Abstract
Aims
To evaluate the effects of intensive insulin therapy alone and with added pioglitazone on body weight, fat distribution, lean body mass (LBM) and liver-fat in type 2 diabetic patients.
Materials/Methods
25 insulin-treated, obese patients with type-2 diabetes were randomized to addition of pioglitazone 45mg(n=12) or placebo(n=13) and treated intensively for 12-16 weeks. DEXA /abdominal CT scans were performed before/after treatment. LBM, visceral/subcutaneous adipose tissue (VAT/SAT) and liver/spleen attenuation ratios were measured pre/post-treatment (a ratio<1 represents fatty liver).
Results
Intensive insulin alone and insulin+pioglitazone significantly improved glycemic control (7.8±0.3 to 7.2±0.3% and 7.6±0.3 to 7.1±0.4% respectively). Body weight gain was greater with insulin+pioglitazone (4.9±4.5Kg) versus insulin therapy alone (1.7±0.7Kg). SAT increased significantly with pioglitazone+insulin therapy (393.9±48.5cm2 to 443.2±56.7 cm2, p<0.01) compared to a non-significant increase with insulin therapy alone 412.9±42.5cm2 to 420.8±43.8cm2. VAT decreased non-significantly in both groups (240.3±41.7cm2 to 223.8±38.1cm2 with insulin+pioglitazone and 266.6±27.4cm2 to 250.5±22.2cm2 with insulin therapy). LBM increased significantly by 1.92±0.74 Kg with insulin+pioglitazone treatment. The liver/spleen attenuation ratio in the placebo+insulin group decreased from 1.08±0.1 to 1.04±0.1 (p=ns), and increased from 1.00±0.1 to 1.08±0.05 (p=0.06) in the pioglitazone+insulin group.
Conclusions
Intensification of insulin therapy in type 2 diabetic patients causes modest weight gain and no change in body fat distribution, LBM or liver fat. In contrast, the addition of pioglitazone, at equivalent glycemia, increases weight gain, fat mass and SAT; increases LBM and tends to decrease liver-fat. These changes in fat distribution may contribute to the beneficial effects of pioglitazone, despite greater weight gain.
Keywords: Insulin, pioglitazone, body fat composition, lean body mass, liver fat, glitazones, fat, type 2 diabetes
Introduction
Insulin and thiazolidinediones are commonly used in clinical practice to improve glycemic control. Both these agents effectively reduce blood glucose levels and concomitantly cause varying amounts of weight gain (1,2) The combination of insulin and a thiazolidinedione is usually associated with more weight gain than either agent alone. Although some of the weight gained in patients with diabetes and poor glycemic control can be attributed to the reduction in glucosuria with better glucose control (1), the majority of the weight gained with insulin and thiazolidinedione treatment is due to factors other than reduction in glucosuria. Since weight gain per se is usually associated with increased insulin resistance, it would appear paradoxical that agents which increase body weight are also able to improve insulin sensitivity and lower blood glucose levels. There is now growing evidence that in addition to an absolute increase in body weight, the relative distribution of body fat in various body compartments (subcutaneous and visceral adipose tissue depots and liver fat) plays an important role in insulin resistance. Visceral adipose tissue (VAT) is associated with insulin resistance and the development of type 2 diabetes and atherosclerosis (3,4,5). Liver fat has also been found to correlate with hepatic insulin resistance, independent of overall and intra-abdominal obesity (6). Several studies have demonstrated that the weight gain associated with the thiazolidinediones is mainly due to an increase in subcutaneous adipose tissue and a concomitant decrease in visceral adipose tissue and liver fat (7-10). There is less data available on the effects of intensive insulin therapy on body fat distribution and liver fat content. In studies to date, intensive insulin therapy has not had any significant change in the size of the subcutaneous and visceral fat depots in patients with type 2 diabetes (11,12). However, intensive insulin therapy has been shown to decrease liver fat and improve insulin sensitivity (11). In the present study, we sought to determine the effects of intensive insulin therapy alone versus combined insulin+pioglitazone therapy on weight gain, fat distribution, lean body mass and liver fat in patients with type 2 diabetes. To our knowledge, this is the first randomized, placebo-controlled trial examining body fat distribution with intensification of insulin treatment alone compared with the addition of pioglitazone to intensive insulin treatment.
Research Design and Methods
After approval from the University of California Human Research Protections Program, we randomized 25 obese subjects with a mean age 58±2 years (21 men, 4 women), mean BMI 36.7±1.8 kg/m2 and HbA1c values between 7.5 and 10% on insulin therapy alone to receive either placebo or pioglitazone. Subjects were excluded if they had a history of peripheral edema, cardiac, hepatic or renal problems.
After performing the baseline studies described below, subjects were randomized to either insulin + pioglitazone 45mg (n=12) or insulin + placebo (n=13) and treated intensively for a minimum of 12 weeks and a maximum of 16 weeks depending on the availability of subjects to do the follow-up studies. There was no difference in the characteristics of the subjects who were treated for 12 weeks versus 16 weeks. Patients were assessed weekly for the first month and bi-weekly thereafter by a physician to evaluate glycemic response. Frequent adjustments were made to the insulin regimen to achieve a fasting plasma glucose of approximately 100 mg/dL and a postprandial glucose of <180 mg/dL. Subjects also met with a dietician every two weeks to encourage weight maintenance.
Abdominal Fat Depot Measurements
Each patient had a CT scan before and after treatment at the levels of T12-L1 for optimal liver and spleen views (13), and L3-L4 for optimal subcutaneous and visceral adipose tissue measurements. The level of L3-L4 has been highly correlated in the literature to approximate total visceral adipose tissue volume (14). Scanning was performed at 120kV with a slice thickness of 10 mm at the VA Medical Center CT scanning facility. Exposure and scanning time was one second for the abdomen and 2 seconds for the liver. Subjects were scanned supine with their feet in a neutral, slightly plantar flexed position and with arms stretched over their heads for minimization of artifact. The scanner was calibrated with water at room temperature of 20 degrees Celsius before each examination (Toshiba Aquilion 4-slice multi-slice scanner, Toshiba America Medical Systems, Tustin, CA, 2000). Adipose tissue was calculated using the program Image J set to a threshold of −190 and −30 Hounsfield Units for fat (14). The total abdominal wall circumference and area was calculated using automated tools through Image J (Version 1.36b, National Institutes of Health, USA). The intra-abdominal circumference and area were manually traced and the difference between the total abdominal area and the intra-abdominal area yielded the area of subcutaneous adipose tissue. Visceral fat was calculated by direct measurement of visceral fat within the intra-abdominal cavity. The individual using Image J to make the visceral and subcutaneous fat calculations was blinded to the assignment of study subjects. Additionally, in order to ensure accuracy and reproducibility, five images were selected at random and subcutaneous and visceral fat were recalculated. The coefficient of variance between these measurements and the original measurements was <5%.
Liver/Spleen Attenuation
Liver fat was calculated by assessing liver and spleen attenuation values. Liver attenuation on imaging (CT or Hounsfield unit values – [HU]) varies significantly depending upon its chemical content (storage of glycogen, fat, or iron). In contrast, the spleen is relatively metabolically inactive without large fluctuations in chemical content. However, the spleen lies at approximately the same transverse level as the liver and is affected similarly by CT artifact and hence serves as an internal norm for liver (15). The attenuation or CT value is also a marker of tissue density and carries an inverse relationship to fat: the higher the attenuation value, the greater the density of tissue, and hence the lower the fat content (16). In a normal liver, the liver CT number should always be higher than the spleen with a liver to spleen attenuation ratio of greater than one. Conversely, a fatty liver is represented by a liver to spleen attenuation ratio of less than one. To assess the liver to spleen ratio, six regions of interest were identified within the liver, three on the medial aspect, and three on the lateral aspect. The mean HU within these six regions of interest were averaged to yield a mean liver attenuation value. A similar technique was used for the spleen with six regions of interest identified. The mean HU within these six regions of interest were averaged to yield a mean spleen attenuation value for each patient before and after treatment. The liver/spleen attenuation ratio was then calculated with a ratio of <1 representing fatty liver (13,16).
Body Composition by DEXA
A dual-energy X-ray absorptiometry (DEXA) scan was completed before and after the study for evaluation of body composition on each study subject using the Hologic Delphi W (Serial Number 70872) with analysis software version 11.2. The DEXA scanner performs a series of transverse scans of the patient from head to toe at 1cm intervals. The average skin radiation exposure per scan is 1-3mrad. The results are presented as kg for lean body mass and fat mass. DEXA yields accurate measurements for body composition, and is the preferred method for such estimations (17). Both radiation dose and precision error with DEXA are low. The precision error (SD) of DEXA measurements is 425g for both fat and fat-free mass (18).
Statistical Analysis
All results are expressed as mean ±SEM. We performed between-group comparisons using non-paired Student’s t test and within-group comparisons by paired t tests. All t tests were two tailed and p<0.05 was considered statistically significant.
Results: (Table 1)
Table 1.
Body Weight and Fat Distribution at baseline and after 12-16 weeks treatment with Insulin + Pioglitazone or Insulin Only Therapy
| Intensive Insulin + Pioglitazone Group | Intensive Insulin Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Treatment |
Change from Baseline |
p value |
Baseline | Post Treatment |
Change from Baseline |
p value | p value Between Groups |
|
| HbA1c (%) | 7.6±0.3 | 7.1±0.4 | −0.5 | <0.05 | 7.8±0.3 | 7.2±0.3 | −0.6 | <0.05 | ns |
| Insulin dose (units) | 105±22 | 92±19 | −13±9 | ns | 114±11 | 127±16 | 13±8 | ns | <0.05 |
| Weight (Kg) | 108.4±5 | 113.3±5 | 4.9±4.5 | <0.01 | 110.0±5 | 111.7±6 | 1.7±0.7 | 0.02 | <0.01 |
| BMI (Kg/m2) | 35.2±1.4 | 36.6±1.4 | 1.5±0.2 | <0.01 | 38.2±1.7 | 38.7±1.7 | 0.5±0.2 | <0.05 | <0.01 |
| CT Scan measurements | |||||||||
| Subcutaneous Adipose Tissue (cm3) |
393.9±48.5 | 443.2±56.7 | 49.3±10.1 | <0.01 | 412.9±42.5 | 420.8±43.8 | 7.9±7.4 | ns | <0.01 |
| Visceral Adipose Tissue (cm3) |
240.3±41.7 | 223.8±38.1 | −16.5±8.6 | ns | 266.6±27.4 | 250.5±22.2 | −16.1±11.0 | ns | ns |
| VAT/SAT | 0.72±0.20 | 0.59±0.1 | −0.13±0.04 | 0.01 | 0.77±0.12 | 0.70±0.1 | −0.07±0.04 | ns | ns |
| Liver/Spleen Attenuation | 1.00±0.1 | 1.08±0.05 | 0.08±0.04 | 0.06 | 1.08±0.1 | 1.04±0.1 | −0.04±0.04 | ns | ns |
| DEXA measurements (Kg) | |||||||||
| Total Mass | 105.27±4.14 | 109.68±4.05 | 4.41±0.56 | <0.01 | 103.93±4.75 | 105.65±4.72 | 1.72±0.66 | 0.02 | <0.01 |
| Fat Mass | 34.82±2.63 | 37.33±2.75 | 2.51±0.54 | <0.01 | 37.38±3.16 | 37.80±2.98 | 0.42±0.66 | ns | 0.02 |
| Lean Mass | 67.60±2.81 | 69.52±2.79 | 1.92±0.74 | 0.02 | 63.96±3.07 | 65.25±3.25 | 1.29±0.61 | 0.057 | ns |
| Bone Mineral Content | 2.84±0.14 | 2.81±0.13 | −0.03±0.02 | ns | 2.59±0.12 | 2.59±0.12 | 0.00±0.02 | ns | ns |
| % Body Fat | 32.83±1.83 | 33.80±1.89 | 0.97±0.51 | 0.08 | 37.08±2.53 | 35.56±1.98 | −1.52±1.44 | ns | ns |
Glycemic control and Insulin Use
At the end of the study, as reported previously (19), there were similar, significant decreases in HbA1C in both the study groups, 7.8± 0.3 to 7.2± 0.3% in the insulin only group and 7.6±0.3 to 7.1% ±0.4 with insulin plus pioglitazone (p<0.05 for both groups). To achieve equivalent glycemia, the intensive insulin treatment group needed 9% more insulin - 114±11 to 127±16 units (p=ns), whereas the addition of pioglitazone reduced the insulin dose requirement by 8%, 105±22 to 92±19 units (p=ns). Although the within group difference in insulin dose pre and post-treatment was not statistically significant, the between group difference in insulin dose of 26 units was statistically significant (p<0.05).
Body Weight
As expected and previously reported (19), body weight increased significantly in both treatment groups. The increment in body weight was greater at 4.9±4.5 Kg in the pioglitazone group versus 1.7±0.7 Kg in the insulin only group (between group difference p <0.01).
CT Scan Abdominal Adipose Tissue and Liver/Spleen Attenuation Ratios
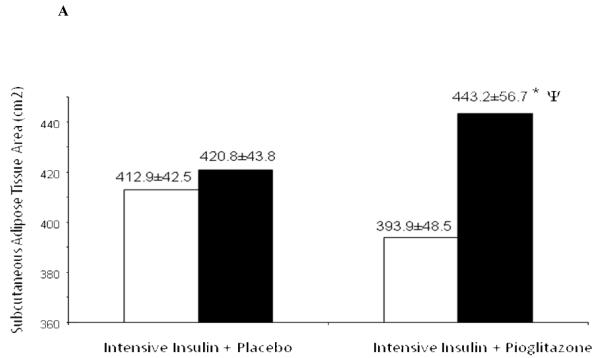
At study end, abdominal subcutaneous adipose tissue (SAT) measured at the L3-L4 level, increased only marginally by about 2% in the intensive insulin group from 412.9±42.5 to 420.8±43.8 cm2. However, the addition of pioglitazone to intensive insulin therapy increased SAT by nearly 13% from 393.9±48.5 to 443.2±56.7 cm2 (p<0.01) Figure 1A. As compared to subcutaneous adipose tissue, visceral adipose tissue measured at the same level did not change significantly and decreased by about 6-7% in both groups (266.6±27.4 to 250.5±22.2 cm2 with insulin alone and 240.3±41.7 to 223.8±38.1 with added pioglitazone, p=ns for both). However, the ratio of VAT to SAT improved significantly with a decrease from 0.72±0.2 to 0.59±0.1 (p=0.01) with insulin plus pioglitazone as compared to a non-significant decrease from 0.77±0.1 to 0.70±0.1 with intensive insulin therapy alone. Liver fat content as measured by the Liver/Spleen attenuation ratio did not change with either intensive insulin therapy (1.08±0.1 to 1.04±0.1, p=ns) or with added pioglitazone (1.00±0.1 to 1.08±0.05, p=0.06). However, the trend in the increase in the liver/spleen attenuation ratio with insulin plus pioglitazone treatment is suggestive of a decrease in liver fat with this therapy. In one study, liver fat content derived by a calibrated CT measurement was correlated with the liver-to-spleen ratio (L/S ratio) obtained by CT scan and these two parameters were linked by a negative linear correlation with a correlation coefficient of -0.86 (p<0.001). In this study, an L/S ratio of 1.0 was equal to a liver fat content of approximately 6% and a ratio of 1.08% was equal to a liver fat content of approximately 4%.
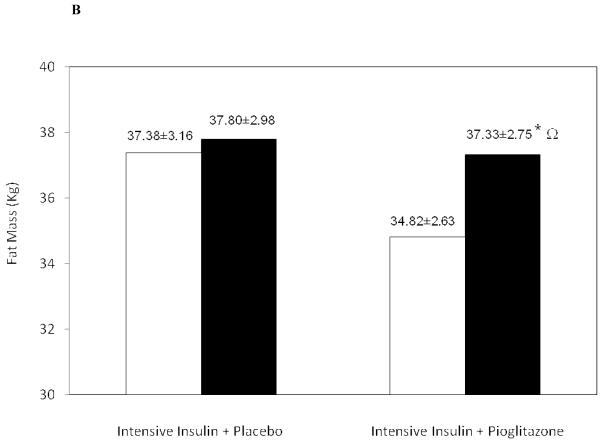
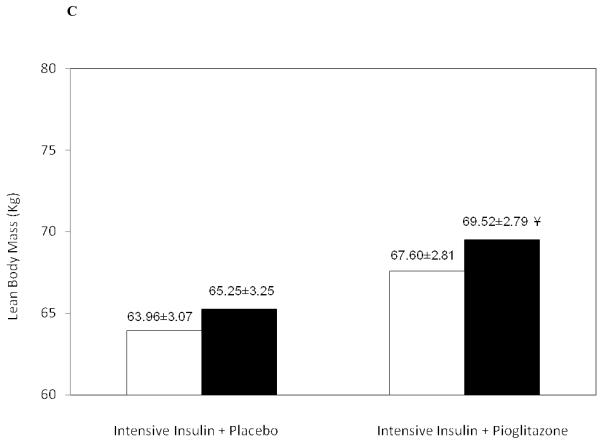
Figure 1.
Increase in (A) Subcutaneous Adipose Tissue, (B) Fat Mass and (C) Lean Body Mass in the Intensive Insulin + Placebo group and the Intensive Insulin + Pioglitazone group.
◻ Pre-Treatment, ∎ Post-Treatment
* p <0.01 pre and post-treatment
Ψ p <0.01 between groups post-treatment
Ω p=0.02 between groups post-treatment
¥ p=0.02 pre and post-treatment
DEXA Lean Body Mass and Body Fat Mass
The increase in body mass (measured on the day of the DEXA scan) was greater at 4.41±0.56 Kg in the pioglitazone group versus 1.72±0.66 Kg in the insulin only group (between group difference p <0.01). Of this increase in total body mass, fat mass accounted for 0.42±0.66 Kg in the insulin alone group (p=ns) and 2.51 Kg±0.54 with added pioglitazone (p<0.01) Figure 1B. Lean body mass also increased in both treatment groups, +1.29±0.61 Kg with intensive insulin treatment alone (p=0.057) and +1.92±0.74 Kg with added pioglitazone (p=0.02) Figure 1C. Of note, Percent body fat measured by DEXA declined from 37.08±2.53% to 35.56±1.98% with intensive insulin treatment alone (p=ns) and increased from 32.83±1.83% to 33.80±1.89 % with added pioglitazone (p=0.08).
Discussion
In this study, we have demonstrated that in patients with type 2 diabetes who are inadequately controlled on insulin therapy, intensification of insulin therapy alone compared to intensification of insulin therapy combined with the addition of pioglitazone, requires a greater dose of insulin (114±11 vs 92±19 units/day) and is associated with less weight gain (1.7±0.7 Kg versus 4.9±4.5 Kg), for the achievement of the same level of glycemia. In addition, while intensification of insulin therapy alone is only associated with a trend towards an increase in LBM and no change in body fat distribution or liver fat accumulation, at equivalent glycemia, the intensification of insulin therapy combined with the addition of pioglitazone is associated with increased LBM, increased SAT, decreased VAT, and a trend towards decreased liver fat. To our knowledge, thisis the first study to evaluate body fat distribution, body composition, and liver fat with intensive insulin/pioglitazone therapy in a randomized, double-blind, placebo-controlled study.
Body fat distribution, especially in the visceral adipose tissue compartment is believed to play an important role in the development and progression of insulin resistance, type 2 diabetes and its macrovascular complications. Our study demonstrates that intensification of insulin therapy alone in a group of obese patients with type 2 diabetes, causes modest weight gain and does not increase abdominal fat deposition in the SAT and VAT depots. These results are in agreement with the findings of Juurinen et al who found that intensive insulin therapy in their patients with type 2 diabetes did not increase fat deposition in SAT or VAT depots. (11). Our study differs from that of Juurinen in that subjects in that study were uncontrolled on baseline metformin therapy, whereas in our study, the patients were uncontrolled on baseline insulin therapy alone and were not on any metformin treatment. Juurinen et al treated their patients for 7 months, whereas the patients in our study were studied for a shorter period. Our findings are also in agreement with those of Elsayed et al, who evaluated 26 patients with type 2 diabetes at baseline and 12 months after instituting insulin therapy. In this observational study, intensive insulin therapy for a year resulted in a decrease in HbA1C from 9% to 7.5% and an increase in BMI from 28.9 to 29.8 kg/m2, with no change in SAT or VAT as assessed by CT scans (12). In that study however, details were not provided regarding the baseline oral agent therapy in these subjects, except that they were not on thiazolidinediones or steroids.
The findings of our study also confirm the findings in the literature regarding the effects of combined insulin and thiazolidinedione therapy on body fat distribution in patients with type 2 diabetes. In a study in 14 patients with poorly controlled type 2 diabetes on large doses of insulin, the addition of rosiglitazone 8 mg/day for 8 months, resulted in an improvement in the HbA1C from 8.9% to 7.8%, a reduction in insulin requirements from 218 to 129 units/day, an increase in body weight from 111 to 114 Kg (+ 3Kg), an increase in SAT and no change in VAT (10). In our study, we used pioglitazone instead of rosiglitazone, achieved a lower HbA1C target of 7.1%, noted lesser reduction in insulin requirements from 105 to 92 units, a greater increase in body weight from 108.4 to 113.3Kg (+ 4.9 kg) and treated our patients for a shorter period. This finding of no change in VAT with intensive insulin plus pioglitazone therapy may help ameliorate some of the concerns regarding the adverse metabolic effect of undue weight gain with thiazolidinedione therapy, especially when used in combination with insulin.
In addition to VAT, increased liver fat could be important in the pathogenesis of type 2 diabetes and has been found to correlate with hepatic insulin resistance, independent of overall and intra-abdominal obesity (6). Several investigators have already demonstrated that thiazolidinedione treatment decreases liver fat (21, 22). However, there are few studies documenting the effects of intensive insulin therapy alone and with added thiazolidinedione on liver fat. Our finding that the addition of pioglitazone to intensive insulin therapy is potentially associated with a decrease in liver fat confirms the findings of Juurinen et al. In their study (using insulin plus rosiglitazone) described above, in addition to finding no change in SAT and VAT with intensive insulin and rosiglitazone therapy, the investigators also noted a significant reduction in liver fat and an improvement in hepatic insulin sensitivity. The main difference is that Magnetic Resonance Spectroscopy was used to study liver fat in that study, while in our study, we used CT scans to study liver fat content. There is a strong correlation between whole-body adipose tissue determinations using results from CT scans, MRI-based scans and DEXA (23).
It is important that in our study, there was no change in liver fat with intensive insulin therapy alone. This finding is discordant with the demonstration of an improvement in liver fat with intensification of insulin therapy in another study (11). However, in that study, glycemic control was improved with intensive insulin treatment in the background of baseline metformin treatment, whereas in our study, the patients were not on any metformin or other oral agent therapy. It should also be mentioned that the patients in the other study were treated for 7 months, whereas the patients in our study were treated for a shorter period. It is possible that a longer period of treatment with intensive insulin may improve hepatic liver fat.
In regards to body mass changes as measured by DEXA, our study is the first study to evaluate the effects of intensive insulin alone and with added pioglitazone on lean body mass and fat mass. In our study, improvement of glycemia with intensification of insulin treatment in patients with diabetes resulted in a significant increase in total body mass of 1.72±0.66 Kg, comprising an increase in fat mass of 0.42±0.66 Kg and an increase in lean body mass of 1.29±0.61 Kg. Thus, with intensification of insulin treatment, most of the increase in body mass (about 75%) occurs in the lean body mass compartment. On the other hand, the addition of pioglitazone to intensive insulin therapy (at equivalent glycemia), not only results in a greater increase in body mass of 4.41±0.56 Kg, and an increase in body fat of 2.51±0.54 Kg, but also a significant increase in lean body mass of 1.92±0.74 Kg. These results suggest that the addition of pioglitazone to intensive insulin treatment not only increases body fat as expected, but also results in an increase in lean body mass, which contributes to about 44% of the total increase in body mass. Our findings corroborate the findings in the literature demonstrating a significant increase in lean body mass with pioglitazone therapy in non-diabetic PCOS subjects (24) and subjects with impaired glucose tolerance (25). In diabetic subjects, Smith et al noted a non-significant trend towards increased lean body mass with pioglitazone therapy (26).
A limitation of the present study is the small number of study subjects and the duration of the study. A larger sample size and a longer duration of the study may have clarified further the study parameters. Since this was a single-center study, the logistics of closely following a large number of subjects who needed close monitoring of the blood glucose levels and frequent changes in insulin dose requirements did not permit us to recruit a larger sample size and follow them for a longer period of time. However, a major strength of this study is that it is a randomized, double-blind, placebo controlled study in which we evaluated the effects of intensive insulin therapy alone and with added pioglitazone, on several parameters of body fat distribution, in the absence of the confounding effect of any other oral anti-diabetic medication.
In conclusion, in patients with type 2 diabetes who are inadequately treated with insulin, the intensification of insulin therapy alone, compared with the intensification of insulin therapy and the combination of pioglitazone, requires larger doses of insulin, but is associated with less weight gain. In addition, intensification of insulin therapy alone is not associated with any change in liver fat accumulation or body fat distribution (except a trend towards increased LBM), whereas, intensification of insulin therapy combined with the addition of pioglitazone, at equivalent glycemia, is associated with increased SAT, decreased VAT, increased LBM and a trend towards decreased liver fat.
Acknowledgements
This study was funded by the Veterans Medical Research Foundation, Department of Veteran Affairs and the VA San Diego Healthcare System, the University of California San Diego General Clinical Research Center NIH Grant MO1 RR00827 and Takeda Pharmaceuticals, North America, Inc.
Footnotes
Disclosure Summary: Robert Henry and Vanita Aroda received research grant support from Takeda Pharmaceuticals.
Conflict of Interest Study design: Sunder Mudaliar, Robert Henry, Anna Chang, Vanita Aroda Conduct/data collection: Priya Shah, Sunder Mudaliar, Anna Chang, Vanita Aroda, Michael Andre, Paivi Burke, Robert Henry Analysis: Priya Shah, Sunder Mudaliar, Anna Chang, Vanita Aroda, Michael Andre, Robert Henry Writing manuscript: Priya Shah, Sunder Mudaliar, Anna Chang, Vanita Aroda, Robert Henry No competing interests for all authors.
References
- 1.Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;65(Suppl 1):S23–7. doi: 10.1016/j.diabres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 3.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–8. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 4.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284(6):E1065–71. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 6.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87(7):3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 7.Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care. 1999;22(2):288–93. doi: 10.2337/diacare.22.2.288. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Funahashi T, Yamashita S, Nishida M, Nishida Y, Takahashi M, Hotta K, Kuriyama H, Kihara S, Ohuchi N, Nishimura T, Kishino BI, Ishikawa K, Kawamoto T, Tokunaga K, Nakagawa C, Mineo I, Watanabe F, Tarui S, Matsuzawa Y. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation--double-blind placebo-controlled trial. Diabetes Res Clin Pract. 2001;54(3):181–90. doi: 10.1016/s0168-8227(01)00319-9. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87(6):2784–91. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 10.Juurinen L, Kotronen A, Granér M, Yki-Järvinen H. Rosiglitazone reduces liver fat and insulin requirements and improves hepatic insulin sensitivity and glycemic control in patients with type 2 diabetes requiring high insulin doses. J Clin Endocrinol Metab. 93(1):118–24. doi: 10.1210/jc.2007-1825. [DOI] [PubMed] [Google Scholar]
- 11.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E829–35. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]
- 12.Elsayed A, ElGebely S, Galal A. Insulin therapy induced adiposity evaluated by computed tomography is no visceral. Pak J Med Sci. 2007;23(2):161–166. [Google Scholar]
- 13.Davidson LE, Kuk JL, Church TS, Ross R. Protocol for measurement of liver fat by computed tomography. J Appl Physiol. 2006;100(3):864–8. doi: 10.1152/japplphysiol.00986.2005. [DOI] [PubMed] [Google Scholar]
- 14.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord. 1993;17(4):187–96. [PubMed] [Google Scholar]
- 15.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137(3):727–9. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 16.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285(4):E906–16. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 17.Prior BM, Cureton KJ, Modlesky CM, Evans EM, Sloniger MA, Saunders M, Lewis RD. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol. 1997;83(2):623–30. doi: 10.1152/jappl.1997.83.2.623. [DOI] [PubMed] [Google Scholar]
- 18.Kelly TL, Berger N, Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49(5-6):511–3. doi: 10.1016/s0969-8043(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 19.Mudaliar S, Chang AR, Aroda VR, Chao E, Burke P, Baxi S, Griver KA, O’Connor DT, Henry RR. Effects of intensive insulin therapy alone and with added pioglitazone on renal salt/water balance and fluid compartment shifts in type 2 diabetes. Diabetes Obes Metab. 2010;12(2):133–8. doi: 10.1111/j.1463-1326.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 20.Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, Crocè LS, Paoletti S, de Bernard B, Tiribelli C, Dalla Palma L. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997 Jul;27(1):108–13. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, Bray GA, Greenway FL, Martin CK, Johnson WD, Smith SR. Pioglitazone, but not metformin, reduces liver fat in Type-2 diabetes mellitus independent of weight changes. J Diabetes Complications. 2010;24(5):289–96. doi: 10.1016/j.jdiacomp.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53(8):2169–76. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 23.Longo R, Ricci C, Masutti F, Vidimari R, Crocé LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993 Apr;28(4):297–302. [PubMed] [Google Scholar]
- 24.Ibáñez L, López-Bermejo A, Díaz M, Enríquez G, Del Río L, De Zegher F. Low-dose pioglitazone, flutamide, metformin plus an estro-progestagen for non-obese young women with polycystic ovary syndrome: increasing efficacy and persistent safety over 30 months. Gynecol Endocrinol. 2010 May 26; doi: 10.3109/09513590.2010.487589. 2010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Rasouli N, Raue U, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288(5):E930–4. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. 2005;54(1):24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]