Abstract
Walking gait is generally held to reach maturity, including walking at adult-like velocities, by 7–8 years of age. Lower limb length, however, is a major determinant of gait, and continues to increase until 13–15 years of age. This study used a sample from the Fels Longitudinal Study (ages 8–30 years) to test the hypothesis that walking with adult-like velocity on immature lower limbs results in the retention of immature gait characteristics during late childhood and early adolescence. There was no relationship between walking velocity and age in this sample, whereas the lower limb continued to grow, reaching maturity at 13.2 years in females and 15.6 years in males. Piecewise linear mixed models regression analysis revealed significant age-related trends in normalized cadence, initial double support time, single support time, base of support, and normalized step length in both sexes. Each trend reached its own, variable-specific age at maturity, after which the gait variables’ relationships with age reached plateaus and did not differ significantly from zero. Offsets in ages at maturity occurred among the gait variables, and between the gait variables and lower limb length. The sexes also differed in their patterns of maturation. Generally, however, immature walkers of both sexes took more frequent and relatively longer steps than did mature walkers. These results support the hypothesis that maturational changes in gait accompany ongoing lower limb growth, with implications for diagnosing, preventing, and treating movement-related disorders and injuries during late childhood and early adolescence.
Keywords: gait, maturation, growth, lower limb, late childhood
1. Introduction
Maturation of spatiotemporal gait properties occurs in stages across childhood alongside neural and musculoskeletal growth and development. Neurological control of the primary determinants of mature gait is established as early as 4 years old [1], after which children tend to walk at adult velocities [2]. Adult-like walking velocities in young children, however, are out of harmony with the still-immature lower limb, which lengthens considerably during childhood. Accordingly, gait parameters continue to change and mature as the lower limb grows.
Gait is commonly considered to reach maturity by ages 7–8 years [2–4] according to five criteria: duration of single-limb stance, walking velocity, cadence, step length, and the ratio of pelvic span to ankle spread (or base of support) [5]. There is, however, a substantial temporal offset between ages 7–8 years and the cessation of lower limb linear growth (abbreviated below as lower limb maturity), which continues until roughly 13 years old in girls and 15 years old in boys [6], just after peak growth velocity occurs. Given close relationships between adult lower limb length and gait characteristics [7], it seems reasonable that walking with adult-like velocity on immature lower limbs should involve the retention of immature gait patterns during late childhood and early adolescence. Existing evidence suggests that ankle and foot kinematics remain immature at 7 years old [8–10], and that spatiotemporal variables differ from the adult condition up to 11–13 years of age [11–13]. Still, few studies have examined gait across the age range during which the lower limb matures [10,14,15], whereas most studies have not [2–5,8,11–13,16]. None of these studies has evaluated the specific relationship between age-related changes in spatiotemporal gait parameters and continued lower limb growth during late childhood and early adolescence.
Here, using a sample from the Fels Longitudinal Study, we test the hypothesis that spatiotemporal aspects of gait undergo age-related changes as long as adult-like absolute walking velocities are coupled with immature lower limbs. More specifically, we test whether normalized gait parameters [17,18] exhibit significant relationships with age prior to lower limb maturity, after which the slopes reach plateaus and do not differ from zero. To test this hypothesis, we determine age at lower limb maturity in each sex, and evaluate trends in lower limb length and spatiotemporal gait variables by testing for significant age-related slopes that reach mature plateaus. Where such patterns do exist, age at maturity for each gait variable is compared to that of the lower limb. We also test the secondary hypothesis that the sexes differ in ages at maturity and rates of change in lower limb length and gait.
2. Methods
2.1 Participants and data screening
The sample is a subset of participants in the Fels Longitudinal Study, the world’s longest-running longitudinal study of human growth and development [19]. Participants are primarily of European descent and generally live in or near southwest Ohio. All participants in the sample were considered normal and healthy, and were not selected for any disease or gait-related trait. All procedures were approved by the Institutional Review Board at Wright State University, and participants provided informed consent before testing.
A total of 528 observations of walking gait were collected on 246 individual participants (128 females, 118 males) between the (rounded) ages of 8 and 30 years. Each individual had 1 to 6 independent observations (median=2). The minimum age was chosen because gait is well-characterized in children younger than 8 years old. The maximum age was selected so as to include enough adults to establish accurate estimates of mature gait parameters. Participants were excluded for prescription shoe inserts; chronic musculoskeletal conditions; toe-walking; lower limb, pelvic, or vertebral skeletal injury ≤ 5 years prior to testing; lower limb, pelvic, or back soft tissue injury ≤ 1 year prior to testing; obesity (affects gait [20,21]): children, body mass index (BMI) ≥95th percentile [22]; adults, BMI ≥ 30.0.
2.2 Data collection and processing
Data collection occurred at the Lifespan Health Research Center (LHRC), Wright State University Boonshoft School of Medicine. Anthropometric measurements, taken using standard methods [23,24] on barefoot participants barefoot wearing light clothing, included stature (to nearest 0.1 cm; stadiometer), body weight (to nearest 0.1 kg; scale), sitting height (to nearest 0.1 cm; stadiometer and chair), and bicristal breadth (to nearest 0.1 cm; sliding caliper, Holtain, Ltd.). Body mass index (BMI) was calculated as kg/m2. Lower limb length (cm) was calculated as stature − sitting height.
For walking tests, participants wore socks and walked at self-selected normal velocity along a 15m walkway in the LHRC’s Motion Analysis Laboratory. The laboratory is equipped with six high-speed cameras (Motion Analysis Corp., Santa Rosa, CA) directed at the walkway and synchronized with three embedded force plates (two AMTI OR6-7-1000, Advanced Mechanical Technology, Inc., Watertown, MA; one Kistler Type 9281B11, Kistler Instruments, Winterthur, Switzerland). Cameras captured the movement of external passive reflective markers placed on each participant at major joints and body segments according to the Helen Hayes Marker System [25]. Several trials were recorded and the best three trials (clean force plate strikes for both feet, high-fidelity marker recognition) were entered into OrthoTrak software (Motion Analysis Corp., Santa Rosa, CA) to extract forward velocity (cm/s); cadence (steps/min); percent of the gait cycle spent in initial double support and single support; step width (cm); and step length (cm). For each variable, participant averages across three trials were used in the analysis.
2.3 Statistical analysis
All analyses were performed using SAS version 9.2 (SAS Inc., Cary, NC, USA), and were two-sided with α=0.05 as the significance level. Sexes were analyzed separately. Gait variables were normalized by expressing temporal measures as percentages of a single gait cycle, adjusting spatial measures for lower limb length, and calculating base of support by dividing bicristal breath by step width [5,17,18]. These transformations removed the effects of lower limb length as a confounder of age effects, since, at any age, lower limb length influences raw values of gait parameters. Age was thus a proxy for degree of lower limb maturity, so that gait parameters could be compared between different maturational stages on a per-unit-of-lower-limb-length basis. We did not, however, normalize forward velocity, because our hypothesis specifically predicts that similarities in absolute velocity between immature and mature walkers, coupled with lower limb immaturity in the former, underlie differences in gait.
Descriptive statistics were computed for each variable and normality of distributions was assessed using the Shapiro-Wilk test. Rather than analyzing left and right limbs separately, participant-specific average values of both limbs were calculated for each variable. Prior to averaging, left and right limbs were compared (paired-sample t-test or Wilcoxon signed rank sum test, depending on distribution), with no significant asymmetry in any variable except for step length (females: P=0.04; males: P<0.01). The step length asymmetries, however, were not particularly meaningful, since they were ≤1% of sex-specific single-leg means (females: right=0.845, left=0.839; males: right=0.824, left=0.815). Thus the average of left and right step lengths was used, consistent with the other variables. For all variables, differences in means between age groups (defined below) and between sexes were assessed using two-sample t-tests.
Piecewise linear mixed models regression, fit using SAS PROC NLMIXED, was used to determine ages at lower limb length and gait variable maturity. The procedure estimated rates of change in lower limb length and gait variables, and the ages at which these rates became zero, providing an empirically-based estimation of the ages at which lower limb length and each gait parameter stabilized. Slopes after the ages at maturity were assumed to be zero; that is, it was assumed that lower limb length and gait parameters attained adult levels at a given age and then remained constant (within the age range of the sample). To check this assumption, the model was first allowed to include non-zero slopes before and after plateau ages. None of the post-maturity slopes, however, were significantly or meaningfully different from zero, validating our assumption. In contrast to other variables, the rate of change in forward velocity did not differ between childhood and adulthood: thus a simple linear fit was sufficient and obviated piecewise regression for forward velocity. As an iterative procedure, PROC NLMIXED relies on user-supplied starting values; a range of starting values was used to check the sensitivity of the analysis, and the results did not change.
3. Results
Sample characteristics for each sex subdivided by estimated ages at lower limb maturity are shown in Table 1. Sexes differed significantly (P≤0.05) for mean age at lower limb maturity: 13.2 ± 0.1 years in females; 15.6 ± 0.1 years in males. Numerous anthropometric and gait variable means also differed significantly between sexes, and between immature and mature age groups defined, respectively, as younger or older than the age at lower limb maturity (Table 1).
Table 1.
Sample characteristics: mean ± SD (range)
| Females (n=128) | Males (n=118) | |||||||
|---|---|---|---|---|---|---|---|---|
| Immature: <13.2 years | Mature: ≥13.2 years | Immature: <15.6 years | Mature: ≥15.6 years | |||||
| Age (years)*°† | 10.3 ± 1.5 | (7.7 – 13.1) | 19.7 ± 4.7 | (13.3 – 29.8) | 12.0 ± 2.2 | (7.8 – 15.3) | 20.2 ± 3.8 | (15.8 – 29.5) |
| Weight (kg)*°†‡ | 40.3 ± 12.4 | (18.4 – 77.4) | 61.6 ± 9.9 | (44.1 – 96.5) | 44.7 ± 16.1 | (21.0 – 90.7) | 72.3 ± 12.0 | (45.6 – 118.5) |
| BMI (kg/m2)*° | 18.8 ± 3.4 | (12.4 – 28.3) | 22.5 ± 3.4 | (14.7 – 33.1) | 18.9 ± 3.7 | (13.4 – 28.6) | 22.3 ± 3.1 | (15.2 – 29.5) |
| Stature (cm)*°†‡ | 144.5 ± 12.2 | (117.8 – 179.9) | 165.4 ± 7.0 | (150.1 – 185.0) | 151.4 ± 15.4 | (119.0 – 184.5) | 179.8 ± 7.1 | (159.4 – 200.6) |
| Sitting height (cm)*°†‡ | 76.1 ± 5.7 | (63.4 – 92.9) | 88.1 ± 3.9 | (78.6 – 98.4) | 78.8 ± 7.4 | (64.1 – 96.5) | 94.1 ± 3.8 | (84.9 – 103.6) |
| Lower limb length (cm)*°†‡ | 68.4 ± 6.9 | (54.4 – 87.1) | 77.2 ± 4.2 | (68.6 – 89.9) | 72.6 ± 8.4 | (53.5 – 92.1) | 85.7 ± 4.3 | (74.6 – 97.0) |
| Forward velocity (cm/s)* | 127.3 ± 13.1 | (100.1 – 164.3) | 123.0 ± 15.1 | (88.9 – 156.6) | 123.6 ± 17.5 | (85.0 – 164.1) | 121.2 ± 14.7 | (89.6 – 162.6) |
| Normalized cadence*°†‡ | 0.567 ± 0.039 | (0.490 – 0.705) | 0.536 ± 0.034 | (0.424 – 0.607) | 0.549 ± 0.049 | (0.428 – 0.704) | 0.526 ± 0.037 | (0.453 – 0.619) |
| Initial double support time (%)*°† | 9.6 ± 1.4 | (6.9 – 13.6) | 11.5 ± 1.4 | (8.1 – 14.9) | 10.1 ± 1.7 | (6.2 – 14.1) | 11.8 ± 1.3 | (8.3 – 15.5) |
| Single support time (%)*°†‡ | 40.3 ± 1.4 | (36.3 – 43.2) | 38.5 ± 1.4 | (35.0 – 42.1) | 39.9 ± 1.6 | (36.0 – 43.9) | 38.1 ± 1.3 | (34.5 – 41.9) |
| Base of support*‡ | 2.53 ± 0.50 | (1.60 – 4.28) | 2.96 ± 0.66 | (1.81 – 5.58) | 2.48 ± 0.53 | (1.50 – 4.71) | 2.58 ± 0.63 | (1.44 – 4.56) |
| Normalized step length*°†‡ | 0.873 ± 0.067 | (0.733 – 1.046) | 0.830 ± 0.070 | (0.672 – 1.03) | 0.845 ± 0.080 | (0.645 – 1.049) | 0.793 ± 0.064 | (0.636 – 0.987) |
Means differ significantly between age groups within females (two-sample t-test; p≤0.01).
Means differ significantly between age groups within males (two-sample t-test; p≤0.01).
Means differ significantly between sexes within immature age class (two-sample t-test; p≤0.05).
Means differ significantly between sexes within mature age class (two-sample t-test; p≤0.05).
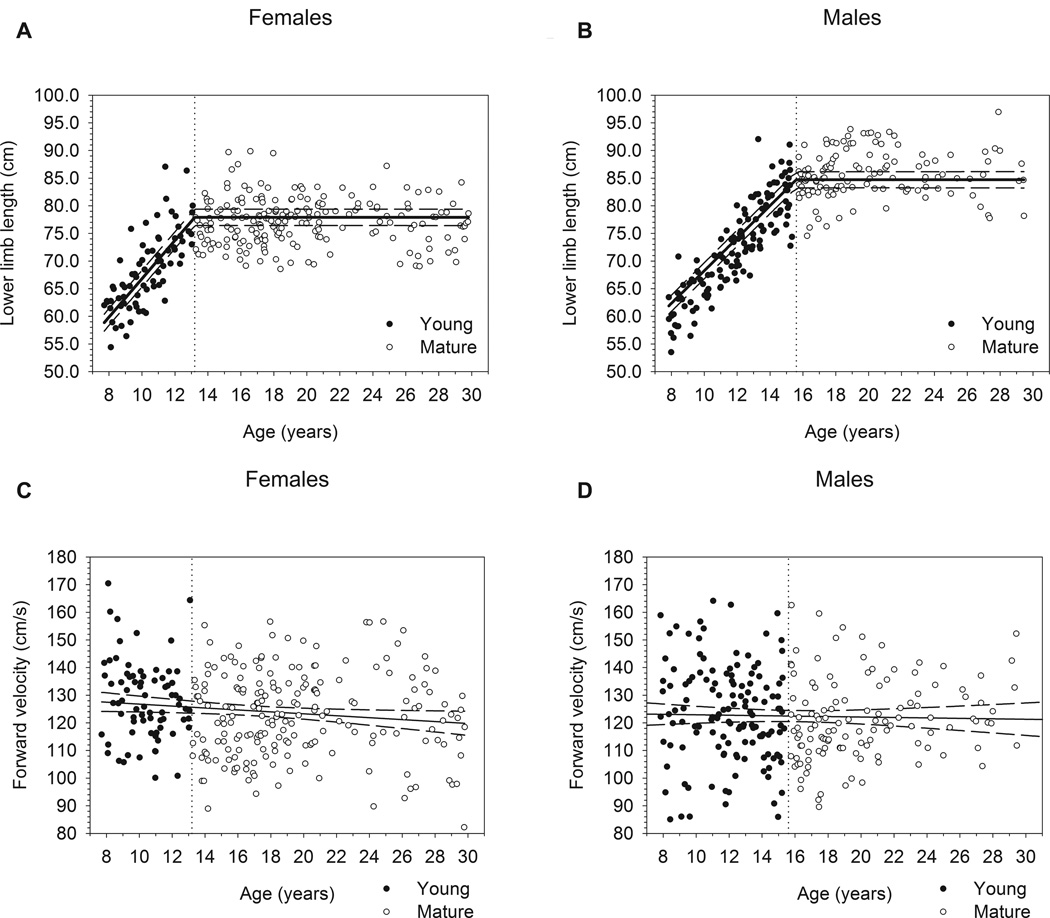
Piecewise regression statistical results are presented in Table 2. Slopes for the increase in lower limb length with age were significantly greater than zero (P≤0.01) in both immature females (b=3.470) and immature males (b=2.944), but did not differ significantly between sexes (P=0.06; see Fig. 1a–b). In males, forward velocity neither differed (on average) between immature and mature groups (P=0.25), nor did it change significantly with age (Fig. 1d; P=0.69). Although immature females on average walked 4.3 cm/s faster than the mature females as a group (P≤0.01), the relationship between age and velocity was not statistically significant (Fig. 1c; P=0.16).
Table 2.
Piecewise regression results prior to age at maturity
| Sex | Dependent Variable | Age (years) at maturity ± SE |
b ± sb | p | 95% C.I. of b | Average value after age at maturity ± SE |
|
|---|---|---|---|---|---|---|---|
| lower | upper | ||||||
| Females | Lower limb length | 13.2 ± 0.1 * | 3.470 ± 0.151 | <0.01 | 3.171 | 3.770 | 77.9 ± 0.4 *** |
| Normalized cadence | 14.2 ± 0.7 | −0.008 ± 0.002 | <0.01 | −0.012 | −0.005 | 0.536 ± 0.003 | |
| Initial double support time | 14.9 ± 0.6 * | 0.440 ± 0.057 ** | <0.01 | 0.328 | 0.552 | 11.6 ± 0.1 | |
| Single support time | 14.7 ± 0.6 * | −0.447 ± 0.060 ** | <0.01 | −0.567 | −0.328 | 38.4 ± 0.1 | |
| Base of support | 14.3 ± 1.2 | 0.092 ± 0.027 | <0.01 | 0.038 | 0.146 | 2.91 ± 0.1 *** | |
| Normalized step length | 12.5 ± 0.6 * | −0.019 ± 0.005 ** | <0.01 | −0.029 | −0.010 | 0.828 ± 0.006 *** | |
| Males | Lower limb length | 15.6 ± 0.1 | 2.944 ± 0.157 | <0.01 | 2.633 | 3.256 | 84.7 ± 0.5 |
| Normalized cadence | 14.1 ± 0.5 | −0.008 ± 0.002 | <0.01 | −0.012 | −0.004 | 0.530 ± 0.004 | |
| Initial double support time | 17.9 ± 0.9 | 0.270 ± 0.041 | <0.01 | 0.188 | 0.352 | 11.8 ± 0.2 | |
| Single support time | 17.9 ± 0.9 | −0.267 ± 0.036 | <0.01 | −0.339 | −0.195 | 38.2 ± 0.2 | |
| Base of support | 15.8 ± 1.5 | 0.059 ± 0.019 | <0.01 | 0.022 | 0.096 | 2.63 ± 0.1 | |
| Normalized step length | 16.4 ± 1.1 | −0.010 ± 0.002 | <0.01 | −0.015 | −0.005 | 0.797 ± 0.008 | |
Females differ significantly from males for age at maturity (p <0.01).
Females differ significantly from males for slope (p <0.05).
Females differ significantly from males for average value after age at maturity (p <0.01).
Fig. 1.
Relationships between lower limb length and age (a: females, b: males), and forward velocity and age (c: females, d: males) in this sample. Dotted vertical lines in plots (a) and (b) demarcate ages at maturity for females (13.2 years) and males (15.6 years), respectively. Plots (a) and (b) include regression lines (solid lines) and their 95% prediction intervals (dashed lines) for Immature and Mature subgroups. Plots (c) and (d) include regression lines fit across all ages in each sub-sample.
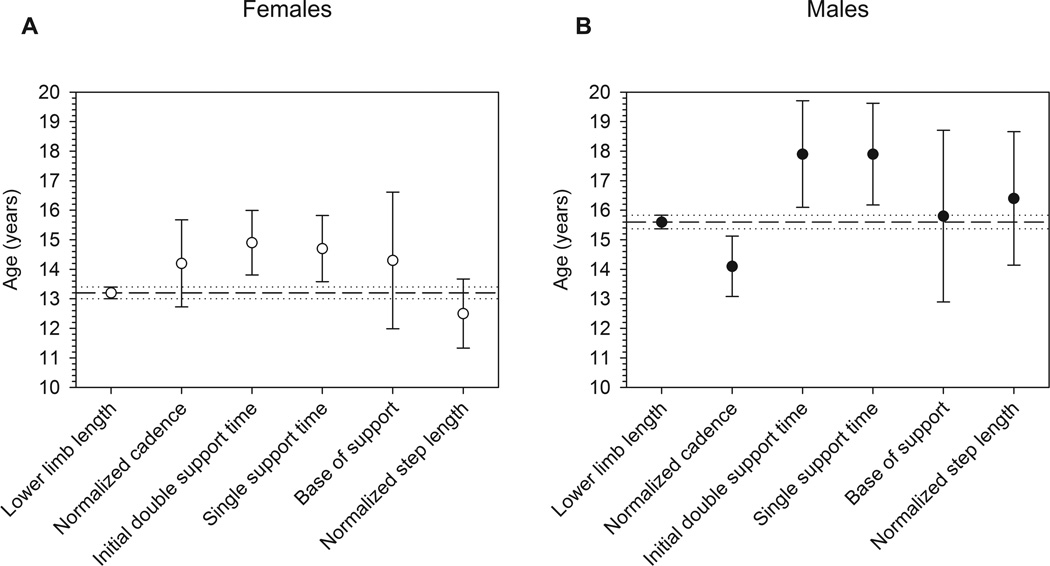
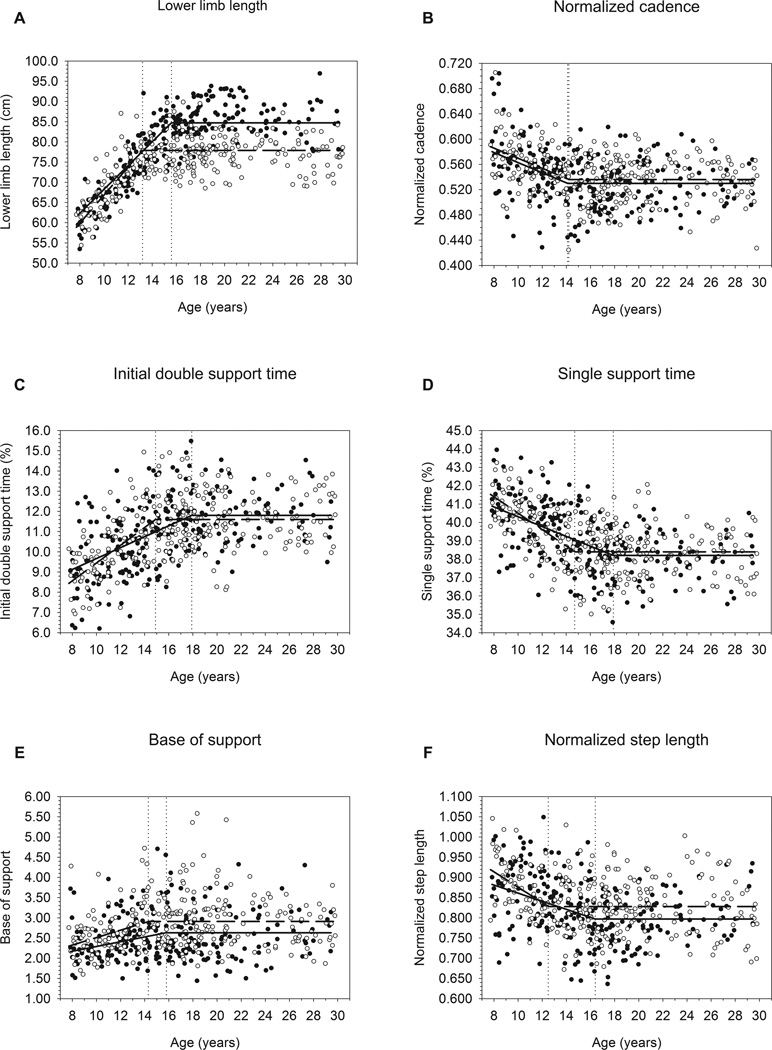
The remaining gait variables exhibited patterns analogous to lower limb length, with significant age-related changes occurring until reaching plateaus at variable-specific ages at maturity. Ages at maturity (± S.E.) for females and males, respectively (see Table 2 and Fig. 2), were as follows: normalized cadence: 14.2 ± 0.7, 14.1 ± 0.5; initial double support time: 14.9 ± 0.6, 17.9 ± 0.9; single support time: 14.7 ± 0.6, 17.9 ± 0.9; base of support: 14.3 ± 1.2, 15.8 ± 1.5; normalized step length: 12.5 ± 0.6, 16.4 ± 1.1. Slopes for all of these variables vs. age were significant prior to maturity, but did not differ significantly from zero after maturity (see Fig. 3). Slopes (± S.E.) for immature females and immature males were, respectively: normalized cadence: −0.008 ± 0.002, −0.008 ± 0.002; initial double support time: 0.440 ± 0.057, 0.270 ± 0.041; single support time: −0.447 ± 0.060, −0.267 ± 0.036; base of support: 0.092 ± 0.027, 0.059 ± 0.019; normalized step length: −0.019 ± 0.005, −0.010 ± 0.002. Sexes differed significantly for age at maturity and immature slopes for initial double support time, single support time, and normalized step length (P<0.05).
Fig. 2.
Mean ages at maturity with their 95% confidence intervals for lower limb length and gait variables in (a) females and (b) males, as derived from piecewise regression analysis.
Fig. 3.
Regression lines for (a) lower limb length, (b) normalized cadence, (c) initial double support time, (d) single support time, (e) base of support, and (f) normalized step length vs. age, comparing females and males. Dotted vertical lines demarcate ages at maturity for each sex, and cross their sex-specific regression lines at the flexion points between Immature and Mature age subgroups.
4. Discussion
Our results support the hypothesis that gait parameters continue to change past 7–8 years of age, maturing alongside continued lower limb growth. Lower limb length increased gradually with age in our sample up to sex-specific ages at maturity, 13.2 and 15.6 years in females and males, respectively. These values were quite similar to previously published ages at lower limb linear growth cessation [6], and the rates of change paralleled previously reported maturational changes in limb proportions and mass distribution during late childhood and early adolescence [26,27]. Sutherland and colleagues [5] listed five determinants of mature gait, which we hypothesized would remain immature prior to the lower limb reaching adult length: duration of single-limb stance, walking velocity, cadence, step length, and the ratio of pelvic span to ankle spread. In our sample, self-selected walking velocity did not change with age in children or adults, and was thus considered mature by 8 years of age. Because velocity was stable as the lower limbs continued to grow, the other four determinants of gait maturation all continued to change until roughly the age at lower limb length maturity.
The patterns of maturation and rates of change in these gait variables in immature subjects were gradual. Percentages of time spent in single and double support each changed by ~3% in both sexes from age 8 years until reaching their plateaus, and cadence decreased by approximately 10% from 8 years to its sex-specific plateaus. In females, step lengths decreased from ~94% of lower limb length at age 8 years, to ~83% of lower limb length at plateau age. In males, step length decreased from ~87% to ~80% of lower limb length from age 8 years to the mature plateau. Base of support narrowed (i.e. step width decreased relative to pelvic breadth) by ~25% in females and ~20% in males between 8 years old and the age at plateau.
There was considerable overlap in 95% confidence intervals for ages at maturity of the lower limb and for the gait variables, but average ages at maturity were offset from one another. Initial double support and single support times were delayed by 1.6–2.3 years relative to lower limb maturity in both sexes. Base of support matured at almost the same age as the lower limb in males, but was slightly delayed relative to the lower limb in females, possibly relating to female-specific patterns of pelvic widening during puberty [28].
Cadence matured just past 14 years old in both sexes, but was offset from lower limb maturity in opposite directions in females and males. In females, cadence matured after the lower limb. Because velocity is the product of step length and cadence, maintaining adult-like velocities at high, immature cadences requires shorter steps. Accordingly, normalized step length in females matured prior to cadence and lower limb length to produce adult-like velocity with immature cadence. Conversely, in males, cadence reached maturity prior to the lower limb: males took relatively fewer steps to maintain adult-like velocities while the lower limb still grew. Consistent with this offset, normalized step length in males remained immature until just past the age at lower limb maturity, so that males took longer steps for a given lower limb length to compensate for relatively early maturation of cadence. It is unclear from the present study what factors underlie these sex-specific gait-maturation patterns. Many sensorimotor functions related to coordination mature during early/mid-adolescence, with few sex differences [29,30], so it is conceivable that sensorimotor changes drive the sex-independent maturation of cadence. Meanwhile, step length depends more directly on lower limb length [14] and thus on sex differences in skeletal development. Factors such as these may generate the observed age at maturity offsets, but further research is needed to explore this possibility.
Broadly speaking, our results show that older children and early adolescents take relatively longer, wider, and more frequent steps than do mature adolescents and adults. These traits allow immature walkers to maintain adult-like velocities on shorter lower limbs, and are consistent with previous findings that both children and adults adjust cadence and stride length to accommodate faster walking speeds [13,15]. The age-related decrease in time spent in single support and the corresponding age-related increase in time spent in initial double support make sense, since higher cadences require greater single support and less double support [31]. As such, self-selected velocity in late childhood and early adolescence is nearer to the walk-run transition than in adults and mature adolescents, which, combined with relatively long and wide steps, likely translates to lower energetic efficiency of walking [32] prior to lower limb maturity.
Two limitations of this study call for further research to confirm our findings. First, participants wore socks during trials, whereas most studies analyze barefoot walking. Socks-wearing may induce a more cautious gait than in the barefoot condition, such that our subjects may walk more slowly than barefoot walkers. Although this difference may constrain direct comparisons between this and other studies’ data, the present study’s internal methodological consistency supports the validity of the results and our interpretation of them. Second, while we include longitudinal data for many participants, not every individual has repeated observations across the entire age range sampled. Thus, our findings are limited to population average patterns in maturation rates and ages at maturity. A truly longitudinal sample is needed to investigate individual-level variation in associations between gait parameters and lower limb maturational changes.
This study presents strong evidence that gait parameters continue to mature during late childhood and early adolescence as the lower limb continues to lengthen. It is clinically important to understand normal processes of age-related gait development in order to better diagnose pathological gait. The present study suggests that using adult norms for gait parameters may bias interpretations of gait in late childhood and early adolescence. Moreover, the retention of faster cadence and longer steps, and sex-specific patterns in gait maturation, may underlie or exacerbate risks for common lower limb musculoskeletal conditions of late childhood and early adolescence. The information presented here may also be useful for establishing normative targets for gait rehabilitation following childhood injury.
Supplementary Material
Research Highlights.
-
!
Children and adolescents walk with adult-like velocity despite immature lower limbs
-
!
Spatiotemporal aspects of gait remain immature while lower limb growth continues
-
!
Gait parameter ages at maturity are somewhat offset from lower limb age at maturity
-
!
Males and females differ in rates of change and ages at maturity of gait parameters
-
!
Immature gait patterns approach the walk-run transition at self-selected velocity
ACKNOWLEDGEMENTS
This work was supported by grants from Wright State University Boonshoft School of Medicine (gait testing and analysis) and National Institutes of Health R01HD012252 (anthropometry). Funding sources had no role in the study design, in the collection, analysis or interpretation of data, in the writing of this manuscript or in the decision to submit it for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no financial or personal relationships with other people or organizations that could inappropriately influence or bias their work.
Contributor Information
Andrew W. Froehle, Division of Morphological Sciences and Biostatistics, Lifespan Health Research Center, Department of Community Health, Boonshoft School of Medicine, Wright State University, 3171 Research Blvd., Dayton, OH 45420-4014, Phone: 937.775.1413, andrew.froehle@wright.edu.
Ramzi W. Nahhas, Division of Morphological Sciences and Biostatistics, Lifespan Health Research Center, Department of Community Health, Boonshoft School of Medicine, Wright State University, 3171 Research Blvd., Dayton, OH 45420-4014, Phone: 937.775.1421, ramzi.nahhas@wright.edu.
Richard J. Sherwood, Division of Morphological Sciences and Biostatistics, Lifespan Health Research Center, Department of Community Health and Department of Pediatrics, Boonshoft School of Medicine, Wright State University, 3171 Research Blvd., Dayton, OH 45420-4014, Phone: 937.775.1462, richard.sherwood@wright.edu.
Dana L. Duren, Division of Morphological Sciences and Biostatistics, Lifespan Health Research Center, Department of Community Health and Department of Orthopaedic Surgery, Boonshoft School of Medicine, Wright State University, 3171 Research Blvd., Dayton, OH 45420-4014, Phone: 937.775.1454, dana.duren@wright.edu.
References
- 1.Sutherland D. The development of mature gait. Gait & Posture. 1997;6:163–170. [Google Scholar]
- 2.Sutherland D, Olshen RA, Biden EN, Wyatt MP. The Development of Mature Walking. London: Mac Keith Press; 1988. [Google Scholar]
- 3.Dusing SC, Thorpe DE. A normative sample of temporal and spatial gait parameters in children using the GAITRite (R) electronic walkway. Gait & Posture. 2007;25:135–139. doi: 10.1016/j.gaitpost.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Holm I, Tveter AT, Fredriksen PM, Vollestad N. A normative sample of gait and hopping on one leg parameters in children 7–12 years of age. Gait & Posture. 2009;29:317–321. doi: 10.1016/j.gaitpost.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland DH, Olshen R, Cooper L, Woo SLY. The Development of Mature Gait. Journal of Bone and Joint Surgery-American Volume. 1980;62:336–353. [PubMed] [Google Scholar]
- 6.Dimeglio A. Growth in pediatric orthopaedics. Journal of Pediatric Orthopaedics. 2001;21:549–555. [PubMed] [Google Scholar]
- 7.Hof AL, Zijlstra W. Normalization of temporal-distance parameters in pediatric gait - Comment. Journal of Biomechanics. 1997;30:299. doi: 10.1016/s0021-9290(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 8.Cupp T, Oeffinger D, Tylkowski C, Augsburger S. Age-related kinetic changes in normal pediatrics. Journal of Pediatric Orthopaedics. 1999;19:475–478. doi: 10.1097/00004694-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ganley KJ, Powers CM. Gait kinematics and kinetics of 7-year-old children: a comparison to adults using age-specific anthropometric data. Gait & Posture. 2005;21:141–145. doi: 10.1016/j.gaitpost.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Menkveld SR, Knipstein EA, Quinn JR. Analysis of Gait Patterns in Normal School-Aged Children. Journal of Pediatric Orthopaedics. 1988;8:263–267. doi: 10.1097/01241398-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lythgo N, Wilson C, Galea M. Basic gait and symmetry measures for primary school-aged children and young adults. II: Walking at slow, free and fast speed. Gait & Posture. 2011;33:29–35. doi: 10.1016/j.gaitpost.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Lythgo N, Wilson C, Galea M. Basic gait and symmetry measures for primary school-aged children and young adults whilst walking barefoot and with shoes. Gait & Posture. 2009;30:502–506. doi: 10.1016/j.gaitpost.2009.07.119. [DOI] [PubMed] [Google Scholar]
- 13.Hillman SJ, Stansfield BW, Richardson AM, Robb JE. Development of temporal and distance parameters of gait in normal children. Gait & Posture. 2009;29:81–85. doi: 10.1016/j.gaitpost.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Norlin R, Odenrick P, Sandlund B. Development of Gait in the Normal-Child. Journal of Pediatric Orthopaedics. 1981;1:261–266. doi: 10.1097/01241398-198111000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Grieve DW, Gear RJ. Relationships Between Length of Stride Step Frequency Time of Swing and Speed of Walking for Children and Adults. Ergonomics. 1966;9 doi: 10.1080/00140136608964399. 379-&. [DOI] [PubMed] [Google Scholar]
- 16.Stansfield BW, Hillman SJ, Hazlewood ME, Robb JE. Regression analysis of gait parameters with speed in normal children walking at self-selected speeds. Gait & Posture. 2006;23:288–294. doi: 10.1016/j.gaitpost.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Hof AL. Letter to the editor: Scaling gait data to body size. Gait & Posture. 1996;4:222–223. [Google Scholar]
- 18.Stansfield BW, Hillman SJ, Hazlewood ME, Lawson AM, Mann AM, Loudon IR, Robb JE. Normalisation of gait data in children. Gait & Posture. 2003;17:81–87. doi: 10.1016/s0966-6362(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 19.Roche AF. Growth, Maturation and Body Composition: The Fels Longitudinal Study 1929–1991. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 20.Shultz SP, Browning RC, Schutz Y, Maffeis C, Hills AP. Childhood obesity and walking: guidelines and challenges. International Journal of Pediatric Obesity. 2011;6:332–341. doi: 10.3109/17477166.2011.590202. [DOI] [PubMed] [Google Scholar]
- 21.Shultz SP, Hills AP, Sitler MR, Hillstrom HJ. Body size and walking cadence affect lower extremity joint power in children's gait. Gait & Posture. 2010;32:248–252. doi: 10.1016/j.gaitpost.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. International. Journal of Obesity. 2005;29:1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 23.Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Publishers; 1988. [Google Scholar]
- 24.Chumlea WC, Wisemandle W, Guo SS, Siervogel RM. Relations between frame size and body composition and bone mineral status. American Journal of Clinical Nutrition. 2002;75:1012–1016. doi: 10.1093/ajcn/75.6.1012. [DOI] [PubMed] [Google Scholar]
- 25.Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GVB. Repeatability of Kinematic, Kinetic, and Electromyographic Data in Normal Adult Gait. Journal of Orthopaedic Research. 1989;7:849–860. doi: 10.1002/jor.1100070611. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RK. Body Segment Mass, Radius and Radius of Gyration Proportions of Children. Journal of Biomechanics. 1986;19:359–368. doi: 10.1016/0021-9290(86)90012-6. [DOI] [PubMed] [Google Scholar]
- 27.Jensen RK. Changes in Segment Inertia Proportions Between 4 and 20 Years. Journal of Biomechanics. 1989;22:529–536. doi: 10.1016/0021-9290(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 28.Moerman ML. Sex-Differences in Adolescent Growth of the Human Pelvis. American Journal of Physical Anthropology. 1982;57:211. [Google Scholar]
- 29.Quatman-Yates CC, Quatman CE, Meszaros AJ, Paterno MV, Hewett TE. A systematic review of sensorimotor function during adolescence: a developmental stage of increased motor awkwardness? British Journal of Sports Medicine. 2011;46:649–655. doi: 10.1136/bjsm.2010.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L, Sheehy A, Gasser T. Neuromotor development from 5 to 18 years. Part 1: timed performance. Developmental Medicine and Child Neurology. 2001;43:436–443. doi: 10.1017/s0012162201000810. [DOI] [PubMed] [Google Scholar]
- 31.Whittle MW. Gait Analysis. An introduction. Edinburgh: Butterworth-Heinemann; 2003. [Google Scholar]
- 32.Kuo AD, Donelan JM. Dynamic Principles of Gait and Their Clinical Implications. Physical Therapy. 2010;90:157–174. doi: 10.2522/ptj.20090125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.