed higher gene expressions of MMP-1, -2 and -12 in injured ACL than those in MCL fibroblasts. The data were recorded after 0 (control), 2, 6, 12 and 24 h following 5 ng/ml IL-1β treatments. The data for each sample were normalised to GAPDH mRNA. Data (means ± SD, n = 4) were represented as the fold change in expression compared to control. *p < 0.05. b The mRNA of MMP-3 in ACL and MCL cells were too low to detect using quantitative real-time PCR by the △Ct method loading 1 μl cDNA coordinate with other MMPs. Through GAPDH using semi-quantitative PCR with 38 cycles, increased gene expressions reached value peaks at 6 h after 5 ng/ml IL-1β in injured ACL and MCL. Notably, the ratio of MMP-3 mRNA (injured ACL to MCL) was high. The data were the mean of four different experiments (n = 4). *sSgnificant difference with respect to control (p < 0.05)
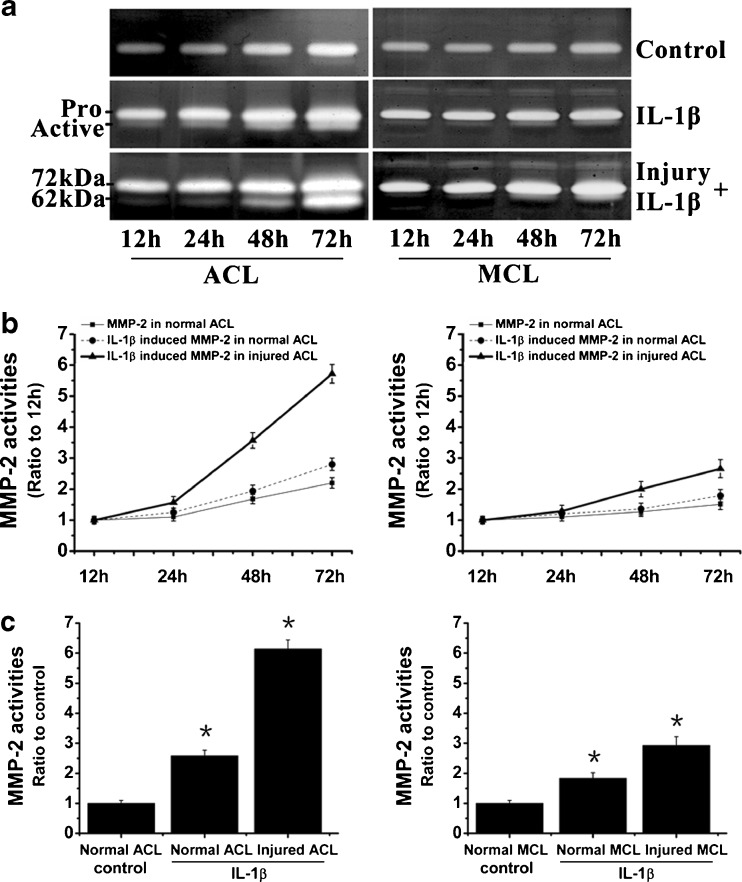
We then collected culture media at 12, 24, 48 and 72 hours after IL-1β treatments in normal and injured ACL/MCL fibroblasts. Activities of MMP-2 were detected using zymography (Fig. 5). We found IL-1β-induced MMP-2 activities in normal and injured ACL were prominently higher than MCL. Specifically, at 72 hours, IL-1β-induced MMP-2 activity in injured ACL was up to 6.14 ± 0.32-fold higher compared to control, while in injured MCL, activity of MMP-2 was 2.92 ± 0.28-fold higher than control.
Fig. 5.
IL-1β induced higher activities of MMP-2 in injured ACL than those in injured MCL fibroblasts. a Zymography showed different expressions of MMP-2 in normal and injured ACL/MCL fibroblasts. The gels shown were representative of four different experiments (n = 4). b Quantification of MMP-2 activities showed time-dependent increases of MMP-2 activities in both normal and injured ACL/MCL. Quantification was done with Quantity One 4.6.3 software. Optical densities of the pro-MMP-2 and active-MMP-2 bands were added as the total value of activity for MMP-2. Then, the values of 24, 48 and 72 h were compared to the values of 12 h. c The indicated quantitative data refer to 72-h time points of control and treated groups, respectively. Besides, the band 62 kDa active form MMP-2 was calculated as 10 times density of the 72 kDa pro-MMP-2 band as described previously [5, 8]. The data were the mean of four different experiments (n = 4). *Significant difference with respect to control (p < 0.05)
Discussion
An essential feature of wound healing is controlled proteolytic degradation and remodelling of the ECM [31, 38, 39]. Wound repair of the connective tissue matrix also undergoes this process including the inflammatory phase, the reparative phase and the remodelling phase [9, 16]. Furthermore, the interactions between the dynamically altered matrix and the inflammatory, reparative and remodelling responses are reciprocal [16].
Inflammation is the initial response to any tissue injury. The inflammatory cytokines play an important role in the wound healing mechanism, especially during the inflammatory phase [34–36]. The acute inflammatory response usually lasts between 24 and 48 hours and may persist for up to two weeks in some cases [11, 37]. In ligament fibroblasts, a high level of inflammatory factor IL-1β appeared within 24 h of ACL and MCL injury, and there were variable changes in IL-1β through the following days [5, 8].
More and more evidence has indicated the relationship between LOXs and wound healing [15, 18, 45–47]. The role of each family member towards ECM cross-linkage has been consecutively identified [45, 48–51]. However, the relationship between IL-1β and LOXs in ligament healing was barely recorded. From our data, we could see IL-1β up-regulated expressions of LOXs in normal and injured ACL/MCL fibroblasts. The results were coincident with those previously reported in adult skin fibroblasts [23] and human lung fibroblasts [24]. Furthermore, the up-regulated LOXs induced by IL-1β were higher in MCL than those in ACL at normal and injured states, respectively (Figs. 1, 2 and 3). Additionally, in ACL fibroblasts, IL-1β-induced LOXs were higher in normal cells compared to injured cells, while in MCL, IL-1β promoted injured cells to express more LOXs than normal cells. In other words, after IL-1β treatment, the injured MCL could express much more mRNAs of the LOXs compared with injured ACL fibroblasts. Since LOX enzymes participate in ECM formation and repair, the result may provide an explanation concerning the better healing response of MCL injury in comparison to that of ACL.
MMPs are Zn2+-dependent endoproteinases, which were initially classified into three groups based on their substrate specificities [40]. The three groups are collagenases (such as MMP-1), gelatinases (MMP-2 and MMP-9) and stromelysins (such as MMP-12). Our previous data [41] showed that, after injury, MMP-1 increased up to 2.76-fold in ACL, but showed no significant change in MCL cells. MMP-2 showed up to 4.29-fold increase in ACL compared with MCL and activities showed a time-dependent manner. MMP-3 and MMP-12 seem without significant change in both ACL and MCL. In this study, we found that, after injury, IL-1β could induce higher expressions of MMPs above. MMP-1 increased up to 9.3-fold in ACL, while 5.2-fold in MCL; MMP-2 up to 6.7-fold in ACL and 3.9-fold in MCL; MMP-12 up to 11.8-fold in ACL and 5.9-fold in MCL (Fig. 4a). Then we detected MMP-3; we found that although there was no significant change in injured ACL and MCL, in vitro IL-1β enhanced injured ACL and MCL to express MMP-3, the ratio of mRNAs (ACL to MCL) was up to 2.1-fold (Fig. 4b).
Previously, our lab has also reported that MMP-2 production is directly related to ACL fibroblast repair [8]. Two forms (62 and 72 kDa) carry out the same enzymatic reaction, but the 72 kDa MMP-2 has approximately 10 % the activity of the 62 kDa MMP-2 [5]. Here we found that after treatment with 5 ng/ml IL-1β, there was a significant time-dependent increase in the production of the 72 kDa MMP-2 in normal and injured ACL/MCL fibroblasts (Fig. 5a). The increased MMP-2 activities were obviously higher in injured ACL than in injured MCL, and the active form (62 kDa) of injured ACL was more prominent compared to injured MCL. The high levels of MMPs in injured ACL may provide another explanation of its poor self-healing ability.
We concentrated on the influence of pro-inflammatory cytokine IL-1β on LOXs and MMPs in injured ACL and MCL, but this only partially mimicked real injury conditions. The ligament-specific factors including cellular properties [42] and actual environmental factors including nutrient delivery, biomechanical forces and synovial fluid [5, 8, 36] may modulate the levels of LOXs and MMPs released from injured ACL and MCL. In the actual injury state, all factors may exert the effects together to induce LOX and MMP expressions during the injury cascade.
In summary, pro-inflammatory cytokine IL-1β induced higher expressions of MMPs, and relatively lower LOXs in injured ACL compared with those in injured MCL fibroblasts might explain the poor self-healing capacity of ACL. Seek factors or combined factors which could inhibit the activities of MMPs and promote activities of LOXs might increase the strength of the repaired injured ACL to a level where there would be no need for reconstructive surgeries.
Acknowledgments
We thank Wei hang, M.D., Ph.D. (Department of Orthopaedics, Chongqing University of Medical Sciences, Chongqing 400000, China), Rongfu Chen, M.D., Chen Chen, M.D. and Chunfeng Fu, M.D. for the tissue material supply.
This study was supported by project 111 (B06023, China) and NIH AR45635 (USA).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21:696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Duthon VB, Barea C, Abrassart S, et al. Anatomy of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14:204–213. doi: 10.1007/s00167-005-0679-9. [DOI] [PubMed] [Google Scholar]
- 3.Sung K-LP, Yang L, Whittemore DE, et al. The differential adhesion forces of anterior cruciate and medial collateral ligament fibroblasts: effects of tropomodulin, talin, vinculin and alpha-actinin. Proc Natl Acad Sci U S A. 1996;93:9182–9187. doi: 10.1073/pnas.93.17.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannus P. Long-term results of conservatively treated medial collateral ligament injuries of the knee joint. Clin Orthop Relat Res. 1988;226:103–112. [PubMed] [Google Scholar]
- 5.Zhou D, Lee HS, Villarreal F, et al. Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23:949–957. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 7.Jomha NM, Borton DC, Clingeleffer AJ, et al. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res. 1999;358:188–193. doi: 10.1097/00003086-199901000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Tang ZY, Yang L, Wang YQ, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27:243–248. doi: 10.1002/jor.20763. [DOI] [PubMed] [Google Scholar]
- 9.Steffensen B, Häkkinen L, Larjava H. Proteolytic events of wound-healing—coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- 10.D’Ambrosia P, King K, Davidson B, et al. Pro-inflammatory cytokines expression increases following low- and high-magnitude cyclic loading of lumbar ligaments. Eur Spine J. 2010;19:1330–1339. doi: 10.1007/s00586-010-1371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzheimer RG, Steinmetz WG. Local and systemic concentrations of pro- and anti-inflammatory cytokines in human wounds. Eur J Med Res. 2000;5:347–355. [PubMed] [Google Scholar]
- 12.Liechty KW, Crombleholme TM, Cass DL, et al. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–84. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 13.Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Ohshima T. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med. 1996;108:231–236. doi: 10.1007/BF01369816. [DOI] [PubMed] [Google Scholar]
- 15.Xie J, Jiang JH, Zhang YJ, et al. Up-regulation expressions of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts induced by transforming growth factor-beta 1. Int Orthop. 2012;36:207–213. doi: 10.1007/s00264-011-1261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerman RH, Apstein CS, Kagan HM, et al. Myocardial healing and repair after experimental infarction in the rabbit. Circ Res. 1983;53:378–388. doi: 10.1161/01.RES.53.3.378. [DOI] [PubMed] [Google Scholar]
- 18.Kagan HM, Li WD. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 19.Kim YH, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270:7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- 20.Saito H, Papaconstantinou J, Sato H, et al. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272:8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 21.Jang W, Hua A, Spilson SV, et al. Comparative sequence of human and mouse BAC clones from the mnd2 region of chromosome 2p13. Genome Res. 1999;9:53–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Mäki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–496. doi: 10.1016/S0945-053X(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 23.Cenizo V, André V, Reymermier C, et al. LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Exp Dermatol. 2006;15:574–581. doi: 10.1111/j.1600-0625.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 24.Roy R, Polgar P, Wang YY, et al. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: interactions among TGF-beta, IL-1 beta, and prostaglandin E2. J Cell Biochem. 1996;62:411–417. doi: 10.1002/(SICI)1097-4644(199609)62:3<411::AID-JCB11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Emonard H, Grimaud JA. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36:131–153. [PubMed] [Google Scholar]
- 26.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 27.Allan JA, Docherty AJP, Barker PJ, et al. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J. 1995;309:299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creemers LB, Jansen IDC, Docherty AJP, et al. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 1998;17:35–46. doi: 10.1016/S0945-053X(98)90123-8. [DOI] [PubMed] [Google Scholar]
- 29.Bramono DS, Richmond JC, Weitzel PP, et al. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res. 2004;428:272–285. doi: 10.1097/01.blo.0000144166.66737.3a. [DOI] [PubMed] [Google Scholar]
- 30.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/S0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 32.Chandler S, Cossins J, Lury J, et al. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Com. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- 33.Gronski TJ, Martin RL, Kobayashi DK, et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189–12194. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- 34.Mooney DP, O’Reilly M, Gamelli RL. Tumor necrosis factor and wound healing. Ann Surg. 1990;211:124–129. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt CC, Georgescu HI, Kwoh CK, et al. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res. 1995;13:184–190. doi: 10.1002/jor.1100130206. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4:293–298. doi: 10.1007/s007760050106. [DOI] [PubMed] [Google Scholar]
- 37.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/S0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 38.Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 39.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–640. doi: 10.1016/S0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 40.Gaire M, Magbanua Z, McDonnell S, et al. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J Biol Chem. 1994;269:2032–2040. [PubMed] [Google Scholar]
- 41.Tang Z, Yang L, Xue R, et al. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: involvement of the p65 subunit of NF-kappaB. Wound Repair Regen. 2009;17:709–716. doi: 10.1111/j.1524-475X.2009.00529.x. [DOI] [PubMed] [Google Scholar]
- 42.Nagineni CN, Amiel D, Green MH, et al. Characterization of the intrinsic properties of anterior cruciate ligament and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465–475. doi: 10.1002/jor.1100100402. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Harwood FL, Akeson WH, et al. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J. 1998;18:19–25. [PMC free article] [PubMed] [Google Scholar]
- 44.Lee AA, Delhaas T, McCulloch AD, et al. Differential responses of adult rat cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol. 1999;31:1833–1843. doi: 10.1006/jmcc.1999.1017. [DOI] [PubMed] [Google Scholar]
- 45.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/S0945-053X(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 46.Kumarasamy A, Schmitt I, Nave AH, et al. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med. 2009;180:1239–1252. doi: 10.1164/rccm.200902-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colwell A, Krummel T, Longaker M, et al. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plast Reconstr Surg. 2005;118:1125–1129. doi: 10.1097/01.prs.0000221056.27536.db. [DOI] [PubMed] [Google Scholar]
- 48.Liu XQ, Zhao Y, Gao JG, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 49.Kim YM, Kim EC, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38:145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 50.Yu QL, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- 51.Kim MS, Kim SS, Jung ST, et al. Expression and purification of enzymatically active forms of the human lysyl oxidase-like protein 4. J Biol Chem. 2003;278:52071–52074. doi: 10.1074/jbc.M308856200. [DOI] [PubMed] [Google Scholar]