Abstract
The re-emerging importance of type 2 diabetes mellitus (DM) to tuberculosis (TB) control is of growing concern, but the basis for this relationship is poorly understood. Given the importance of mononuclear phagocytes for TB control and the reported alterations in monocytes of DM patients, we evaluated whether the initial interaction between both was affected in diabetics. M. tuberculosis-naïve individuals with and without DM were group matched by age and gender and the efficiency of M. tuberculosis association (attachment and ingestion) with their monocytes was assessed in the presence of autologous serum. The association of M. tuberculosis with monocytes was significantly lower in diabetics (19.2±6.1) than non-diabetics (27.5±7.9; p=0.02). Multivariate analysis controlling for host sociodemographics, DM characteristics and serum lipids indicated that male gender (p=0.04) and poorly-controlled DM (high HbA1c and hyperglycemia; p=0.01) were significantly associated with the lower interaction of M. tuberculosis with monocytes. Serum heat-inactivation reduced the association of M. tuberculosis to similar levels in both study groups (p=0.69) suggesting alterations in the complement pathway of DM patients. These findings suggest an altered route of entry of the pathogen in DM patients that may influence the downstream activation of signaling pathways in the monocyte and the survival of mycobacteria.
Keywords: tuberculosis, diabetes, complement, monocyte, mycobacterium
1. Introduction
The current pandemic of type 2 diabetes mellitus (DM) is accelerating in a world where approximately one third of the population is latently infected with Mycobacterium tuberculosis.1;2 Adults with DM have at least a 3-fold higher risk of developing tuberculosis (TB).3;4 In order to develop strategies to prevent TB among DM patients, we must understand the mechanism(s) by which DM increases the risk for TB.
The risk of TB can be stratified into 1) the risk of becoming infected with M. tuberculosis and 2) the risk of progression to active TB disease, but the impact of DM on the natural history of TB is unknown.5 The higher susceptibility of DM patients to TB may occur at both stages based on very limited data. In the present study we explored the impact of type 2 DM on the initial encounter between M. tuberculosis and the host innate immune system, based on indirect support from studies in humans6;7 and mice.8;9 Furthermore, studies in type 1 and 2 DM patients unrelated to TB also suggest compromised phagocyte immunity, including abnormalities in chemotaxis, phagocytosis, respiratory burst and altered expression of cytokines, adhesins and receptors (e.g. complement receptor 3, toll-like receptors).10–18 However, studies to date have varied in their findings, likely as a result of differences in study design and difficulty in controlling for a variety of associated factors.
M. tuberculosis is an intracellular bacterium that has adapted to the human host and evolved the ability to survive in mononuclear phagocytes. These cells can also limit intracellular M. tuberculosis growth under certain conditions. The ability of M. tuberculosis to survive inside phagocytes may depend on the strategy used by the bacterium to enter the host cell, i.e. receptor-ligand interactions that mediate phagocytosis.19 In the present study we focused on the initial interaction between M. tuberculosis and the host phagocyte to begin elucidating alterations in DM patients. We specifically evaluated the impact of DM on M. tuberculosis association (attachment and ingestion) with blood monocytes where entry is largely dependent on two processes. The first is the opsonization of M. tuberculosis by serum components, with the two most common being the C3b complement protein (and its breakdown product iC3b)20;21 and natural antibodies to mycobacteria.22 The second is the binding of these opsonins to complement receptors (mainly CR1 and CR321) or, in the setting of immune serum, Fcγ receptors (FcγRI, FcγRII, FcγRIII) on the monocyte, which is followed by phagocytosis.19
Based on the current literature regarding TB patients and mice with DM, we hypothesized that the initial encounter between M. tuberculosis and the monocyte would be altered in DM patients with no previous exposure to the bacterium. There were two possible outcomes: the first would be that the higher susceptibility of DM patients to TB would be reflected by a higher rate of M. tuberculosis association with monocytes, which could lead directly to enhanced intracellular replication. The second possible outcome would be that M. tuberculosis association with monocyes is reduced, as has been reported in studies with other bacteria.10;15;23 This result would suggest that host cell recognition is altered, with the potential to induce a dysfunctional response that facilitates replication of ingested bacteria. In the present study we conducted experiments to explore which of these possibilities occurs in DM patients.
2. Materials and methods
2.1 Participant enrollment and characterization
Healthy volunteers from South Texas (Hidalgo and Cameron counties) between the ages of 25 and 61 years (range 27–61 in DM and 25–56 in no DM) were identified in the community or at the Joslin Diabetes Center affiliated with Doctors Hospital at Renaissance. Those with no history of TB or knowledge of a positive tuberculin skin test (TST) were invited to participate according to a protocol approved by Committee for the Protection of Human subjects of UTHealth. Individuals were interviewed to assess risk factors for TB, history of DM and other factors that could affect their immune response. History of previous BCG vaccination was recorded. Blood was drawn at enrollment to measure glucose, glycated hemoglobin (HbA1c), triglycerides and total and HDL cholesterol. Individuals with a history of TB or positive for the TSpot-TB test once enrolled (Oxford Immunotec, Oxford, UK) were excluded to focus on innate immune function in naïve hosts. Participants taking metformin, corticosteroids, aspirin or TNF blockers were also excluded due to possible alterations in immune function. Height and weight were recorded to calculate body mass index [(weight in pounds * 703)/(height in inches)2].
2.2 Diabetes classification
Hyperglycemia was defined as fasting glucose ≥126 mg/dl or random glucose ≥200 mg/dl, impaired fasting glucose as levels between 110 and 125 mg/dl under fasting conditions, and normoglycemia as glucose below 110 mg/dl for fasting or 200 mg/dl for random. Chronic hyperglycemia was based on an HbA1c >6.5%. Individuals with hyperglycemia, self-reported DM or chronic hyperglycemia were classified with DM following the American Diabetes Association 2010 guidelines 24. DM patients were further classified into those with DM- well controlled (DMwc; normal HbA1c and normoglycemia), DM-borderline (DMb; high HbA1c or hyperglycemia but not both), or DM-poor control (DMp; high HbA1c and hyperglycemia or impaired fasting glucose).
2.3 Monocytes and serum
PBMCs were isolated from heparinized blood on a Ficoll-sodium cushion (GE Health, Piscataway, NJ) and cultured in teflon wells (Savillex Corp., Minnesota, MN) overnight in 20% unheated AB serum (1.5–2.0 × 106 cells/ml) 25. The PBMCs (2×106 cells/well) were then washed and incubated with RPMI, 2% HEPES and 20% autologous serum in 24-well flat-bottom plates containing glass coverslips. After incubation for 2h at 37°C in 5% CO 2 the non-adherent cells were removed. More than 90% of the remaining adherent cells on glass coverslips were monocytes based on Wright staining. Serum with intact complement was obtained from the blood of the participants as described previously, and heat-inactivation in some aliquots was performed by incubation at 56°C for 30 min. 26
2.4 M. tuberculosis preparation
The reference M. tuberculosis H37Rv strain expressing Green Fluorescence Protein (M. tuberculosis-GFP) was used in all experiments. For each experiment, an aliquot of a frozen stock was grown in Difco™ Middlebrook 7H9 broth with glycerol (BD Diagnostic Systems, Sparks, MD) to an OD600 of 0.5. Single cell suspensions were prepared using a modified version of a previously reported protocol.21;27 Liquid cultures were vortexed intermittently for 2 min in the presence of glass beads and sonicated for 30 s at power 9 (Ultrasonic Dismembrator, Fisher Scientific) to reduce mycobacterial clumps and submitted to a brief 1000 × g force centrifugation to remove bacterial clumps. The upper bacterial suspension was used for quantification with a Petroff-Hausser chamber which also verified single cell suspensions.
2.5 Monocyte-M. tuberculosis association assays
M. tuberculosis was added to the monocyte monolayer on coverslips in tissue culture wells or slides at a 10:1 multiplicity of infection (MOI) and incubated in RPMI-HEPES media containing various concentrations of autologous serum (0, 5, 10 or 20%) for 2h at 37°C. The first 30 min were conducted under mild shaking at 37°C to optimize uniform disp ersion of bacilli, and then the trays were transferred to 5% CO2 at 37°C standing for 90 min. Non-adhered bacteria were washed and the remaining monocytes with associated mycobacteria were fixed with 10% formalin. Monocyte nuclei were stained with DAPI. The percentage of monocytes with at least one associated (attached or ingested) M. tuberculosis was calculated by counting at least 200 monocytes using fluorescence microscopy.
2.6 Statistics
Univariate analysis was conducted with two sample t tests to compare continuous variables and chi-squared or Fisher’s exact for categorical variables. Pearson correlations coefficients were established between continuous variables. The variables with p values ≤ 0.1 by univariate analysis were selected for multivariate analysis. When DM was the independent variable of interest, HbA1c and glucose levels were also excluded from multivariate analysis. P values ≤ 0.05 were considered significant.
3. Results
3.1 Lower association of M. tuberculosis with monocytes from DM patients
To determine if there is a difference in opsonin-dependent association of M. tuberculosis with monocytes from DM versus non-DM participants, we assayed for M. tuberculosis association with monocytes under low serum concentrations (5%) where classical complement activation is more prominent, versus high serum concentrations (20%) where the alternative pathway predominates 28. We evaluated the monocytes and autologous serum from eight participants without DM and nine with DM (four DMb and five DMp). For this, M. tuberculosis was incubated with the monocytes from each participant in 5% or 20% autologous serum and after 2h the percentage of monocytes containing at least one associated (attached or phagocytosed) M. tuberculosis was calculated (Fig 1A). The monocytes from DM patients tended to have a lower association of M. tuberculosis at both serum concentrations, but the difference was more marked with 20% versus 5% serum (Fig. 1B). To expand on the apparent difference between study groups with 20% serum, additional participants were evaluated under these conditions. The results confirmed that M. tuberculosis association was higher for monocytes from healthy controls (28%) when compared to those with DM (19%; p=0.019; Table 1). Stratification of the DM patients suggested that the monocytes from DMb (n=4) had an intermediate level of M. tuberculosis association (23%) when compared to those with DMp (n=7; 18%; data not shown).
Figure 1. M. tuberculosis association with monocytes from participants with or without DM.
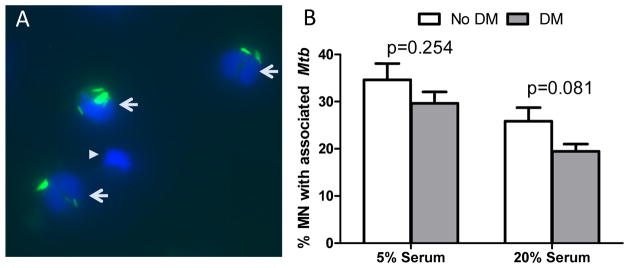
M. tuberculosis-GFP was incubated with the monocytes from eight participants without DM and nine with DM, either borderline (n=4) or poorly-controlled DM (n=5). After 2h the monocytes with associated mycobacteria were fixed and monocyte nuclei stained with DAPI. The percentage of monocytes with at least one associated M. tuberculosis (green) was calculated by counting at least 200 cells (blue) using fluorescence microscopy. A. Representative high power field (63×) showing three monocytes with associated M. tuberculosis (arrows) and one with no M. tuberculosis (arrowhead) B. The mean and standard deviation (error bars) are shown for M. tuberculosis association with monocytes from no DM (open bar) and DM (gray bar) under culture conditions with 5% or 20% autologous serum. Differences between no DM and DM were assessed by the student’s t-test.
Table 1.
M. tuberculosis association with monocytes in 20% autologous serum from participants by diabetes status
| Diabetes classification | Participant ID | HbA1c (%)a | Blood glu interpretationb | Monocytes with ≥ 1 associated M. tuberculosis (%) | Mean (SD) | P value |
|---|---|---|---|---|---|---|
| No diabetes | 5–105 | 4.9 | Normoglycemia | 21.5 | 27.5 (7.9) | Reference |
| 5–107 | 5.4 | Normoglycemia | 36.4 | |||
| 5–120 | 6.0 | Normoglycemia | 19.7 | |||
| 5–133 | 5.2 | Normoglycemia | 31.2 | |||
| 5–135 | 5.1 | Normoglycemia | 21.0 | |||
| 5–141 | 5.1 | Normoglycemia | 32.0 | |||
| 5–147 | 5.7 | Normoglycemia | 29.0 | |||
| 5–149 | 5.7 | Normoglycemia | 39.5 | |||
| 5–153 | 5.3 | Normoglycemia | 17.5 | |||
|
| ||||||
| Diabetes-DMwc | 5–119 | 6.2 | Normoglycemia | 16.2 | 19.2 (6.1) | 0.019 |
| Diabetes-DMb | 5–134 | 12.9 | Normoglycemia | 24.9 | ||
| 5–137 | 7.4 | Normoglycemia | 31.0 | |||
| 5–143 | 6.9 | Normoglycemia | 23.5 | |||
| 5–150 | 8.2 | Normoglycemia | 12.0 | |||
| Diabetes- DMp | 5–101 | 11.5 | Hyperglycemia | 12.6 | ||
| 5–114 | 6.6 | Impaired fasting glucose | 10.4 | |||
| 5–139 | 11.5 | Hyperglycemia | 19.0 | |||
| 5–140 | 9.1 | Hyperglycemia | 20.0 | |||
| 5–142 | 11.4 | Impaired fasting glucose | 23.5 | |||
| 5–146 | 11.2 | Hyperglycemia | 22.0 | |||
| 5–154 | 6.6 | Impaired fasting glucose | 15.3 | |||
HbA1c > 6.5% = chronic hyperglycemia (bold font).
Cutoff for glucose levels are described in Methods, with abnormal values (impaired fasting glucose and hyperglycemia) shown in bold; Diabetes patients comprise DMwc, well-controlled diabetes (n=1); DMb, borderline control of diabetes (n=4); DMp, poor control of diabetes (n=7; see Methods for definitions).
During the course of study each experiment was conducted with no more than four participants where DM patients were generally matched to non-DM controls by gender and similar age. However, the final analysis with the combined data from all participants (Table 1) could be affected by host factors other than DM. To assess this we compared the socio demographics, history of BCG vaccination, characteristics that define DM, and dyslipidemia in the 21 participants evaluated for M. tuberculosis association in 20% serum (Table 2). First, we explored if there were differences between the participants with or without DM, and found that the only significant variations were the higher HbA1c and glucose levels in DM, which was expected. Second, we evaluated if there was a relationship between host characteristics and degree of M. tuberculosis association with monocytes by univariate analysis. DM was significantly associated with lower M. tuberculosis association with monocytes (p=0.03), and a similar but non-significant trend was observed for male gender (p=0.07) and higher BMI (p=0.09). To expand on this we conducted a multivariate analysis and found that DM remained significantly associated with lower M. tuberculosis association, along with male gender. Together, these findings confirmed that DM status has a significant and independent effect on the lower association of M. tuberculosis with monocytes.
Table 2.
Participant socio demographics and their association with M. tuberculosis-monocyte interaction
| Characteristics by DM status
|
Association or correlation between host characteristics and percent of monocytes with associated M. tuberculosis
|
||||||
|---|---|---|---|---|---|---|---|
| Host characteristics | Totala (n=21) | DMa (N=12) | no DMa (n=9) | P value | Kappa or mean(SD) | P value | Adjusted P (DM)b |
| Age in years (mean,SD) | 43 (12) | 46 (12) | 40 (11) | 0.230 | −0.220 | 0.34 | |
| Gender | |||||||
| Male | 5 (24) | 3 (25) | 2 (22) | 1.000 | 17%(4) | 0.013 | 0.039 |
| Female | 16 (76) | 9 (75) | 7 (78) | 25%(8) | |||
| BCG vaccination | |||||||
| No or don’t know | 11 (52) | 7 (58) | 4 (44) | 0.669 | 21%(7) | 0.32 | |
| Yes | 10 (48) | 5 (42) | 5 (56) | 25%(9) | |||
| Diabetes classification | |||||||
| No diabetes | 9 (43) | 0 (100) | 9 (100) | NA | 20%(6) | 0.025 | 0.010 |
| Diabetes | 12 (57) | 12 (100) | 0 (100) | 28% (8) | |||
| HbA1c (mean,SD) | 7.5 (2.6) | 9.1 (2.4) | 5.4 (0.4) | <0.001 | −0.304 | 0.180 | |
| Glucose levels (mean,SD) | 123 (57) | 153 (59) | 83 (18) | 0.003 | −0.347 | 0.123 | |
| BMI (mean,SD) | 34 (15) | 35 (8) | 33 (21) | 0.860 | −0.539 | 0.014 | 0.118 |
| Total cholesterol (mean,SD) | 182 (41) | 178 (49) | 187 (30) | 0.618 | 0.051 | 0.825 | |
| HDL cholesterol (mean,SD) | 55 (14) | 52 (14) | 58 (14) | 0.331 | 0.327 | 0.147 | |
| Triglycerides (mean,SD) | 131 (64) | 140 (56) | 120 (76) | 0.536 | −0.355 | 0.114 | |
Data expressed as n(column %) for discrete or mean(standard deviation, SD) for continuous variables;
P values adjusted after controlling for gender, diabetes and BMI (variables with p≤0. 1, excluding HbA1c and glucose which define diabetes);
NA, not applicable; BMI, body-mass index; HDL, High-density cholesterol;
Diabetes (independent variable of interest) and the other host characteristics with p values ≤ 0.1 by univariate analysis were selected for multivariate analysis (DM, BMI and gender), HbA1c and glucose levels were excluded since these are the defining characteristics of diabetes.
3.2 Contribution of serum to M. tuberculosis association with monocytes from DM patients
Efficient association of M. tuberculosis with monocytes is largely dependent on bacterial opsonization with either serum C3 breakdown products like C3b and iC3b, or natural anti-mycobacterial antibodies that can enhance complement opsonization 19;29. These complexes are recognized by monocyte complement and occasionally Fcγ receptors, respectively. We evaluated whether a defect in the serum components of DM participants explained their reduced M. tuberculosis association with monocytes. Even though our study participants had no evidence of latent TB infection, most individuals in any population will have low titers of natural antibodies that cross-react with mycobacteria 22. To assess the relative contribution of the heat-labile serum components (largely complement proteins) versus heat stable components (including natural anti-mycobacterial antibodies), the association of M. tuberculosis with monocytes was assessed in the presence of 20% serum that was either intact or heat-inactivated. The inactivation of serum reduced the association of M. tuberculosis with monocytes by 74% in healthy controls when compared to 38% in DM patients (p=0.06; Table 3). Despite the small sample size for statistical analysis, there was a trend for reduction in M. tuberculosis association as DM control deteriorated (74% in no DM, 60% in DMb and 38% in DMp). In contrast, the residual M. tuberculosis:monocyte association attributed to heat stable components in serum was not significantly different between the study groups (range 7.4 to 10.1%). These findings suggested that M. tuberculosis association with monocytes in 20% serum was mainly dependent (74% of total binding) on heat-labile components (presumably complement), and that this factor(s) was defective in DM patients. In contrast, M. tuberculosis association via heat-stable opsonins (such as natural antibodies) was apparently intact in DM patients.
Table 3.
Effect of serum heat-inactivation on M. tuberculosis association with monocytes from participants with and without diabetes
| Diabetes classification | PID |
M. tuberculosis association (% monocytes infected)
|
Fraction of association attributed to heat-labile factora | P values
|
|||
|---|---|---|---|---|---|---|---|
| Fresh serum | Heat-inactivated serum | Fresh serum | Heated serum | Association due to heat-labile factor | |||
| No DM | 5–133 | 31.2 | 6.9 | 78% | REF | REF | REF |
| No DM | 5–135 | 21.0 | 3.0 | 86% | |||
| No DM | 5–141 | 32.0 | 19.5 | 39% | |||
| No DM | 5–147 | 29.0 | 0.0 | 100% | |||
| No DM | 5–149 | 39.5 | 8.9 | 77% | |||
| No DM | 5–153 | 17.5 | 6.0 | 66% | |||
|
| |||||||
| Mean (SD) | 28.4(8) | 7.4(6.7) | 74.3%(20.6) | ||||
|
| |||||||
| DMb | 5–134 | 24.9 | 6.0 | 76% | 0.04 | 0.69 | 0.06 |
| DMb | 5–137 | 31.0 | 9.0 | 71% | |||
| DMb | 5–143 | 23.5 | 9.0 | 62% | |||
| DMb | 5–150 | 12.0 | 6.0 | 50% | |||
| DMp | 5–101 | 11.9 | 9.2 | 23% | |||
| DMp | 5–114 | 10.4 | 7.1 | 32% | |||
| DMp | 5–139 | 19.0 | 17.0 | 11% | |||
| DMp | 5–140 | 20.0 | 12.5 | 38% | |||
| DMp | 5–142 | 23.5 | 5.5 | 77% | |||
| DMp | 5–146 | 22.0 | 4.0 | 82% | |||
|
| |||||||
| Mean (SD) | 17.8(5.4) | 10.1(5) | 37.6%(33) | ||||
Mycobacteria was added at a 10:1 MOI in 20% fresh or heated autologous serum for 2h (see Methods);
Fraction of association=1-(%monocyte association in Heat-inactivated serum/%monocyte association in fresh serum);
DMb, diabetes with borderline glucose control; DMp, diabetes with poor glucose control; PID, participant identification; M. tuberculosis, M. tuberculosis.
4. Discussion
We conducted the first in vitro studies aimed at identifying alterations in the initial interaction between human monocytes from DM patients and M. tuberculosis. Our findings provide evidence for reduced association of M. tuberculosis with monocytes from DM patients. This observation was strongest under higher serum concentrations (20% versus 5%), and appeared to involve heat-labile serum components, most likely complement. Together, these findings suggest a difference in the mechanism by which M. tuberculosis binds to and enters monocytes from DM patients, which may lead to alterations in the intracellular fate of the bacterium resulting in increased susceptibility of DM patients to TB.
The relationship between reduced association of M. tuberculosis with monocytes from DM patients and heat-labile serum components suggests defects in complement factors required for M. tuberculosis opsonization, or the complement receptors on monocytes. Differences in the mechanism of entry between DM and non-DM individuals may affect downstream cell activation and intracellular fate of the bacterium. Data from healthy (non-DM) hosts provide evidence that particle phagocytosis mediated by CR3 can reduce IL-12 secretion.30;31 A recent study showed that monocytes from DM patients express reduced IL-12 and IFN-γ secretion following M. tuberculosis infection in vitro, but the relationship of this finding with altered host-bacterial interactions was not evaluated.12 These independent studies provide the foundation for further research aimed at understanding how differential ligand-receptor usage ultimately affects the downstream activation of host cells and the fate of intracellular mycobacteria in the setting of DM.
Despite evidence for immune dysfunction in type 1 and type 2 DM, studying immunity in DM is complex and sometimes yields conflicting results. This is likely due to the marked heterogeneity of diabetics where altered immunity can be attributed to: 1) DM characteristics such as hyperglycemia, chronic hyperglycemia (HbA1c) and years with DM, 2) the older age and higher obesity of type 2 diabetics, and 3) the frequent co-morbidities with administration of multiple medications.24 We took these considerations into account and found that self-reported years with diabetes, current use of insulin, BMI or dyslipidemia based on cholesterol and triglyceride levels were not associated with M. tuberculosis association (Table 2 and data not shown). In contrast, DMp (the combination of diabetes with high HbA1c and hyperglycemia) was a predictor of M. tuberculosis association. This suggests that glucose control is linked to immune dysfunction in DM, but hyperglycemia or chronic hyperglycemia alone are not precise markers of this defect. HbA1c may not always predict monocyte or C3 function because it reflects glucose control in past three months, while the half-life of monocytes in circulation is 3–5 days and that of C3 is about 9 days 32. This may explain why HbA1c and immune dysfunction are associated in many, but not all studies. The fructosamine assay which indicates glucose control in the past two weeks may be a more precise predictor of monocyte and complement function in DM.24;33
We recognize potential study limitations. First, our sample was relatively small. However, significant differences were detected between study groups, which are likely due to our group matching by age and gender, and the fact that most DM patients had poor glucose control. We anticipate that a larger sample size would provide power to detect significant differences between the subset of DM patients with DMp versus DMb. Second, we excluded DM patients taking metformin due to its reported effect on immunity.34–36 The impact of insulin or other medications taken by the DM participants could not be ruled out even though self-reported use of insulin was not associated with M. tuberculosis-monocyte interaction (data not shown).
Our findings using M. tuberculosis are consistent with previous studies showing lower phagocytosis to other pathogens in type 1 and type 2 DM. Several studies have shown reduced phagocytosis of neutrophils to extracellular pathogens, including Streptococcus pneumonia, Staphylococcus aureus, Escherichia coli, S. epidermidis and Candida spp.17;37–39 In contrast, there are few studies with monocytes, but all show reduction in phagocytosis to different pathogens (opsonized Eschelichia coli, Candida albicans and Listeria monocytogenes)10;15;23 Whether the underlying defect for phagocytosis is in type 1 or type 2 DM, or in PMN versus monocytes is unclear, with alterations attributed to serum factors, the phagocyte, or both, depending on the study. Our findings suggest that reduced M. tuberculosis association may be related to altered complement in serum or complement receptors in monocytes. There are data supporting alterations in complement receptors or complement function in DM or hyperglycemia. In diabetics there appears to be a lower percentage of CR3-positive monocytes, and in a separate study anti-PspA antibodies to S. pneumonia displayed reduced capacity for C3 deposition that was proportional to glucose control16;17 Hyperglycemia has been associated with induction of structural changes on C3 that inhibit C3-mediated effector functions to S. aureus.40 We are beginning to assess if there are defects in the complement components in serum and monocytes that explain the reduced association of M. tuberculosis, but we cannot exclude abnormalities in other heat-labile components of the immune system, such as cytokines.
In summary, as epidemiological studies are highlighting the re-emerging contribution of DM to TB, there is a growing interest in understanding the nature of the association between both diseases. DM patients provide a new opportunity for TB researchers to expand knowledge on the complex interaction between M. tuberculosis and phagocytes. Our findings warrant future studies aimed at elucidating the biological basis for reduced M. tuberculosis association with monocytes, and how these initial interactions may influence the intracellular fate of the bacteria and ultimate progression to TB.
Acknowledgments
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R21AI079624-01A1). The sponsor had no role in the study design, collection, analysis or interpretation of data.
We are grateful to Ms. Damaris Garcia and Ms. Izelda Zarate for field and laboratory support and to the staff at the Joslin-Doctors Hospital at Renaissance for assistance with participant enrollment.
Footnotes
Conflict of Interest: The authors have no financial or personal conflicts of interest in the present study.
Ethical approval: Participants signed an informed consent previously approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center Houston.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diana I. Gomez, Email: Diana.i.gomez@gmail.com.
Marcel Twahirwa, Email: m.twahirwa@dhr-rgv.com.
Larry S. Schlesinger, Email: larry.schlesinger@osumc.edu.
References
- 1.World Health Organization. Tuberculosis Global Facts 2011/2012. 2011 Available at: http://www.who.int/tb/publications/2011/factsheet_tb_2011.pdf.
- 2.International Diabetes Federation. Diabetes Atlas. 2009 Available at: http://www.diabetesatlas.org/
- 3.Restrepo B, Camerlin A, Rahbar M, Restrepo M, Zarate I, Wing R, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis patients. Bull World Health Organ. 2011;89:352–359. doi: 10.2471/BLT.10.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:1091–1101. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society, Infectious Diseases Society of America, Centers for Disease Control and Prevention. Controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 6.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van d V, Ottenhoff TH, van der Meer JW, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Curiel I, Castaneda-Delgado J, Lopez-Lopez N, Araujo Z, Hernandez-Pando R, Gandara-Jasso B, et al. Differential expression of antimicrobial peptides in active and latent tuberculosis and its relationship with diabetes mellitus. Hum Immunol. 2011;72:656–662. doi: 10.1016/j.humimm.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis Susceptibility of Diabetic Mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komura T, Sakai Y, Honda M, Takamura T, Matsushima K, Kaneko S. CD14+ monocytes are vulnerable and functionally impaired under endoplasmic reticulum stress in patients with type 2 diabetes. Diabetes. 2010;59:634–643. doi: 10.2337/db09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE. Activation of RAGE induces elevated O2- generation by mononuclear phagocytes in diabetes. J Leukoc Biol. 2007;81:520–527. doi: 10.1189/jlb.0406262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan KS, Lee KO, Low KC, Gamage AM, Liu Y, Tan GY, et al. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J Clin Invest. 2012;122:2289–2300. doi: 10.1172/JCI57817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass EJ, Stewart J, Matthews DM, Collier A, Clarke BF, Weir DM. Impairment of monocyte “lectin-like” receptor activity in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1987;30:228–231. doi: 10.1007/BF00270420. [DOI] [PubMed] [Google Scholar]
- 14.Hill HR, Augustine NH, Rallison ML, Santos JI. Defective monocyte chemotactic responses in diabetes mellitus. J Clin Immunol. 1983;3:70–7. doi: 10.1007/BF00919141. [DOI] [PubMed] [Google Scholar]
- 15.Katz S, Klein B, Elian I, Fishman P, Djaldetti M. Phagocytotic activity of monocytes from diabetic patients. Diabetes Care. 1983;6:479–82. doi: 10.2337/diacare.6.5.479. [DOI] [PubMed] [Google Scholar]
- 16.Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28:137–46. doi: 10.1016/0168-8227(95)00168-8. [DOI] [PubMed] [Google Scholar]
- 17.Mathews CE, Brown EL, Martinez PJ, Bagaria U, Nahm M, Burton RL, et al. Impaired function of antibodies to Pneumococcal surface protein A and not to Capsular Polysaccharide in Mexican American Adults with Type 2 Diabetes Mellitus. Clin Vaccine Immunol. 2012;19:1360–1369. doi: 10.1128/CVI.00268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noritake M, Katsura Y, Shinomiya N, Kanatani M, Uwabe Y, Nagata N, et al. Intracellular hydrogen peroxide production by peripheral phagocytes from diabetic patients. Dissociation between polymorphonuclear leucocytes and monocytes. Clin Exp Immunol. 1992;88:269–74. doi: 10.1111/j.1365-2249.1992.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlesinger LS. Entry of Mycobacterium tuberculosis into mononuclear phagocytes. Curr Top Microbiol Immunol. 1996;215:71–96. doi: 10.1007/978-3-642-80166-2_4. [DOI] [PubMed] [Google Scholar]
- 20.Schlesinger LS. Mycobacterium tuberculosis and the complement system. Trends Microbiol. 1998;6:47–49. doi: 10.1016/S0966-842X(97)01203-1. [DOI] [PubMed] [Google Scholar]
- 21.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 22.Bardana EJ, Jr, McClatchy JK, Farr RS, Minden P. Universal occurrence of antibodies to tubercle bacilli in sera from non-tuberculous and tuberculous individuals. Clin Exp Immunol. 1973;13:65–77. [PMC free article] [PubMed] [Google Scholar]
- 23.Geisler C, Almdal T, Bennedsen J, Rhodes JM, Kolendorf K. Monocyte functions in diabetes mellitus. Acta Pathol Microbiol Immunol Scand [C] 1982;90:33–7. doi: 10.1111/j.1699-0463.1982.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of Medical Care in Diabetes-2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 26.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 27.Zhang M, Gong J, Lin Y, Barnes PF. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun. 1998;66:794–799. doi: 10.1128/iai.66.2.794-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun. 2004;72:2564–2573. doi: 10.1128/IAI.72.5.2564-2573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst JD. Macrophage Receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 2004;27 (Suppl 1):S91–S93. doi: 10.2337/diacare.27.2007.s91. [DOI] [PubMed] [Google Scholar]
- 34.Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther. 2010;334:206–213. doi: 10.1124/jpet.109.164970. [DOI] [PubMed] [Google Scholar]
- 35.Labuzek K, Liber S, Gabryel B, Adamczyk J, Okopien B. Metformin increases phagocytosis and acidifies lysosomal/endosomal compartments in AMPK-dependent manner in rat primary microglia. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:171–186. doi: 10.1007/s00210-009-0477-x. [DOI] [PubMed] [Google Scholar]
- 36.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagdade JD, Nielson KL, Bulger RJ. Reversible abnormalities in phagocytic function in poorly controlled diabetic patients. Am J Med Sci. 1972;263:451–6. doi: 10.1097/00000441-197206000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 39.Davidson NJ, Sowden JM, Fletcher J. Defective phagocytosis in insulin controlled diabetics: evidence for a reaction between glucose and opsonising proteins. J Clin Pathol. 1984;37:783–786. doi: 10.1136/jcp.37.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hair PS, Echague CG, Rohn RD, Krishna NK, Nyalwidhe JO, Cunnion KM. Hyperglycemic conditions inhibit C3-mediated immunologic control of Staphylococcus aureus. J Transl Med. 2012;10:35. doi: 10.1186/1479-5876-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


