Abstract
Purpose
Postictal generalized EEG suppression (PGES) seems to be a pathophysiological hallmark in ictal recordings of sudden unexpected death in epilepsy (SUDEP). It has recently been suggested that presence and duration of PGES might be predictors of SUDEP risk. Little is known about the aetiology of PGES.
Methods
We conducted a retrospective case-control study in 50 people with convulsive seizures (CS) recorded on digital video-EEG. One CS per individual was reviewed for presence and duration of PGES by two independent observers: Pre- and postictal heart rate (HR) (1 minute before seizure onset and 1, 3, 5, 15 and 30 minutes after seizure end) and frequency domain measures of heart rate variability (HRV) including the ratio of low versus high frequency power were analyzed. The relationship between PGES and peri-ictal autonomic changes was evaluated, as well as its association with several clinical variables.
Key Findings
Thirty seven (74%) individuals exhibited PGES and 13 (26%) did not. CS resulted in a significant increase of peri-ictal HR and the LF/HF ratio. PGES was associated with neither peri-ictal HR (mean HR difference between PGES+ and PGES− seizures: −2 bpm, 95% CI −10 to +6 bpm) nor HRV change. There was no association between the duration of PGES and peri-ictal HR change. People with PGES were more likely to be asleep prior to seizure onset (OR 4.7, 95% CI 1.2-18.3) and had a higher age of onset of epilepsy (median age 15 vs. 4 years).
Significance
PGES was not associated with substantial changes in measures of cardiac autonomic instability but was more prevalent in CS arising from sleep.
Keywords: Epilepsy, Postictal generalized EEG suppression (PGES), Sudden unexpected death in epilepsy (SUDEP), Heart rate (HR), Heart rate variability (HVR)
Introduction
Sudden unexpected death in epilepsy (SUDEP) is the most common cause of death directly related to epilepsy and most frequently occurs in people with chronic epilepsy. SUDEP is thought to result from the fatal concurrence of several predisposing and triggering factors with cardiac arrhythmias or respiratory depression as the most likely pathomechanisms (Tomson et al., 2008; Surges et al., 2009). The majority of cases are unwitnessed and ictal recordings are scarce. Postictal generalized EEG suppression (PGES) seems to be a pathophysiological hallmark in ictal recordings of SUDEP (Purves et al., 1992; Bird et al., 1997; Lee et al., 1998; McLean & Wimalaratna, 2007; Lhatoo et al., 2010; Bateman et al., 2010; Tao et al., 2010). Recently, PGES was associated with a higher risk of later SUDEP, which appeared to increase in tandem with PGES duration (Lhatoo et al., 2010). In a subsequent study, PGES occurred more frequently after secondarily convulsive seizures (CS), but its association with SUDEP was not confirmed. (Surges et al., 2011). People with PGES exhibited fewer spontaneous movements and needed more emergency aid (upper airway suction, administration of oxygen) from the attending nurses in the postictal period (Semmelroch et al., 2011). Accordingly, PGES following a CS was associated with more severe ictal respiratory hypoxemia (Seyal et al., 2012). The precise mechanisms of PGES and its relationship with SUDEP are still poorly understood (Lhatoo et al., 2010; Surges et al., 2011, Surges and Sander, 2012). Notably, the duration of CS did not affect either the occurrence or duration of PGES (Lhatoo et al., 2010; Surges et al., 2011; Semmelroch et al., 2011; Seyal et al., 2012).
As PGES is a potential marker for fatal seizures and severe ictal hypoxemia, accompanying signs of cardiac autonomic instability may also be expected; this has not yet been formally assessed. PGES and ictal tachycardia are both well known predictors of treatment response to electroconvulsive therapy (ECT) for major depression (Azuma et al., 2011). The exact relationship between postictal EEG-suppression and ictal heart rate (HR) was not assessed, but these findings suggest that they are closely associated.
The prevalence of PGES in children with CS seems lower, but it is unclear whether there is a direct relationship or whether age, or age at onset of epilepsy, acts as a proxy for different aetiologies of epilepsy (Kim et al., 2006). Another potential clinical determinant of PGES is sleep; most victims of SUDEP die in their sleep and the presence of nocturnal seizures has recently been identified as a possible SUDEP risk factor (Hitiris et al., 2007; Lamberts et al., 2011). To further our understanding of PGES, we performed a systematic analysis of cardiac autonomic changes and clinical determinants such as sleep, age, and age of onset in people with CS.
Subjects and methods
Patient selection
All presurgical video-EEG reports of people >15 years old recorded between January 2003 and August 2011 at a tertiary epilepsy referral centre were reviewed and those mentioning a recorded CS selected. In each individual the first CS on video-EEG was analyzed. In total, 53 CS were identified. EEGs with less than five minutes of postictal recording time (n=1) and those of insufficient quality (e.g. disruption of entire EEG signal due to falls, n=2) were discarded. Therefore a total of 50 people remained. In six people only limited postictal EEG recording time (<15 minutes) was available, thus HR could not be recorded in all periods.
Collection of variables
Various seizure characteristics were collected from the digital video-EEGs: arousal state prior to the seizure (awake/asleep), sleeping stage (REM/NREM I-III), duration of the seizure, duration of the tonic-clonic phase, and location of the ictal EEG onset (temporal/extratemporal). The following clinical details were obtained from the medical records:gender, age of onset of epilepsy, duration of epilepsy, age at time of EEG recording, epilepsy classification (symptomatic or cryptogenic/idiopathic), lesion on MRI (yes/no), and frequency of CS. After completion of the study, medical records were reviewed to ensure that none had died of SUDEP. This study was approved by the local ethics committee; due to its retrospective nature informed consent was not required.
Evaluation of PGES
Conventional scalp EEG recordings (International 10-20 System) were performed at a sampling rate of 200 Hz. A modified lead-I ECG (adhesive electrode placed below clavicle) was simultaneously recorded.
PGES was defined as the immediate postictal (within 30 seconds), generalized absence of electroencephalographic activity >10 μV in amplitude, allowing for muscle, movement, breathing, and electrode artefacts (Lhatoo et al., 2010). Only PGES of > 1 second in duration was scored (Surges et al., 2011).
Two board-certified clinical neurophysiologists (DNV, RDT), who were blinded to the ECG signal, analyzed all digital video-EEGs for presence (cases) or absence of PGES (controls). In case of disagreement, the two reviewers discussed to reach consensus. Interobserver agreement on the presence of PGES was good (Cohen’s κ=0.8), and estimations of duration did not differ significantly between reviewers: paired t-test p = 0.24.
In five individuals the video recording in the postictal phase was terminated while the EEG was still suppressed.
In these people the PGES duration may have been underestimated since no reliable distinction could be made between passive movement artefacts (e.g. caused by an attending nurse) and the recovery of cerebral activity. Additional spectral EEG analysis was performed using Gabor time-frequency representation to provide an objective measure of postictal EEG activity [Methods 1]. The average spectral power of the signal between 4 and 35 Hz in the Cz-REF channel was determined in the pre-ictal (last minute before seizure onset) and postictal period (1st minute after seizure end (Qian & Chen, 1996; Flandrin, 1999). The median standard deviation (SD) for each period was calculated and then used to determine the ratio of postictal versus pre-ictal activity with the following formula: (median SD postictal-median SD pre-ictal)/ (median SD postictal+ median SD pre-ictal).
HR analysis
HR was analyzed during various one-minute time periods at baseline (one minute ECG segment with patient awake and supine) and periods surrounding the seizure event: pre-ictal one minute before seizure onset), and the 1st, 3rd, 5th, 15th, and 30th minute after seizure end. HR variability (HRV) was analyzed through the Hilbert-Huang transform using frequency domain methods during the last 2 minutes before seizure onset and a postictal period of similar length (Tavares et al., 2011) [Methods 2]. Analysis was focussed on the low (LF: 0.04-0.15 Hz) and high frequency (HF: 0.15-0.4 Hz) bands, as likely markers of sympathetic and parasympathetic-respiratory activity respectively.
Statistical analysis
Relationships between PGES and clinical or seizure-related variables were analyzed with Fisher’s exact probability test, χ2- or Mann-Whitney U tests, where appropriate. A linear mixed model was used to compare peri-ictal changes in either HR or HRV measures in individuals with and without PGES before and after adjustment of covariates (HR: baseline HR; HRV: age) and their relationship with PGES duration and spectral EEG power.
Statistical analysis was performed with PWAS software (version 17). Two-sided p-values < 0.05 were considered significant.
Results
The relationship of PGES with other variables
PGES occurred in 37/50 (74%) of seizures and had a median duration of 28s. Details of other clinical and seizure-related characteristics are presented in Table 1. PGES appeared more frequently after CS that started during sleep: OR 4.7 (95% CI 1.2-18.3). Age at onset of epilepsy was higher in people with PGES: median age 15y vs. 4y (p<0.01). No other significant associations were found.
Table 1.
Characteristics of cases and controls
| Variables | PGES+, n=37 | PGES−, n=13 | Test |
|---|---|---|---|
| Sex | χ2, p=0.34 | ||
| Male | 20 (54%) | 9 (69%) | |
| Female | 17 (46%) | 4 (31%) | |
|
| |||
| Age at onset of epilepsy, yr (Median; Range) |
15 (1-51) | 4 (0-19) | MW, p<0.001 |
|
| |||
| Age at time of EEG, yr (Median; Range) |
33 (15-61) | 32 (16-43) | MW, p=0.15 |
| Duration of epilepsy, yr (Median; Range) |
19 (2-46) | 21 (4-42) | MW, p=0.31 |
|
| |||
| Epilepsy classification | F,p=0.25 | ||
| Symptomatic | 27 (73%) | 12 (92%) | |
| Cryptogenic/idiopathic | 10 (27%) | 1 (8%) | |
|
| |||
| Lesion on MRI | F,p=0.3 | ||
| Yes | 25 (68%) | 11 (85%) | |
| No | 12 (32%) | 2 (15%) | |
|
| |||
| Frequency of CS | χ2, p=0.99 | ||
| 1-2 CS/year | 20 (54%) | 7 (54%) | |
| ≥3 CS/year | 17 (46%) | 6 (46%) | |
|
| |||
| Duration of seizure, s (Median, Range) |
108 (63-444) | 116 (45-828) | MW, p=0.86 |
|
| |||
| Duration of TC phase, s (Median;Range) |
68 (32-118) | 65 (35-100) | MW, p=0.723 |
|
| |||
| Ictal EEG onset | χ2, p=0.42 | ||
| Temporal | 19 (51%) | 5 (38%) | |
| Non-temporal | 18 (49%) | 8 (62%) | |
|
| |||
| State of wakefulness | χ2, p=0.02 | ||
| Asleep | 25 (68%) | 4 (31%) | |
| Awake | 12 (32%) | 9 (69%) | |
|
| |||
| Sleeping stage | |||
| NREM1 | 2 (8%) | 0 (0%) | |
| NREM2 | 16 (64%) | 3 (75%) | |
| NREM3 | 7(28%) | 1 (25%) | |
|
| |||
| Duration of PGES, s (Median; Range) |
53 (2-252) | NA | |
| PGES>20s | 32 | NA | |
| PGES>50s | 20 | NA | |
MW=Mann-Whitney U test
F=Fisher’s exact probability test
TC=tonic-clonic
NA= not applicable
The effect of seizures and PGES on HR
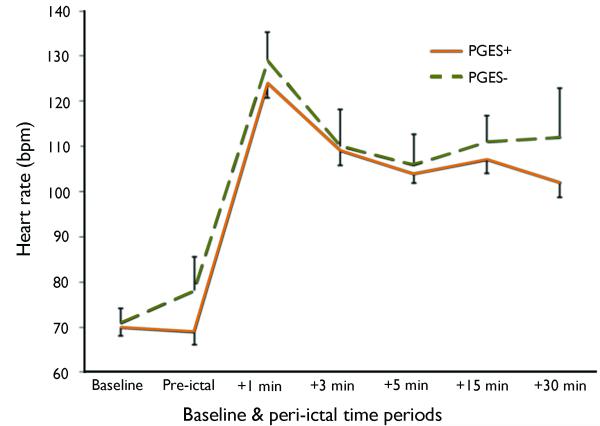
HR increased significantly over the course of the CS (Figure 1). Seizure-induced HR rise was not affected by the duration of the entire seizure (p=0.37) or the tonic clonic phase (p=0.70). People with higher baseline HR were likely to have larger peri-ictal HR increases (p<0.001). The presence of PGES did not affect peri-ictal HR change: mean HR difference between PGES+ and PGES− seizures: −2 bpm, 95% CI −10 to +6 bpm. PGES duration was not associated with peri-ictal HR change (p=0.62). No association was found between peri-ictal spectral EEG and HR changes (p=0.56).
Fig 1.
Time course of peri-ictal heart rate (HR) change in 50 people with CS. Standard errors are displayed for PGES+ (bottom lines) and PGES− (top lines). No significant difference in peri-ictal HR change between PGES+ (n=37; solid line) and PGES− seizures (n=13; dotted line) was found: −2 bpm; 95% CI −10 to +6 bpm.
The effect of seizures and PGES on HRV
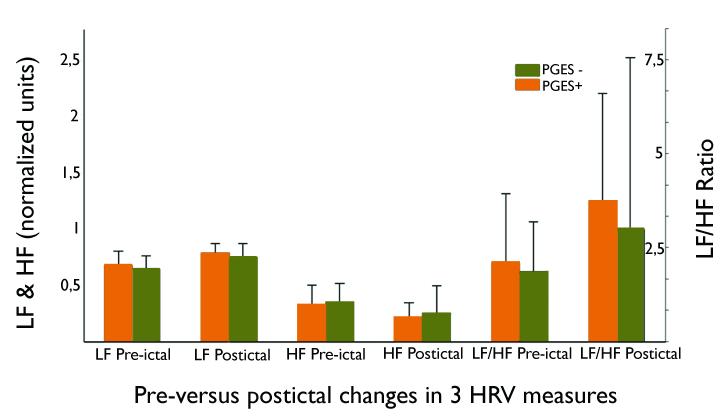
Irrespective of the occurrence of PGES, there was a significant increase in sympathetic LF (p=0.02) and a decrease in parasympathetic HF (p=0.02) over the course of the CS, resulting in an increased LF/HF ratio (p=0.02): see Figure 2. Age was not associated with peri-ictal change in LF/HF ratio (p=0.13). There was no significant difference in peri-ictal HRV change of normalized LF power (p=0.37), HF power (p=0.37), or LF/HF ratio (p=0.70) between people with PGES+ and PGES− seizures.
Figure 2.
Comparison of peri-ictal heart rate variability (HRV) in 50 people with CS. Measures are expressed as normalised units (nu) for low (LF) and high frequency (HF) power and displayed as medians with the 75th percentile range. An overall seizure-related increase in low frequency (p=0.02), and a decrease in high frequency power (p=0.02) is found, resulting in an elevated LF/HF ratio (p=0.02). No significant difference in peri-ictal change of the LF/HF ratio was found between PGES+ (n=37; orange bars) and PGES− seizures (n=13; green bars): p=0.70.
Discussion
We addressed the potential relationship between PGES and peri-ictal cardiac autonomic instability in 50 people with CS. No substantial differences in seizure-related HR or HRV changes between those with and without PGES were seen. PGES was, however, more frequent in CS that arose from sleep. In addition, those with PGES+ seizures had a higher age of onset of epilepsy.
The effects of CS on HR
We found that CS were associated with a sustained tachycardia of >100 bpm that lasted up to 30 minutes after seizure end. Peri-ictal HR changes were not affected by seizure duration and associated with a marked increase in sympathetic and a decrease in parasympathetic power. These findings are in line with previous work (Surges et al., 2010; Toth et al., 2010; Poh et al., 2012). Interestingly, peri-ictal HR increase was most marked in people with a higher resting HR. This could be explained by an alteration of autonomic balance. Compared to healthy controls, people with refractory epilepsy have a higher resting HR (Evrengul et al., 2005) and a lower HRV (Ansakorpi et al., 2002) with increased sympathetic and/or decreased parasympathetic tone (Lotufo et al., 2012). An altered sympathovagal balance may facilitate unopposed tachycardia during sympathetic activation. It is therefore conceivable that those with the highest resting HR are more vulnerable to the sympathetic surge caused by CS.
PGES and HR(V)
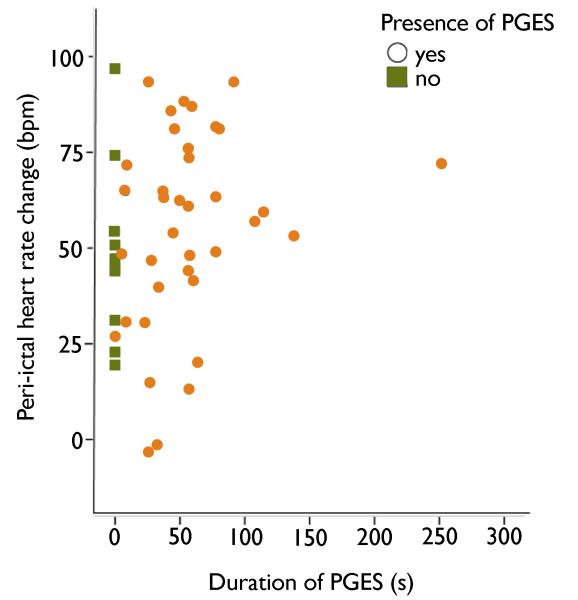
We did not find a significant difference in either peri-ictal HR or HRV change between PGES+ and PGES− seizures. We assessed PGES-related HR(V) changes in the immediate postictal phase (within 30 minutes after seizure end). A small pilot study of six patients and eleven CS suggested that PGES duration is associated with long term (2 h) effects on autonomic control: a higher electrodermal response and a decrease of HF activity was found indicating sympathetic hyperactivation and parasympathetic suppression (Poh et al., 2012). We could not confirm these findings. As indicated by Figure 3 no trend towards HR increase is seen in our study, even in those with PGES of long duration. It is questionable whether the differences between acute and long-term effects of CS with PGES on autonomic balance can solely be attributed to PGES itself. In all ictal recordings of SUDEP signs of cardiac instability appeared within minutes after seizure end, and cardiac function irreversibly ceased within 30 minutes (Purves et al., 1992; Bird et al., 1997; Lee et al., 1998; McLean & Wimalaratna, 2007; Lhatoo et al., 2010; Bateman et al., 2010; Tao et al., 2010). We therefore believe acute postictal HRV changes are more relevant to SUDEP pathophysiology.
Figure 3.
The difference in heart rate between the first postictal and the last pre-ictal minute and PGES duration in 50 people with CS. No significant relationship was found between PGES and peri-ictal heart rate change: p=0.62.
The frequency of PGES is highly variable with a reported prevalence of 27% (Semmelroch et al., 2011), 40% (Surges et al., 2011), 65% (Lhatoo et al., 2010), and 74% in our study. In children, it seems to be a rarer phenomenon: six (8%) of 200 seizures in children (Kim et al., 2006). This age effect may also explain the relative low prevalence of 27% reported in one study (Semmelroch et al., 2011), as it included children (>8 years old) and adults.
Seizure type is another possible confounder with a higher prevalence of PGES found after CS than complex partial seizures (Surges et al., 2011). This may in part explain the lower prevalence in the paediatric PGES study as a mixture of both focal and generalised seizures were studied (Kim et al., 2006).
For this reason, we confined this study exclusively to CS. Another possible confounder is sleep, given the strong relationship between PGES and sleep we found. Our study population had a relatively high percentage (68%) of seizures that started during sleep, which might explain the relatively high prevalence of PGES. The wide variation in prevalence of PGES might also be attributed to the use of different definitions regarding the minimum duration of the PGES: >2 s (Seyal et al., 2012) vs. >1s (Surges et al., 2011) or unspecified (Lhatoo et al., 2010; Semmelroch et al., 2011). To avoid potential interpretation bias in our study, the EEGs were assessed independently by two observers, resulting in a good interobserver agreement. An additional analysis using quantitative EEG parameters was performed, confirming our findings. Therefore, we believe the overall conclusions of this study are valid.
A high frequency of CS is the strongest risk factor for SUDEP (Hesdorffer et al., 2011), yet even in these people only a small minority will die. Presumably a complex interplay of various clinical and peri-ictal factors determines whether or not SUDEP will occur. PGES is the most frequently reported pathomechanism in the few ictal recordings of SUDEP (Purves et al., 1992; Bird et al., 1997; Lee et al., 1998; McLean & Wimalaratna, 2007; Lhatoo et al., 2010; Bateman et al., 2010; Tao et al., 2010). It usually starts immediately after seizure end and is thought to precede cardiac asystole and apnea. It is, however, important to stress that the precise sequence of events is uncertain since all reported cases lacked pulse oximetry measurements and only a few cases included video assessment of the respiratory excursions (Lhatoo et al., 2010; Bateman et al., 2010; Tao et al., 2010).
Several theories exist regarding the aetiology of PGES (Purves et al., 1992; Bird et al., 1997; Lee et al., 1998; McLean & Wimalaratna, 2007; Lhatoo et al., 2010; Bateman et al., 2010; Tao et al., 2010). In accordance with previous work we did not find a relationship between seizure duration and PGES, which renders it unlikely that PGES results from neuronal exhaustion (Lhatoo et al., 2010; Surges et al., 2011; Semmelroch et al., 2011; Seyal et al., 2012). It has also been postulated that PGES causes respiratory or cardiac dysfunction (Lhatoo et al., 2010; Surges et al., 2011). We did not, however, find substantial changes in measures of cardiac autonomic instability in PGES. Our study lacked peri-ictal blood pressure assessments so we cannot exclude the possibility that PGES is caused by systemic hypotension as seen in syncope (Wieling et al., 2009). It is unlikely, however, that seizure-induced hypotension occurred in our cases given the fact that PGES was not accompanied by HR changes. If hypotension results from syncope, then hypotension is likely to coincide with either bradycardia/asystole or compensatory tachycardia (van Dijk et al., 2009).
Recently, an association between PGES and severe ictal respiratory hypoxemia was found (Seyal et al; 2012). End-tidal CO2 concentration was also elevated in people with PGES (Seyal et al., 2012). These respiratory changes were already apparent during the ictal phase. Thus, as in our study, PGES is unlikely to trigger either postictal respiratory or postictal cardiac dysregulation. Instead, PGES may represent the direct effect of seizure-induced hypoxia on the brain or may result from excessive neuronal inhibition in response to severe hypoxemia. CS with PGES were more likely to arise from sleep. This finding may explain the relationship between SUDEP and sleep; most SUDEP cases are found in or by the bed and a history of nocturnal seizures was found to increase SUDEP risk (Hitiris et al., 2007; Lamberts et al., 2011).
The mechanisms underlying the association between PGES and sleep require further study. It has been postulated that in parallel with sudden infant death syndrome, sleep- and seizure-related dysfunction of serotonergic neurons may facilitate respiratory depression and hereby SUDEP (Richerson & Buchanan, 2011). Dysfunction of these midbrain neurons results in a lack of respiratory increase and a diminished arousal response to hypoxemia (Buchanan & Richerson, 2009). The association between PGES and sleep could thus be explained by a higher frequency of ictal hypoxemia following CS. It is not known, however, whether CS that arise from sleep are associated with more pronounced hypoxemia. An earlier study of seizure-related respiratory dysfunction did not find a higher frequency of ictal hypoxemia during sleep, but this study predominantly analysed partial seizures (Bateman et al., 2008). It would therefore be of great interest to explore further the association between sleep, PGES and respiratory measures. Alternatively, excessive neuronal inhibition may explain the relationship between PGES and sleep, since sleep is associated with the activation of inhibitory networks.
Age of onset was higher in those with PGES than in controls. As we did not find a relationship with either age at time of EEG or duration of epilepsy, it seems likely that this is related to aetiology. It has previously been suggested that PGES was more frequently seen in children with idiopathic, cryptogenic, or acute symptomatic aetiologies (“normal” brains) than in those with remote or progressive symptomatic aetiologies (“abnormal” brains) (Kim et al., 2006). We could not confirm these findings, but it should be noted that most of our cases had a symptomatic aetiology and only a few cases had cryptogenic or idiopathic aetiologies.
In conclusion, our findings suggest that PGES is not associated with measures of autonomic instability but seems more prevalent in CS arising from sleep. This would support the notion that PGES itself is not a trigger of postictal cardiac dysfunction. Instead, it could be a consequence of severe ictal hypoxemia as has recently been suggested (Seyal et al., 2012). The higher age of seizure onset found in those with PGES might reflect the effect of different epilepsy aetiologies on PGES development.
Supplementary Material
Acknowledgements
This work was partly undertaken at UCLH/UCL, which receives a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. RDT and JWS are supported by the Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie (Nederland) and the Dutch National Epilepsy Fund (project number 10-07) . JWS receives research support from the Epilepsy Society, Dr. Marvin Weil Epilepsy Research Fund, Eisai, UCB, the Wellcome Trust, the World Health Organization, National Health and Medical Research Council (Australia), the National Institutes of Health (NIH), Tuberous Sclerosis Association, and the Brain Research Trust. RDT and JWS are part of PRISM – the Prevention and Risk Identification of SUDEP Mortality Consortium which is funded by the NIH (NBIH/NINDS –1P20NS076965-01). The authors are grateful to Dr. R Wolterbeek for providing statistical assistance, and to Dr. GS Bell for critically reviewing the manuscript.
Footnotes
Disclosure of Conflicts of Interest: RDT has received fees for lectures from Medtronic. JWS has been consulted by and received fees for lectures from GSK, Medtronic, Viropharma and UCB. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information: Additional supporting information may be found in the online version of this article:
Methods 1. More detailed information of the methods used to perform the EEG spectral power analysis.
Methods 2. More detailed information of the methods used to perform the HRV analysis.
References
- Ansakorpi H, Korpelainen JT, Huikuri HV, Tolonen U, Myllylä VV, Isojärvi JIT. Heart rate dynamics in refractory and well controlled temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2002;72:26–30. doi: 10.1136/jnnp.72.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Yamada A, Shinagawa Y, Nakano Y, Watanabe N, Akechi T, Furukawa TA. Ictal physiological characteristics of remitters during bilateral electroconvulsive therapy. Psychiatry Res. 2011;185:462–464. doi: 10.1016/j.psychres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia. 2010;51:916–920. doi: 10.1111/j.1528-1167.2009.02513.x. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity, and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird JM, Dembny KAT, Sandeman D, Butler S. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia. 1997;38(Suppl. 11):52–56. [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, Brodie MJ, Hauser A. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52(6):1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Role of chemoreceptors in mediating dyspnea. Respir Physiol Neurobiol. 2009;167:9–19. doi: 10.1016/j.resp.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrengul H, Tanriverdi H, Dursunoglu D, Kaftan A, Kuru O, Unlu U, Kilic M. Time and frequency domain analyses of heart rate variability in patients with epilepsy. Epilepsy Res. 2005;63:131–139. doi: 10.1016/j.eplepsyres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Flandrin P. Time-frequency scale analysis. Academic Press; San Diego, London, Boston, New York, Sidney, Tokyo, Toronto: 1999. [Google Scholar]
- Hitiris N, Suratman S, Kelly K, Stephen LJ, Sills GJ, Brodie MJ. Sudden unexpected death in epilepsy: a search for risk factors. Epilepsy Behav. 2007;10(1):138–141. doi: 10.1016/j.yebeh.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Kuroda MM, Nordli DR. Abruptly attenuated terminal ictal pattern in pediatrics. J Clin Neurophysiol. 2006;23:532–550. doi: 10.1097/01.wnp.0000229045.28725.5c. [DOI] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia. 2011;53:253–257. doi: 10.1111/j.1528-1167.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- Lee MA. EEG video recording of sudden unexpected death in epilepsy (SUDEP) Epilepsia. 1998;39(Suppl. 6):123–124. [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53:272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- McLean BN, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatry. 2007;78:1395–1397. doi: 10.1136/jnnp.2006.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78:1868–1876. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves SJ, Wilson-Young M, Sweeney VP. Sudden death in epilepsy: single case report with video-EEG documentation. Epilepsia. 1992;33(Suppl. 3):123. [Google Scholar]
- Qian S, Chen D. Joint time-frequency analysis. Prentice Hall; New Jersey: 1996. [Google Scholar]
- Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(Suppl. 1):28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmelroch M, Elwes RDC, Lozsadi DA, Nashef L. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia. 2011;53:e21–e24. doi: 10.1111/j.1528-1167.2011.03296.x. [DOI] [PubMed] [Google Scholar]
- Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia. 2012;53:825–831. doi: 10.1111/j.1528-1167.2012.03443.x. [DOI] [PubMed] [Google Scholar]
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology. 2010;74:421–426. doi: 10.1212/WNL.0b013e3181ccc706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges R, Strelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electric encephalographic suppression is associated with generalized seizures. Epilepsy Behav. 2011;21:271–274. doi: 10.1016/j.yebeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Curr Opin Neurol. 2012;25:201–207. doi: 10.1097/WCO.0b013e3283506714. [DOI] [PubMed] [Google Scholar]
- Tao JX, Qian S, Baldwin M, Chen XJ, Rose S, Ebersole SH, Ebersole JS. SUDEP, suspected positional airway obstruction, and hypoventilation in postictal coma. Epilepsia. 2010;51:2344–2347. doi: 10.1111/j.1528-1167.2010.02719.x. [DOI] [PubMed] [Google Scholar]
- Tavares C, Martins RC, Laranjo S, Rocha I. Computational tools for assessing cardiovascular variability. EMBS/IEEE. 2011 10.1109/ENBENG.2011.6026082, E-ISBN : 978-1-4577-0521-2. [Google Scholar]
- Toth V, Hejjel L, Fogarasi A, Gyimesi C, Orsi G, Szucs A, et al. Periictal heart rate variability analysis suggests long-term postictal autonomic disturbance in epilepsy. Eur J Neurol. 2010;17:780–787. doi: 10.1111/j.1468-1331.2009.02939.x. [DOI] [PubMed] [Google Scholar]
- Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol. 2009;5:438–448. doi: 10.1038/nrneurol.2009.99. [DOI] [PubMed] [Google Scholar]
- Wieling W, Thijs RD, van Dijk N, Wilde AA, Benditt DG, van Dijk JG. Symptoms and signs of syncope: a review of the link between physiology and clinical clues. Brain. 2009;132:2630–2642. doi: 10.1093/brain/awp179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.