Abstract
Purpose
The positioning of the femoral cup in hip resurfacing is essential for the survival of the implant. We described a technique in 2005 to position the femoral cup guided by fluoroscopy independent of the approach performed. The main objectives were to study the positioning of the femoral components of the implant and the accuracy of the technique.
Methods
Between 2003 and 2011 we conducted a prospective study of 160 consecutive hip resurfacings all operated with this fluoroscopic-guided technique. Three independent observers performed a radiographic analysis at the pre-operative planning stage and on postoperative radiographs using OsiriX software. The statistical analysis was based on comparison of two groups by Student’s t test.
Results
The entire implant was positioned in valgus, with an average of 7.816° valgus (p <0.001). All implants were positioned in neutral or anteverted with a mean of 1.98° (p <0.001). The risk of malpositioning on the antero-posterior plane was less than 1.41° with p <0.019. The risk of profile positioning error was lower than 0.80° with p <0.047.
Conclusion
This study validates a technique of femoral implant positioning for resurfacing. It is simple, precise and independent of the approach performed.
Introduction
Correct positioning of the femoral implant in hip resurfacing arthroplasty is essential for the survival of a hip replacement and for good functional results. An excess of varus or valgus position can cause femoral head fracture [1, 2]. Depending on the series, this type of complication varies from 0 % to 17 % [3]. We described a technique in 2005 [4] for positioning the femoral cup guided by fluoroscopy and independent of the surgical approach chosen.
Our null hypothesis stated that with our technique of femoral implant positioning, we could reproducibly and accurately put the femoral implants in the position chosen during the pre-operative planning.
To test this hypothesis, we defined two main objectives—to determine the correct positioning of the femoral implant and to study the accuracy of the technique to reproduce the pre-operative planning.
Material and methods
Material
Institutional review board approval was obtained prior to the initiation of the study.
Between 2003 and 2011 we conducted a single centre prospective study on 160 cases of hip resurfacing (BHR® Smith & Nephew, Memphis, Tennessee and Durom® Zimmer, Warsaw, Indiana) performed consecutively.
Our inclusion criteria were: patients with primary or secondary osteoarthritis, regardless of aetiology, or osteonecrosis of a volume less than 30 % of the femoral head, age <65 years for men and <60 years for women without major morphological abnormalities of the upper end of the femur or acetabulum, good bone quality, absence of history of allergy to nickel. Women of childbearing age were excluded.
The average age of patients at surgery was 46.8 years ± 7.2 years with a minimum age of 37 years and maximum 60 years. Patients were predominantly males with a 95 % male dominance and a ratio of 20 to one.
Etiological distribution showed 76.8 % (123 patients) were coxa-arthrosis cases: primitive in 58.6 % (72 patients), secondary to epiphysiolysis in 27.6 % (34 patients), secondary to femoro-acetabular impingement in 13.8 % (17 patients), and aseptic osteonecrosis of the femoral head in 23.2 % of cases (37 patients).
Surgical technique
Planning was made according to the following criteria: an implant should be in valgus < 10°, its stem parallel to the longitudinal trabecular system, and it should avoid notching the supero-lateral neck. It should be centred in the neck on frog-leg views. The correct positioning of the femoral cup was assessed on an antero-posterior pelvis radiograph with a femoral internal rotation of 10° and on a lateral radiograph of hip with 45° of flexion, 45° of abduction, and 30° of external rotation [5]. The axis of the stem corresponded to the axis of the guide pin. The entry point on the of the upper end side of the femur was identified in relation to the position of the lesser trochanter (high - medium - low). The exit point of the pin at the top of the head was also identified.
On a technical aspect, the patient in the operating room was placed in a lateral position, with the image intensifier positioned perpendicular to the patient from above. In this configuration, it was possible to obtain a radiograph of the hip with internal rotation of 10° and a position of 45° flexion, 45° abduction, and 30° external rotation without moving the image intensifier (Figs. 1 and 2). The entry point of the pin was marked using a metallic marker and an image intensifier. A 1 cm skin incision was made 2 cm below the point marked for the insertion of the pin in the centre of the femur in the anteroposterior plane. The soft tissues were bluntly dissected until the femur was exposed. An oblique hole was drilled in the axis of the neck with a 4.5-mm diameter drill. This drill was directed, under the control of successive radiographs (Fig. 2), until the subchondral bone of the head was breached. The drill was then removed and replaced with a single guide pin. Guided by this pin, a canulated drill corresponding to the diameter of the guide bar was used. This allowed the drill to guide a tunnel in the desired axis. Breaching of the subchondral bone and cartilage was the endpoint and was performed under image intensifier.
Fig. 1.

Patient placed in lateral decubitus position
Fig. 2.

Intraoperative radiographic control of drill positioning
Whichever approach was used, we found it possible after the hip was dislocated to position the guide bar and to finish the preparation of the femoral head with the appropriate reamers and jigs without the need for an image intensifier.
In our series, we used a minimally invasive anterolateral approach which preserved both the muscles and vascularity of the femoral head.
Methods
Radiographic evaluation
Three independent observers performed double-blinded, randomized analysis of the radiographs at an interval of two weeks. All radiological parameters were studied using OsiriX software (OsiriX, Switzerland) [6] after calibration on anteroposterior digital pelvic radiographs with the hip in 20° internal rotation and on lateral hip radiographs such as the frog-leg 45/45/30 view [5]. Conforming with strict quality criteria was required, and this was verified before any hip measurements were performed, since even a minimal change in rotation leads to major differences in measurement [7].
On pre-operative radiographs and planned radiographs the following parameters were studied: the neck-shaft angle (NSA), the planned stem-shaft angle (planSSA), and the planned anteversion angle of the implant stem in relation to the axis of the neck.
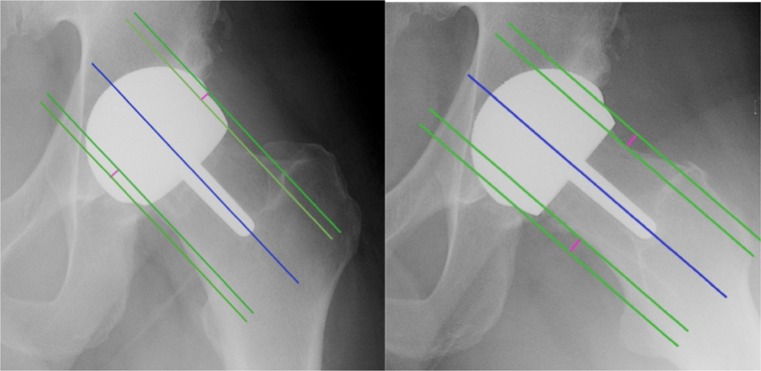
On postoperative radiographs, the following parameters were studied: the stem-shaft angle (SSA); superior and inferior offset, which ratio was used to evaluate anterior centring of the implant; the anteversion angle of the implant stem in relation to the axis of the neck; anterior and posterior offset, of which the ratio was used to evaluate the lateral centring of the implant; the presence of supero-lateral notching of the femoral neck; and leg length discrepancies with the tear drop line method [8] (Figs. 3 and 4).
Fig. 3.
The SSA angle and anteversion were measured using the trapezoid technique for determining the axes. This technique considers the femoral neck as a quadrangle. The axis of the femoral neck is determined by taking the midpoint of two segments of the femoral neck and by drawing a straight line through these two points. The axis of the femoral shaft or of the stem of the femoral implant can easily be determined using the same technique
Fig. 4.
Vertical and horizontal offsets were measured as follows: first a line was drawn along the axis of the femoral neck, then a second line is drawn parallel and tangent to one edge of the neck and a third line is drawn parallel and tangent to the external edge of the femoral implant (Fig. 2). The distance between the second and third line was the offset
To verify our null hypothesis, we defined two main objectives. The first one was to assess the correct positioning of the femoral implants according to the following criteria: the implant positioned in valgus (<10°) (not in varus), the stem parallel to the longitudinal trabeculae and without notching of the supero-lateral cortices of the femoral neck. The leg-length discrepancy should be less than 5 mm. It should also be centred in the axis of the neck on a frog-leg lateral view.
The second objective was to study the accuracy of the technique which was defined by a difference in positioning of the femoral implant compared to preoperative planning on lateral and antero-posterior (AP) radiographs; it should be less than a 2° angle for the SSA angle which evaluated the orientation of the implant in the AP plane, and less than 1° for the anteversion angle of the femoral implant in the lateral plane.
Clinical evaluation
The total duration of the procedure and the duration of the positioning of the guide wire was assessed. The fluoroscopy time in seconds was recorded for each intervention to appreciate the radiation exposure to the patients. Failures of the technique and complications during the procedure and at the last follow-up were recorded.
Statistical analysis
Gaussian distribution of continuous variables was verified using the Shapiro-Wilk test [9] and equality of variances using the Fisher F-test and the Levene test [10] to check the condition of homoscedasticity required for the use of parametric tests. To check intraobserver validity of the measurements, we carried out correlation matrices with Pearson correlation tests. These tests were interpreted according to the recommendations of Landis and Koch [11] and calculated the strength of the correlation (between −1 and +1) between two metric measurements and their significance. We carried out a Fisher F-test (analysis of variance) to ensure that there were no significant differences between the results obtained by each observer, and thus study interobserver reproducibility.
The postoperative radiographic parameters such as angle SSA, the anteversion angle and offsets ratio were compared with preoperative measurements using parametric Student t-tests. Differences in absolute terms between these variables and post/ pre-operative values were calculated and presented in degrees ± standard deviation in parentheses along with the confidence interval.
Postoperative radiographic parameters were compared with preoperative planning by a paired Student’s t-test, with the null hypothesis: H0 = SSA − planSSA < 2° and H0 = anteversion angle − angle of anteversion planned < 1°.
Radiological parameters of the first 20 resurfacings performed were compared to the rest of the series using Student’s t-test to analyse the learning curve.
A p value < 0.05 and an ICC > 95 % were considered significant. Statistical analysis was carried out by an independent statistician using EXCEL (Microsoft Inc, Redmond, WA) and SPSS software (SPSS Inc, Chicago, IL).
Results
Positioning of the femoral implant
In comparison to the preoperative neck-shaft angle NSA, the value of the angle of the implant stem-shaft postoperative SSA showed an increase of 7.816° ± 4.85 (5.29–10.34; p <0.001). All the implants were positioned in valgus and always inferior than 11°.
The anteversion angle of the implant relative to the axis of the neck averaged 1.98° ± 1.68 (1.43–2.55; p <0.001). All implants were positioned in neutral or anteverted with a minimum angle of 0° and 5° max.
The ratio of vertical offset averaged 0.90 ± 0.44 (0.76–1.05; p <0.001). The horizontal offset ratio averaged 0.92 ± 0.28 (0.82–1.01; p <0.001). None of the implants demonstrated supero-lateral notching of the femoral neck. Mean lengthening was 1.5 mm (−6, 8.5) (p <0.001).
Accuracy of the technique
Comparison between the measurements of the postoperative angle SSA and SSA planned angle showed a mean difference of 1.41° ± 1.3 (0.12–1.87; p <0.019; Fig. 5).
Fig. 5.
Difference between postoperative SSA / planSSA
The difference between the angle of anteversion of the implant after surgery and the planned anteversion angle averaged 0.805° ± 0.695 (0.01–0.98; p <0.047). The risk of profile positioning error was lower than 1° with p <0.047 and alpha <0.05.
Learning curve
There was no statistically significant difference between the values of radiological parameters of the first 20 resurfacings performed and the rest of the series (Table 1).
Table 1.
Learning curve effect
| Measurement | 20 firsts | Rest of the series | Difference |
|---|---|---|---|
| NSA-SSA | 7.4° ± 4.6° | 7.8° ± 4.8° | 0.6°, p< 0.23 |
| Anteversion | 2.0° ± 1.3° | 1.96° ± 1.6° | 0.04°, p< 0.46 |
| Vertical ratio offset | 0.89 ± 0.45 | 0.90 ± 0.44 | 0.01, p< 0.17 |
| Horizontal ratio offset | 0.91 ± 0.26 | 0.92 ± 0.28 | 0.01, p<0.17 |
Reproducibility of measurements
Intra-observer reproducibility was very good, as for all radiological parameters the Pearson correlation coefficient was greater than 0.85 (Table 2).
Table 2.
Intraobserver reproducibility measured by Pearson’s correlation coefficient and the corresponding P value
| Observer | Parameter | SSA | Offset > | Offset < | Anteversion | Anterior offset | Posterior offset |
|---|---|---|---|---|---|---|---|
| Obs 1 | r | 0.991** | 0.872** | 0.976** | 0.993** | 0.594 | 0.943** |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.054 | 0.000 | |
| Obs 2 | r | 0.965** | 0.754** | 0.965** | 0.942** | 0.923** | 0.975** |
| P | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Obs 3 | r | 0.951** | 0.963** | 0.877** | 0.827** | 0.948** | 0.972** |
| P | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | |
| Mean | r | 0.969 | 0.863 | 0.939 | 0.920 | 0.821 | 0.963 |
* Significant result
Interobserver reproducibility was also very good. We carried out a Fisher test (analysis of variance) which showed there were no significant differences between the various observers, except for the superior offset variable (Table 3).
Table 3.
Interobserver reproducibility measured by analysis of variance using the Fisher F-test
| Observer | Measure | Femoral implant diameter (mm) | SSA | Offset > | Offset < | Anteversion | Anterior offset | Posterior offset |
|---|---|---|---|---|---|---|---|---|
| First analysis | ||||||||
| Observer 1 | Mean | 47.273 | 141.648 | 0.531 | 0.591 | 7.436 | 0.597 | 0.707 |
| Standard deviation | 2.87 | 4.89 | 0.08 | 0.18 | 5.71 | 0.16 | 0.23 | |
| Observer 2 | Mean | 47.273 | 141.011 | 0.667 | 0.655 | 8.551 | 0.659 | 0.770 |
| Standard deviation | 2.87 | 4.83 | 0.18 | 0.24 | 5.26 | 0.22 | 0.24 | |
| Observer 3 | Mean | 47.273 | 142.556 | 0.459 | 0.560 | 6.928 | 0.579 | 0.581 |
| Standard deviation | 2.87 | 5.08 | 0.12 | 0.16 | 3.04 | 0.15 | 0.14 | |
| Total | Mean | 47.273 | 141.738 | 0.552 | 0.602 | 7.638 | 0.612 | 0.686 |
| Standard deviation | 2.78 | 4.82 | 0.16 | 0.20 | 4.71 | 0.18 | 0.22 | |
| F | 0.000 | 0.272 | 6.938 | 0.673 | 0.327 | 0.595 | 2.318 | |
| P | 1.000 | 0.763 | 0.003 | 0.518 | 0.724 | 0.558 | 0.116 | |
| Significance | ns | ns | sig. | ns | ns | ns | ns | |
| Second analysis | ||||||||
| Observer 1 | Mean | 47.273 | 141.869 | 0.551 | 0.603 | 7.161 | 0.544 | 0.800 |
| Standard deviation | 2.87 | 4.59 | 0.09 | 0.19 | 5.25 | 0.22 | 0.32 | |
| Observer 2 | Mean | 47.273 | 141.982 | 0.582 | 0.625 | 7.975 | 0.663 | 0.769 |
| Standard deviation | 2.87 | 4.71 | 0.13 | 0.19 | 4.73 | 0.28 | 0.26 | |
| Observer 3 | Mean | 47.273 | 142.759 | 0.447 | 0.579 | 6.032 | 0.620 | 0.679 |
| Standard deviation | 2.87 | 5.03 | 0.13 | 0.15 | 3.63 | 0.20 | 0.19 | |
| Total | Mean | 47.273 | 142.203 | 0.526 | 0.602 | 7.056 | 0.609 | 0.749 |
| Standard deviation | 2.78 | 4.65 | 0.13 | 0.18 | 4.52 | 0.23 | 0.26 | |
| F | 0.000 | 0.113 | 3.700 | 0.179 | 0.497 | 0.720 | 0.644 | |
| P | 1.000 | 0.893 | 0.037 | 0.837 | 0.613 | 0.495 | 0.532 | |
| Significance | ns | ns | sig. | ns | ns | ns | ns | |
ns not significant, sig. significant
Secondary endpoints
The mean duration of surgery was 117.8 minutes ± 19.6 minutes with a minimum of 70 minutes and a maximum of 150 minutes. The mean duration of the procedure for positioning the pin was 16.4 minutes ± 7.4 minutes with a minimum of ten minutes and maximum 35 minutes.
The average fluoroscopy time was 20.1 seconds ± 10.9 seconds with a minimum of seven seconds and a maximum of 43 seconds.
No intra-operative complication related to the technique or failure has been identified. At a mean follow-up of 47.10 months ± 2.56 (12–108), we observed four femoral neck fractures, due to high energy trauma in two cases and to avascular necrosis in the other two. One case of ALVAL due to acetabular cup malpositioning (60° of inclination) was observed and required surgical revision.
Discussion
Our series statistically confirms and allows us to validate the accuracy and reliability of the technique for placement of the femoral implants. The results of this study also highlight the excellent intra-observer reproducibility and interobserver measurement of radiographic parameters to assess the positioning of a resurfacing implant. Indeed intraobserver reproducibility for the Spearman correlation coefficient r was whatever the parameters studied and whatever the observer averaged 0.913 (0.754 min – max 0.993). The interobserver study by analysis of variance highlighted excellent reproducibility since no significant difference could be detected except for the measurement of superior offset.
Furthermore, this study did not assess the intra and interoperator reproducibility. However, the results did include the learning curve of this technique. The results of the first 20 resurfacings were compared to the rest of the series and showed no statistically significant difference. In other words, even when the operator was untrained, this technique gave reliable and reproducible results. These results are encouraged by those of Hurst and Millett [12].
The assessment of positioning of the implant on using radiographs could be criticised; nonetheless, Olsen reported good intra and interobserver reproducibility when measuring the SSA angle [13]. In fact, a three-dimensional CT evaluation would have been of preference to assess precisely the correct positioning of implants. However, because of the stringent radiological quality criteria selected, we had a radiological analysis with very good intra and interobserver reproducibility.
In our series we chose to position the femoral implants in valgus parallel to the longitudinal axis of the trabeculae. Many studies have confirmed the importance of this for implant positioning [14, 15]. In an anatomical and biomechanical study, Freeman et al. [16] highlighted that the compressive strength of the femoral head depends on the medial trabecular system which runs through the head at approximately 20° to the vertical plane. Long et al. [17] conducted a finite element analysis of the effects of femoral implant positioning, which found a significant decrease in the risk of loosening and fracture, with valgus positioning of the implants. In addition to those ex-vivo studies, many clinical studies have shown the importance for the future of the resurfaced hip in accordance with the valgus positioning of femoral implant and absence of supero lateral femoral neck notching [1, 15, 18]. The correct positioning is essential but remains a difficult task even for experienced surgeons [19].
Many techniques have been developed to shorten the learning curve for surgeons and improve the ease and accuracy of femoral implant placement [20]. The work done on many navigation systems “free image” or systems-based on CT tend to show a decrease in the learning curve and a better positioning of the implants. Seyler, in a study comparing the accuracy of implantation of femoral components based on the experience of the surgeon, found an average accuracy compared to the planned angle SSA of 0.9–1.5 ° (p <0.17) [21]. Ganapathi et al. found a difference of position with navigation less than 1° compared to planning in a retrospective study comparing 51 hips operated with navigation and 88 by the conventional technique [22]. These results are comparable to those of our technique and in some cases even better.
Our technique has the advantage of positioning the femoral implant without influencing the surgical approach to hip resurfacing. Indeed, even if Myers et al. [23] and McBryde et al. [24] suggest that the surgical approach slightly modifies the position of the femoral implant and survival, it is vital for the surgeon to preserve the vascularity of the femoral head and neck [25].
Conclusion
This study validates a technique for resurfacing femoral implant positioning which is simple, accurate, independent of the approach, and does not require heavy physical logistics.
Acknowledgments
Conflict of interest
None.
References
- 1.De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158–1163. doi: 10.1302/0301-620X.90B9.19891. [DOI] [PubMed] [Google Scholar]
- 2.Kim PR, Beaulé PE, Laflamme GY, Dunbar M. Causes of early failure in a multicenter clinical trial of hip resurfacing. J Arthroplast. 2008;23(6 Suppl 1):44–49. doi: 10.1016/j.arth.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Pailhé R, Sharma A, Reina N, Cavaignac E, Chiron P, Laffosse J-M. Hip resurfacing: a systematic review of literature. Int Orthop. 2012;36(12):2399–2410. doi: 10.1007/s00264-012-1686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiron P. Use of a guide wire in hip resurfacing arthroplasty. Osteologie. 2005;14(suppl 2):65–67. [Google Scholar]
- 5.Cavaignac E, Chiron P, Espié A, Reina N, Lepage B, Laffosse J-M. Experimental study of an original radiographic view for diagnosis of cam-type anterior femoroacetabular impingement. Int Orthop. 2012;36(9):1783–1788. doi: 10.1007/s00264-012-1550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17(3):205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannast M, Zheng G, Anderegg C, Burckhardt K, Langlotz F, Ganz R, Siebenrock KA. Tilt and rotation correction of acetabular version on pelvic radiographs. Clin Orthop Relat Res. 2005;438:182–190. doi: 10.1097/01.blo.0000167669.26068.c5. [DOI] [PubMed] [Google Scholar]
- 8.Sayed-Noor ASA, Hugo AA, Sjödén GOG, Wretenberg PP. Leg length discrepancy in total hip arthroplasty: comparison of two methods of measurement. CORD Conf Proc. 2009;33(5):1189–1193. doi: 10.1007/s00264-008-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royston P. Estimating departure from normality. Stat Med. 1991;10(8):1283–1293. doi: 10.1002/sim.4780100811. [DOI] [PubMed] [Google Scholar]
- 10.Won S, Morris N, Lu Q, Elston RC. Choosing an optimal method to combine P-values. Stat Med. 2009;28(11):1537–1553. doi: 10.1002/sim.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 12.Hurst JM, Millett PJ. A simple and reliable technique for placing the femoral neck guide pin in hip resurfacing arthroplasty. J Arthroplast. 2010;25(5):832–834. doi: 10.1016/j.arth.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Olsen M, Gamble P, Chiu M, Tumia N, Boyle RA, Schemitsch EH. Assessment of accuracy and reliability in preoperative templating for hip resurfacing arthroplasty. J Arthroplast. 2010;25(3):445–449. doi: 10.1016/j.arth.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Beaulé PE, Lee JL, Le Duff MJ, Amstutz HC, Ebramzadeh E. Orientation of the femoral component in surface arthroplasty of the hip. A biomechanical and clinical analysis. J Bone Joint Surg Am. 2004;86-A(9):2015–2021. doi: 10.2106/00004623-200409000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Richards CJ, Giannitsios D, Huk OL, Zukor DJ, Steffen T, Antoniou J. Risk of periprosthetic femoral neck fracture after hip resurfacing arthroplasty: valgus compared with anatomic alignment. A biomechanical and clinical analysis. J Bone Joint Surg Am. 2008;90(Supplement 3):96–101. doi: 10.2106/JBJS.H.00444. [DOI] [PubMed] [Google Scholar]
- 16.Freeman MA. Some anatomical and mechanical considerations relevant to the surface replacement of the femoral head. Clin Orthop Relat Res. 1978;134:19–24. [PubMed] [Google Scholar]
- 17.Long JP, Bartel DL. Surgical variables affect the mechanics of a hip resurfacing system. Clin Orthop Relat Res. 2006;453:115–122. doi: 10.1097/01.blo.0000238873.09390.6f. [DOI] [PubMed] [Google Scholar]
- 18.Laffosse JM, Aubin K, Lavigne M, Roy A, Vendittoli PA. Radiographic changes of the femoral neck after total hip resurfacing. Orthop Traumatol Surg Res. 2011;97(3):229–240. doi: 10.1016/j.otsr.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Steffen R-T, Foguet PR, Krikler SJ, Gundle R, Beard DJ, Murray DW. Femoral neck fractures after hip resurfacing. J Arthroplast. 2009;24(4):614–619. doi: 10.1016/j.arth.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Witjes S, Smolders JMH, Beaulé PE, Pasker P, van Susante JLC. Learning from the learning curve in total hip resurfacing: a radiographic analysis. Arch Orthop Trauma Surg. 2009;129(10):1293–1299. doi: 10.1007/s00402-009-0875-z. [DOI] [PubMed] [Google Scholar]
- 21.Seyler TM, Lai LP, Sprinkle DI, Ward WG, Jinnah RH. Does computer-assisted surgery improve accuracy and decrease the Learning curve in hip resurfacing? A radiographic analysis. J Bone Joint Surg Am. 2008;90(Supplement 3):71–80. doi: 10.2106/JBJS.H.00697. [DOI] [PubMed] [Google Scholar]
- 22.Ganapathi M, Vendittoli P-A, Lavigne M, Günther K-P. Femoral component positioning in hip resurfacing with and without navigation. Clin Orthop Relat Res. 2009;467(5):1341–1347. doi: 10.1007/s11999-008-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers GJC, Morgan D, Mcbryde CW, O’Dwyer K. Does surgical approach influence component positioning with Birmingham hip resurfacing? Int Orthop. 2009;33(1):59–63. doi: 10.1007/s00264-007-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBryde CW, Revell MP, Thomas AM, Treacy RB, Pynsent PB. The influence of surgical approach on outcome in Birmingham hip resurfacing. Clin Orthop Relat Res. 2008;466(4):920–926. doi: 10.1007/s11999-008-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hananouchi T, Nishii T, Lee SB, Ohzono K, Yoshikawa H, Sugano N. The vascular network in the femoral head and neck after hip resurfacing. J Arthroplast. 2009;25(1):146–151. doi: 10.1016/j.arth.2008.09.014. [DOI] [PubMed] [Google Scholar]