Abstract
Purpose
The aim of this study was to investigate whether cationised gelatin and hyaluronic acid (CH) coating could induce polyethylene terephthalate (PET) artificial ligament graft osseointegration in the bone tunnel.
Methods
Surface modification of PET artificial ligament graft was performed by layer-by-layer (LBL) self-assembly CH coating. Six pigs underwent anterior cruciate ligament (ACL) reconstruction on the right knees, with three pigs receiving the CH-coated PET grafts and the other three pigs non-CH-coated PET grafts as controls. They were sacrificed at three months after surgery and the graft-bone complexes were acquired for computed tomography (CT) scan and histological examination.
Results
CT scans showed a significant difference at the distal femoral site (p = 0.031) or at the distal tibial site (p = 0.0078), but no significant difference in the bone tunnel areas’ enlargement at other sites (p > 0.05) between the CH group and the control group. Histologically, application of CH coating induced new bone formation between graft and bone at three months compared with the controls at the distal site. The interface width of the CH group was significantly lower than that of the control group at the distal femoral site (p = 0.0327) and at the distal tibial site (p = 0.0047).
Conclusions
The study has shown that CH coating on the PET artificial ligament surface has a positive biological effect in the induction of artificial ligament osseointegration within the bone tunnel at the distal site of the bone tunnel.
Introduction
The LARS artificial ligament (Ligament Advanced Reinforcement System, Surgical Implants and Devices, Arc-sur-Tille, France), made of polyethylene terephthalate (PET), has been accepted as a graft choice for anterior cruciate ligament (ACL) reconstruction by many surgeons all over the world [1–3]. It is a valid alternative for treating some acute and serious cases [4]. As this type of PET ligament has hydrophobicity and chemical inertia, the graft-bone healing of the LARS artificial ligament is one of the most important concerns after implantation. By analysing ruptured ACL artificial ligaments, Guidoin et al. [5] observed a chronic inflammatory reaction with macrophages and giant cells in the polyester ligaments. In a three to five year follow-up multicentre study, Gao et al. [6] reported that seven of 156 cases of ACL reconstruction using the LARS ligament were noted to exhibit graft failure at the bone tunnel, and they found that an interposed layer of fibrous scar tissue appears at the graft-bone interface at the second arthroscopic revision surgery. It was presumed that the inflammation at the graft-bone interface might induce the scar tissue formation and hamper the graft osseointegration within the bone tunnel. This raises questions about whether we could improve the artificial ligament graft healing in the bone tunnel through alleviating the inflammation and promoting graft biocompatibility.
Hyaluronic acid has been used to make the artificial graft a more desirable biomaterial scaffold for ligament tissue engineering [7, 8]. As a component of the extracellular matrix, hyaluronic acid has been demonstrated to accelerate the healing of rabbit flexor tendons [9]. Particularly, hyaluronic acid has the high capacity to suppress and alleviate inflammation [10–12]. Cationised gelatin, as a positively charged polymer, has long been used as a highly valuable carrier system as it provides a universally effective way to enhance biocompatibility of the polymers [13–15]. Previously, it has been demonstrated that the cationised gelatin and hyaluronic acid coating (CH coating) on PET artificial ligaments has a positive effect by inhibiting inflammatory cell infiltration and promoting graft biocompatibility [16].
As reported, the layer-by-layer (LBL) technique was very useful to make a surface coating on PET artificial ligaments [16, 17]. Therefore, the aim of this study was to promote graft osseointegration using LBL self-assembly CH coating. Moreover, a porcine ACL reconstruction model was applied to evaluate the effect of this CH coating in vivo. We hypothesised that the surface coating of CH would result in less fibrous scar tissue formation and better graft osseointegration at the graft-bone interface when compared with that of the blank controls at three months after operation.
Methods
Study design and operative procedure
In this study, PET artificial ligament grafts were modified by LBL self-assembly CH coatings. The detailed modification procedure has been illustrated in another report [16]. The pure PET graft without coating was used for the control group.
The animal experiment was approved by the Animal Care and Use Committee of our college. ACL reconstruction experiments were also performed in six healthy GuangXi male pigs (mean weight 43.4 ± 8.5 kg). After successful anaesthesia (induced by 25 mg/kg sodium pentobarbitone), the native ACL was exposed and removed from the insertion sites by sharp dissection. Next, a 4.5-mm diameter tunnel was drilled in the femoral and tibial insertion sites of the ACL. The graft was pulled manually into the bone tunnel. The graft with LBL coating was implanted into the right knees of three pigs, while the graft with no coating was implanted into the right knees of the other three pigs. At the bone portal, the graft ends were sutured with the adjacent periosteum and soft tissue with no. 5 Ethibond sutures. The wounds were closed in layers. Post-operatively, the animals were then returned to the animal care facilities. Three pigs in each group were sacrificed at three months after surgery for the following examinations.
Computed tomography
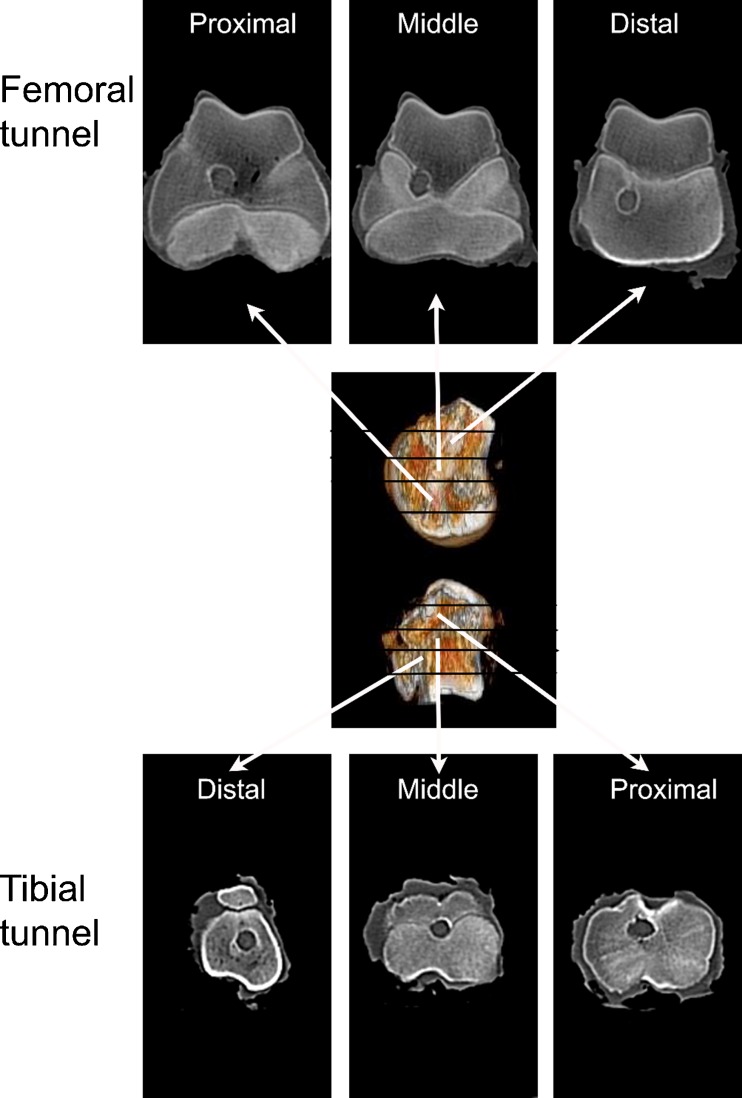
Immediately after sacrifice, the graft-tibia complex samples (n = 3) underwent computed tomography (CT, SOMATOM Sensation 64, Siemens, Forchheim, Germany) scan to measure the area of bone tunnel (bone window data, width 1,500; level 450). The tunnel areas on the axial plane were estimated and measured with Image-Pro Plus 6.0 software (Media Cybernetics Corp, Rockville, MD, USA). The sections of each sample were averaged for statistical estimation. The bone tunnel of the femoral tunnel or the tibial tunnel was respectively divided into three parts according to the distance from the knee joint: distal part, middle part and proximal part (Fig. 1).
Fig. 1.
CT scan of the knee joint. The bone tunnel of the femoral tunnel or the tibial tunnel was respectively divided into three parts according to the distance from the knee joint: distal part, middle part and proximal part
Histological examination
Graft-bone complexes (n = 3 limbs for each group) were prepared for histological analysis of the graft-bone interface. Immediately after sacrifice, the graft-bone complex specimens were fixed in 10 % formalin for 48 h, then decalcified in ethylenediaminetetraacetic acid (EDTA) solution and then embedded in paraffin wax. The samples were sectioned perpendicular to the longitudinal axis of the tibial tunnel with a thickness of 5 μm using a microtome (SM2500, Leica, Wetzlar, Germany). These sections were stained with haematoxylin and eosin (H&E) for routine histological evaluation. The slides were examined to visualise the graft-bone interface with inverted light microscopy (IX71SBF-2, Olympus Co., Tokyo, Japan). Digital images were taken using a DP Manager (Olympus Optical Co., Tokyo, Japan). The two investigators performing the histological analysis were blinded to animal treatment.
Histomorphometric analysis
Each section was divided into four quadrants to determine the graft-bone interface width. In each of the four quadrants, the interface width was measured as the distance between the edge of the bone tunnel and the outer graft determined under ×200 magnification. Four separate measurements were made in each of the quadrants for a total of 16 measurements for each specimen. The average interface width for each specimen was then determined by averaging these values obtained from each specimen. The two investigators who performed the histomorphometric analysis were blinded to the type of animal treatment.
Statistical analysis
Data analysis was performed using Stata 9.0 software (StataCorp, College Station, TX, USA) and then data were reported as mean and standard deviation for description. A statistical analysis of quantitative results was carried out with the paired Student’s t test or two-sample Wilcoxon rank sum test. The statistical significance level was set at 0.05.
Results
Computed tomography
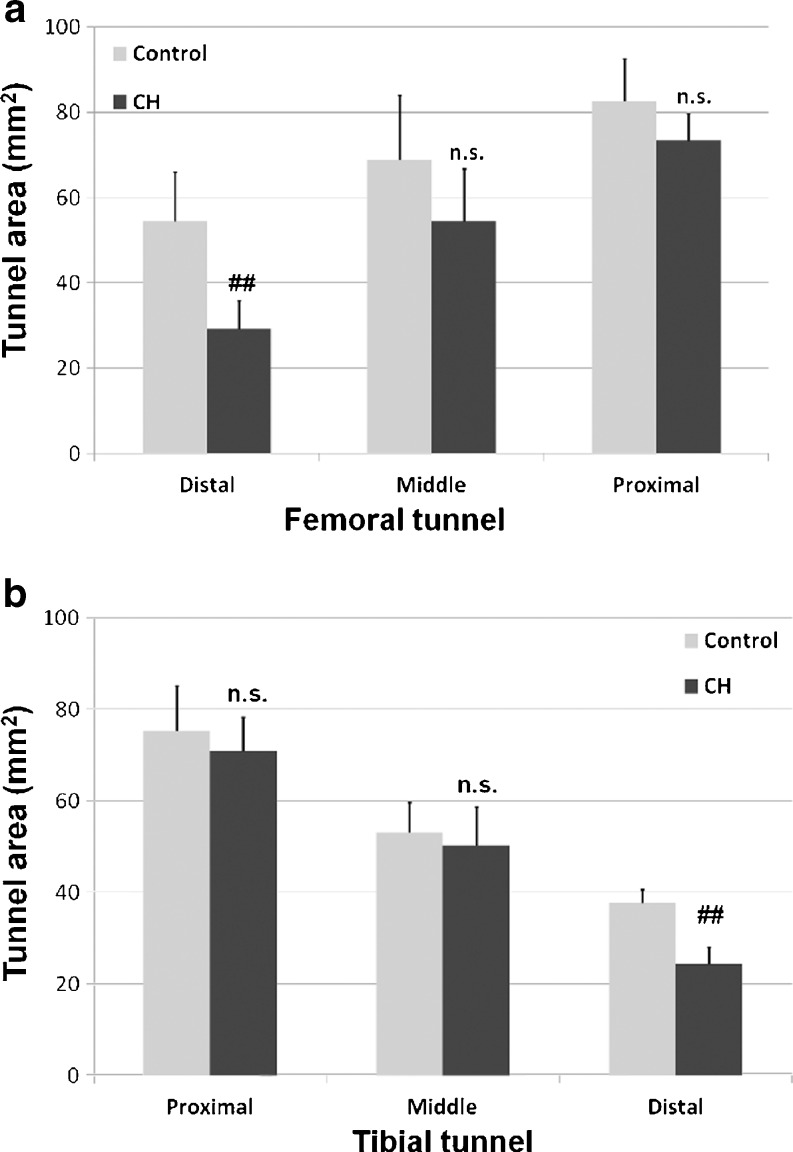
For the femoral tunnel, the average bone tunnel areas of the control group were 54.4 ± 11.6 mm2 (distal site), 68.9 ± 15.1 mm2 (middle site) and 82.7 ± 9.8 mm2 (proximal site), while the average bone tunnel areas of the CH group were 29.1 ± 6.7 mm2 (distal site), 54.4 ± 12.4 mm2 (middle site) and 73.3 ± 6.4 mm2 (proximal site) (Fig. 2a). In the femoral tunnel, there were significant differences of tunnel area at the distal site between the control group and the CH group (p = 0.031), and there were no differences of the tunnel area between the control group and the CH group at the proximal site (p > 0.05) or at the middle site (p > 0.05). For the tibial tunnel, the average bone tunnel areas of the control group were 75.2 ± 9.8 mm2 (proximal site), 53.1 ± 6.5 mm2 (middle site) and 37.8 ± 2.9 mm2 (distal site), while the average bone tunnel areas of the CH group were 70.9 ± 7.4 mm2 (proximal site), 50.3 ± 8.4 mm2 (middle site) and 24.4 ± 3.7 mm2 (distal site) (Fig. 2b). There was a significant difference of the bone tunnel area between the control group and the CH group at the distal tibial site (p = 0.0078). No significant difference of tunnel area was detected between groups at the proximal site (p > 0.05) or at the middle site (p > 0.05).
Fig. 2.
a The average bone tunnel area of the femoral tunnel between the CH group and the control group. b The average bone tunnel area of the tibial tunnels between the CH group and the control group. ## significant difference, NS no significant difference, CH cationised gelatin and hyaluronic acid coating
Histological findings
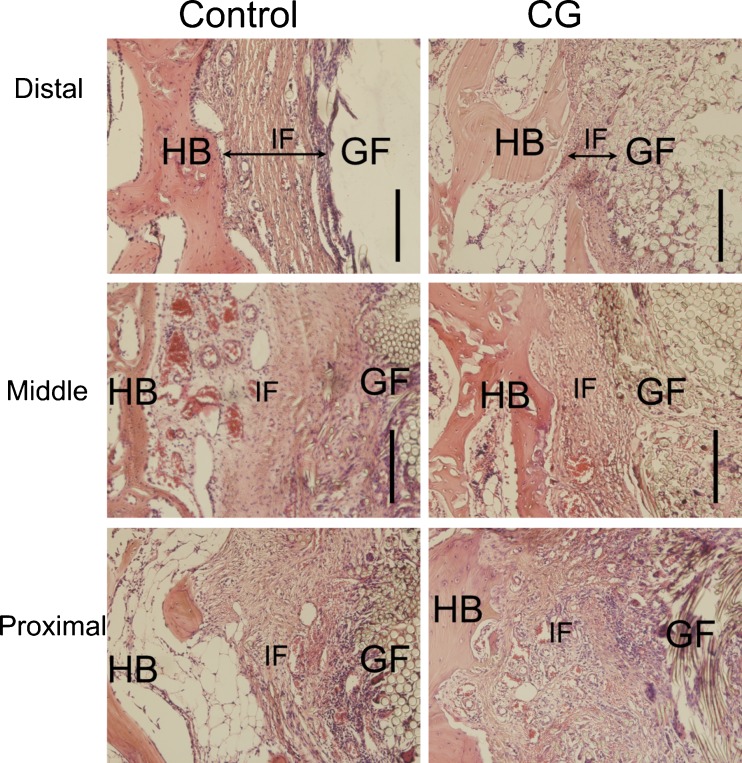
For the femoral tunnel (Fig. 3), the graft-bone interface of the control group was filled with granulation tissue at the proximal and middle site, and a thick fibrous scar tissue was formed at the graft-bone interface at the distal site. In the group with CH coating, the graft-bone interface also appeared somewhat messy with fibrous tissue at the proximal site. At the middle site in the CH group, interface width appeared much narrower and host bone grew onto to the graft. When it comes to the distal site of the CH group, there was less scar tissue formation at the graft-bone interface, and the interface between the graft and host bone was very narrow.
Fig. 3.
Histological characterisation of the femoral graft-bone sample between the control group and the CH-coated group at each site. HB host bone, IF interface, GF graft fibres. Bar = 200 μm
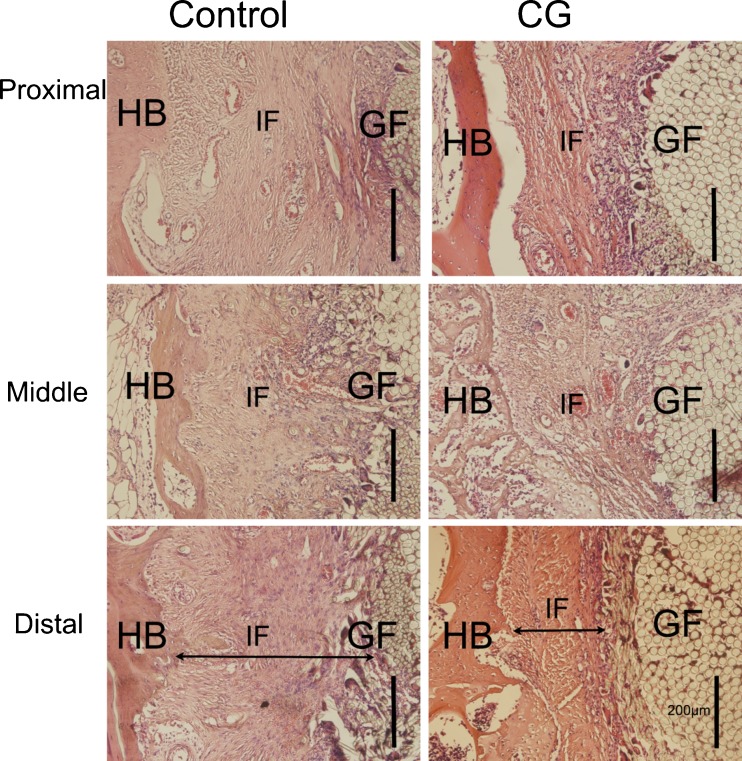
In the tibial tunnel (Fig. 4), both the control group and CH group had fibrous scar tissue at the interface of the proximal site and the middle site, potentially arousing an inflammatory response. In the distal site of the control group, there was apparent scar tissue formation at the interface, whereas there was much progressive new matrix formation and bone ingrowth at the distal tibial site of the CH group. Treatment with the CH-coated grafts resulted in less apparent scar tissue formation at the graft-bone interface than did the non-coated grafts.
Fig. 4.
Histological characterisation of the tibial graft-bone sample between the control group and the CH-coated group at each site. HB host bone, IF interface, GF graft fibres. Bar = 200 μm
Histomorphometric analysis
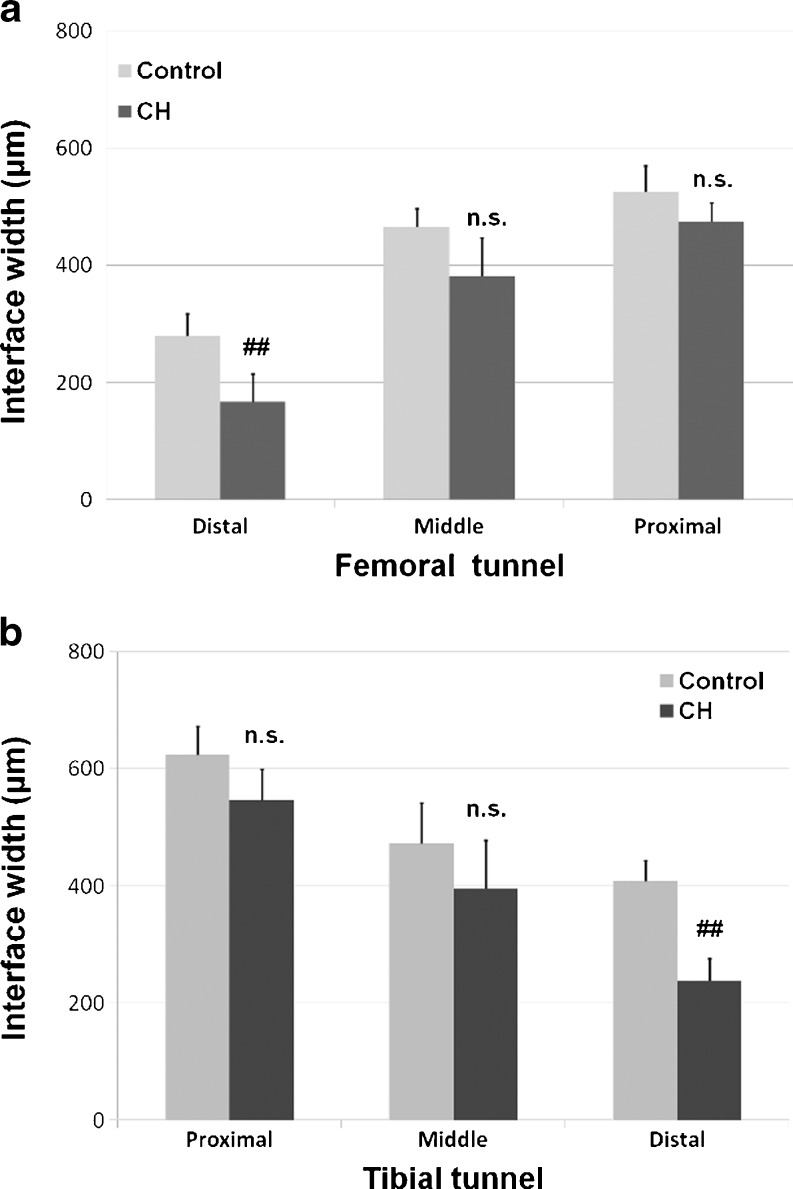
In the femoral tunnel, there were no differences of the interface width between the control group and the CH group at the proximal site (p > 0.05) or at the middle site (p > 0.05). However, there was a significant difference of interface width between the control group (279.7 ± 37.6 μm) and the CH group (167.0 ± 47.8 μm) at the distal site (p = 0.0327). Likewise in the tibial tunnel, the mean interface width of the CH-coated group was significantly lower than that of the control group at the distal site (238.7 ± 37.0 μm vs 407.7 ± 35.7 μm, p = 0.0047). No significant difference of interface width was detected between groups at the proximal site (p > 0.05) or at the middle site (p > 0.05) (Fig. 5).
Fig. 5.
Comparison of the graft-bone interface width between the control group and the CH group in the femoral tunnel (a) and in the tibial tunnel (b). ## significant difference, NS no significant difference, CH cationised gelatin and hyaluronic acid coating
Discussion
Tendon healing in a bone tunnel is a priority concern in the orthopaedic and biomedical field after ACL reconstruction [18–23]. Fibrous scar tissue formation has been shown to be an incomplete healing response [24]. This kind of immature granulation fibrous layer is extremely weak and will significantly influence the graft stability in the bone tunnel. Thus, the importance of understanding and improving graft-bone healing, particularly in ACL reconstructions, has fuelled a great increase in research on the topic.
In this study, the PET artificial ligament was modified with a multiple layer of CH coating in order to promote the graft osseointegration in the bone tunnel. Both the CT scan and the histological examination revealed that the CH group had a smaller bone tunnel area and a thinner interface width than the control group in the distal site of the bone tunnels. These results indicated that CH coating had a positive effect by enhancing the graft osseointegration in the distal site of the bone tunnel though promoting the graft biocompatibility and enhancing new bone formation at the graft-bone interface.
Generally speaking, the tendon-bone healing process is divided into four stages: (1) inflammation, (2) proliferation, (3) matrix synthesis and (4) matrix remodelling. During this process, mechanical and biological factors are involved in the healing process of the reconstructed tendons [25–29]. In the distal part of the bone tunnel, the mechanical effect was relatively low and the graft-bone healing was influenced mainly by the biological environment. CH coating is able to exert a biological effect, promoting the graft biocompatibility and alleviating inflammation in the bone tunnel, thus decreasing the formation of fibrous tissue at the graft-bone interface of the distal bone site. Therefore, it was presumed that the CH coating came into play at the distal bone site with not much mechanical influence.
Iorio et al. [30] also used CT to analyse bone tunnel enlargement and concluded that a less aggressive rehabilitation process influenced the amount of tunnel enlargement after ACL reconstruction. Vadalà et al. [31] also used CT to exactly determine the diameters of both femoral and tibial tunnels at various levels of the lateral femoral condyle and proximal tibia and demonstrated that early post-operative knee motion increases the diameters of the tibial and femoral bone tunnels. Interestingly, in the proximal site of the femoral tunnel or the tibial tunnel in our study, we found no significant difference of the bone tunnel area between groups on the CT images. Both groups revealed enlarged bone tunnels at the proximal site. Tunnel enlargement indicated that the natural graft-bone healing is not satisfactory with a fibrous scar tissue layer formation at the graft-bone interface [24, 32, 33]. As demonstrated, tunnel widening or enlargement frequently occurred due to excessive graft-tunnel motion, so as to delay graft incorporation in the bone tunnel [34, 35]. The effect of mechanical load influences the cellular and molecular cascade of tendon-to-bone healing. It has been illustrated that the closer the fixation to the joint, the lower the incidence of tunnel widening [36, 37]. The graft motion influenced the proximal site of the graft more greatly than the distal site. Therefore, the proximal site had poorer graft healing in the bone tunnel compared with the distal site, although the CH coating might afford some positive biological effect.
Conclusion
This study demonstrated that the CH coating had a positive biological effect by enhancing graft-bone healing at the distal site of the bone tunnel. This biological technique of LBL self-assembly CH coating may open a new door for orthopaedic surgeons to accelerate artificial ligament graft healing after implantation in the bone tunnel, thereby promoting rapid recovery and a quicker return to sports activity. To be noted, the biological effect of CH coating might be influenced by the biomechanical factors. Investigation of the biomechanical effect should be undertaken in a larger sample size before the CH coating is used in clinical application.
Acknowledgments
This work was supported by the Grants from 973 Project from the Ministry of Science and Technology of China (No. 2009CB930000), the Young Project of National Natural Science Foundation of China (81000816) and the Project of Shanghai Municipal Science and Technology Commission (11JC1401700).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Liu ZT, Zhang XL, Jiang Y, Zeng BF. Four-strand hamstring tendon autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Int Orthop. 2010;34:45–49. doi: 10.1007/s00264-009-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen G, Xu Y, Dong Q, Zhou H, Yu C. Arthroscopic posterior cruciate ligament reconstruction using LARS artificial ligament: a retrospective study. J Surg Res. 2012;173:75–82. doi: 10.1016/j.jss.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Hamido F, Misfer AK, Al Harran H, Khadrawe TA, Soliman A, Talaat A, Awad A, Khairat S. The use of the LARS artificial ligament to augment a short or undersized ACL hamstrings tendon graft. Knee. 2011;18:373–378. doi: 10.1016/j.knee.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Ranger P, Renaud A, Phan P, Dahan P, De Oliveira E, Jr, Delisle J. Evaluation of reconstructive surgery using artificial ligaments in 71 acute knee dislocations. Int Orthop. 2011;35:1477–1482. doi: 10.1007/s00264-010-1154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidoin MF, Marois Y, Bejui J, Poddevin N, King MW, Guidoin R. Analysis of retrieved polymer fiber based replacements for the ACL. Biomaterials. 2000;21:2461–2474. doi: 10.1016/S0142-9612(00)00114-9. [DOI] [PubMed] [Google Scholar]
- 6.Gao K, Chen S, Wang L, Zhang W, Kang Y, Dong Q, Zhou H, Li L. Anterior cruciate ligament reconstruction with LARS artificial ligament: a multicenter study with 3- to 5-year follow-up. Arthroscopy. 2010;26:515–523. doi: 10.1016/j.arthro.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Funakoshi T, Majima T, Iwasaki N, Yamane S, Masuko T, Minami A, Harada K, Tamura H, Tokura S, Nishimura S. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J Biomed Mater Res A. 2005;74:338–346. doi: 10.1002/jbm.a.30237. [DOI] [PubMed] [Google Scholar]
- 8.Irie T, Majima T, Sawaguchi N, Funakoshi T, Nishimura S, Minami A. Biomechanical and histologic evaluation of tissue engineered ligaments using chitosan and hyaluronan hybrid polymer fibers: a rabbit medial collateral ligament reconstruction model. J Biomed Mater Res A. 2011;97:111–117. doi: 10.1002/jbm.a.32938. [DOI] [PubMed] [Google Scholar]
- 9.de Wit T, de Putter D, Tra WM, Rakhorst HA, van Osch GJ, Hovius SE, van Neck JW. Auto-crosslinked hyaluronic acid gel accelerates healing of rabbit flexor tendons in vivo. J Orthop Res. 2009;27:408–415. doi: 10.1002/jor.20730. [DOI] [PubMed] [Google Scholar]
- 10.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]
- 11.Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59:519–530. doi: 10.1007/s00011-010-0156-x. [DOI] [PubMed] [Google Scholar]
- 12.Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26:1032–1037. doi: 10.1002/jor.20558. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Capito RM, Spector M. Delivery of plasmid IGF-1 to chondrocytes via cationized gelatin nanoparticles. J Biomed Mater Res A. 2008;84:73–83. doi: 10.1002/jbm.a.31372. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Hu X, Yang F, Bei J, Wang S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide) Biomaterials. 2007;28:4219–4230. doi: 10.1016/j.biomaterials.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Chen JP, Su CH. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011;7:234–243. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Chen C, Zhang S, Jiang J, Tao H, Xu J, Sun J, Zhong W, Chen S (2012) The use of layer by layer self-assembled coatings of hyaluronic acid and cationized gelatin to improve the biocompatibility of poly(ethylene terephthalate) artificial ligaments for reconstruction of the anterior cruciate ligament. Acta Biomater 8:4007–4019. doi:10.1016/j.actbio.2012.07.008 [DOI] [PubMed]
- 17.Li H, Ge Y, Zhang P, Wu L, Chen S. The effect of layer-by-layer chitosan-hyaluronic acid coating on graft-to-bone healing of a poly(ethylene terephthalate) artificial ligament. J Biomater Sci Polym Ed. 2012;23:425–438. doi: 10.1163/092050610X551989. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH. Graft healing in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:21. doi: 10.1186/1758-2555-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekdahl M, Wang JH, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:935–947. doi: 10.1007/s00167-008-0584-0. [DOI] [PubMed] [Google Scholar]
- 20.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 21.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 22.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597–604. doi: 10.1177/0363546506296312. [DOI] [PubMed] [Google Scholar]
- 23.Hettrich CM, Beamer BS, Bedi A, Deland K, Deng XH, Ying L, Lane J, Rodeo SA. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res. 2012;30:769–774. doi: 10.1002/jor.22006. [DOI] [PubMed] [Google Scholar]
- 24.Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36:1290–1297. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 25.Lui P, Zhang P, Chan K, Qin L. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5:59. doi: 10.1186/1749-799X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto T, Kubo S, Sasaki K, Kawakami Y, Oka S, Sasaki H, Takayama K, Tei K, Matsushita T, Mifune Y, Kurosaka M, Kuroda R. Acceleration of tendon-bone healing of anterior cruciate ligament graft using autologous ruptured tissue. Am J Sports Med. 2012;40:1296–1302. doi: 10.1177/0363546512439026. [DOI] [PubMed] [Google Scholar]
- 27.Mutsuzaki H, Kanamori A, Ikeda K, Hioki S, Kinugasa T, Sakane M. Effect of calcium phosphate-hybridized tendon graft in anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med. 2012;40:1772–1780. doi: 10.1177/0363546512449618. [DOI] [PubMed] [Google Scholar]
- 28.Bedi A, Kovacevic D, Fox AJ, Imhauser CW, Stasiak M, Packer J, Brophy RH, Deng XH, Rodeo SA. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010;92:2387–2401. doi: 10.2106/JBJS.I.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brophy RH, Kovacevic D, Imhauser CW, Stasiak M, Bedi A, Fox AJ, Deng XH, Rodeo SA. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. J Bone Joint Surg Am. 2011;93:381–393. doi: 10.2106/JBJS.I.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iorio R, Vadalà A, Argento G, Di Sanzo V, Ferretti A. Bone tunnel enlargement after ACL reconstruction using autologous hamstring tendons: a CT study. Int Orthop. 2007;31:49–55. doi: 10.1007/s00264-006-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadalà A, Iorio R, De Carli A, Argento G, Di Sanzo V, Conteduca F, Ferretti A. The effect of accelerated, brace free, rehabilitation on bone tunnel enlargement after ACL reconstruction using hamstring tendons: a CT study. Knee Surg Sports Traumatol Arthrosc. 2007;15:365–371. doi: 10.1007/s00167-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 32.Petersen W, Laprell H. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2000;8:26–31. doi: 10.1007/s001670050006. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki K, Kuroda R, Ishida K, Kubo S, Matsumoto T, Mifune Y, Kinoshita K, Tei K, Akisue T, Tabata Y, Kurosaka M. Enhancement of tendon-bone osteointegration of anterior cruciate ligament graft using granulocyte colony-stimulating factor. Am J Sports Med. 2008;36:1519–1527. doi: 10.1177/0363546508316282. [DOI] [PubMed] [Google Scholar]
- 34.Lind M, Feller J, Webster KE. Bone tunnel widening after anterior cruciate ligament reconstruction using EndoButton or EndoButton continuous loop. Arthroscopy. 2009;25:1275–1280. doi: 10.1016/j.arthro.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Kamelger FS, Onder U, Schmoelz W, Tecklenburg K, Arora R, Fink C. Suspensory fixation of grafts in anterior cruciate ligament reconstruction: a biomechanical comparison of 3 implants. Arthroscopy. 2009;25:767–776. doi: 10.1016/j.arthro.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Sabat D, Kundu K, Arora S, Kumar V. Tunnel widening after anterior cruciate ligament reconstruction: a prospective randomized computed tomography–based study comparing 2 different femoral fixation methods for hamstring graft. Arthroscopy. 2011;27:776–783. doi: 10.1016/j.arthro.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Giron F, Aglietti P, Cuomo P, Mondanelli N, Ciardullo A. Anterior cruciate ligament reconstruction with double-looped semitendinosus and gracilis tendon graft directly fixed to cortical bone: 5-year results. Knee Surg Sports Traumatol Arthrosc. 2005;13:81–91. doi: 10.1007/s00167-004-0553-1. [DOI] [PubMed] [Google Scholar]